Abstract
Dok-3 is a Dok-related adaptor expressed in B cells and macrophages. Previously, we reported that Dok-3 is an inhibitor of B-cell activation in A20 B cells and that it associates with SHIP-1, a 5′ inositol-specific lipid phosphatase, as well as Csk, a negative regulator of Src kinases. Here, we demonstrate that Dok-3 suppresses B-cell activation by way of its interaction with SHIP-1, rather than Csk. Our biochemical analyses showed that the Dok-3-SHIP-1 complex acts by selectively inhibiting the B-cell receptor (BCR)-evoked activation of the Jun N-terminal protein kinase (JNK) cascade without affecting overall protein tyrosine phosphorylation or activation of previously described SHIP-1 targets like Btk and Akt/PKB. Studies of B cells derived from SHIP-1-deficient mice showed that BCR-triggered activation of JNK is enhanced in the absence of SHIP-1, implying that the Dok-3-SHIP-1 complex (or a related mechanism) is a physiological negative regulator of the JNK cascade in normal B cells. Together, these data elucidate the mechanism by which Dok-3 inhibits B-cell activation. Furthermore, they provide evidence that SHIP-1 can be a negative regulator of JNK signaling in B cells.
B-cell maturation and activation are initiated by interactions between soluble antigens and the B-cell receptor (BCR) for antigen (3, 8, 25, 36). Upon antigen binding, the BCR transduces intracellular signals that are initiated by protein tyrosine phosphorylation as a result of an association with Igα and Igβ, two subunits bearing immunoreceptor tyrosine-based activation motifs (ITAMs). ITAMs function by recruiting several classes of cytoplasmic protein tyrosine kinases (PTKs), which phosphorylate intracellular enzymes and adaptor molecules. Such phosphorylation events cause increased levels of intracellular calcium, activation of phosphatidylinositol (PI) 3-kinase, cytoskeletal reorganization, transcriptional activation, and, finally, B-cell maturation, proliferation, and antibody secretion.
Given the high sensitivity of B cells to BCR triggering, several mechanisms exist to prevent inappropriate B-cell activation and avoid autoreactive antibodies and autoimmune diseases (7, 34, 45). These regulatory mechanisms include a large group of receptors carrying intracytoplasmic tyrosine-based inhibitory motifs termed ITIMs (immunoreceptor tyrosine-based inhibitory motifs). Such inhibitory receptors make up PD-1, which recruits Src homology 2 (SH2) domain-containing protein tyrosine phosphatases (PTPs), as well as FcγRIIB, which binds the SH2 domain-bearing 5′ inositol phosphatase SHIP-1. These two classes of phosphatases prevent B-cell activation by inhibiting critical steps in the BCR signaling cascade.
SHIP-1 is expressed mostly in hemopoietic cells, including cells of lymphoid and myeloid lineages (6, 24, 37). It acts by hydrolyzing inositol metabolites phosphorylated at the 5′ position of the inositol ring, namely, PI(3,4,5)P3 and I(1,3,4,5)P4. The membrane-bound PI(3,4,5)P3 is critical for binding and membrane recruitment of pleckstrin homology (PH) domain-containing molecules like the PTK Btk, a pivotal effector of B-cell activation, and the serine-threonine-specific protein kinase Akt/PKB, a prosurvival factor. By converting PI(3,4,5)P3 to PI(3,4)P2, SHIP-1 precludes activation of these PH domain-bearing effectors and can prevent B-cell activation. In support of this idea, it has been reported that B cells freshly isolated from SHIP-1-deficient mice exhibited augmented BCR-induced proliferation (5, 12, 27). Moreover, in vivo B-cell maturation is accelerated in SHIP-1−/− animals.
The primary mode of recruitment of SHIP-1 in activated B cells is believed to involve FcγRIIB (31, 32). Engagement of FcγRIIB by the Fc portion of immunoglobulin G (IgG) present in immune complexes (which are generated as a consequence of productive B-cell activation) results in tyrosine phosphorylation of the ITIM of FcγRIIB, thus triggering binding of the SHIP-1 SH2 domain and membrane translocation of SHIP-1. Analyses of ex vivo B cells or B-cell lines lacking SHIP-1 have provided evidence that FcγRIIB-associated SHIP-1 inhibits B-cell activation by preventing BCR-induced PI(3,4,5)P3 accumulation, activation of Btk and Akt/PKB, calcium fluxes, and Erk activation (2, 4, 20, 27, 32, 39). There are also FcγRIIB-independent mechanisms for recruiting SHIP-1 in B cells. In agreement with this, it has been reported that SHIP-1-deficient B cells display enhanced BCR-elicited PI(3,4,5)P3 generation and Akt activation even in the absence of FcγRIIB coligation (5, 20, 27). While the exact mechanism of recruitment of SHIP-1 in this setting is not known, it likely involves interactions with other molecules. This view is also consistent with the finding that SHIP-1 can associate with intracellular adaptor molecules like Shc and Dok-related polypeptides (13, 26).
Cong et al. (10) and Lemay et al. (26) previously reported the identification of Dok-3, a member of the Dok family of adaptors expressed in B cells and macrophages. Like its relatives Dok-1 and Dok-2, Dok-3 possesses an amino-terminal PH domain, a phosphotyrosine-binding (PTB) region, and a long carboxyl-terminal segment with potential sites of tyrosine phosphorylation. Dok-3 becomes rapidly tyrosine phosphorylated in response to B-cell activation and associates by way of tyrosines in its carboxyl-terminal segment with the SH2 domains of SHIP-1 and the PTK Csk, an inhibitor of Src-related PTKs (26). Our studies demonstrated that overexpression of Dok-3 in the A20 B-cell line caused an inhibition of BCR-induced release of interleukin (IL)-2. An opposite effect was seen with expression of a mutant of Dok-3 (Dok-3 4F), in which the four carboxyl-terminal tyrosines were replaced by phenylalanines. Since this mutant was also incapable of binding SHIP-1 and Csk, it was estimated that Dok-3 4F is a dominant-interfering form of Dok-3 that blocks the action of endogenous wild-type Dok-3. Coupled with the findings that wild-type Dok-3 and Dok-3 4F were also able to regulate BCR-induced proliferation of normal B cells (J. D. Robson and A. Veillette, unpublished results), these findings led to the idea that Dok-3 is an inhibitor of B-cell activation on the basis of its capacity to recruit SHIP-1, Csk, or both.
In this study, we examined the mechanism of Dok-3-mediated inhibition in B cells. The results of our investigation showed that the inhibitory effect of Dok-3 is mediated through SHIP-1 rather than Csk. We found that the Dok-3-SHIP-1 complex functions by selectively suppressing the Jun N-terminal protein kinase (JNK) signaling cascade without affecting the activation of known targets of SHIP-1 like Akt/PKB. Finally, we demonstrated that BCR-triggered activation of JNK is enhanced in B cells lacking SHIP-1, implying that Dok-3-mediated recruitment of SHIP-1 or an analogous mechanism is a physiologically relevant mode of JNK inhibition.
MATERIALS AND METHOD
cDNAs and constructs
The cDNAs encoding wild-type Dok-3 and a mutant in which all four carboxyl-terminal tyrosines were replaced by phenylalanines (Dok-3 4F) have been described previously (26). Additional cDNAs in which individual tyrosines or combinations of tyrosines were changed to phenylalanines were generated by PCR. cDNAs encoding chimeric proteins in which the PH and PTB domains of Dok-3 (amino acids 1 to 255) were fused with the catalytic domain of SHIP-1 (amino acids 401 to 900) (cDNA provided by Gerry Krystal, Vancouver, British Columbia, Canada) (13) or Csk (amino acids 162 to 440) were created by PCR. A chimera bearing an inactive version of the catalytic region of SHIP-1 (D672A mutant) was also generated. All cDNAs were verified by sequencing to ensure that they contained no unwanted mutation (data not shown). The construct coding for constitutively activated FynT (FynT Y528F) has been described elsewhere (14).
For transient transfections, cDNAs were cloned into the vector pXM139, which contains the simian virus 40 origin of replication and the adenovirus major late promoter. For stable transfection in A20 cells, cDNAs were inserted into the mammalian expression vector pNT-Neo, which bears an SRα-based promoter and the neo gene.
Cells and transfections
Cos-1 cells were grown in alpha minimal essential medium supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. They were transfected by the DEAE-dextran method as described elsewhere (16). A20 B cells were propagated in RPMI medium supplemented with 10% FBS and antibiotics. Stable and transient transfections of A20 were achieved as outlined previously (26). Splenic B cells were purified by negative selection (Stem Cell Technologies, Vancouver, Canada) from 4- to 8-week-old C57BL/6 mice. The purity of the preparations was confirmed by flow cytometry, using surface IgM and IgD as markers. In all experiments, greater than 90% of cells were positive for surface immunoglobulins (data not shown). Purified B cells were then propagated in RPMI medium supplemented with 10% FBS, antibiotics, and lipopolysaccharide (LPS; 20 μg/ml) prior to experimentation (see below).
Antibodies
Rabbit antisera reacting against Dok-3, SHIP-1, Csk, and FynT have been reported elsewhere (9, 14, 26). A mouse monoclonal antibody (MAb) directed against the FLAG epitope tag (M2) was purchased from Sigma-Aldrich (Oakville, Canada). Polyclonal rabbit antibodies directed against Erk-1 and Erk-2, JNK, p38, phospho-Erk (pT180/pY182), phospho-JNK (pT183/pY185), phospho-p38 (pT180/pY182), ATF-2, phospho-ATF-2 (pT69/pT71), Rac-1, and phospho-Btk (pY223) were purchased from Cell Signaling Technologies (Beverly, Mass.). Anti-phosphotyrosine MAb 4G10 was obtained from Upstate Biotechnologies (Lake Placid, N.Y). Anti-Btk MAb G149-11 was purchased from Pharmingen.
Cell stimulation
A20 B cells (2 × 107 cells/ml) were stimulated with the indicated concentrations of F(ab′)2 fragments of sheep anti-mouse (SAM) IgG (Jackson Immunoresearch Laboratories, West Grove, Pa.). After stimulation, cells were lysed in TNE buffer (1× TNE is 50 mM Tris [pH 8.0], 1% Nonidet P-40, and 2 mM EDTA) supplemented with protease and phosphatase inhibitors, as described in an earlier report (26). Lysates were processed for immunoprecipitation, in vitro binding assays, or immunoblotting. Mouse splenic B cells (2 × 107 cells/ml) were stimulated for the indicated periods of time with F(ab′)2 fragments of goat anti-mouse (GAM) IgM (5 or 10 μg/ml) (Jackson Immunoresearch Laboratories) and lysed by addition of boiling 2× sodium dodecyl sulfate sample buffer. In some cases, A20 B cells or ex vivo B cells were stimulated with phorbol myristate acetate (PMA; 50 ng/ml).
Immunoprecipitations and immunoblots
Immunoprecipitations and immunoblots were performed as described in earlier reports (14, 44). Immunoreactive products were detected using 125I-protein A (Amersham Pharmacia Biotech), horseradish peroxidase-coupled protein A (Amersham Pharmacia Biotech), 125I-rabbit anti-mouse IgG (ICN Biochemicals), or horseradish peroxidase-SAM IgG (Amersham Pharmacia Biotech). Radioactive signals were quantitated using a phosphorimager (BAS2000; Fuji).
In vitro binding assays
Glutathione S-transferase (GST) fusion proteins encompassing the SH2 domain of SHIP-1 or Csk have been described elsewhere (26). A construct coding for GST fused to the Rac-binding domain of Pak was obtained from Sylvain Meloche, IRCM, Montreal, Canada. Production and purification of fusion proteins, as well as in vitro binding assays, were performed according to previously described protocols (33).
BCR-triggered IL-2 production
Pools of three independent A20 B-cell clones (105 cells in 200 μl) were stimulated for 24 h at 37°C in 96-well plates in the presence of various concentrations of F(ab′)2 fragments of SAM IgG. As a control, cells were stimulated with a combination of PMA (100 ng/ml) and ionomycin (1 μM). The resulting production of IL-2 was measured using a bioassay, in agreement with an established protocol (1). All cytokine secretion assays were performed in triplicate and were repeated at least three times. Representative results are shown.
BCR-induced proliferation
Ex vivo splenic B cells were stimulated for 48 h in the presence of either F(ab′)2 fragments of GAM IgM or LPS (20 μg/ml). They were then labeled for 6 h with tritiated thymidine to measure proliferation according to standard procedures (1).
BCR-induced activation of IL-2 promoter
A20 cells (10 × 106) were transfected by electroporation with 20 μg of pIL-2 promoter-luciferase in combination with 5 μg of the indicated plasmids and 1 μg of pXM139-β-galactosidase (to standardize for transfection efficiency). After 40 h, 2 × 106 cells were stimulated for 6 h with 10 μg of F(ab′)2 fragments of SAM IgG/ml or with a combination of 100 ng of PMA/ml and 1 μM ionomycin. Cells were then lysed and assayed for luciferase activity using the luciferase reporter assay system (Promega) and a luminometer (EG&G Berthold). Luciferase activity was calculated either as the percentage of PMA plus ionomycin-induced activity or as the increase (n-fold) over what was seen with unstimulated cells. β-Galactosidase activity was determined using a β-gal assay system (Tropix, Bedford, Mass.). To verify expression of the Dok-3 polypeptides, equal numbers of viable cells were lysed in boiling sample buffer and probed by immunoblotting with anti-Dok-3 or anti-FLAG antibodies.
BRC-induced calcium fluxes
A20 cells were loaded with the calcium indicator dye fura-2/AM. They were subsequently resuspended in calcium assay buffer (140 mM NaCl, 5 mM KCl, 5 mM glucose, 1 mM CaCl2, 1 mM MgCl2, 2 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4]), and intracellular calcium levels were measured using a Perkin-Elmer LS50 luminescence spectrometer. After 30 s of incubation at 37°C to establish baseline values, cells were activated by addition of F(ab′)2 fragments of SAM IgG (10 μg/ml). The response of cells to the calcium ionophore ionomycin (1 μM) was later tested as a control.
RESULT
The inhibitory potential of Dok-3 in B cells requires tyrosines 325 and 343
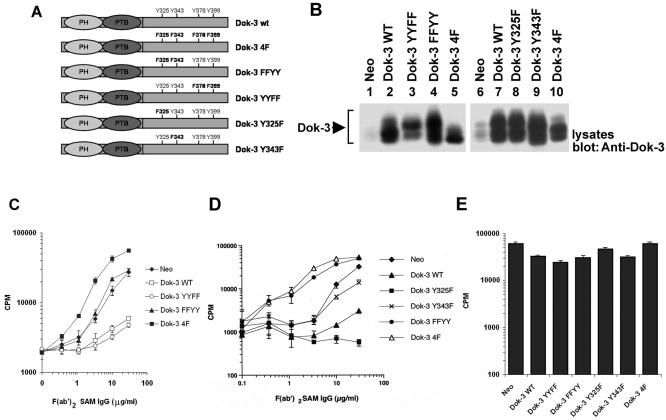
To elucidate the mechanism of action of Dok-3, we first determined which of the four carboxyl-terminal tyrosines were required for its ability to inhibit B-cell activation. To this end, mutants of Dok-3 in which the carboxyl-terminal tyrosines were replaced alone or in combination by phenylalanines were created (Fig. 1A). A20 B cells, which express IgG at their surface (29), were stably transfected with constructs encoding these polypeptides. Monoclonal cell lines were generated and tested for Dok-3 overexpression by immunoblotting with anti-Dok-3 antibodies (data not shown) (Fig. 1B). Expression levels were quantitated using a phosphorimager (data not shown). All clones selected for our experiments expressed amounts of Dok-3 that were four to five times greater than for parental A20 cells (Fig. 1B), and they exhibited unaltered levels of BCR, FcγRIIB, CD45, and CD40 (data not shown).
FIG. 1.
Tyrosines 325 and 343 are required for the inhibitory effect of Dok-3 in A20 B cells. (A) Primary structures of the Dok-3 mutants created for this study. The positions of the PH and PTB domains, as well as of the four carboxyl-terminal tyrosines, are highlighted. (B) Expression of the various Dok-3 polypeptides in A20 B cells. The expression of Dok-3 in pools of three independent transfectants for each of the Dok-3-encoding constructs was measured by immunoblotting of equivalent amounts of total cell proteins with anti-Dok-3. (C) Impact of Dok-3 YYFF and Dok-3 FFYY on BCR-induced IL-2 secretion. Pools of three transfectants were activated for 24 h with the indicated amounts of F(ab′)2 fragments of SAM IgG. IL-2 release was determined in a bioassay using the IL-2-dependent T-cell line HT-2. Assays were done in triplicate, and average values with standard deviations are shown. (D) Effect of Dok-3 Y325F and Dok-3 Y343F on BCR-triggered IL-2 production. The experiment was conducted as outlined for panel C, except that other transfectants were tested. (E) Influence of Dok-3 polypeptides on responsiveness to PMA plus ionomycin. Cells were stimulated for 24 h with PMA and ionomycin. IL-2 secretion was subsequently measured as described for panel C.
Pools of at least three independent transfectants of each type were tested for their ability to respond to BCR stimulation (Fig. 1C and D). Cells were stimulated with F(ab′)2 fragments of SAM IgG (anti-IgG), and the release of IL-2 in the supernatant was measured using a bioassay. In agreement with the findings of Lemay et al. (26), clones expressing wild-type Dok-3 exhibited a pronounced reduction of BCR-evoked IL-2 production compared with cells expressing the neomycin resistance marker alone (Neo). In contrast, clones containing Dok-3 4F demonstrated enhanced cytokine secretion. More importantly, these studies also revealed that mutation of the first two tyrosines, Y325 and Y343 (Dok-3 FFYY mutant), abolished the inhibitory potential of Dok-3 (Fig. 1C). In some experiments, cells expressing this mutant actually showed a markedly enhanced responsiveness in comparison to control Neo cells (Fig. 1D). In contrast, replacement of the last two tyrosines, Y378 and Y399 (Dok-3 YYFF mutant), had no impact. The various cells responded equally well to the combination of PMA and ionomycin (Fig. 1E).
To ascertain whether Y325, Y343, or both were responsible for the inhibitory influence of Dok-3, single point mutants were analyzed (Fig. 1D). We found that mutation of Y325 alone did not prevent the inhibitory effect of Dok-3. Whereas alteration of Y343 alone alleviated to some extent the inhibitory influence of Dok-3, the impact of this mutation was not as marked as that of mutation of both Y325 and Y343. Therefore, these findings indicated that both Y325 and Y343 were involved in the inhibitory function of Dok-3 in A20 cells. It is noteworthy, though, that mutation of Y325 alone seemed to augment the capacity of Dok-3 to inhibit IL-2 production (Fig. 1D) (data not shown). While the basis for this effect is not known, this observation raised the possibility that Y325 may also carry out a stimulatory function under certain conditions.
The inhibitory effect of Dok-3 in B cells correlates with binding to SHIP-1 but not Csk
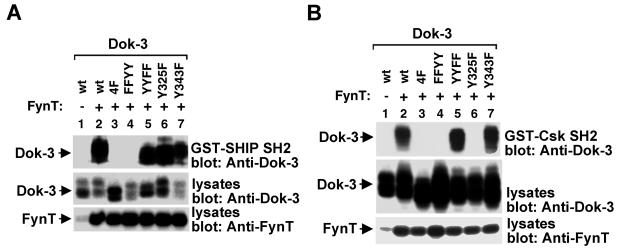
To clarify the biochemical mechanism implicated in the inhibitory function of Dok-3, the tyrosine residues of Dok-3 responsible for mediating the interaction with the SH2 domain of SHIP-1 or Csk were identified. The various Dok-3 mutants detailed above were transiently expressed in Cos-1 cells in the presence or absence of an activated version of the Src-related PTK FynT to allow Dok-3 tyrosine phosphorylation. After cell lysis, the ability of the Dok-3 variants to interact with the SHIP-1 (Fig. 2A) or Csk (Fig. 2B) SH2 domain was revealed by in vitro binding assays. As reported elsewhere (26), wild-type Dok-3 interacted with the SH2 domain of SHIP-1 (Fig. 2A) or Csk (Fig. 2B) when expressed in the presence (lanes 2), but not in the absence (lanes 1), of FynT. Mutation of all four carboxyl-terminal tyrosines (lanes 3) abolished these associations. In addition, these experiments revealed that replacement of Y325 (Fig. 2A, lane 6) or Y343 (lane 7) alone was insufficient to abolish binding of Dok-3 to the SHIP-1 SH2 domain. This interaction was eliminated only when both residues were replaced (lane 4). Mutation of the two other tyrosines (Y378 and Y399, lane 5) had no effect on binding. In the case of the Csk SH2 domain, substitution of Y325 alone (Fig. 2B, lane 6), but not Y343 alone (lane 7), was found to eliminate the association with Dok-3. Therefore, these results showed that while the SHIP-1 SH2 domain bound to Y325 and Y343, the Csk SH2 region interacted only with Y325. Since mutation of Y325 alone was insufficient to abolish the inhibitory effect of Dok-3 (Fig. 1D), it is unlikely that the inhibitory function of Dok-3 in B cells was mediated by Csk. Rather, it correlated with the capacity of Dok-3 to bind SHIP-1 through Y325 and Y343.
FIG. 2.
Differential binding of the SHIP-1 or Csk SH2 domain to tyrosine-phosphorylated Dok-3. Cos-1 cells were transfected with the indicated dok-3 cDNAs in the presence or absence of FynT Y528F. Equivalent amounts of total cell proteins were then incubated with either GST-SHIP-1 SH2 domains (A) or GST-Csk SH2 domains (B). After several washes, the association with Dok-3 was detected by immunoblotting with anti-Dok-3 (top blocks). The expressions of Dok-3 (middle blocks) and FynT (bottom blocks) were verified by immunoblotting of total cell lysates with anti-Dok-3 and anti-FynT, respectively.
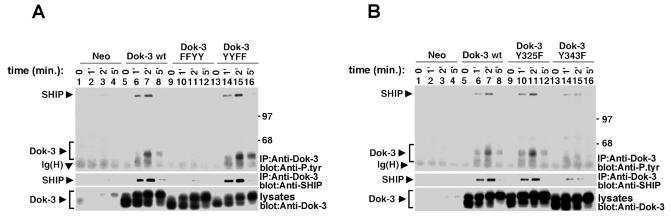
To add support to this possibility, the ability of the various Dok-3 mutants to associate with SHIP-1 was examined in A20 B cells (Fig. 3). Cells were stimulated for the indicated periods of time with F(ab′)2 fragments of anti-IgG, and Dok-3 was immunoprecipitated from cell lysates by using a rabbit anti-Dok-3 serum. The presence of associated SHIP-1 was revealed by immunoblotting with anti-phosphotyrosine (Fig. 3, top blocks in panels A and B) or anti-SHIP-1 (middle blocks) antibodies. As described previously (26), overexpression of wild-type Dok-3 (Fig. 3A, top block, lanes 5 to 8) resulted in an increase in BCR-induced tyrosine phosphorylation of Dok-3 and its associated SHIP-1 compared to what was seen with control Neo cells (lanes 1 to 4). The amount of SHIP-1 found in Dok-3 immunoprecipitates was also increased (middle block). More significantly, this study showed that mutation of both Y325 and Y343 (Fig. 3A, lanes 9 to 12, Dok-3 FFYY) also abolished BCR-triggered Dok-3 tyrosine phosphorylation (top block) and association with SHIP-1 (middle block). In comparison, mutation of both Y378 and Y399 (lanes 13 to 16, Dok-3 YYFF) had no impact. The effect of mutation of either Y325 or Y343 alone was examined next (Fig. 3B). This analysis demonstrated that mutation of Y325 (lanes 9 to 12) had no influence on the ability of Dok-3 to undergo tyrosine phosphorylation (top block) and associate with SHIP-1 (middle block). Moreover, replacement of Y343 (lanes 13 to 16) caused an incomplete, although appreciable, decrease in Dok-3 tyrosine phosphorylation (top block) and association with SHIP-1 (top and middle blocks). Hence, these observations implied that both Y325 and Y343 participated in the interaction between Dok-3 and SHIP-1 in activated B cells.
FIG. 3.
Tyrosines 325 and 343 are required for the association between Dok-3 and SHIP-1 in B cells. Pools of three independent transfectants of the indicated A20 derivatives were stimulated for various periods of time with F(ab′)2 fragments of SAM IgG. Following lysis, Dok-3 was immunoprecipitated from equivalent quantities of total cell proteins and probed by immunoblotting with anti-phosphotyrosine (Anti-P.tyr; top blocks) or anti-SHIP-1 (middle blocks). The presence of Dok-3 was confirmed by immunoblotting of equivalent amounts of total cell lysates with anti-Dok-3 (bottom blocks). (A) Comparison of Dok-3 FFYY and Dok-3 YYFF. Note that a small amount of Dok-3-associated SHIP-1 could be seen upon longer autoradiographic exposures in cells expressing the neo gene or Dok-3 FFYY (data not shown). This is presumably due to an interaction between endogenous Dok-3 and SHIP-1. (B) Comparison of Dok-3 Y325F and Dok-3 Y343F.
The catalytic activity of SHIP-1 is sufficient to mediate the inhibitory effect of Dok-3
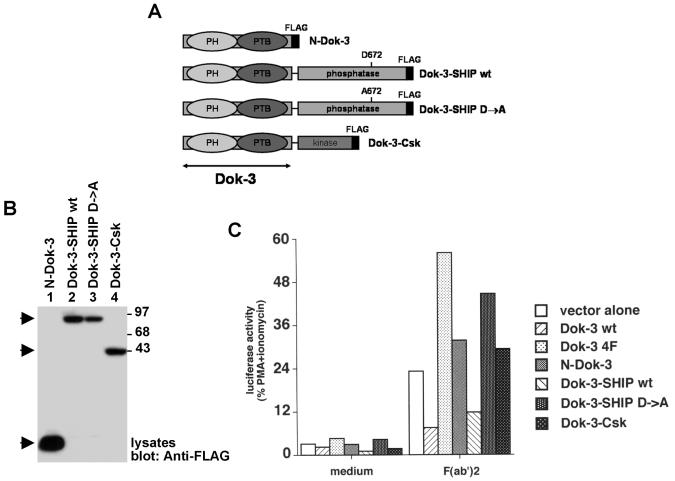
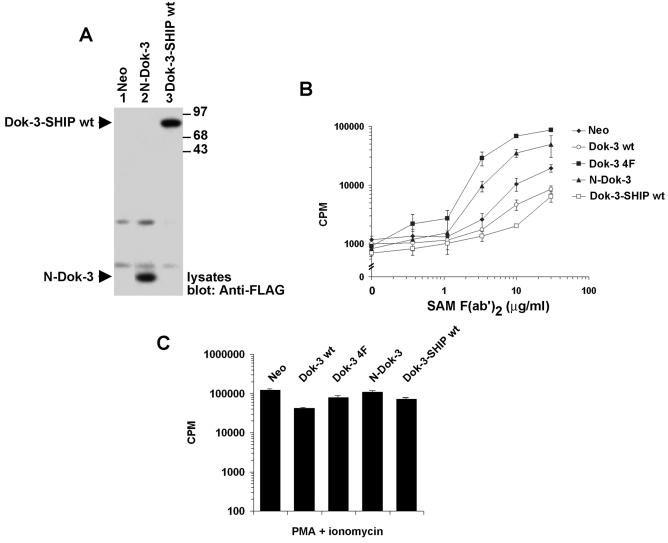
Collectively, these results support the idea that the inhibitory potential of Dok-3 is mediated by SHIP-1 rather than Csk. To demonstrate this notion more clearly, we tested whether the function of Dok-3 could be regained by creating a chimera in which the PH and PTB domains of Dok-3 are linked to the catalytic region of SHIP-1 (Fig. 4A). This fusion, termed Dok-3-SHIP-1, would presumably allow recruitment of the catalytic domain of SHIP-1 to the intracellular locales targeted by the Dok-3 PH and PTB domains. As controls, constructs in which the PH and PTB domains of Dok-3 were expressed in isolation (N-Dok-3), or connected to an inactive version of the catalytic domain of SHIP-1 (Dok-3-SHIP-1 D→A) or to the kinase domain of Csk (Dok-3-Csk), were produced. All fusions carried a FLAG epitope tag at the carboxyl terminus in order to allow comparison of levels of expression.
FIG. 4.
The catalytic activity of SHIP-1, but not Csk, is sufficient to mediate the inhibitory impact of Dok-3 on IL-2 promoter activation. (A) Schematic representation of the chimeric proteins used for this experiment (see text for details). All chimeras contained a FLAG epitope tag at the carboxyl terminus. (B) Expression of Dok-3 chimeras in transiently transfected A20 cells. Cells were transfected with the indicated cDNAs in the presence of an IL-2 promoter-luciferase reporter. After 40 h, expression of the chimeric proteins was revealed by immunoblotting of equivalent amounts of total cell proteins with anti-FLAG. (C) Luciferase assays. Transfected cells were stimulated or not for 6 h with F(ab′)2 fragments of SAM IgG. Luciferase was then measured, as described in Materials and Methods. Values are presented as percentages of the luciferase activity induced by stimulation with PMA plus ionomycin. Similar conclusions were reached when values were calculated as the increase in induction (n-fold) over unstimulated controls (data not shown).
We initially tested the function of these chimeras in transient transfection assays (Fig. 4B and C). A20 cells were transfected with the different constructs in the presence of an IL-2 promoter-driven luciferase reporter plasmid. An immunoblot of cell lysates with anti-FLAG antibodies indicated that all the chimeras were expressed in comparable amounts (Fig. 4B). After 40 h, cells were stimulated or not for 6 h with F(ab′)2 fragments of anti-IgG and analyzed for luciferase activity (Fig. 4C). In keeping with the results obtained with stable transfections (26), we found that wild-type Dok-3 inhibited activation of the IL-2 promoter-luciferase reporter in response to BCR stimulation. Conversely, this response was enhanced by expression of Dok-3 4F. More importantly, we also found that the active Dok-3-SHIP-1 chimera caused a repression of BCR-induced activation of IL-2 promoter activity. In comparison, N-Dok-3 and Dok-3-Csk had little or no effect, while inactive Dok-3-SHIP-1 induced an increase in activity.
To expand on these findings, the active Dok-3-SHIP-1 chimera and N-Dok-3 were stably expressed in A20 cells (Fig. 5). An immunoblot analysis of representative clones by using anti-FLAG antibodies showed that the two polypeptides were expressed in equal amounts in these cells (Fig. 5A). Upon stimulation with F(ab′)2 fragments of anti-IgG (Fig. 5B), cells expressing Dok-3-SHIP-1 exhibited a reduced BCR-triggered cytokine secretion in comparison to Neo cells in a manner analogous to that of clones overexpressing wild-type Dok-3. In contrast, reminiscent of cells bearing Dok-3 4F, those containing N-Dok-3 had a moderately augmented response. Nevertheless, all cells responded equally to PMA and ionomycin (Fig. 5C). Thus, combined with the results shown in Fig. 4, these findings indicated that the inhibitory impact of Dok-3 in A20 cells was mimicked by replacing the carboxyl-terminal region of Dok-3 with the active catalytic domain of SHIP-1 but not with that of Csk.
FIG. 5.
The catalytic region of SHIP-1 is sufficient to mediate the inhibitory effect of Dok-3 on BCR-triggered cytokine production. Pools of stable A20 transfectants expressing Dok-3-SHIP-1 or N-Dok-3 were tested. (A) Expression levels. The expression levels of the chimeric proteins were ascertained by immunoblotting of equivalent quantities of cell proteins with anti-FLAG. (B) BCR-induced IL-2 secretion. Antigen receptor-triggered IL-2 secretion was tested as outlined for Fig. 1C. Assays were done in triplicate and average values with standard deviations are shown. (C) Responsiveness to PMA plus ionomycin. Cells were tested as detailed for Fig. 1E. Assays were done in triplicate, and average values with standard deviations are shown.
Selective inhibition of JNK pathway by the Dok-3-SHIP-1 complex in B cells
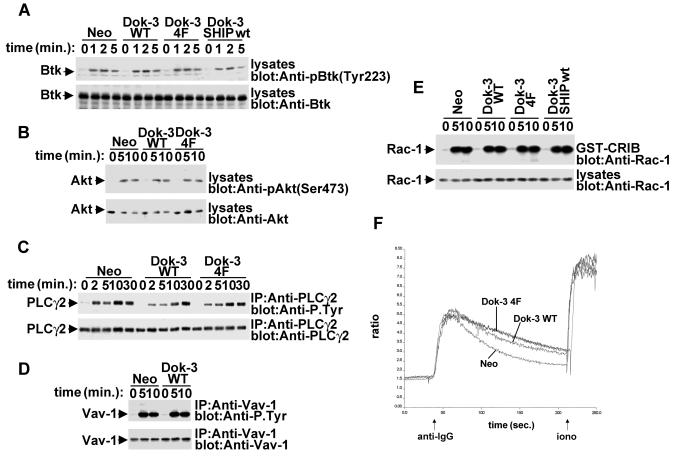
Previous studies have shown that by converting PI(3,4,5)P3 into PI(3,4)P2, SHIP-1 can inhibit the membrane recruitment and activation of Btk and Akt/PKB in B cells (2, 4, 5, 39). This inhibitory effect suppresses BCR-triggered phospholipase C gamma (PLC-γ) tyrosine phosphorylation, calcium fluxes, and cell survival. To ascertain whether Dok-3 is acting on these targets, we examined the impact of wild-type Dok-3, Dok-3 4F, and Dok-3-SHIP-1 on these parameters (Fig. 6 and data not shown). In repeated experiments, we were unable to demonstrate that Dok-3 or Dok-3-SHIP-1 had an effect on BCR-induced tyrosine phosphorylation of Btk, PLC-γ2, or Vav-1, induction of calcium fluxes, or activation of Akt/PKB. Likewise, no impact was noted on BCR-triggered clustering and internalization of the antigen receptor complex (data not shown). Thus, these findings indicate that the Dok-3-SHIP-1 complex may inhibit BCR signaling by acting on targets distinct from those previously shown to be regulated by SHIP-1.
FIG. 6.
Lack of influence of Dok-3 on multiple signaling pathways in A20 B cells. A20 derivatives were stimulated for the indicated periods of time with F(ab′)2 fragments of SAM IgG. (A) BCR-induced Btk activation. Lysates were probed by immunoblotting with phosphospecific anti-Btk antibodies (anti-pY223) (upper block). The abundance of Btk was verified by reprobing of the immunoblot membrane with anti-Btk (lower block). (B) BCR-triggered Akt/PKB activation. Lysates were probed by immunoblotting with phosphospecific anti-Akt antibodies (anti-pS473) (upper block). The abundance of Akt was verified by reprobing of the immunoblot membrane with anti-Akt (lower block). (C) BCR-induced PLC-γ2 tyrosine phosphorylation. PLC-γ2 was immunoprecipitated from cell lysates and probed by immunoblotting with anti-phosphotyrosine (upper block). The abundance of PLC-γ2 was verified by reprobing of the membrane with anti-PLC-γ2. (D) BCR-elicited Vav-1 tyrosine phosphorylation. Vav-1 was immunoprecipitated from cell lysates and probed by immunoblotting with anti-phosphotyrosine (upper block). The abundance of Vav-1 was verified by reprobing of the membrane with anti-Vav-1. (E) BCR-induced Rac-1 activation. Activated Rac-1 was recovered from cell lysates by using a GST fusion protein encompassing the Rac-1-binding region of PAK (GST-CRIB). Associated Rac-1 was detected by immunoblotting with anti-Rac-1. The abundance of Rac-1 in the A20 derivatives was verified by immunoblotting of total cell lysates with anti-Rac-1. (F) BCR-evoked calcium fluxes. Cells were stimulated and calcium fluxes were monitored, as outlined in Materials and Methods.
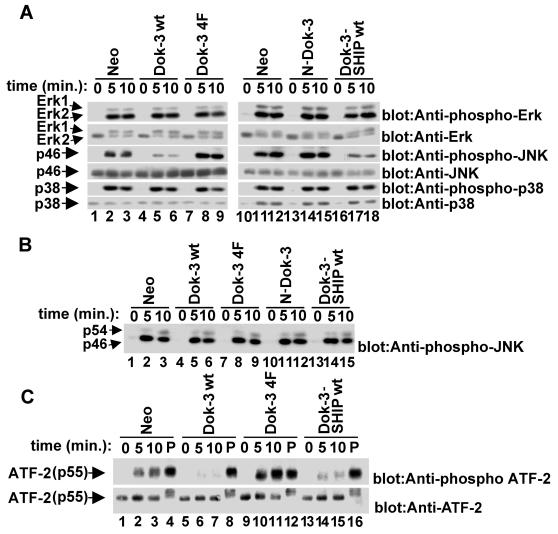
In an attempt to identify these targets, we examined the impact of wild-type Dok-3, Dok-3 4F, Dok-3-SHIP-1, and N-Dok-3 on the activation of various mitogen-activated protein kinases (MAPKs), including Erk-1, Erk-2, JNK (p54 and p46), and p38 (Fig. 7). A20 derivatives were stimulated with F(ab′)2 fragments of anti-IgG, and lysates were probed by immunoblotting with antibodies recognizing the activated forms of Erk-1 and Erk-2 (Fig. 7A, top blocks), JNK (third blocks from top), and p38 (fifth blocks). The abundance of the MAPKs was also verified by reprobing of the immunoblot membranes with antibodies recognizing the total amounts of these enzymes (second, fourth, and sixth blocks). This study demonstrated that none of the Dok-3 variants (lanes 4 to 9 and 13 to 18) had an influence on BCR-mediated activation of Erk-1, Erk-2, and p38 (first and fifth blocks). However, expression of either wild-type Dok-3 (lanes 4 to 6) or Dok-3-SHIP-1 (lanes 16 to 18) clearly inhibited (approximately threefold; estimated using a phosphorimager) the activation of JNK, especially p46JNK (third blocks) in comparison to what was seen with expression of the neomycin resistance marker alone (lanes 1 to 3 and 10 to 12). By contrast, Dok-3 4F (lanes 7 to 9) had a stimulatory effect on JNK activation. These variations in JNK activity were not caused by changes in the abundance of JNK, as all cells expressed equivalent amounts of JNK protein (fourth blocks). Furthermore, the effect of Dok-3 on BCR-induced JNK activation did not reflect a global defect in JNK responsiveness, as cells responded in an equal manner to PMA (Fig. 7B).
FIG. 7.
Selective inhibition of the JNK cascade by the Dok-3-SHIP-1 complex. (A) Effect of Dok-3 polypeptides on BCR-induced MAPK activation. Pools of A20 derivatives expressing the indicated polypeptides were stimulated for various periods of time with F(ab′)2 fragments of SAM IgG (3 μg/ml). After lysis, the activation of Erk-1 and Erk-2 (top blocks), JNK (third blocks from top), and p38 (fifth blocks) was assayed by immunoblotting of equivalent amounts of total cell proteins with antibodies specific for the activated forms of MAPKs. Levels of expression of MAPKs were confirmed by immunoblotting with anti-Erk (second blocks), anti-JNK (fourth blocks), and anti-p38 (sixth blocks). (B) Impact of Dok-3 polypeptides on PMA-induced activation of JNK. The experiment was conducted as detailed for panel A, except that cells were stimulated with PMA (100 nM). (C) Effect of Dok-3 polypeptides on activation of transcription factor ATF-2. Pooled cell lines were stimulated for the indicated times with F(ab′)2 fragments of SAM IgG (3 μg/ml) or PMA (100 nM; lanes P). The activation of ATF-2 was assayed by immunoblotting of equivalent quantities of cellular proteins with anti-phospho-ATF-2 (pT69/T71).
The JNK subclass of MAPKs is involved in a variety of biological processes, including stress-induced responses and receptor-mediated proliferation and differentiation (15, 23). Upon activation, JNKs phosphorylate and activate transcription factors such as c-Jun and activation transcription factor 2 (ATF-2). In immune cells, ATF-2 has been shown to be involved in the regulation of genes like c-jun as well as gamma interferon and tumor necrosis factor alpha (35, 42, 43). To examine whether Dok-3 has an impact on a known downstream target of JNK, lysates from resting and activated A20 derivatives were probed by immunoblotting with an antibody recognizing ATF-2 polypeptides dually phosphorylated at threonines 69 and 71, the known sites of JNK-mediated phosphorylation (Fig. 7C, upper block) (28). This experiment revealed that, compared with expression of the neo gene alone (lanes 1 to 3), overexpression of wild-type Dok-3 (lanes 5 to 7) caused an inhibition (approximately threefold) of BCR-triggered ATF-2 phosphorylation. An analogous change was observed in cells containing the Dok-3-SHIP-1 chimera (lanes 13 to 15), whereas an opposite effect was seen in cells bearing Dok-3 4F (lanes 9 to 11). No differences in ATF-2 phosphorylation were observed when cells were exposed to PMA (lanes 4, 8, 12, and 16), and all cells expressed equivalent quantities of ATF-2 (lower block). Thus, these data showed that the Dok-3-SHIP-1 complex was involved in selective inhibition of the JNK signaling cascade in A20 B cells.
Enhanced JNK activation in B cells derived from SHIP-1-deficient mice
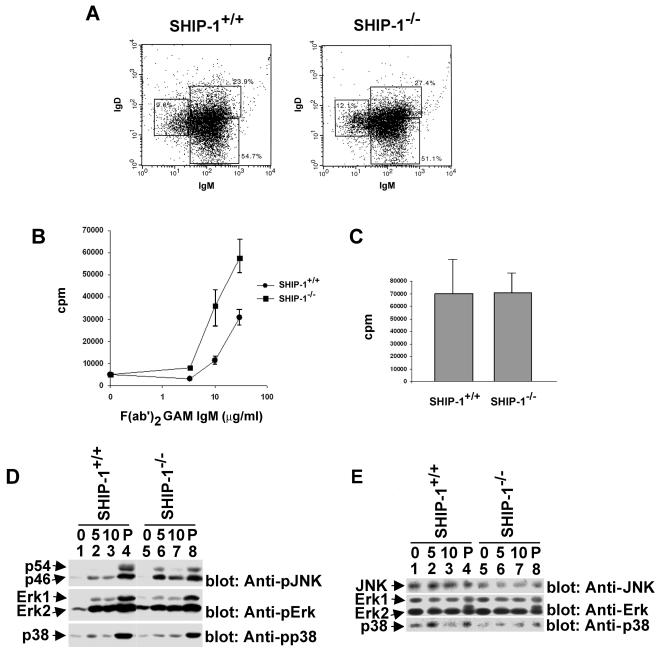
To examine the physiological relevance of these findings, we assessed whether lack of SHIP-1 in B cells had an impact on the extent of JNK activation induced by BCR stimulation by using B cells derived from SHIP-1-deficient mice (Fig. 8). It should be pointed out first that a relative increase in the abundance of mature (IgMlo/IgDhi) over immature (IgMhi/IgDlo) splenic B cells is found in SHIP-1−/− animals (5, 20). One potential problem stemming from this difference is that the B-cell populations isolated from SHIP-1-deficient mice are not exactly comparable to those from wild-type animals. To circumvent this problem, splenic B cells from SHIP-1+/+ and SHIP-1−/− mice were subjected to an initial in vitro stimulation with LPS. Our preliminary studies had indicated that this treatment corrected the difference in relative abundance of immature and mature B cells between the two types of animals (Fig. 8A) (Robson and Veillette, unpublished). Whereas the basis for this effect remains to be clarified, it allows a more adequate comparison of B cells from wild-type and SHIP-1-deficient mice.
FIG. 8.
SHIP-1 is required for JNK regulation in ex vivo mouse B cells. B cells were purified from SHIP-1+/+ and SHIP-1−/− mice and then propagated for 60 h in LPS-containing culture medium as detailed in Materials and Methods. (A) Flow cytometry. After propagation in LPS-containing medium, B-cell populations were identified by flow cytometry using antibodies against surface IgM and surface IgD. Percentages of cells in the various populations are shown. (B) BCR-induced proliferation. LPS-propagated cells were stimulated for 48 h with the indicated concentrations of F(ab′)2 fragments of GAM IgM. Proliferation was then assessed by measuring tritiated thymidine incorporation. Assays were done in triplicate. Average values with standard deviations are shown. (C) LPS-induced proliferation. The experiment was conducted as detailed for panel B, except that cells were stimulated with LPS (20 μg/ml). Assays were done in triplicate, and average values with standard deviations are shown. (D) BCR-triggered activation of MAPKs. Cells were stimulated and lysates were analyzed as detailed for Fig. 7. (E) Levels of MAPKs in ex vivo mouse B cells. The abundance of the various MAPKs in the cells used in panel D was assessed by immunoblotting of cell lysates with the indicated antibodies.
First, we tested the impact of SHIP-1 deficiency on BCR-mediated proliferation of LPS-propagated B cells (Fig. 8B). To study the FcγRIIB-independent function of SHIP-1, B cells were stimulated with F(ab′)2 fragments of anti-IgM, which trigger the antigen receptor without engaging FcγRIIB. After 48 h, proliferation was determined by measuring tritiated thymidine incorporation. This analysis showed that lack of SHIP-1 caused an enhancement of B-cell proliferation in response to BCR engagement. The magnitude of the increase seen in these LPS-propagated B cells was similar to that previously described for freshly isolated SHIP-1-deficient B cells (5, 20, 27). By contrast, SHIP-1 deficiency had no impact on the proliferative response to LPS (Fig. 8C). Therefore, these findings confirmed that SHIP-1 had an inhibitory role in B cells in the absence of ligation of FcγRIIB and established that LPS pretreatment did not alleviate this effect.
Next, LPS-induced B cells were stimulated with F(ab′)2 fragments of anti-IgM and the activation of JNK, Erk, and p38 was monitored as outlined above (Fig. 8D). This study revealed that BCR-evoked activation of p54JNK and, to a lesser extent, p46JNK (top block) was noticeably augmented in B cells from SHIP-1−/− mice (lanes 5 to 7) in comparison to what was seen with cells obtained from SHIP-1+/+ animals (lanes 1 to 3). However, both cell types responded in an equivalent manner to PMA (lanes 4 and 8). Contrary to the impact of SHIP-1 deficiency on JNK activation (Fig. 8D, top block), there was no appreciable effect on Erk (middle block) or p38 (bottom block) activation. The lack of impact of SHIP-1 deficiency on Erk activation was in keeping with the finding of Liu et al. (27). These various effects were not caused by differences in the levels of expression of the kinases, as demonstrated by immunoblotting of parallel cell lysates with the relevant antibodies (Fig. 8E). Thus, in conclusion, these results supported the idea that recruitment of SHIP-1 by one or more mechanisms independent of FcγRIIB is implicated in selective inhibition of JNK in normal activated B cells.
DISCUSSION
In this report, we presented evidence that the ability of Dok-3 to inhibit BCR signaling was dependent on Y325 and Y343, but not Y378 and Y399, in the carboxyl-terminal segment of Dok-3. Y325 and Y343 were also needed for binding of Dok-3 to the SH2 domain of SHIP-1. In contrast, only Y325 was necessary for the interaction of Dok-3 with the Csk SH2 domain. Since mutation of Y325 alone had no impact on the inhibitory potential of Dok-3, these findings implied that Csk is unlikely to play a significant role in the inhibitory function of Dok-3 in B cells. This notion is also supported by the previous finding that the stoichiometry of the Dok-3-Csk association in activated B cells is quite low (26).
These data indicated that the inhibitory role of Dok-3 correlated with the capacity of Dok-3 to bind SHIP-1. To provide more direct evidence supporting this model, various Dok-3-based chimeric proteins were created and studied. We found that fusion of the PH and PTB regions of Dok-3 (the presumed intracellular targeting domains of Dok-3) to the active catalytic domain of SHIP-1, but not to the catalytic region of Csk, was able to reestablish efficiently the inhibitory effect of Dok-3 in A20 cells. Because the Dok-3-SHIP-1 chimera did not encompass regions outside the catalytic domain of SHIP-1, such as its SH2 domain or its sites of tyrosine phosphorylation, it is likely that the impact of Dok-3-SHIP-1 was due to Dok-3-mediated recruitment of the catalytic activity of SHIP-1. In agreement with this, we found that the effect of Dok-3-SHIP-1 was abolished by mutation of a conserved residue required for SHIP-1 catalytic activity (D672A mutation) (22). Furthermore, the biochemical consequences of expression of wild-type Dok-3 and Dok-3-SHIP-1 in A20 cells were identical.
Indeed, our biochemical studies demonstrated that Dok-3 and the Dok-3-SHIP-1 chimera inhibited very specific targets in BCR signaling. Both polypeptides had no effect on BCR-induced overall protein tyrosine phosphorylation or tyrosine phosphorylation of PLC-γ2 and Vav-1 (Fig. 6 and data not shown). Likewise, they failed to have an impact on the BCR-triggered activation of Btk and Akt/PKB or the induction of calcium fluxes, which are known to be regulated by FcγRIIB-associated SHIP-1 (2, 4, 31, 32, 39). Surprisingly, however, Dok-3 and Dok-3-SHIP-1 caused an inhibition of BCR-induced phosphorylation and activation of JNK, while they had no effect on other MAPKs like Erk-1, Erk-2, and p38. Conversely, the dominant-negative Dok-3 4F induced an increase in JNK activity in activated B cells. Similar effects were seen on ATF-2, a known downstream target of JNK. Therefore, these results implied that the Dok-3-SHIP-1 complex was acting by selectively inhibiting the JNK pathway. This notion is also supported by the finding that B cells derived from SHIP-1-deficient mice exhibited an augmentation of BCR-elicited activation of JNK but not Erk-1, Erk-2, and p38. While this last observation did not establish that Dok-3 per se was responsible for recruitment of SHIP-1 leading to JNK regulation in normal B cells, this was nevertheless consistent with the notion that non-FcγRIIB-interacting SHIP-1 was implicated in selective down-regulation of JNK activity.
Like other MAPKs, JNKs are activated through phosphorylation by upstream kinases consisting of MAPK kinases MKK-4 and MKK-7 as well as several MAPK kinase kinases like MEKKs and MLKs (15, 23). However, we were unable to detect an impact of Dok-3 on the phosphorylation state of MKK-4 (data not shown). It has also been reported that PLC-γ2 and Rac-1 can be involved in the activation of JNK in BCR-stimulated B cells (11, 18, 21). Once again, though, we observed no effect of Dok-3 on PLC-γ2 tyrosine phosphorylation or Rac-1 activity. Whereas it is possible that the Dok-3-SHIP-1 complex acts on a restricted pool of these JNK regulators that cannot be identified with the currently available assays, we favor the idea that Dok-3-SHIP-1 affects less conventional or as-yet-unknown activators of the JNK pathway. In particular, it may prevent the PI(3,4,5)P3-dependent membrane recruitment of a PH domain-containing enzyme or scaffold molecule involved in JNK activation. This possibility will deserve future consideration.
The ability of SHIP-1 to bind various types of docking proteins suggests that it carries out diverse functions in immune cells. As reported elsewhere, by interacting with FcγRIIB, SHIP-1 can inhibit the BCR-induced activation of Btk and Akt/PKB, thus causing diminished PLC-γ2 tyrosine phosphorylation and calcium fluxes, as well as reduced cell proliferation and survival (2, 4, 20, 27, 32, 39). Once soluble antibody secretion is initiated during the course of an immune response, this signal is presumed to prevent sustained B-cell activation in vivo. In striking contrast, by way of its association with Dok-3, SHIP-1 selectively inhibits the JNK cascade in activated B cells without measurably affecting the Btk and Akt/PKB pathways (this report). This effect is accompanied by an inhibition of BCR-induced cytokine production and proliferation (26; Robson and Veillette, unpublished). Thus, the Dok-3-SHIP-1 complex constitutes an alternative negative feedback mechanism that may be important for terminating B-cell activation in the absence of FcγRIIB coligation. It is also possible that Dok-3 qualitatively modifies the outcome of B-cell activation. Along these lines, Healy et al. reported that JNK activation is selectively deficient in tolerant, but not naïve, B cells (19). Hence, Dok-3-associated SHIP-1 may promote B-cell tolerance.
One of the most surprising results observed in the present study is that activation of Btk and Akt was not detectably inhibited by Dok-3-associated SHIP-1. Given that binding of the PH domain of Btk and Akt to PIP3 appears to be critical for their activation in response to BCR stimulation, this finding suggests that Btk and Akt may be physically protected from the action of Dok-3-SHIP-1. In all likelihood, Dok-3 and its associated SHIP-1 are targeted to an intracellular compartment distinct from those occupied by Btk and Akt. Although less likely, it is also conceivable that the substrate specificity of Dok-3-associated SHIP-1 is distinct from that of FcγRIIB-interacting SHIP-1. For example, while the SHIP-1 molecules recruited by FcγRIIB seem to target PI(3,4,5)P3, Dok-3-associated SHIP-1 may hydrolyze the 5′ phosphate of an alternative inositol phospholipid implicated in regulation of the JNK pathway. Obviously, future studies are needed to address these issues.
In addition to identifying SHIP-1 as a negative regulator of JNK signaling in B cells, our results provide evidence of the functional importance of JNK in B-cell activation. While little is known about the role of JNK in B cells, others have reported that B-cell differentiation and functions seemed to be unaffected in mice lacking expression of single jnk genes (38, 46). While one interpretation of these findings is that JNK proteins have no significant role in B cells, it is likely that there is functional redundancy between the various JNKs as well as JNK-related molecules such as p38. JNKs may also have a more specialized function in B cells. For instance, as suggested above, they may be involved in protecting B cells against tolerance induction. Clearly, based on these and our findings, more should be done to address the role of JNK in B cells.
SHIP-1 interacts not only with Dok-3 but also with other adaptors such as Dok-1, Dok-2, and Shc (13, 26). Interestingly, it has been reported that, like Dok-3, Dok-1 and Dok-2 can inhibit receptor-mediated signaling events in immune cells, including T cells and B cells (17, 30, 40, 41). In light of this, it is appealing to propose that the inhibitory effect of Dok-1 and Dok-2 in these cells is caused at least in part by SHIP-1 and inhibition of JNK signaling. Although additional evaluations are required to test this possibility, the strong sequence similarities between the Dok-related molecules suggest that this may well be the case.
Acknowledgments
We thank Sylvain Latour for critical reading of the manuscript and Keith Humphries, Cheryl Helgason, and Gerry Krystal for providing the SHIP-1-deficient mouse strain. We also thank Ken Murphy, Gary Nolan, Sylvain Meloche, and Gerry Krystal for providing some of the reagents used in this study.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the National Cancer Institute of Canada. J.D.R. held a Studentship from the CIHR, while A.V. is a Senior Scientist of the CIHR and holds a Canada Research Chair in Immune Cell Signaling.
REFERENCE
- 1.Abraham, N., M. C. Miceli, J. R. Parnes, and A. Veillette. 1991. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature 350:62-66. [DOI] [PubMed] [Google Scholar]
- 2.Aman, M. J., T. D. Lamkin, H. Okada, T. Kurosaki, and K. S. Ravichandran. 1998. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J. Biol. Chem. 273:33922-33928. [DOI] [PubMed] [Google Scholar]
- 3.Benschop, R. J., and J. C. Cambier. 1999. B cell development: signal transduction by antigen receptors and their surrogates. Curr. Opin. Immunol. 11:143-151. [DOI] [PubMed] [Google Scholar]
- 4.Bolland, S., R. N. Pearse, T. Kurosaki, and J. V. Ravetch. 1998. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity 8:509-516. [DOI] [PubMed] [Google Scholar]
- 5.Brauweiler, A., I. Tamir, J. Dal Porto, R. J. Benschop, C. D. Helgason, R. K. Humphries, J. H. Freed, and J. C. Cambier. 2000. Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5′ inositol phosphatase (SHIP). J. Exp. Med. 191:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauweiler, A. M., I. Tamir, and J. C. Cambier. 2000. Bilevel control of B-cell activation by the inositol 5-phosphatase SHIP. Immunol. Rev. 176:69-74. [DOI] [PubMed] [Google Scholar]
- 7.Cambier, J. C. 1995. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J. Immunol. 155:3281-3285. [PubMed] [Google Scholar]
- 8.Cambier, J. C., C. M. Pleiman, and M. R. Clark. 1994. Signal transduction by the B cell antigen receptor and its coreceptors. Annu. Rev. Immunol. 12:457-486. [DOI] [PubMed] [Google Scholar]
- 9.Chow, L. M., M. Fournel, D. Davidson, and A. Veillette. 1993. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature 365:156-160. [DOI] [PubMed] [Google Scholar]
- 10.Cong, F., B. Yuan, and S. P. Goff. 1999. Characterization of a novel member of the DOK family that binds and modulates Abl signaling. Mol. Cell. Biol. 19:8314-8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 12.Cox, D., B. M. Dale, M. Kashiwada, C. D. Helgason, and S. Greenberg. 2000. A regulatory role for Src homology 2 domain-containing inositol 5′-phosphatase (SHIP) in phagocytosis mediated by Fc γ receptors and complement receptor 3 (αMβ2; CD11b/CD18). J. Exp. Med. 193:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damen, J. E., L. Liu, P. Rosten, R. K. Humphries, A. B. Jefferson, P. W. Majerus, and G. Krystal. 1996. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc. Natl. Acad. Sci. USA 93:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson, D., L. M. Chow, M. Fournel, and A. Veillette. 1992. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J. Exp. Med. 175:1483-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 16.Fournel, M., D. Davidson, R. Weil, and A. Veillette. 1996. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J. Exp. Med. 183:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gugasyan, R., C. Quilici, S. T. I, D. Grail, A. M. Verhagen, A. Roberts, T. Kitamura, A. R. Dunn, and P. Lock. 2002. Dok-related protein negatively regulates T cell development via its RasGTPase-activating protein and Nck docking sites. J. Cell Biol. 158:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto, A., H. Okada, A. Jiang, M. Kurosaki, S. Greenberg, E. A. Clark, and T. Kurosaki. 1998. Involvement of guanosine triphosphatases and phospholipase C-γ2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med. 188:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy, J. I., R. E. Dolmetsch, L. A. Timmerman, J. G. Cyster, M. L. Thomas, G. R. Crabtree, R. S. Lewis, and C. C. Goodnow. 1997. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity 6:419-428. [DOI] [PubMed] [Google Scholar]
- 20.Helgason, C. D., C. P. Kalberer, J. E. Damen, S. M. Chappel, N. Pineault, G. Krystal, and R. K. Humphries. 2000. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of B lymphocytes in SHIP−/− mice. J. Exp. Med. 191:781-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiai, M., M. Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity 10:117-125. [DOI] [PubMed] [Google Scholar]
- 22.Jacob, A., D. Cooney, S. Tridandapani, T. Kelley, and K. M. Coggeshall. 1999. FcγRIIb modulation of surface immunoglobulin-induced Akt activation in murine B cells. J. Biol. Chem. 274:13704-13710. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 24.Krystal, G. 2000. Lipid phosphatases in the immune system. Semin. Immunol. 12:397-403. [DOI] [PubMed] [Google Scholar]
- 25.Kurosaki, T. 2000. Functional dissection of BCR signaling pathways. Curr. Opin. Immunol. 12:276-281. [DOI] [PubMed] [Google Scholar]
- 26.Lemay, S., D. Davidson, S. Latour, and A. Veillette. 2000. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 20:2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Q., A. J. Oliveira-Dos Santos, S. Mariathasan, D. Bouchard, J. Jones, R. Sarao, I. Kozieradzki, P. S. Ohashi, J. M. Penninger, and D. J. Dumont. 1998. The inositol polyphosphate 5-phosphatase Ship is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 188:1333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingstone, C., G. Patel, and N. Jones. 1995. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 14:1785-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muta, T., T. Kurosaki, Z. Misulovin, M. Sanchez, M. C. Nussenzweig, and J. V. Ravetch. 1994. A 13-amino-acid motif in the cytoplasmic domain of FcγRIIB modulates B-cell receptor signalling. Nature 368:70-73. [DOI] [PubMed] [Google Scholar]
- 30.Nemorin, J. G., P. Laporte, G. Berube, and P. Duplay. 2001. p62dok negatively regulates CD2 signaling in Jurkat cells. J. Immunol. 166:4408-4415. [DOI] [PubMed] [Google Scholar]
- 31.Ono, M., S. Bolland, P. Tempst, and J. V. Ravetch. 1996. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature 383:263-266. [DOI] [PubMed] [Google Scholar]
- 32.Ono, M., H. Okada, S. Bolland, S. Yanagi, T. Kurosaki, and J. V. Ravetch. 1997. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell 90:293-301. [DOI] [PubMed] [Google Scholar]
- 33.Peri, K. G., F. G. Gervais, R. Weil, D. Davidson, G. D. Gish, and A. Veillette. 1993. Interactions of the SH2 domain of lymphocyte-specific tyrosine protein kinase p56lck with phosphotyrosine-containing proteins. Oncogene 8:2765-2772. [PubMed] [Google Scholar]
- 34.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 290:84-89. [DOI] [PubMed] [Google Scholar]
- 35.Reimold, A. M., J. Kim, R. Finberg, and L. H. Glimcher. 2001. Decreased immediate inflammatory gene induction in activating transcription factor-2 mutant mice. Int. Immunol. 13:241-248. [DOI] [PubMed] [Google Scholar]
- 36.Reth, M., and J. Wienands. 1997. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15:453-479. [DOI] [PubMed] [Google Scholar]
- 37.Rohrschneider, L. R., J. F. Fuller, I. Wolf, Y. Liu, and D. M. Lucas. 2000. Structure, function, and biology of SHIP proteins. Genes Dev. 14:505-520. [PubMed] [Google Scholar]
- 38.Sabapathy, K., Y. Hu, T. Kallunki, M. Schreiber, J. P. David, W. Jochum, E. F. Wagner, and M. Karin. 1999. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 9:116-125. [DOI] [PubMed] [Google Scholar]
- 39.Scharenberg, A. M., O. El-Hillal, D. A. Fruman, L. O. Beitz, Z. Li, S. Lin, I. Gout, L. C. Cantley, D. J. Rawlings, and J. P. Kinet. 1998. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 17:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzu, S., M. Tanaka-Douzono, K. Nomaguchi, M. Yamada, H. Hayasawa, F. Kimura, and K. Motoyoshi. 2000. p56(dok-2) as a cytokine-inducible inhibitor of cell proliferation and signal transduction. EMBO J. 19:5114-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamir, I., J. C. Stolpa, C. D. Helgason, K. Nakamura, P. Bruhns, M. Daeron, and J. C. Cambier. 2000. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immunity 12:347-358. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, E. Y., J. V. Falvo, A. V. Tsytsykova, A. K. Barczak, A. M. Reimold, L. H. Glimcher, M. J. Fenton, D. C. Gordon, I. F. Dunn, and A. E. Goldfeld. 2000. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol. Cell. Biol. 20:6084-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai, E. Y., J. Yie, D. Thanos, and A. E. Goldfeld. 1996. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol. Cell. Biol. 16:5232-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veillette, A., M. A. Bookman, E. M. Horak, and J. B. Bolen. 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301-308. [DOI] [PubMed] [Google Scholar]
- 45.Veillette, A., S. Latour, and D. Davidson. 2002. Negative regulation of immunoreceptor signaling. Annu. Rev. Immunol. 20:669-707. [DOI] [PubMed] [Google Scholar]
- 46.Yang, D. D., D. Conze, A. J. Whitmarsh, T. Barrett, R. J. Davis, M. Rincon, and R. A. Flavell. 1998. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity 9:575-585. [DOI] [PubMed] [Google Scholar]