Abstract
Focal segmental glomerulosclerosis (FSGS) is a glomerular disease characterized by proteinuria, frequent progression to end-stage renal disease, and recurrence after kidney transplantation in ~25% of patients, which negatively impacts long-term allograft survival. Experimental studies suggest that abnormalities in T and, possibly, B cells may represent one initial pathogenic trigger, leading to podocyte injury and progressive loss. New data also support the existence of circulating permeability factors able to damage the podocytes, but no single molecule has been consistently identified as the causal pathogenic element in FSGS recurrence. Unfortunately, major progress from mechanistic studies has not translated into substantial advancements in patient treatment, with plasmapheresis (PP) and high doses of cyclosporine (CsA) remaining the mainstays of therapy. Despite consistent experimental and clinical evidence that treatment of proteinuria slows renal function decline in proteinuric nephropathies, maximal use of antiproteinuric agents such as renin angiotensin system antagonists is not routine in the management of FSGS recurrence. More recently, encouraging results have been reported with anti-CD20 depleting antibody rituximab, but further studies are needed to establish its safety/efficacy profile.
Index words: kidney transplant, FSGS, glomerulonephritis, permeability factor, proteinuria
INTRODUCTION
Gallon et al. recently reported the case of a 27-year-old patient with end stage renal disease (ESRD) caused by primary focal segmental glomerulosclerosis (FSGS) who developed severe nephrotic syndrome shortly after receiving a kidney transplant from his 24-year-old sister (1). A graft biopsy obtained on day 6 showed FSGS recurrence, revealing signs of podocyte foot-process effacement and loss of the interdigitating arrangements. Severe hypoalbuminemia and rapidly deteriorating graft function, together with the development of an intra-abdominal hematoma, led to renal allograft removal on post-transplant day 14. After consulting with the hospital ethics committee and internal review board, the removed kidney was transplanted into a 66-year-old man with ESRD secondary to type 2 diabetic nephropathy. Immediately after retransplantation, the graft regained function, proteinuria decreased, and glomerular lesions regressed, as shown by allograft biopsies performed on days 8 and 25 after retransplantation (1).
This intriguing case emphasizes the role of host factors in initiating recurrent FSGS, and prompts us to review the status of our understanding of the pathophysiology of FSGS recurrence and the currently available therapeutic options for this problematic disorder.
FSGS RECURRENCE IN KIDNEY TRANSPLANT PATIENTS
The global incidence of FSGS has been estimated at 8 cases/million/yr (2). Further there appears to have been a tripling of FSGS incidence, expressed as a fraction of the kidney biopsy population (3). There is a major effect of race/ethnicity, with African descent individuals at increased risk (4). In the USA, the lifetime risk for FSGS has been estimated at 0.2% for European Americans and 0.7% for African Americans (5). Progression to ESRD occurs in 40–60% of FSGS patients within 10 to 20 years from diagnosis, which makes of FSGS the most common primary glomerular disease in dialysis patients in the USA (6).
Five forms of FSGS are currently recognized: genetic, adaptive (post-adaptive), virus-associated, drug-induced, and primary (idiopathic) (7). Genetic FSGS has been associated with mutations in over 20 genes, encoded in the nuclear or mitochondrial genome, and encoding a range of molecules, including those of the slit diaphragm and actin cytokeleton, which appear to be critical for podocyte function. Adaptive FSGS arises due to a mismatch between physiological load (in part dependent upon body size but also other determinants of glomerular blood flow) and glomerular filtration surface (in part dependent on nephron number), and this mismatch leads to podocyte stress, followed by podocyte detachment and loss. Virus-associated FSGS - including, amongst the others, parvovirus B19 and HIV-associated FSGS may occur via direct viral infection of the podocyte, circulating viral proteins, or as a consequence of the inflammatory cytokines released by other infected cells that interact with podocyte receptors. Drug-induced FSGS is due to a short list of medication including those that act on the podocyte (pamidronate, interferon-alpha) and those that damage the tubulointerstitium (e.g. lithium, cyclosporine, tenofovir). Importantly, only primary FSGS generally recurs following kidney transplant.
Primary FSGS patients are thought to display immune and/or cytokine abnormalities that lead to podocyte injury. This provides the rationale for the use of glucocorticoids as initial treatment. However, 20% of patients are resistant to steroids and other immunosuppressants (cyclosporine, tacrolimus, mycophenolate mofetil, or cyclophosphamide) and often progress to ESRD. Unfortunately, up to 50% of patients develop recurrence of proteinuria after kidney transplantation, which can occur within hours to days after grafting, and this increases the risk of renal dysfunction and early graft loss (8). The first three cases of FSGS recurrence were reported by Hoyler et al. in 1972 (9). As described in this initial paper, diffuse podocyte foot process effacement by electron microscopy is usually the only finding in early graft biopsies and may already appear after reperfusion, anticipating proteinuria onset (10). Severity of foot process effacement correlates with the extent of proteinuria, which supports the central role of podocytes in the pathophysiology of this disease (10). Compared to patients with recurrence of other glomerulonephritides, those with FSGS recurrence have a two-fold higher risk of losing the graft over 10 years (11). Risk factors for recurrent FSGS include younger age (especially in children <6 at FSGS onset), nonblack race, a rapid progression to ESRD in the native kidney (<3 years), heavy proteinuria in the period before transplantation, and the loss of previous allografts to recurrence (12).
PATHOPHYSIOLOGY OF FSGS RECURRENCE: INSIGHTS FROM BUFFALO RATS
The Buffalo/Mna rats develop spontaneous proteinuria associated with renal histological features of FSGS. As with their human counterparts, treatment of these rats with glucocorticoids, cyclosporine, or cyclophosphamide is associated with proteinuria reduction. Of even higher interest, when a kidney from a healthy MHC-compatible LEW.1W rat is transplanted into a nephrectomized Buffalo/Mna recipient, FSGS recurs (13). Importantly, detailed sequential analyses of renal histology, infiltrating cell populations and cytokine transcripts excluded the presence of acute or chronic rejection processes in the transplanted kidney and confirmed that primary FSGS and post-transplantation recurrence represent the same entity (14), making Buffalo/Mna rats a suitable model for study FSGS recurrence. Intriguingly, when Buffalo/Mna kidneys are transplanted into normal LEW.1W rats, proteinuria and renal lesions regress, which closely reproduces the case described in the initial vignette (13).
Studies performed in Buffalo/Mna rats showed that the most represented cell lineage in the kidney infiltrate before and during the development of the disease was monocytes/macrophages (15), together with Th2 lymphocytes (15). Importantly, treatment with the deoxyspergualin derivative LF15-0195 was associated with the formation of regulatory T cells that, in turn, were able to reduce proteinuria in the initial kidney disease and to prevent its recurrence after transplant. Taken together, these findings support the importance of T cells in the pathogenesis of FSGS.
In addition to these immune abnormalities, genetic analyses have shown that the development of the proteinuria in Buffalo/MNa rats is associated with an abnormality in the actin cytoskeleton, which suggests the existence an inherited direct podocyte defect (16–18). This finding is consistent with the rare possibility of FSGS recurrence in patients with heterozygous mutations in NPHS2 gene encoding podocin that should be theoretically corrected by the graft (19, 20). Though anti-podocin antibodies have not been found in patients with disease recurrence and mechanisms at the basis of this phenomenon are still obscure, it could be hypothesized that inherited abnormalities in the kidney elicit, in predisposed individuals, an immune response, which could further accelerate glomerulosclerosis.
CIRCULATING FACTORS
The frequent occurrence of proteinuria relapse in the immediate phases after transplantation suggests that podocyte injury is probably caused by a circulating permeability factor, initially thought to be released by T cells. Moreover, the rapid recovery of allograft function after retransplantation into a patient without FSGS further supports this hypothesis. However, despite mounting in vitro and in vivo observations supporting the existence of permeability factor(s), their identification has proven frustratingly elusive.
Many cytokines and other mediators able to increase glomerular albumin permeability in vitro have been found increased in sera of patients with FSGS. This group includes cardiotrophin-like cytokine 1 (CLC-1), a member of the interleukin-6 family, decreases nephrin expression in cultured podocytes and its blockade reverses the permeability effect of sera from FSGS patients (21). However, data on its potential role in the pathogenesis of FSGS are still preliminary.
At present the strongest evidence for a permeability factor relates to soluble urokinase receptor (suPAR), the soluble form of the urokinase type plasminogen activator receptor (uPAR) (22, 23). In mice, circulating suPAR activated podocyte β3 integrin (ITGβ3), causing foot process effacement, proteinuria and FSGS-like glomerulopathy. Intriguingly, the proteinuric effects of suPAR are blocked in vitro by a specific antagonising antibody. Moreover, creating a point mutation in suPAR gene in mice blocked its ability to activate β3 integrin and to produce proteinuria.
This solid experimental evidence of a causative role of suPAR in FSGS, however, is not supported by similarly convincing clinical data. Though serum levels of suPAR in FSGS patients reported by Wei et al. were, on average, significantly higher than in patients with other glomerulopathies, a wide overlap was present amongst different renal diseases (22). High serum levels of suPAR were predictive of FSGS recurrence in transplanted kidneys, and lowering the levels of suPAR by plasmapharesis was associated with disease remission. However, serum levels of suPAR above the suggested threshold of 3000 pg/mL were found in patients without recurrent FSGS (22). Further, plasma suPAR levels are elevated in a wide range of inflammatory conditions, including chronic infections (including tuberculosis and malaria), bacterial pneumonia, bacterial and viral central nervous system infections, sepsis, and various cancers (24). These conditions are not frequently associated with nephrotic proteinuria, casting doubt upon a simple model that relates suPAR plasma levels to podocyte injury and nephrotic proteinuria. HIV infection has been also associated with high suPAR levels, but no evidence has linked suPAR with HIV-associated FSGS.
Therefore, the role of suPAR in human FSGS pathogenesis is unclear and probably less straightforward than initially proposed. The role of suPAR as a potential permeability factor in FSGS was challenged by data showing that, in a single center cohort of 23 patients, serum suPAR levels were not different amongst idiopathic FSGS, secondary FSGS, and minimal change disease (MCD), nor did they predict responsiveness to steroid therapy in patients with idiopathic FSGS or MCD (25). Conversely, an inverse correlation was found between serum suPAR levels and estimated GFR, independently from glomerular disease. These findings raised additional doubts on the potential use of serum suPAR as a marker of primary FSGS. This report has stimulated a reply that points out the critical role of careful phenotyping in FSGS studies (26).
Altogether, despite strong clues in support of its existence, research on the circulating factor has been inconclusive so far, possibly because not a single factor is responsible for all the cases of FSGS. Conversely, in harmony with the protean nature of the disease, multiple factors may act in the same patients together or at different stages of the disease.
PROGRESSION OF FSGS INJURY
Data from experimental and clinical studies converge to support the hypothesis that FSGS is the result of various insults directed to or inherent within the podocyte. Podocyte injury leads to effacement of the foot processes, which is the major structural correlate of nephrotic proteinuria. This change in podocyte shape is the result of cytoskeleton rearrangement, a process that is partially reversible by glucocorticoid and calcineurin inhibitor therapy (7).
In rats with podocytes transgenic for the human diphtheria toxin receptor, Wharram et al. were able to induce precise levels of podocyte depletion by titrating the dose of administered toxin (27). The animals showed dose-dependent structural alterations characteristic of FSGS disease. Podocyte depletion of <20% induced transient proteinuria and mesangial expansion, loss of 20% to 40% of podocytes resulted in persistent proteinuria and focal glomerulosclerosis, but no progressive renal function decline, and >40% podocyte loss caused progressive glomerular failure, indicating the existence of a threshold for disease progression (27) In a chimeric model in which only a subpopulation of podocytes express toxin receptor, podocyte injury and dedifferentiation were shown to spread to neighboring toxin-resistant podocytes that escaped the initial insult (28). Consistently, podocytes are shed into the urine for months after a brief toxin exposure (29). Mechanisms underlying the local propagation of podocyte injury are still ill defined; one possibility is that podocyte loss requires that neighboring podocytes must undergo hypertrophy to cover a larger area of the capillary loop and that hypertrophy, beyond a certain threshold, places stress on the podocyte. Recently, Fukuda et al. (30) showed that in rats with impaired mammalian target of rapamycin (mTOR) signaling (a key determinant of a cell hypertrophic response to nutrients and growth factors), weight gain was associated with accelerated development of glomerular hypertrophy, development of FSGS lesions, and renal failure. This phenomenon was even more striking when renal mass was reduced by uni-nephrectomy. Thus, the process of podocyte hypertrophy is essential in renal compensation, although it may come at a cost.
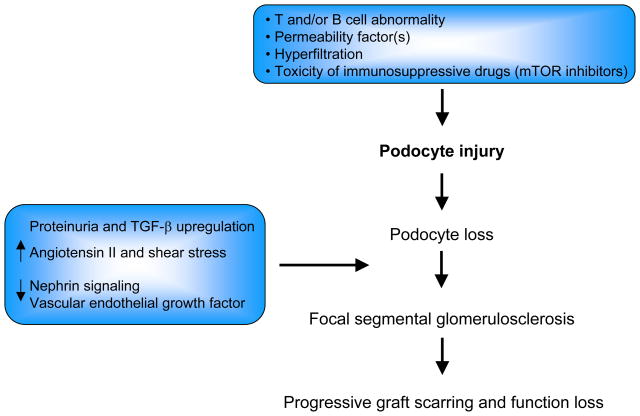
As a consequence of podocyte loss, unremitting proteinuria may be associated with upregulation of transforming growth factor-β and other soluble factors, and downregulation of vascular endothelial growth factor and other soluble factors, which mediates progressive glomerular scarring (31). Additional, non mutually exclusive mechanisms include loss of pro-survival factors such as nephrin signaling or enhanced angiotensin II, shear stress, or cell death gap junction signaling (32) (Figure 1).
Figure 1.
Proposed pathogenic mechanism(s) of FSGS recurrence after transplant.
Therefore, a mismatch between nephron number and metabolic demand represents an important element for progression of FSGS. This could be particularly relevant in kidney transplant patients, where nephron mass provided with a single graft is reduced by the ischemia-reperfusion injury and immunosuppressive agent nephrotoxicity (especially calcineurin and mTOR inhibitors), which could at least partially account for the accelerated loss of renal function in FSGS recurrence compared to primary disease. Thus, recurrent FSGS patients may have components of both primary FSGS and adaptive FSGS from relatively early stages of the disease evolution.
TREATMENT OF FSGS RECURRENCE
The management of patients with recurrent FSGS is challenging, with none of the multiple approaches providing consistent efficacy. Most reports consist of single cases and the few prospective studies are uncontrolled or with limited follow-up periods. Therefore, while experimental studies have provided major advancements in our understanding the pathophysiology of FSGS, treatment of affected patients is still largely empirical.
Plasmapheresis and plasma absorption
Starting from animal experiments showing the potential existence of a circulating permeability factor in a patient with FSGS recurrence, Zimmerman reported in 1985 the first successful use of plasmapheresis (PP) (33). Since that original description, many case reports and case series supported the use of PP for the treatment of recurrent FSGS (34). Otsuba et al. retrospectively reviewed 5 recurrent FSGS patients treated with PP and 11 who were not treated; while outcomes were marginally better in those who received plasma exchange, the results did not reach statistical significance (35). A systematic review of the literature showed that PP was effective in promoting partial or complete remission of proteinuria in 70% of children and 63% of adults with recurrent FSGS (8). However, most studies were flawed by a retrospective, uncontrolled, design and by a short-term follow-up.
Few prospective studies of plasma exchange are available and none have involved a control group, either a non-treated control group or an alternative therapy. Gohh et al. prospectively treated 10 patients deemed to be at high risk for recurrent FSGS with 8 plasma exchanges during the perioperative period; only 3 patients experienced recurrent FSGS, which the authors suggested was less than would have been predicted in the absence of the intervention (36). Interpreting the retrospective literature is complicated by publication bias of cases with positive outcomes that may lead to overestimation of treatment efficacy and by the fact that other therapies in addition to PP were part of the treatment regimen.
Cases and series have suggested that plasma absorption with protein A is effective at reducing proteinuria, and some authors have advocated the use of plasma absorption as an adjunctive treatment to PP, but data in support of this hypothesis are still limited (37, 38). According to a retrospective study of 11 patients with recurrent FSGS, double diffusion PP was able to reduce proteinuria in 7 cases, but its potential benefit over traditional PP needs further evaluation (35).
Despite the absence of good controlled trials, PP is still widely employed to treat recurrent FSGS in kidney transplant recipients. In light of the still limited evidence on the role of permeability factor(s) in the pathogenesis of FSGS, this treatment should probably be considered more critically.
Calcineurin inhibitors
A few small studies have tested the hypothesis that calcineurin inhibitors prevent or treat FSGS recurrence by inhibiting T cells. More recently, the antiproteinuric effect of cyclosporine has been attributed also to the inhibition of calcineurin-mediated dephosphorylation of synaptopodin, a protein critical for stabilizing the actin cytoskeleton in podocytes (39). Though the use of standard oral doses of cyclosporine used to prevent acute rejection has not been associated with reduced incidence of FSGS, higher intravenous doses have been associated with proteinuria reduction (Table 1). The rationale behind maintaining a high cyclosporine blood level is to overcome the effect of high serum cholesterol often seen in patients with nephrotic syndrome due to recurrent FSGS. Indeed, due to the lipophilic nature of cyclosporine, hypercholesterolemia reduces the fraction of the active free drug. In a prospective cohort study, 17 patients with FSGS were treated with intravenous cyclosporine (3 mg/kg/d, converting to the oral route in 3–4 weeks to maintain trough levels between 250 and 350 ng/mL) (40). Fourteen patients had a prompt remission of proteinuria that, in some cases, persisted for years. Other reports showed encouraging results of cyclosporine in treating FSGS recurrence (41–43), but the long-term safety/efficacy of such a therapy remains to be established, especially in light of the severe toxicities associated with high doses of calcineurin inhibitors.
Table 1.
Studies on cyclosporine effect on recurrent FSGS.
| Reference | Study Design | Treatments | Incidence of FSGS recurrence/°Remission Rate |
|---|---|---|---|
| Prevention | |||
| Banfi G, et al. (52) | Retrospective | − Steroids + AZA (n=6) | 2 (33%) |
| − Steroids + CsA (n=19) | 10 (55%) | ||
| Schwarz A, et al. (43) | Retrospective | − Steroids + AZA (n=7) | 1 (14%) |
| − Steroids + CsA (n=8) | 2 (25%) | ||
| Inguilli E Tejani A (53) | Retrospective | − Steroids + AZA (n=22) | 4 (18%) |
| − Steroids + CsA (n=18) | 2 (11%) | ||
| Treatment° | |||
| Ingulli E, et al. (42) | Case report | Progressive uptitration of oral CsA doses | 1 Complete and 1 partial remission |
| Salomon R, et al. (40) | Retrospective | I.v. CsA (n=16). trough levels: 250–350ng/mL | Complete remission: 13 (81%) Partial remission: 2 (13%) |
| Raafat RH, et al. (41) | Retrospective | Oral CsA doses were uptitrated until proteinuria reduction or serum creatinine elevation (n=16) | Complete remission: 11 (65%) Partial remission: 2 (12%) |
| Canaud G, et al. (72) | Prospective cohort | I.v. CsA, combined with high-dose steroids and intensive plasmapheresis (n=10) | Complete remission: 9 (90%) [Incidence of complete remission in a control historical cohort: 5/19 (27%)] |
AZA: azathioprine; CsA: cyclosporine.
Rituximab
Rituximab, a chimeric monoclonal antibody targeting the CD20 antigen of B-cells, was approved by the US Food and Drug Administration in 1997 for the treatment of relapsing or refractory non-Hodgkin’s lymphoma. In 2006, a child with post-transplant recurrent FSGS achieved remission of nephrotic syndrome after receiving six intravenous rituximab administrations to treat a post-transplant lymphoproliferative disease (44). Depletion of a circulating autoantibody or interference with the presentation of B-cell antigens were mechanisms suggested to explain the efficacy of rituximab in this patient. Moreover, recent evidence has been provided that rituximab can directly bind to molecules other than CD20, such as SMPDL-3b, expressed in human podocytes. Intriguingly, in vitro exposure of podocytes to sera of FSGS patients down-regulates SMPDL-3b (a protein implicated implicated in actin remodeling), which can be prevented by rituximab (45).
A recent systematic review of the 39 reported cases (19 pediatric) of FSGS recurrence treated with rituximab showed that complete or partial remission occurred in 64% of patients (46). Multivariate analysis revealed that normal serum albumin at FSGS recurrence and lower age at transplant were associated with response. Intriguingly, fewer number of rituximab infusions was associated with a higher frequency of response. This is in line with what reported in membranous nephropathy, where a single rituximab dose is enough to achieve proteinuria remission (47). Therefore, though rituximab is generally well tolerated, its dosing should be titrated to the minimal level required to obtain B-cell depletion, especially in consideration of the increased risk of opportunistic infections in immunosuppressed transplant patients (48). According to some reports, combined use of PP and rituximab may potentiate the efficacy of both treatments (49) (Table 2). Intriguingly, this treatment strategy is made more appealing by its efficacy in reducing the anti-HLA antibody titer, another major risk factor for long-term graft loss.
Table 2.
Cases of rituximab therapy in FSGS recurrence.
| Reference | Gender, age (y) | Background treatment | Rituximab dose | Follow-up (m)* | Remission | Adverse effects |
|---|---|---|---|---|---|---|
| Adults | ||||||
| Hristea et al.(54) | 1M (22) | Basiliximab induction, Tac, MMF, steroids, PP | 375mg/m2×2 | 24 | Y | No |
| Gossman et al.(55) | 1F (48) | Thymoglobulin induction, Tac, MMF, steroids, PP | 375mg/m2×2 | 12 | Y | No |
| Meyer et al. (56) | 1F (29) | Steroids, Tac, MMF, immunoadsorbption | 375mg/m2×3 | 12 | Y | No |
| Kamar et al. (57) | 2M (25, 47) | MMF, steroids, CsA, PP | 375mg/m2×2 | 10 | - Y (M 27) - No (M 47) |
No |
| Yabu et al. (58) | 3F (41, 43, 47) 1M (41) | FSGS refractory or dependent on PP | 1000mg × 2 375mg/m2×4–6 |
-Y (1M) -No (3W) |
No | |
| El-Firjani, et al. (59) | 1F (48) | Thymoglobulin induction, Tac, MMF, steroids, PP | 375mg/m2×6 | No | No | |
| Apeland T, et al. (60) | 1M (18) | MMF, Tac, steroids, PP | 375mg/m2×3 | 36 | Y | No |
| Freiberger V, et al. (50) | 1F (29) | MMF, Tac, steroids, PP | 375mg/m2×1 | 12 | No | No |
| Children | ||||||
| Grenda R, et al. (61) | 1M (5) | CsA, MMF, steroids, PP | 375mg/m2×2 | 8 | Y | No |
| Sethna C, et al. (62) | 2F (13, 17) | Thymoglobulin induction, steroids, Tac, MMF, PP | 375mg/m2×4 | 22.5 | 3Y, 1 No | No |
| 2M (13, 18) | ||||||
| Prytuła, et al. (63) | 14 cases | N/A | 375mg/m2×1–5 | 5–84 | 6Y, 3 partial, 5No | 2 acute reactions, 1infection |
| Stewart, et al. (64) | 1F (16) | Thymoglobulin induction, steroids, Tac, MMF, PP | 375mg/m2×6 | 12 | Y | No |
| Nozu et al. (65) | 1M (12) | CyA, steroids | 375mg/m2×4 | 36 | Y | No |
| Pescovitz et al. (44) | 1M (7) | Tac, MMF, steroids, PP | 375mg/m2×6 | 8 | Y | No |
| Nakayama et al. (66) | 2F (10, 12) | CyA, steroids, chyclophosphamide Mizoribin | 375mg/m2×3 | 8 2 |
Y | No |
| Marks and McGraw (67) | 2M (6, 10) | Steroids, Tac, AZA, cyclophosphamide, CyA, PP | 375mg/m2×2–4 | No | No | |
| Bayrakci, U. S.(68) | 1M (14) | Daclizumab induction, steroids, CycA, , MMF, PP | 375mg/m2×4 | 8 | Y | |
| Hickson LJ, et al. (69) | 1F (19) | Tac, PP | 375mg/m2×2–4 | 7–30 | Y | No |
| 3M (5, 6,13) | ||||||
| Rodriguez-Ferrero M, et al. (70) | 1M (6), 2F(17, 22) | Steroids, MMF, Tac (n=2), Everolimus (n=1), PP | 375mg/m2×4 | 1 | Significant proteinuria reduction | No |
| Dello Strogolo, et al. (71) | 7 cases (7.3–26.9 years) | Steroids, Tac (n=5), CsA (n=2), MMF, PP , ACE inhibitors | 375mg/m2×1–4 | 2–9 | 3Y, 2 partial, 2No | 2 acute reactions |
| Fornoni, et al. (45) | 41 cases (<25years) | Untreated (n=14), Rituximab to prevent recurrence (n=27) | 375mg/m2×1 | 12 | Untreated=9o Rituximab=7o |
|
ACE: Angiotensing converting enzyme; CsA: Cyclosporine A; MMF: mycophenolate mofetil; PP: Plasmapheresis; RAS: Renin angiotensin system; Tac: tacrolimus.
after rituximab administration.
Cases of FSGS recurrence.
Renin angiotensin system inhibitors
Despite experimental and clinical evidence that activation of the renin angiotensin system (RAS) is involved in FSGS progression, only a few cases have been reported on the use of RAS inhibitors in patients with FSGS recurrence. Recently, Freiberger et al. (50) reported the case of a patient with FSGS recurrence after transplant that safely achieved proteinuria remission with intensified RAS inhibition through the use of triple RAS therapy: ACE inhibitor, an angiotensin II receptor antagonist and a renin inhibitor. Of note, previous treatment with PP and rituximab obtained only transient proteinuria reduction. This finding is in line with retrospective studies showing a beneficial effect of RAS inhibitors in reducing proteinuria and improving graft or patient survival in subjects with chronic rejection. Consistent evidence of the nephroprotective effects of RAS inhibitors should therefore encourage their wider use in transplant patients with proteinuria, including those with nephrotic syndrome associated with FSGS recurrence. Though these drugs have a good safety profile, serum creatinine and potassium levels should be carefully monitored, at least during the up-titration phase.
MANAGEMENT OF FSGS RECURRENCE
In renal transplant recipients with FSGS recurrence, proteinuria should be monitored strictly and treatment should be instituted as soon as the diagnosis of FSGS recurrence is made. Urinary protein excretion should be measured frequently during the early post-transplant phase, when the risk of recurrence is the highest. Ideally, proteinuria should be evaluated on a daily basis during the first one or two weeks after transplant, then the frequency of measurements should be progressively tapered up to once every week or every two weeks (month 3), then to once a month (month 6) and every two months thereafter. Due to the potential late onset of disease recurrence, proteinuria should be evaluated at least every 6 months after the first transplant year. Measurement of urinary protein/creatinine ratio is the preferred way to assess proteinuria, at least during the first months after transplant. Urinary dipstick represents an alternative and more practical screening strategy to identify those patients who require a quantitative measurement of urinary proteins. Once proteinuria is found, a graft biopsy should be performed to confirm that diagnosis. Early graft biopsies may show normal-appearing glomeruli by light microscopy but diffuse foot process effacement by electron microscopy.
Lack of any treatment with proven benefit makes the management of FSGS disease extremely challenging. Despite the mixed results available, a course of PP treatment should be attempted in every patient. Initial schedule should be 2–3 changes per week, using 5% albumin as replacement fluid, and then titrated according to patient response (8). Rituximab should be also administered early, starting with a single 375mg/m2 dose (48). Evidence also exists suggesting that rituximab induction may reduce the rate of FSGS recurrence (45).
Because of the reported association between sirolimus and FSGS, conversion from mTOR to calcineurin inhibitors is recommended (51). The use of high CsA doses should be decided case by case and its potential benefits in proteinuria reduction have to be carefully weighed against the drug-related toxicities, including nephrotoxicity. For patients with a contraindication to CsA, tacrolimus should be used. All patients with FSGS recurrence should receive the maximal tolerated dose of RAS inhibitors to reduce proteinuria and protect the kidney from progressive scarring.
To assess response to treatment, proteinuria should be monitored frequently after any change in the drug regimen or dosing, since most of the aforementioned therapies, when effective, reduce proteinuria rapidly. In case of disease remission, patients should be followed-up in the long-term with proteinuria evaluations every three to six months.
OUTLOOK
Recurrence of FSGS after transplant is frequent and associated with accelerated graft loss. Despite the remarkable progress made in unraveling the pathogenesis of FSGS, outcomes of affected patients have not significantly improved in the last several decades. More recently, studies investigating the immune abnormalities associated with FSGS and the pathophysiology of progressive podocyte loss are opening new avenues for the identification of novel, hypothesis-driven therapies. Investigations on rare, genetic forms of FSGS could provide important clues about more common cases that are not genetically determined. Hopefully, these achievements will translate into more powerful and selective therapies for FSGS patients.
Acknowledgments
This work was supported in part by the Intramural Research Program, NIDDK, NIH.
List of abbreviations
- ACE
Angiotensin converting enzyme
- CLC-1
Cardiotrophin-like cytokine 1
- CsA
Cyclosporin
- ESRD
End stage renal disease
- FSGS
Focal segmental glomerulosclerosis
- HLA
Human Leukocyte antigen
- ITGβ3
podocyte β3 integrin
- MCD
Minimal change disease
- mTOR
Mammalian target of rapamycin
- PP
Plasmapheresis
- RAS
Renin angiotensin system
- suPAR
Soluble form of the urokinase type plasminogen activator receptor
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366(17):1648–1649. doi: 10.1056/NEJMc1202500. [DOI] [PubMed] [Google Scholar]
- 2.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 3.Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44(5):815–825. [PubMed] [Google Scholar]
- 4.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitiyakara C, Kopp JB, Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23(2):172–182. doi: 10.1053/snep.2003.50025. [DOI] [PubMed] [Google Scholar]
- 6.Renal Data System. USRDS 2012 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda: MNIoH, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [Google Scholar]
- 7.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365(25):2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant. 2010;25(1):25–31. doi: 10.1093/ndt/gfp538. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer JR, Vernier RL, Najarian JS, Raij L, Simmons RL, Michael AF. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet. 1972;2(7773):343–348. doi: 10.1016/s0140-6736(72)91734-5. [DOI] [PubMed] [Google Scholar]
- 10.Chang JW, Pardo V, Sageshima J, Chen L, Tsai HL, Reiser J, et al. Podocyte Foot Process Effacement in Postreperfusion Allograft Biopsies Correlates With Early Recurrence of Proteinuria in Focal Segmental Glomerulosclerosis. Transplantation. 2012 doi: 10.1097/TP.0b013e318250234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347(2):103–109. doi: 10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 12.Vinai M, Waber P, Seikaly MG. Recurrence of focal segmental glomerulosclerosis in renal allograft: an in-depth review. Pediatr Transplant. 2010;14(3):314–325. doi: 10.1111/j.1399-3046.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 13.Le Berre L, Godfrin Y, Gunther E, Buzelin F, Perretto S, Smit H, et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J Clin Invest. 2002;109(4):491–498. doi: 10.1172/JCI12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Berre L, Bruneau S, Renaudin K, Naulet J, Usal C, Smit H, et al. Development of initial idiopathic nephrotic syndrome and post-transplantation recurrence: evidence of the same biological entity. Nephrol Dial Transplant. 2011;26(5):1523–1532. doi: 10.1093/ndt/gfq597. [DOI] [PubMed] [Google Scholar]
- 15.Le Berre L, Herve C, Buzelin F, Usal C, Soulillou JP, Dantal J. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005;68(5):2079–2090. doi: 10.1111/j.1523-1755.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama M, Ogiu T, Kontani K, Yamada C, Kawai M, Hiai H, et al. Genetic regulation of the development of glomerular sclerotic lesions in the BUF/Mna rat. Nephron. 1990;54(4):334–337. doi: 10.1159/000185890. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama K, Morita H, Suetsugu S, Kuraba S, Numata Y, Yamamoto Y, et al. Actin -related protein 3 (Arp3) is mutated in proteinuric BUF/Mna rats. Mamm Genome. 2008;19(1):41–50. doi: 10.1007/s00335-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 18.Murayama S, Yagyu S, Higo K, Ye C, Mizuno T, Oyabu A, et al. A genetic locus susceptible to the overt proteinuria in BUF/Mna rat. Mamm Genome. 1998;9(11):886–888. doi: 10.1007/s003359900888. [DOI] [PubMed] [Google Scholar]
- 19.Weber S, Gribouval O, Esquivel EL, Moriniere V, Tete MJ, Legendre C, et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66(2):571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 20.Jungraithmayr TC, Hofer K, Cochat P, Chernin G, Cortina G, Fargue S, et al. Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J Am Soc Nephrol. 2011;22(3):579–585. doi: 10.1681/ASN.2010010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5(11):2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 22.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17(8):952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 24.Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27(3):157–172. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maas RJ, Wetzels JF, Deegens JK. Serum-soluble urokinase receptor concentration in primary FSGS. Kidney Int. 2012;81(10):1043–1044. doi: 10.1038/ki.2012.32. [DOI] [PubMed] [Google Scholar]
- 26.Trachtman H, Gipson DS, Kaskel F, Ghiggeri GM, Faul C, Gupta V, et al. Regarding Maas's editorial letter on serum suPAR levels. Kidney Int. 2012;82(4):492. doi: 10.1038/ki.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16(10):2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 28.Matsusaka T, Sandgren E, Shintani A, Kon V, Pastan I, Fogo AB, et al. Podocyte injury damages other podocytes. J Am Soc Nephrol. 22(7):1275–1285. doi: 10.1681/ASN.2010090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, et al. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol. 2009;20(5):1041–1052. doi: 10.1681/ASN.2007121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, et al. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23(8):1351–1363. doi: 10.1681/ASN.2012030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichikawa I, Ma J, Motojima M, Matsusaka T. Podocyte damage damages podocytes: autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens. 2005;14(3):205–210. doi: 10.1097/01.mnh.0000165884.85803.e1. [DOI] [PubMed] [Google Scholar]
- 32.D'Agati V. Podocyte injury can be catching. J Am Soc Nephrol. 2011;22(7):1181–1183. doi: 10.1681/ASN.2011050486. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman SW. Plasmapheresis and dipyridamole for recurrent focal glomerular sclerosis. Nephron. 1985;40(2):241–245. doi: 10.1159/000183469. [DOI] [PubMed] [Google Scholar]
- 34.Keith DS. Therapeutic apheresis rescue mission: recurrent focal segmental glomerulosclerosis in renal allografts. Semin Dial. 2012;25(2):190–192. doi: 10.1111/j.1525-139X.2011.01031.x. [DOI] [PubMed] [Google Scholar]
- 35.Otsubo S, Tanabe K, Shinmura H, Ishikawa N, Tokumoto T, Hattori M, et al. Effect of post-transplant double filtration plasmapheresis on recurrent focal and segmental glomerulosclerosis in renal transplant recipients. Ther Apher Dial. 2004;8(4):299–304. doi: 10.1111/j.1526-0968.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 36.Gohh RY, Yango AF, Morrissey PE, Monaco AP, Gautam A, Sharma M, et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5(12):2907–2912. doi: 10.1111/j.1600-6143.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 37.Fencl F, Simkova E, Vondrak K, Janda J, Chadimova M, Stejskal J, et al. Recurrence of nephrotic proteinuria in children with focal segmental glomerulosclerosis after renal transplantation treated with plasmapheresis and immunoadsorption: case reports. Transplant Proc. 2007;39(10):3488–3490. doi: 10.1016/j.transproceed.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 38.Belson A, Yorgin PD, Al-Uzri AY, Salvatierra O, Higgins J, Alexander SR. Long-term plasmapheresis and protein A column treatment of recurrent FSGS. Pediatr Nephrol. 2001;16(12):985–989. doi: 10.1007/s004670100008. [DOI] [PubMed] [Google Scholar]
- 39.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14(9):931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon R, Gagnadoux MF, Niaudet P. Intravenous cyclosporine therapy in recurrent nephrotic syndrome after renal transplantation in children. Transplantation. 2003;75(6):810–814. doi: 10.1097/01.TP.0000055215.20367.21. [DOI] [PubMed] [Google Scholar]
- 41.Raafat RH, Kalia A, Travis LB, Diven SC. High-dose oral cyclosporin therapy for recurrent focal segmental glomerulosclerosis in children. Am J Kidney Dis. 2004;44(1):50–56. doi: 10.1053/j.ajkd.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Ingulli E, Tejani A, Butt KM, Rajpoot D, Gonzalez R, Pomrantz A, et al. High-dose cyclosporine therapy in recurrent nephrotic syndrome following renal transplantation. Transplantation. 1990;49(1):219–221. doi: 10.1097/00007890-199001000-00050. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz A, Krause PH, Offermann G, Keller F. Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine A. Am J Kidney Dis. 1991;17(5):524–531. doi: 10.1016/s0272-6386(12)80493-8. [DOI] [PubMed] [Google Scholar]
- 44.Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med. 2006;354(18):1961–1963. doi: 10.1056/NEJMc055495. [DOI] [PubMed] [Google Scholar]
- 45.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araya CE, Dharnidharka VR. The factors that may predict response to rituximab therapy in recurrent focal segmental glomerulosclerosis: a systematic review. J Transplant. 2011;2011:374213. doi: 10.1155/2011/374213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2(5):932–937. doi: 10.2215/CJN.01180307. [DOI] [PubMed] [Google Scholar]
- 48.Cravedi P, Ruggenenti P, Remuzzi G. Low-dose rituximab for posttransplant recurrent membranous nephropathy. Am J Transplant. 2010;10(5):1336. doi: 10.1111/j.1600-6143.2010.03029.x. [DOI] [PubMed] [Google Scholar]
- 49.Tsagalis G, Psimenou E, Nakopoulou L, Laggouranis A. Combination treatment with plasmapheresis and rituximab for recurrent focal segmental glomerulosclerosis after renal transplantation. Artif Organs. 2011;35(4):420–425. doi: 10.1111/j.1525-1594.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 50.Freiberger V, Amann K, Heemann U, Frank H. Effect of a triple blockade of the renin-angiotensin-system in recurrent focal segmental glomerulosclerosis after kidney transplantation. Transpl Int. 2009;22(11):1110–1113. doi: 10.1111/j.1432-2277.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 51.Cravedi P, Ruggenenti P, Remuzzi G. Sirolimus for calcineurin inhibitors in organ transplantation: contra. Kidney Int. 78(11):1068–1074. doi: 10.1038/ki.2010.268. [DOI] [PubMed] [Google Scholar]
- 52.Banfi G, Colturi C, Montagnino G, Ponticelli C. The recurrence of focal segmental glomerulosclerosis in kidney transplant patients treated with cyclosporine. Transplantation. 1990;50(4):594–596. doi: 10.1097/00007890-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Ingulli E, Tejani A. Incidence, treatment, and outcome of recurrent focal segmental glomerulosclerosis posttransplantation in 42 allografts in children--a single-center experience. Transplantation. 1991;51(2):401–405. doi: 10.1097/00007890-199102000-00025. [DOI] [PubMed] [Google Scholar]
- 54.Hristea D, Hadaya K, Marangon N, Buhler L, Villard J, Morel P, et al. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl Int. 2007;20(1):102–105. doi: 10.1111/j.1432-2277.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 55.Gossmann J, Scheuermann EH, Porubsky S, Kachel HG, Geiger H, Hauser IA. Abrogation of nephrotic proteinuria by rituximab treatment in a renal transplant patient with relapsed focal segmental glomerulosclerosis. Transpl Int. 2007;20(6):558–562. doi: 10.1111/j.1432-2277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 56.Meyer TN, Thaiss F, Stahl RA. Immunoadsorbtion and rituximab therapy in a second living-related kidney transplant patient with recurrent focal segmental glomerulosclerosis. Transpl Int. 2007;20(12):1066–1071. doi: 10.1111/j.1432-2277.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 57.Kamar N, Faguer S, Esposito L, Guitard J, Nogier MB, Durand D, et al. Treatment of focal segmental glomerular sclerosis with rituximab: 2 case reports. Clin Nephrol. 2007;67(4):250–254. doi: 10.5414/cnp67250. [DOI] [PubMed] [Google Scholar]
- 58.Yabu JM, Ho B, Scandling JD, Vincenti F. Rituximab failed to improve nephrotic syndrome in renal transplant patients with recurrent focal segmental glomerulosclerosis. Am J Transplant. 2008;8(1):222–227. doi: 10.1111/j.1600-6143.2007.02021.x. [DOI] [PubMed] [Google Scholar]
- 59.El-Firjani A, Hoar S, Karpinski J, Bell R, Deschenes MJ, Knoll GA. Post-transplant focal segmental glomerulosclerosis refractory to plasmapheresis and rituximab therapy. Nephrol Dial Transplant. 2008;23(1):425. doi: 10.1093/ndt/gfm616. [DOI] [PubMed] [Google Scholar]
- 60.Apeland T, Hartmann A. Rituximab therapy in early recurrent focal segmental sclerosis after renal transplantation. Nephrol Dial Transplant. 2008;23(6):2091–2094. doi: 10.1093/ndt/gfn099. [DOI] [PubMed] [Google Scholar]
- 61.Grenda R, Jarmuzek W, Piatosa B, Rubik J. Long-term effect of rituximab in maintaining remission of recurrent and plasmapheresis-dependent nephrotic syndrome post-renal transplantation - case report. Pediatr Transplant. 2011;15(6):E121–125. doi: 10.1111/j.1399-3046.2010.01303.x. [DOI] [PubMed] [Google Scholar]
- 62.Sethna C, Benchimol C, Hotchkiss H, Frank R, Infante L, Vento S, et al. Treatment of recurrent focal segmental glomerulosclerosis in pediatric kidney transplant recipients: effect of rituximab. J Transplant. 2011;2011:389542. doi: 10.1155/2011/389542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prytula A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, et al. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol. 2011;25(3):461–468. doi: 10.1007/s00467-009-1376-6. [DOI] [PubMed] [Google Scholar]
- 64.Stewart ZA, Shetty R, Nair R, Reed AI, Brophy PD. Case report: successful treatment of recurrent focal segmental glomerulosclerosis with a novel rituximab regimen. Transplant Proc. 2011;43(10):3994–3996. doi: 10.1016/j.transproceed.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 65.Nozu K, Iijima K, Fujisawa M, Nakagawa A, Yoshikawa N, Matsuo M. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20(11):1660–1663. doi: 10.1007/s00467-005-2013-7. [DOI] [PubMed] [Google Scholar]
- 66.Nakayama M, Kamei K, Nozu K, Matsuoka K, Nakagawa A, Sako M, et al. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol. 2008;23(3):481–485. doi: 10.1007/s00467-007-0640-x. [DOI] [PubMed] [Google Scholar]
- 67.Marks SD, McGraw M. Does rituximab treat recurrent focal segmental glomerulosclerosis post-renal transplantation? Pediatr Nephrol. 2007;22(1):158–160. doi: 10.1007/s00467-006-0260-x. [DOI] [PubMed] [Google Scholar]
- 68.Bayrakci US, Baskin E, Sakalli H, Karakayali H, Haberal M. Rituximab for post-transplant recurrences of FSGS. Pediatr Transplant. 2009;13(2):240–243. doi: 10.1111/j.1399-3046.2008.00967.x. [DOI] [PubMed] [Google Scholar]
- 69.Hickson LJ, Gera M, Amer H, Iqbal CW, Moore TB, Milliner DS, et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87(8):1232–1239. doi: 10.1097/TP.0b013e31819f12be. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Ferrero M, Ampuero J, Anaya F. Rituximab and chronic plasmapheresis therapy of nephrotic syndrome in renal transplantation patients with recurrent focal segmental glomerulosclerosis. Transplant Proc. 2009;41(6):2406–2408. doi: 10.1016/j.transproceed.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 71.Dello Strologo L, Guzzo I, Laurenzi C, Vivarelli M, Parodi A, Barbano G, et al. Use of rituximab in focal glomerulosclerosis relapses after renal transplantation. Transplantation. 2009;88(3):417–420. doi: 10.1097/TP.0b013e3181aed9d7. [DOI] [PubMed] [Google Scholar]
- 72.Canaud G, Zuber J, Sberro R, Royale V, Anglicheau D, Snanoudj R, et al. Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: a pilot study. Am J Transplant. 2009;9(5):1081–1086. doi: 10.1111/j.1600-6143.2009.02580.x. [DOI] [PubMed] [Google Scholar]