Abstract
Objective
To identify muscle physiological properties that may contribute to post-exertional fatigue and malaise in women with fibromyalgia (FM).
Methods
Healthy postmenopausal women with (n=11) and without (n=11) fibromyalgia, age 51–70 years, participated in this study. Physical characteristics along with self-reported questionnaires were evaluated. Strength loss and tissue oxygenation in response to a fatiguing exercise protocol were used to quantify fatigability and the local muscle hemodynamic profile. Muscle biopsies were obtained to assess between-group differences in baseline muscle properties using histochemical, immunohistochemical and electron microscopic analyses.
Results
No significant difference in muscle fatigue in response to exercise was apparent between healthy controls and subjects with FM. However, self-reported fatigue and pain were correlated to prolonged loss of strength following 12-min of recovery in subjects with FM. Although there was no difference in percent SDH positive (type I) and SDH negative (type II) fibers or in mean fiber cross-sectional area between groups, subjects with FM showed greater size variability and altered fiber size distribution. Only in healthy controls, fatigue-resistance was strongly correlated with the size of SDH positive fibers and hemoglobin oxygenation. By contrast, subjects with FM with the highest percentage of SDH positive fibers recovered strength most effectively, which was correlated to capillary density. However, overall, capillary density was lower in subjects with FM.
Conclusion
Peripheral mechanisms i.e. altered muscle fiber size distribution and decreased capillary density may contribute to post-exertional fatigue in subjects with FM. Understanding these defects in fibromyalgic muscle may provide valuable insight for treatment.
INTRODUCTION
Fatigue is a hallmark of functional somatic syndromes including fibromyalgia (FM) (1–3). FM is a common disorder with epidemiological studies showing that FM is found in approximately 2–5% of the U.S. population, with women having prevalence rates nine times higher than men (4, 5). The condition is characterized by chronic widespread musculoskeletal pain, tenderness, fatigue, unrefreshing sleep, dyscognition, and multiple other somatic symptoms (6–8). Although there has been considerable research in recent years, there is no consensus on a single etiopathogenesis of FM, and current theories include both central and peripheral mechanisms (9).
Although somewhat controversial, peripheral defects have been reported in FM that may contribute to both pain and fatigue, including muscle fiber dysfunction due to oxidative damage, chronic inflammation, and vasomotor dysregulation (10–12). Mitochondrial abnormalities have been described in FM and include shape, volume, orientation, and distribution that occur very rarely in healthy subjects (13–15). Mitochondrial function may also be reduced, as lower levels of ATP and phosphocreatine have been found in the muscle of persons with FM (16). Defects in capillary microcirculation (17), lower capillary density per muscle fiber, reduced capillary permeability, and changes in the capillary endothelium thickness have been described as well (18, 19). The current study was designed to test the hypothesis that reduced muscle oxidative capacity associated with decreased capillary density contributes to exercise-induced fatigue and malaise in FM.
MATERIALS AND METHODS
Subjects
Thirty seven post-menopausal women age 51–70 years participated in this study representing the racial and ethnic makeup of Kentucky. Informed written consent was obtained prior to participation, and all experimental procedures were in accordance with the University of Kentucky’s Institutional Review Board. Subjects included 23 healthy women and 14 women diagnosed with FM by a rheumatologist in accordance with the 1990 criteria set forth by the American College of Rheumatology (8). FM was characterized by number of tender points, subjective fatigue and pain using the multidimensional assessment of fatigue (MAF) (20), Borg rating of perceived exertion (RPE) 6–20 scale (21), visual analog scale (VAS) for fatigue (22) and pain (23), global rating of change scale (GRCS) (24), and the Fibromyalgia Impact Questionnaire (FIQ) (25) (Table 1).
Table 1.
Subject characteristics (n=11/group).
| Healthy Controls | Subjects with FM | P | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 59.2 ± 5.2 | 60.9 ± 5.9 | 0.489 |
| Height (cm) | 164.5 ± 5.5 | 163.2 ± 6.4 | 0.615 |
| Weight (kg) | 69.9 ± 8.3 | 69.4 ± 17.9 | 0.942 |
| Tender Points | 2.0 ± 3.4 | 15.8 ± 1.7 | <0.001* |
| Body Composition | |||
| BMI | 25.9 ± 3.1 | 25.9 ± 5.4 | 0.988 |
| % Body Fat | 37.1 ± 6.6 | 38.9 ± 9.1 | 0.604 |
| Thigh Mineral-Free Lean Mass (kg) | 3.9 ± 0.5 | 3.6 ± 0.7 | 0.244 |
| Baseline Questionnaires | |||
| IPAQ | 4,758.0 (3,424.8/6,102.8) | 5,055.0 (1,671.0/12,219.4) | 1.000 |
| FIQ | 12.2 ± 12.2 | 55.5 ± 15.6 | <0.001* |
| VAS-Pain Score Pre-exercise (mm) | 7.3 ± 12.6 | 49.1 ± 26.4 | <0.001* |
| Self-reported Exertion, Fatigue, and Pain Levels with Exercise | |||
| RPE | 13.4 ± 1.0 | 16.2 ± 2.8 | 0.005* |
| MAS of Fatigue | 11.5 ± 9.2 | 36.8 ± 6.4 | 0.001* |
| VAS-Fatigue Score (mm) | 24.5 ± 20.1 | 63.6 ± 28.7 | 0.001* |
| VAS-Pain Score Post-exercise (mm) | 15.8 ± 18.6 | 57.0 ± 28.3 | <0.001* |
| VAS-Pain Score 12-min of Recovery (mm) | 6.5 ± 13.3 | 52.5 ± 27.2 | <0.001* |
| GRCS-Pain Post-exercise | −0.5 ± 0.8 | −2.3 ± 2.5 | 0.046* |
| Strength Assessment | |||
| MVIC pre-exercise (Nm) | 131.5 ± 30.5 | 114.5 ± 20.1 | 0.137 |
| MVIC post-exercise (Nm) | 96.2 ± 29.2 | 79.5 ± 13.7 | 0.149 |
| MVIC-12 min of recovery (Nm) | 109.4 ± 29.1 | 92.0 ± 15.0 | 0.131 |
BMI, Body Mass Index;
IPAQ, International Physical Activity Questionnaire: median scores reported in MET·min·wk−1, higher scores show higher physical activity levels, n = 9 healthy controls, n = 9 FM;
FIQ, Fibromyalgia Impact Questionnaire: 0 (low impact or problems related to FM) to 100 (high impact or greater problems related to FM);
VAS-Pain or Fatigue, Visual Analog Scale: from 0 mm (lowest pain and fatigue) to 100 mm (greatest pain and fatigue);
RPE, Rating of Perceived Exertion: from 6 (easy or little to no effort) to 20 (maximum exertion);
MAS, Multidimensional Assessment of Fatigue: measuring global fatigue from 0 (low fatigue) to 50 (highest fatigue);
GRCS, Global Rating of Change Scale: from −7 (feeling a great deal worse after exercise) to 0 (no change) to +7 (feeling a great deal better after exercise);
MVIC, Maximal Voluntary Isometric Contraction.
All Data were expressed as mean ± SD except IPAQ Total Physical Activity Score that was expressed as median (25%ile/75%ile).
A Dual Energy X-Ray Absorptiometry (DXA) scan was performed for assessment of body composition including total percent body fat and thigh mineral-free lean mass (26). The control and FM groups reported here were limited to n=11 each due to inadequate quality of biopsied muscle from some subjects and to match groups for age, height, weight, and BMI. Further, subjects were assessed for the frequency, intensity, type, and duration of weekly exercise during the consenting process using the International Physical Activity Questionnaire (IPAQ) (27) to closely match for activity levels between the two groups (Table 1).
Subjects with FM taking non-steroidal anti-inflammatory drugs (2 of 11) were instructed to discontinue these medications at least 3 days prior to the muscle biopsy. Several subjects were taking fish oil supplements (3 of 11) and low-dose aspirin (2 of 11) which were also discontinued at least 3 days prior to the procedure. Compliance with these instructions was confirmed prior to the biopsy. Other medications for treatment of fibromyalgia were continued and included anti-depressants (9 of 11), medications for sleep (5 of 11), opioid-containing analgesics (4 of 11), anti-epileptic/alpha-2-delta ligands (3 of 11), and stimulants (1 of 11).
Maximal Isometric Strength Testing
Prior to strength testing, subjects participated in a session to become familiar with the performance of maximal voluntary isometric contractions (MVIC). Following familiarization, the subject’s central activation was quantified as previously described [central activation ratio (CAR) = MVIC/total force; total force = MVIC + superimposed involuntary twitch] (28). CAR did not differ between the healthy controls and subjects with FM (0.99 ± 0.01 VS. 0.99 ± 0.02) suggesting that subjects in both groups were able to maximally and voluntarily activate their quadriceps muscle.
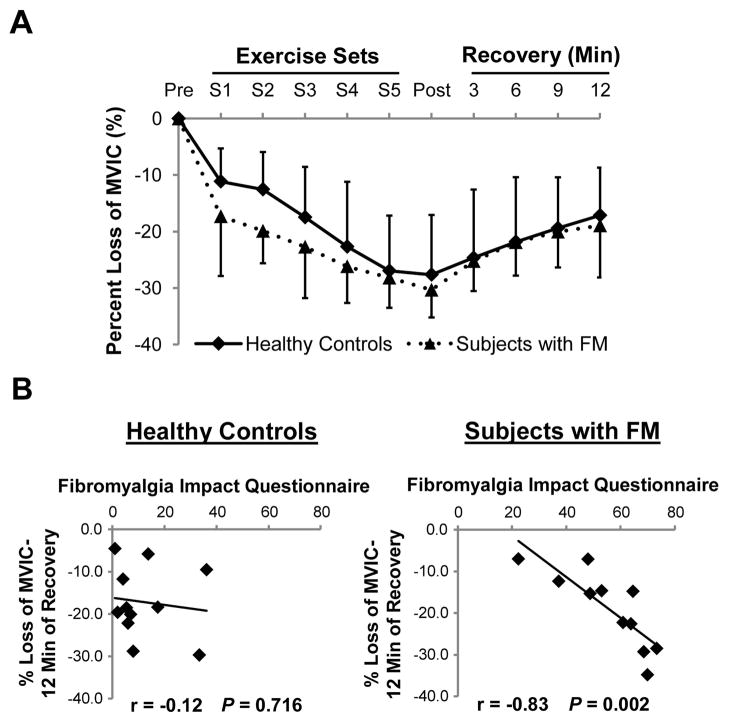
On the test day, subjects were asked to warm-up for five minutes on a semi-recumbent elliptical (Biostep 2, Biodex Medical Systems) at a comfortable rate and workload. After the warm-up period, MVIC was determined in the subject’s right leg using a Biodex dynamometer (Biodex System 4 Quick-Set) in a seated position (seat angle 85°) with the lateral femoral epicondyle aligned to the center of the dynamometer shaft. To minimize the use of muscles other than the knee extensors, subjects were stabilized with two shoulder straps and a waist strap. Three maximal practice trials were performed followed by three maximal test trials. MVIC (knee angle 90°) force was recorded as the highest force generated over three trials held for four seconds with a three-minute rest period between each attempt. MVIC force was acquired at baseline, every two minutes during the fatigue protocol (see below), immediately after exercise, and at time points 3, 6, 9, and 12 minutes during recovery (Figure 1A).
Figure 1. Percent loss in MVIC following the fatiguing exercise protocol is correlated with self-reported assessment in subjects with FM.
(A) Maximum voluntary isometric contractions (MVICs) were recorded and plotted against time periods (pre-exercise, after each exercise set (S1–5), and during recovery. (B) Correlation between fibromyalgia impact questionnaire score and % loss of MVIC following 12-min of recovery from fatiguing exercise was observed in subjects with FM but not healthy controls.
Fatiguing Exercise Protocol
After performing maximal isometric strength testing, subjects immediately started a fatiguing exercise protocol. Subjects were asked to perform 6 sets of 12 isometric contractions with a 40% duty cycle (4-sec contraction and 6-sec relaxation) with each set followed by a MVIC as described above. The initial intensity was set at 20% MVIC and increased 10% every set, eventually reaching an intensity of 70% MVIC. Thus, there were 78 total contractions over the period of exercise. Percent loss in MVIC and repetition to exhaustion during the fatigue protocol served as the primary and secondary measures of fatigability.
Tissue Blood Oxygenation
The optical sensor placed on the quadriceps was connected to a commercial near-infrared tissue-oximeter (Imagent, ISS Inc) for tissue blood oxygenation measurements. Absolute deoxy-hemoglobin [Hb] and oxy-hemoglobin [HbO2] concentrations were extracted from the measured tissue blood absorption coefficients at the two wavelengths (830 and 690 nm) and 4 source-detections (2.0, 2.5, 3.0, and 3.5 cm) (29). The absolute values of [Hb] and [HbO2] during the first 6 seconds following exercise were normalized to baseline (before exercise) to calculate relative change (r[Hb] and r[HbO2]).
Muscle Biopsy
A muscle biopsy from the vastus lateralis muscle of the non-dominant, non-exercised, leg was obtained, approximately 15 cm above the proximal patella edge, using a 5 mm Bergstrom biopsy needle with applied suction within 60 minutes of recovery from the fatiguing exercise protocol. Standard local anesthesia and aseptic procedures were used. Approximately 50 mg of vastus lateralis muscle tissue was mounted and oriented in tragacanth gum, snap frozen in isopentane pre-cooled with liquid nitrogen, and stored at −80°C for histochemical analysis. Approximately 10 mg of muscle was fixed and embedded for electron microscopy (EM) analysis. Baseline physiologic properties of muscle that may contribute to fatigue were examined that were not expected to be altered by the single bout of acute exercise.
Histochemical and Immunohistochemical Analyses
Frozen muscle samples were sectioned with a cryostat (Microm HM 525) at 7 μm thickness and used for succinate dehydrogenase (SDH), fiber typing, lectin, nitrotyrosine, and dystrophin detection. All steps were performed at room temperature unless noted. SDH activity in the muscle sections was visualized by incubation in a mixed solution containing nitrotetrazolium blue (Sigma), succinate acid disodium (Sigma), and 0.2 M of phosphate-buffered saline (PBS) at 37°C for 1 h in a dark chamber. Sections were rinsed with acetone (30%-60%-30%) for 1 min each, distilled water, and mounted with Vectashield mounting medium (Vector Laboratories).
For fiber typing, sections were rehydrated in PBS and incubated with three different isoform-specific myosin heavy chain (MyHC) antibodies. Because the primary antibodies were of different isotypes, they were applied in combination for 90 min. BA.D5 IgG2b (Developmental Studies Hybridoma Bank) (1:75), SC.71 IgG1 hybridoma supernatant (provided by Stefano Schiaffino, University of Padova, Padua, Italy), and 6H1 IgM hybridoma supernatant (provided by J.Y. Hoh, Sydney University, Sydney Australia) were used for MyHC type I, IIA, and IIX expression, respectively. Goat anti-mouse IgG2b Alexa Fluor 647 conjugated (Invitrogen) (1:250), goat anti-mouse IgG1 Alexa Fluor 488 conjugated (Invitrogen) (1:500), and goat anti-mouse IgM biotin conjugated (Invitrogen) (1:150) secondary antibodies were applied together and incubated for 1 h. Thereafter, strepavidin-Texas Red (Vector Laboratories) (1:150) was applied for 15 min. Sections were post-fixed in methanol for 5 min and mounted with Vectashield mounting medium.
For identifying capillaries, sections were rehydrated in PBS and blocked in 2.5% normal horse serum (Vector Laboratories) for 30 min. Primary lectin TRITC-conjugated (1:50) (Sigma) was incubated for 1 h. Sections were washed 3 times with Tris-buffered saline (TBS) and mounted with Vectashield mounting medium.
For nitrotyrosine and dystrophin detection, sections were rehydrated in PBS and permeabilized with 0.3% Triton X-100 (Sigma) for 5 min, then blocked in 10% normal goat serum (Jackson Lab). Primary mouse anti-dystrophin (Vector Laboratories) (1:100) and rabbit anti-nitrotyrosine (Millipore) (1:100) were applied for 2 h. Goat anti-mouse Texas Red (Rockland Immunochemicals Inc.) (1:200) and goat anti-rabbit Alexa Fluor 488 (Invitrogen) (1:500) secondary antibodies were applied for 1 h. Sections were post-fixed with 4% paraformaldehyde for 10 min and counterstained with Vectashield mounting medium containing DAPI (Vector Laboratories). As a positive control for the nitrotyrosine staining, oxidative stress was induced in mouse muscle by mechanical overloading the plantaris muscle. Briefly, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and synergist ablation was performed as previously described (30); muscle was dissected 24 h after overloaded and snap frozen. All histochemical and immunohistochemical images were captured with a Zeiss upright microscope (Axio Imager.M1) and analyzed with AxioVision Rel software (v4.8).
Electron Microscopy
The muscle biopsy samples were fixed in buffer containing 0.1 M Cacodylate, pH 7.4 with 3.5% glutaraldehyde and 4% paraformaldehyde for 2 h at 4°C. The samples were post-fixed with 1% OsO4 for 2 h at 4°C, dehydrated with a series of graded ethanol, 10 min each, and then propylene oxide, 2×20 min. Finally, the samples are incubated with a 1:1 dilution of resin with accelerator and propylene oxide overnight, placed into pure resin with accelerator ×2 for 1 h, and then embedded in resin with accelerator and placed into a 60°C oven for 48 h. Thin cross-sections were cut (60–90 nm) and 20–30 muscle fibers per subject were analyzed to determine the density of interfibrillar mitochondria by point counting as described in standard stereological methods (31) with 10×10 grids (132 points/image) using MetaMorph Advanced Image Analysis software, version 7.7.3.0 (Molecular Device). The large number of fibers counted per subject randomizes the effect of fiber type. Images were captured at ×13,000 magnification and data represented as % fiber volume on each image occupied by mitochondria. The % of fibers from each subject that demonstrated mitochondrial clumping was also determined.
Data Analyses
All of the data are presented as mean ± SD. The statistical significant difference was set at α level < 0.05. Repeated measure ANOVA on rank test, independent t-test, and Mann-Whitney Rank sum test were used to analyze the difference between groups where appropriate. The Kolmogorov-Smirnov test was used to test the distribution analyses and the correlation analyses were tested with Pearson’s correlation. Statistics were performed using SigmaPlot software version 12.0, Build 12.0.0.182 and SAS software version 9.3.
RESULTS
Subject characteristics
Age, height, weight, BMI, % body fat, thigh mineral-free lean mass, and IPAQ total physical activity score were not different between the subject groups (Table 1). Subjects with FM demonstrated significantly more tender points, higher fibromyalgia impact score, higher subjective fatigue scores (MAF and VAS-fatigue), greater perceived exertion during the fatiguing exercise task (RPE), as well as a greater sensation of pain change or malaise post exercise (GRCS), and pain pre- and post-exercise (VAS-pain score) compared to healthy control subjects (Table 1).
Strength loss with fatiguing exercise
To investigate muscle fatigue in response to acute exercise, subjects performed a fatiguing exercise protocol with strength loss quantified over time. Percent loss of strength, evaluated by MVIC before, during and after exercise, demonstrated the effectiveness of the protocol in inducing fatigue in both healthy controls and subjects with FM (−27.7 ± 10.5 % vrs. −30.3 ± 4.9 %, P = 0.453, Figure 1A). There was a trend towards a more rapid onset of fatigue in subjects with FM, indicated by the decrease in MVIC during the early stages of fatiguing exercise (sets 1–2), suggesting possible differences in physiologic muscle properties in subjects with FM. However, there was no significant difference in the percent loss of strength following the fatiguing exercise protocol or recovery period, or in time to failure to achieve MVIC (data not shown) between subjects with FM and healthy controls. In addition, the absolute MVICs (pre-exercise, immediately post-exercise, and following 12 min of recovery) appeared lower in subjects with FM but did not reach significance (Table 1). The trend towards lower absolute strength may be due to the fact that there was a trend for reduced muscle mass of the thigh in subjects with FM measured by DXA, although other intrinsic muscle properties may also contribute.
Self-reported assessment of fatigue and pain correlate with strength loss only in subjects with FM
Although there was no significant difference in MVICs following fatiguing exercise and recovery between the groups, correlation analyses revealed that subjects with FM who showed the greatest prolonged strength loss after 12-min of recovery had the highest scores on the fibromyalgia impact questionnaire (r = −0.83, P = 0.002) (Figure 1B). Similar correlations were also apparent between multidimensional assessment of fatigue (r = −0.70, P = 0.017) and VAS-pain scores (r = −0.83, P = 0.002) and fatigue after 12-min of recovery in subjects with FM. No such correlations between fatigue and fibromyalgia impact questionnaire (r = −0.12, P = 0.716), multidimensional assessment of fatigue (r = 0.03, P = 0.930), nor VAS-pain scores (r = 0.17, P = 0.624) were apparent in healthy subjects, suggesting that mechanisms underlying fatigue may differ between healthy subjects and those with FM.
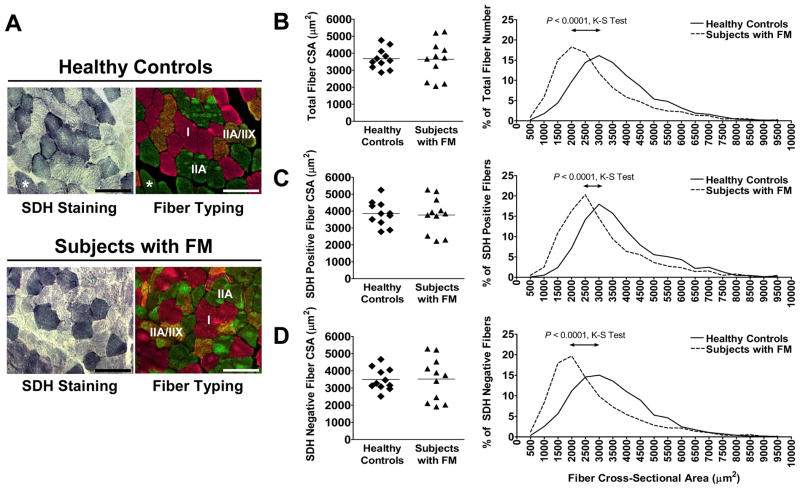
Differences in fiber size and metabolic activity between healthy controls and subjects with FM
To evaluate muscle oxidative capacity between the groups, two different assays were performed on serial cryosections of muscle biopsies (Figure 2A): succinate dehydrogenase (SDH) staining as an indicator of mitochondrial activity and fiber typing to distinguish slow, type I fibers and fast, type IIA and IIX fibers, using fiber type-specific myosin heavy chain antibodies. These analyses showed that oxidative, SDH positive fibers were type I fibers in both healthy controls and subjects with FM. However, rarely, type IIA fibers demonstrated high SDH activity in healthy controls (Figure 2A, asterisks). The majority of SDH negative fibers co-expressed IIA and IIX myosin heavy chains in both healthy controls and subjects with FM.
Figure 2. Defects in fiber size distribution and loss of correlation to fatigue in subjects with FM.
(A) SDH positive fibers (blue) were strongly correlated with type I fibers (pink fibers in right panels) in both healthy controls and subjects with FM. Rarely, type IIA fibers (green in right panel of healthy control) were SDH positive (asterisks). Most IIX fibers also expressed IIA myosin heavy chain (orange staining in right panels) in both groups. Scale bars = 100 μm. Mean fiber cross-sectional area (CSA) and size distribution of total (B); SDH positive (C) and SDH negative (D) fibers. The distribution of fiber sizes differed significantly as determined by the Komogorov-Smirnov (K-S) test, with more small fibers present in subjects with FM. A total of 3,737 fibers for healthy controls (1,425 SDH positive fibers and 2,312 SDH negative fibers) and 3,556 fibers for subjects with FM (1,498 SDH positive fibers and 2,058 SDH negative fibers) were measured for CSA quantification.
Quantification of the number of oxidative fibers in healthy controls and subjects with FM showed no difference in the percentage of SDH positive (40.4 ± 11.3 % vrs. 43.3 ± 9.5 %, P = 0.529) and negative (59.6 ± 11.3 % vrs. 56.7 ± 9.5 %, P = 0.529) fibers. These results were supported by no difference in the percentage of type I and type IIA + IIA/IIX fibers between the groups (Supplementary Table 1). In addition, no significant difference was observed in the mean cross-sectional area (CSA) of total fibers (SDH positive fibers + SDH negative fibers) (Figure 2B), SDH positive fibers (Figure 2C), or SDH negative fibers (Figure 2D). Although the mean fiber CSA was not different between groups, higher variation in fiber CSA of subjects with FM was apparent (Figure 2B–D); very small and very large fibers were present in subjects with FM. More detailed examination of fiber size distribution showed that subjects with FM had significantly more small fibers, indicated by the leftward shift in fiber size distribution (Figure 2B). The leftward shift was most pronounced in SDH negative fibers (Figure 2D) compared to SDH positive fibers (Figure 2C). Distribution analyses confirmed that total fiber CSA, SDH positive fiber CSA, and SDH negative fiber CSA were significantly different between healthy controls and subjects with FM (Figure 2B–D, P < 0.0001).
Dissociation of fatigue and oxidative fiber size in subjects with FM
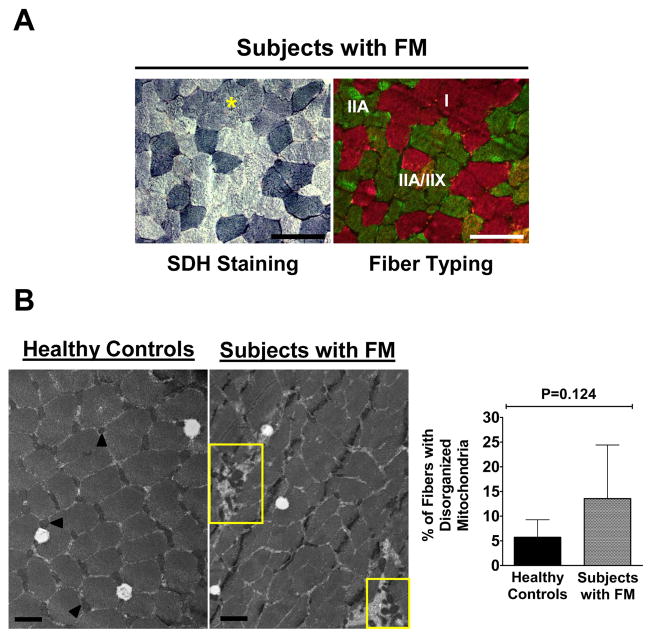
Fatigue resistance largely depends on the oxidative capacity of muscle fibers. SDH positive fiber CSA was positively correlated with both fatigue resistance (data not shown) and strength recovery 12-min after exercise in healthy controls (r = 0.75, P = 0.008) but not in subjects with FM (r = −0.08, P = 0.808, Supplementary Figure 1).
In addition to the dissociation of oxidative fiber size and fatigue-resistance in subjects with FM, many type I fibers in subjects with FM showed relatively weak SDH staining (asterisk, Figure 3A). To determine if weak SDH activity was related to mitochondrial defects in subjects with FM, mitochondrial volume density was quantified in parallel with mitochondrial distribution by electron microscopy. No difference in mitochondrial density was apparent between healthy controls (2.39 ± 0.95 %) and subjects with FM (2.59 ± 0.65 %). Abnormal clumping of mitochondria appeared more frequently in subjects with FM (Figure 3B, yellow rectangles); however, this did not reach significance (5.70 +/− 3.57% for healthy controls and 13.54 +/− 10.86% for subjects with FM, bar graph, Figure 3B).
Figure 3. Weak SDH positive fiber staining in type I fibers in FM muscle and mitochondrial distribution.
(A) Representative weak SDH positive fibers (asterisk, left panel) in a cluster of type I fibers (pink fibers, right panel) in subjects with FM. Images were captured at ×200, scale bars = 100 μm. (B) Representative electron micrographs (EM) showing normal interfibrillar mitochondria in a healthy control (arrows, left panel), compared to disrupted organization in a subject with FM (yellow rectangles). Images were captured at ×13,000, scale bars = 1 μm. Bar graph represents quantification of the frequency of fibers with disorganized mitochondria. Values are means ± SD (n=6/group).
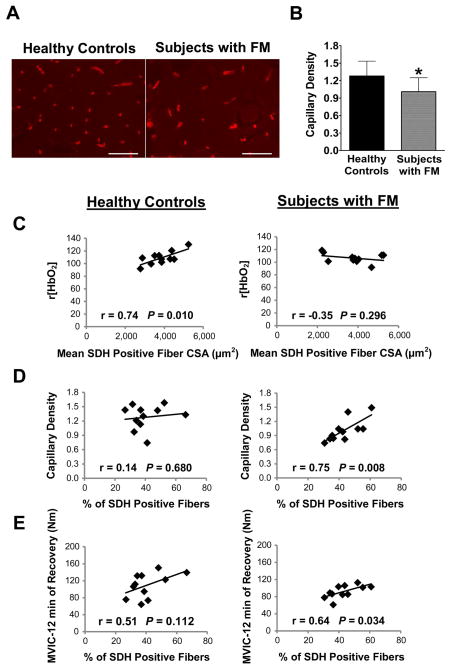
Lower capillary density is associated with altered oxygen delivery in subjects with FM
To investigate oxygen delivery to the muscle in subjects with FM, the number of capillaries per fiber (capillary density) was determined. Subjects with FM had significantly lower capillary density compared to healthy controls as shown in the representative images of Figure 4A, and quantified in Figure 4B (1.28 ± 0.25 vrs. 1.01 ± 0.24 capillaries per fiber, P < 0.05). Near-infrared tissue-oximetry revealed that although tissue blood oxygenation was not different between the groups, the relative change in oxy-hemoglobin concentration r[HbO2] following exercise was strongly correlated with the size of oxidative, SDH positive fibers in healthy controls (r = 0.74, P = 0.010), but not in subjects with FM (r = −0.35, P = 0.296) (Figure 4C). On the other hand, capillary density was positively correlated with the percent of type I, SDH positive fibers only in the subjects with FM (r = 0.75, P = 0.008) but not healthy controls (r = 0.14, P = 0.680) (Figure 4D). Finally, subjects with FM who had a high percentage of type I, SDH positive fibers also demonstrated higher MVICs following 12-min of recovery (r = 0.64, P = 0.034, Figure 4E). Taken together, these results show that fatigue-resistance in subjects with FM was associated with the percent of type I, SDH positive fibers, which was strongly correlated with capillary density. In healthy controls, overall capillary density was higher and the size of SDH positive fibers predicted fatigue-resistance (see Supplementary Figure 1).
Figure 4. Reduced capillary density and altered correlations with tissue oxygenation, oxidative fibers and strength recovery in FM muscle.
(A) Capillaries were identified by lectin staining (red). Scale bars = 100 μm. (B) A significant decrease in capillaries per fiber in subjects with FM compared to healthy controls was detected. * indicates P < 0.05, values are means ± SD (n=11/group). (C) Correlation between r[HbO2] and mean SDH positive fiber CSA was observed in healthy controls but not in subjects with FM. (D) A correlation between capillary density and % SDH positive fibers was observed in subjects with FM but not in healthy controls. (E) A weak correlation between strength following 12-min of recovery, measured by maximum voluntary isometric contractions (MVICs), and % SDH positive fibers was observed in subjects with FM.
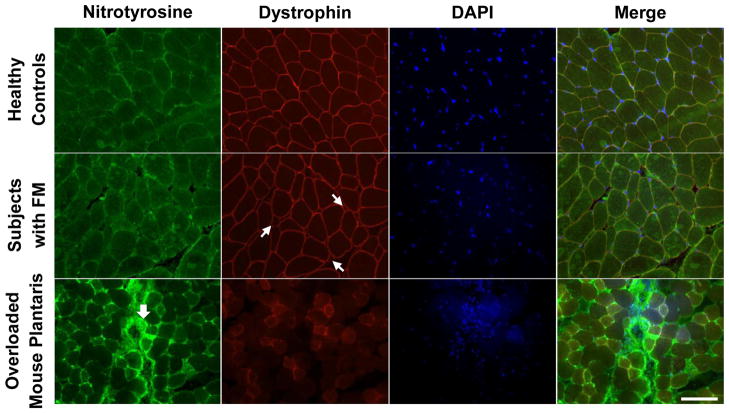
No apparent muscle morphologic defects in subjects with FM
Oxidative stress was investigated by using nitrotyrosine as a marker of nitrogen species-induced oxidative stress as shown in Figure 5. No difference in accumulation of nitrotyrosine between healthy control and FM muscles was apparent. An overloaded mouse muscle (see Methods under Histochemical and Immunohistochemical Analyses) was used as a positive control, showing strong nitrotyrosine staining in an area of cell infiltration (large arrow). No cellular infiltration or any sign of inflammation was observed in FM muscle. In addition, an antibody against dystrophin was used to double stain with anti-nitrotyrosine to delineate muscle fibers. Small fibers were apparent in FM muscle (small arrows), as noted above, but no difference in muscle membrane integrity between groups was apparent.
Figure 5. No apparent muscle morphologic defects in subjects with FM.
Anti-nitrotyrosine (green) and anti-dystrophin (red) antibodies, together with DAPI staining (blue) were used to visualize muscle fiber structure and nuclei. Damaged mouse muscle from overloading was used as a positive control for nitrotyrosine staining. Small arrows represent small fibers in fibromyalgic muscle and large arrow denotes nitrotyrosine accumulation in overloaded mouse plantaris muscle. Scale bar = 100 μm.
DISCUSSION
The goal of this study was to identify muscle properties that may contribute specifically to the post-exertional fatigue and malaise associated with FM. Although the post-menopausal women with FM in this study self-reported experiencing higher levels of fatigue than healthy controls, loss of strength in response to a fatiguing exercise protocol did not differ significantly between the groups. These results were similar to those previously reported in pre-menopausal women with FM, that no difference in isometric strength and neuromuscular activation during fatiguing resistance exercise were observed (32). However, muscle fatigue, measured as the percent strength loss at the end of the exercise protocol and the magnitude of the deficit 12 minutes after completion of exercise, were highly correlated with self-reported pain and fatigue only in subjects with FM. These results suggest a central component, contributes to fatigue in FM, as was observed in hemodialysis patients using the same type of fatiguing protocol (33).
Examination of muscle phenotype from biopsies showed that whereas the percentage and mean fiber cross-sectional area of type I and type II fibers were not different in FM compared to control muscle, consistent with an earlier report (19), muscle fibers in women with FM were more variable in size and significantly different in size distribution than fibers in healthy controls. Type I fibers are oxidative, as indicated by high SDH activity, whereas type II fibers are more glycolytic, involved in rapid force generation. The percentage and size of type I fibers are the primary determinants of oxidative capacity and fatigue resistance. For healthy controls, those individuals with the largest type I, SDH positive fibers were the most fatigue resistant. This correlation was not apparent in subjects with FM. The loss of type I fiber size as a contributor to fatigue resistance in FM muscle may have physiologic relevance in terms of exercise prescription (see below).
Oxidative capacity of individual muscle fibers is determined by mitochondrial activity, which may be affected in FM. Mitochondrial abnormalities have been described in FM (13–15). Assessment of mitochondrial volume density by electron microscopy showed no difference between groups, however, a trend towards abnormal mitochondrial distribution was seen in subjects with FM. No indicators of oxidative stress or inflammation were apparent at the light microscope level in muscle from subjects with FM, consistent with a previous report (34).
The most pronounced difference in muscle between subjects with FM and healthy controls observed in this study was reduced capillary density in FM. Evidence also exists for reduced capillary permeability and structural changes in the capillary endothelium in subjects with FM (18, 19). Altered microcirculation may result in lower oxygen delivery and waste product clearance, contributing directly to pain and fatigue in FM. This idea is supported by the work of Lund et al. who found a pathological distribution of tissue oxygen pressure in subjects with FM using an oxygen multi-point electrode (17). Further, blood flow response during exercise has been reported to be lower in subjects with FM using contrast media enhanced Doppler ultrasound (35). Using a near-infrared tissue-oximeter, we found that the relative concentration of oxygenated hemoglobin immediately following exercise correlated significantly with SDH positive fiber size in healthy controls but not in subjects with FM, suggesting that impaired oxygen delivery may limit the size of oxidative fibers in FM. Interestingly, we also observed that capillary density was strongly correlated with the percentage of type I, SDH positive fibers only in subjects with FM, which was associated with fatigue-resistance. Thus, oxygen availability may dictate muscle fiber metabolic phenotype in FM, altering mitochondrial oxidative capacity, even limiting the size of oxidative fibers, which may contribute directly to post-exertional muscle fatigue. Taken together, these results suggest that approaches to promote angiogenesis and increase muscle vascular density may hold promise in treatment of FM.
Although exercise has become a common key component in the management of FM, variability in study design and exercise training programs have made appropriate exercise prescription unclear. Our results suggest combined aerobic and resistance exercise should be prescribed to effectively combat the primary muscle physiologic deficits in FM: variation in muscle fiber size and reduced capillary density. Exercise in general has been shown to be beneficial for subjects with FM (36–38), producing higher effect sizes than pharmacologic treatments (39, 40). Aerobic training improves physical function and fitness indices in subjects with FM [reviewed in (36)], however, effects on pain, tender points and fatigue are equivocal. Our findings suggest that aerobic training programs designed specifically to increase capillary density (41) should be employed to maximize beneficial effects in this population.
Much less research has examined the effects of resistance training on FM symptoms. Some reports suggest that those with FM have similar trainability to healthy subjects, significantly increasing strength, neuromuscular function, and power (42–44). While some report that subjects with FM can tolerate traditional resistance exercise programs designed for muscle hypertrophy that focus on high loads and intensities (45), others report poor adherence and adverse events [reviewed in (36)]. Interestingly, recent research shows that exercise performed at 30% of one repetition maximum (1 RM) to momentary fatigue was equally as effective as exercise performed at 90% 1 RM in stimulating myofibrillar protein synthesis (46) and that a slow lifting movement (increased time under tension) stimulated muscle protein synthesis even further (47). In addition, similar hypertrophic responses were observed in young men utilizing 30% compared to 80% 1 RM, as long as the muscle was taxed to momentary failure (48). Thus, we hypothesize that exercise training incorporating multiple resistance exercise sets at a relatively low intensity (30–40% 1 RM) to permit a higher number of slow-lifting repetitions, performed to failure, may be appropriate for individuals with FM. This type of resistance exercise prescription, combined with concurrent aerobic exercise, would be most effective in improving both muscular strength and endurance deficits observed in individuals with FM, while minimizing adverse events and poor adherence. Such an approach may also avoid the delayed onset of muscle relaxation between contractions reported in subjects with FM (49), as well as increased co-activation of the antagonist muscle that may also contribute to the pain and fatigue experienced after exercise (50).
In conclusion, understanding peripheral mechanisms that affect muscle function provides valuable insight for fibromyalgia treatment and symptom management. Further studies on the effectiveness of concurrent aerobic and resistance training are required.
Supplementary Material
Correlation between strength following 12-min of recovery, measured by maximum voluntary isometric contractions (MVICs), and mean SDH positive fiber CSA was observed only in healthy controls.
Acknowledgments
The authors thank Rod A. Erfani, Jyothi Mula, James G. Begley, and Mary G. Engle for the technical assistance on histochemical and electron microscopy techniques and Catherine P. Starnes and Dr. Heather M. Bush for consultation on statistical analyses. We also thank University of Kentucky Center for Clinical and Translational Sciences for assistance with muscle biopsies and body composition analyses, Dr. Karyn A. Esser for providing the overloaded mouse muscle to use as a positive control for nitrotyrosine staining, and Dr. Leigh Ann Callahan for helpful discussions in the early phases of the project.
Grant support: This project was supported by Grant Number R21 AG34279 from NIA/NIH and by NCRR/NCATS/NIH, through Grant Number UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Author Contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Peterson has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Srikuea, Long, Yu, Crofford, Peterson
Acquisition of data. Srikuea, Symons, Long, Lee, Shang, Chomentowski, Crofford
Analysis and interpretation of data. Srikuea, Long, Lee, Crofford, Peterson
References
- 1.Abeles AM, Pillinger MH, Solitar BM, Abeles M. Narrative review: the pathophysiology of fibromyalgia. Ann Intern Med. 2007;146:726–34. doi: 10.7326/0003-4819-146-10-200705150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Cho HJ, Skowera A, Cleare A, Wessely S. Chronic fatigue syndrome: an update focusing on phenomenology and pathophysiology. Curr Opin Psychiatry. 2006;19:67–73. doi: 10.1097/01.yco.0000194370.40062.b0. [DOI] [PubMed] [Google Scholar]
- 3.Mease P, Arnold LM, Bennett R, Boonen A, Buskila D, Carville S, et al. Fibromyalgia syndrome. J Rheumatol. 2007;34:1415–25. [PubMed] [Google Scholar]
- 4.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 5.Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gend Specif Med. 2002;5:42–7. [PubMed] [Google Scholar]
- 6.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Niermann KJ, Olsen N. Evidence for metabolic abnormalities in the muscles of patients with fibromyalgia. Curr Rheumatol Rep. 2000;2:131–40. doi: 10.1007/s11926-000-0053-3. [DOI] [PubMed] [Google Scholar]
- 10.Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, et al. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int. 2005;25:188–90. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 11.Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses. 2007;69:517–25. doi: 10.1016/j.mehy.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Sackner MA, Gummels EM, Adams JA. Say NO to fibromyalgia and chronic fatigue syndrome: an alternative and complementary therapy to aerobic exercise. Med Hypotheses. 2004;63:118–23. doi: 10.1016/j.mehy.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Kalyan-Raman UP, Kalyan-Raman K, Yunus MB, Masi AT. Muscle pathology in primary fibromyalgia syndrome: a light microscopic, histochemical and ultrastructural study. J Rheumatol. 1984;11:808–13. [PubMed] [Google Scholar]
- 14.Sprott H, Salemi S, Gay RE, Bradley LA, Alarcon GS, Oh SJ, et al. Increased DNA fragmentation and ultrastructural changes in fibromyalgic muscle fibres. Ann Rheum Dis. 2004;63:245–51. doi: 10.1136/ard.2002.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yunus MB, Kalyan-Raman UP, Kalyan-Raman K, Masi AT. Pathologic changes in muscle in primary fibromyalgia syndrome. Am J Med. 1986;81:38–42. doi: 10.1016/0002-9343(86)90872-7. [DOI] [PubMed] [Google Scholar]
- 16.Bengtsson A, Henriksson KG, Larsson J. Reduced high-energy phosphate levels in the painful muscles of patients with primary fibromyalgia. Arthritis Rheum. 1986;29:817–21. doi: 10.1002/art.1780290701. [DOI] [PubMed] [Google Scholar]
- 17.Lund N, Bengtsson A, Thorborg P. Muscle tissue oxygen pressure in primary fibromyalgia. Scand J Rheumatol. 1986;15:165–73. doi: 10.3109/03009748609102084. [DOI] [PubMed] [Google Scholar]
- 18.Grassi W, Core P, Carlino G, Salaffi F, Cervini C. Capillary permeability in fibromyalgia. J Rheumatol. 1994;21:1328–31. [PubMed] [Google Scholar]
- 19.Lindh M, Johansson G, Hedberg M, Henning GB, Grimby G. Muscle fiber characteristics, capillaries and enzymes in patients with fibromyalgia and controls. Scand J Rheumatol. 1995;24:34–7. doi: 10.3109/03009749509095152. [DOI] [PubMed] [Google Scholar]
- 20.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995;22:639–43. [PubMed] [Google Scholar]
- 21.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 22.Wolfe F. Fatigue assessments in rheumatoid arthritis: comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol. 2004;31:1896–902. [PubMed] [Google Scholar]
- 23.Altan L, Bingol U, Aykac M, Koc Z, Yurtkuran M. Investigation of the effects of pool-based exercise on fibromyalgia syndrome. Rheumatol Int. 2004;24:272–7. doi: 10.1007/s00296-003-0371-7. [DOI] [PubMed] [Google Scholar]
- 24.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 25.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–33. [PubMed] [Google Scholar]
- 26.Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol. 1999;87:1513–20. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–9. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Shang Y, Zhao Y, Cheng R, Dong L, Irwin D, Yu G. Portable optical tissue flow oximeter based on diffuse correlation spectroscopy. Opt Lett. 2009;34:3556–8. doi: 10.1364/OL.34.003556. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Hitomi Y, Kizaki T, Ohno H, Haga S, Takemasa T. Contribution of the calcineurin signaling pathway to overload-induced skeletal muscle fiber-type transition. J Physiol Pharmacol. 2004;55:751–64. [PubMed] [Google Scholar]
- 31.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–94. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 32.Hakkinen A, Hakkinen K, Hannonen P, Alen M. Force production capacity and acute neuromuscular responses to fatiguing loading in women with fibromyalgia are not different from those of healthy women. J Rheumatol. 2000;27:1277–82. [PubMed] [Google Scholar]
- 33.Johansen KL, Doyle J, Sakkas GK, Kent-Braun JA. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am J Physiol Regul Integr Comp Physiol. 2005;289:R805–13. doi: 10.1152/ajpregu.00187.2005. [DOI] [PubMed] [Google Scholar]
- 34.Drewes AM, Andreasen A, Schroder HD, Hogsaa B, Jennum P. Pathology of skeletal muscle in fibromyalgia: a histo-immuno-chemical and ultrastructural study. Br J Rheumatol. 1993;32:479–83. doi: 10.1093/rheumatology/32.6.479. [DOI] [PubMed] [Google Scholar]
- 35.Elvin A, Siosteen AK, Nilsson A, Kosek E. Decreased muscle blood flow in fibromyalgia patients during standardised muscle exercise: a contrast media enhanced colour Doppler study. Eur J Pain. 2006;10:137–44. doi: 10.1016/j.ejpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35:1130–44. [PubMed] [Google Scholar]
- 37.Gowans SE, deHueck A. Effectiveness of exercise in management of fibromyalgia. Curr Opin Rheumatol. 2004;16:138–42. doi: 10.1097/00002281-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Jones KD, Adams D, Winters-Stone K, Burckhardt CS. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005) Health Qual Life Outcomes. 2006;4:67. doi: 10.1186/1477-7525-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossy LA, Buckelew SP, Dorr N, Hagglund KJ, Thayer JF, McIntosh MJ, et al. A meta-analysis of fibromyalgia treatment interventions. Ann Behav Med. 1999;21:180–91. doi: 10.1007/BF02908299. [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388–95. doi: 10.1001/jama.292.19.2388. [DOI] [PubMed] [Google Scholar]
- 41.Shono N, Urata H, Saltin B, Mizuno M, Harada T, Shindo M, et al. Effects of low intensity aerobic training on skeletal muscle capillary and blood lipoprotein profiles. J Atheroscler Thromb. 2002;9:78–85. doi: 10.5551/jat.9.78. [DOI] [PubMed] [Google Scholar]
- 42.Figueroa A, Kingsley JD, McMillan V, Panton LB. Resistance exercise training improves heart rate variability in women with fibromyalgia. Clin Physiol Funct Imaging. 2008;28:49–54. doi: 10.1111/j.1475-097X.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 43.Valkeinen H, Alen M, Hannonen P, Hakkinen A, Airaksinen O, Hakkinen K. Changes in knee extension and flexion force, EMG and functional capacity during strength training in older females with fibromyalgia and healthy controls. Rheumatology (Oxford) 2004;43:225–8. doi: 10.1093/rheumatology/keh027. [DOI] [PubMed] [Google Scholar]
- 44.Valkeinen H, Hakkinen A, Hannonen P, Hakkinen K, Alen M. Acute heavy-resistance exercise-induced pain and neuromuscular fatigue in elderly women with fibromyalgia and in healthy controls: effects of strength training. Arthritis Rheum. 2006;54:1334–9. doi: 10.1002/art.21751. [DOI] [PubMed] [Google Scholar]
- 45.Hakkinen A, Hakkinen K, Hannonen P, Alen M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann Rheum Dis. 2001;60:21–6. doi: 10.1136/ard.60.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PloS One. 2010;5:e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burd NA, Andrews RJ, West DW, Little JP, Cochran AJ, Hector AJ, et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol. 2012;590:351–62. doi: 10.1113/jphysiol.2011.221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, et al. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol. 2012;113:71–7. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elert JE, Rantapaa-Dahlqvist SB, Henriksson-Larsen K, Lorentzon R, Gerdle BU. Muscle performance, electromyography and fibre type composition in fibromyalgia and work-related myalgia. Scand J Rheumatol. 1992;21:28–34. doi: 10.3109/03009749209095059. [DOI] [PubMed] [Google Scholar]
- 50.Jegede AB, Gilbert C, Tulkin SR. Muscle characteristics of persons with fibromyalgia syndrome. NeuroRehabilitation. 2008;23:217–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between strength following 12-min of recovery, measured by maximum voluntary isometric contractions (MVICs), and mean SDH positive fiber CSA was observed only in healthy controls.