Abstract
Background
To analyze how lidocaine 40 mg mixed prevents injection pain of propofol affects the onset time of rocuronium, tracheal intubating conditions and intubation related hemodynamic changes.
Methods
This study consisted of 70 patients with an American Society of Anesthesiologists (ASA) physical status class 1 or 2 for general anesthesia. All the patients were randomly allocated into two groups: propofol 2 mg/kg plus normal saline 2 ml (Group C) and propofol 2 mg/kg plus 2% lidocaine 40 mg (Group L). Each group was administrated intravenously during induction and the patient was intubated 1 minute after an injection of 0.6 mg/kg of rocuronium. The time at disappearance of the first twitch and intubation scores were recorded. Also, blood pressure and heart rate were measured at the baseline, after intravenous injection of propofol, before intubation, and at 0, 1, 2, 3 and 5 minutes after intubation.
Results
There were no significant differences between group C and L (P > 0.05).
Conclusions
40 mg of lidocaine mixed with propofol to prevent injection pain did not affect the onset time of rocuronium, intubating conditions and intubation related hemodynamic changes.
Keywords: Lidocaine, Neuromuscular blockade, Propofol, Rocuronium
Introduction
Rapid onset, possibility of continuous injection and fast recovery has made propofol one of the most widely used agents for induction of general anesthesia. However, 28-90% of patients have complained of pain during injection [1,2].
Various methods are being used to reduce the injection pain of propofol. To date, adding lidocaine to propofol is the most popular method. Studies show that lidocaine mixed with propofol is more effective than lidocaine pretreatment [3] and 40 mg of lidocaine is the optimal dose typically used in order to reduce the intravenous injection pain in adults [4].
Interaction between local anesthetics and neuromuscular blockers has been observed in experimental studies, and clinical studies have been reported, resulting in potentiation of the effects of neuromuscular blockers by local anesthetics [5-7]. Braga et al. [8] reported that the rocuronium-induced neuromuscular blockade with preperations exposed to local anesthetics was significantly greater than that produced by rocuronium in an experiment using the phrenic nerve-diaphragm of rats. Also, Yorukoglu et al. [9] has compared intubating conditions provided by succinylcholine with those after roucuronium (0.6 mg/kg) associated or not lidocaine (1.5 mg/kg). They reported that the combination of lidocaine with rocuronium showed similar intubation conditions to succinylcholine within 60 seconds and better than rocronium alone.
Reports on the effect of lidocaine on neuromuscular blockade of rocuronium are rare and most clinical studies have been conducted with dose of 1.5 mg/kg, the amount of lidocaine that stabilizes hemodynamic responses due to endotracheal intubation by inhibiting the activation of the symphathetic nerve system.
Mixing 40 mg of lidocaine has become a standard practice in reducing injection pain of propofol. Therefore, the aim of this study was to analyze whether or not affected neuromuscular blockade of rocuronium or the hemodynamic response of endotracheal intubation.
Materials and Methods
This study was conducted with 70 patients who were planned for elective surgeries under general anesthesia, with ages from 17 to 65 years, and met the American Society of Anesthesiology classifications 1 and 2. Exclusion criteria included the presence of an abnormal airway, expectation of difficult intubation, disease of the cardiovascular respiratory, neuromuscular, hepatic or renal system, and concurrent administration of any drug known or suspected to interfere with neuromuscular transmission. Patients with lidocaine hypersensitivity were also excluded. Written consent was obtained from each subject, and the institutional Human Investigation Committee approved the protocol of this study.
The patients were allocated randomly to two groups: the group that received propofol (2 mg/kg) mixed with normal saline 2 ml, called Group C (n = 35); and the group that received propofol (2 mg/kg) mixed with 2% lidocaine 40 mg, called Group L (n = 35).
All patients were premedicated 30 minutes prior to the arrival at the operating room with midazolam 2 mg, glycopyrrolate 0.2 mg via muscular injection and with famotidine 20 mg via intravenous injection. Monitoring was performed upon arrival in the operating room, including ECG, non-invasive automatic blood pressure monitor and pulse oximeter. Heart rate, blood pressure and peripheral oxygen saturation was continuously measured. In order to measure the level of muscle relaxation, two electrodes from the nerve stimulator (TOF-Watch®, Organon teknika, Netherlands) were attached over the ulna nerve of the arm with the i.v. fluid tube, but without the blood pressure monitor cuff; the other remaining fingers were fixated with tape to a rigid plate, and were monitored for the contraction reaction of adductor pollicis.
The hand skin temperature was maintained above 32℃. Propofol 2 mg/kg mixed with normal saline 2 ml or propofol 2 mg/kg mixed with 2% lidocaine 40 mg was administered via intravenously for 10 seconds. After confirming the loss of eyelash reflexes; patients were ventilated with 100% oxygen by a face mask. After the reference point was verified through a nerve stimulator correction, rocuronium 0.6 mg/kg was slowly intravenously injected for 10 seconds. Tracheal intubation was performed 1 minute after rocuronium injection. A train of four (TOF, 2 Hz, 0.2 msec) was used at the supramaximal stimulation and was repeated every 15 seconds after intravenous injection of rocuronium. The point at disappearance of the first twitch was recorded as the onset time of rocuronium on adductor pollicis. Intubation conditions were assessed by the scoring system used by Debaene et al. [10], classified as Excellent, Good, Poor, and Inadequate by another investigator, who was blinded to the protocol (Table 1). Immediately after tracheal intubation, sevoflurane 2%, O2 50%, and medical air 50% were used to maintain anesthesia. Systolic, diastolic blood pressure and heart rate were measured and recorded as follow; before inducing anesthesia, after intravenous injection of propofol, just before intubation, immediately after intubation, 1, 2, 3 and 5 minutes after intubation.
Table 1.
Intubating Condition Score

Adopted from Debaene et al. [10].
Based on a preliminary study, a power analysis indicated that 35 patients per group would be sufficient to detect a 30-sec (SD: 69.07, 29.58) difference of the mean onset time between the groups, with a power greater than 80% at the significance level of 0.05.
Data was expressed as the means ± SD or as numbers of patients, and the statistical significance analysis was done using SPSS (version 17.0, SPSS Inc., IL, USA). Age, height, weight, muscle relaxation onset time, blood pressure and heart rate was compared with a unpaired t-test, and intubation conditions were compared by using the Fisher's exact test. We used a repeated measure ANOVA for continuous variables. A P vlaue < 0.05 was considered statistically significant.
Results
A total of 70 patients were enrolled, and all completed the study. There were no demographic differences between the two groups, in respect to age, weight or height (Table 2).
Table 2.
Demographic Data
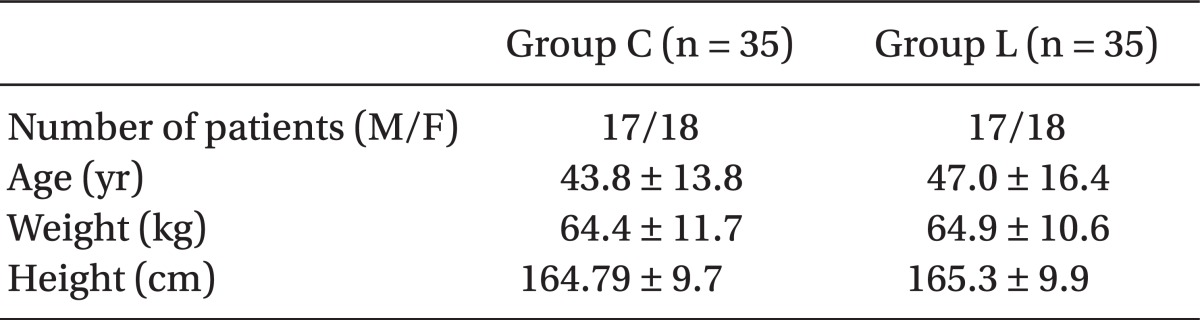
Values are expressed as mean ± SD. Group C: propofol 2 mg/kg & normal saline 2 ml mixed, Group L: propofol 2 mg/kg & lidocaine 40 mg mixed. The measured values for patients' age, weight and height did not differ significantly between the two groups.
The onset time of muscle relaxation was 164.7 ± 77.9 seconds for Group L, which was quicker than the 186.6 ± 71.0 seconds for Group C, but did not differ statistically.
In the condition of endotracheal intubation assessment, except for one case of a "Poor" in Group C, both groups were either Excellent or Good, and did not differ significantly (Table 3).
Table 3.
Intubating Condition Scores
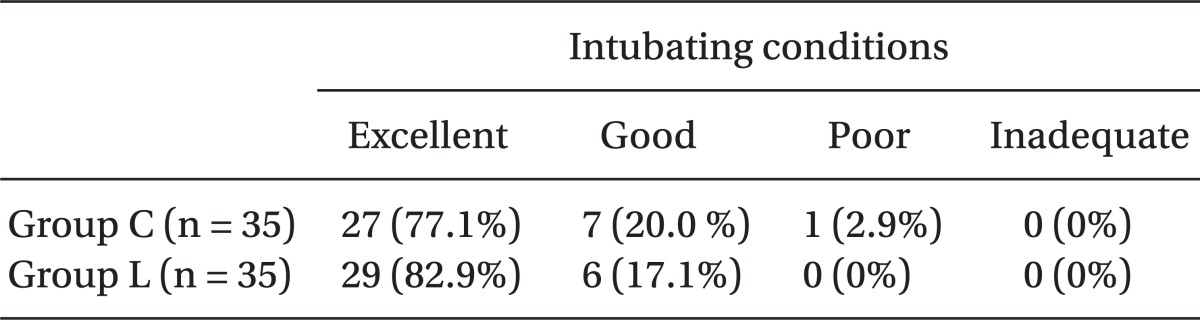
Values are number of patients (%). Group C (propofol 2 mg/kg & normal saline 2 ml mixed), Group L (propofol 2 mg/kg & lidocaine 40 mg mixed). The data are presented as number of patients. No statistically significant difference was seen between the two groups.
Systolic, diastolic blood pressures and heart rate did not show any significant differences before inducing anesthesia, after intravenous injection of propofol, just before intubation, immediately after intubation, 1, 2, 3 and 5 minutes after intubation, between the two groups.
According to time, each group had significantly lower systolic, diastolic blood pressure and heart rates values after intravenous injection of propofol and higher systolic, diastolic blood pressure and heart rates values immediately after intubation, compared to the baseline.
Discussion
It is confirmed that local anesthetics produce neuromuscular block and increase neuromuscular responses to non-depolarizing neuromuscular blockers. The interaction between local anesthetics and neuromuscular blockers is not fully elucidated, and several mechanisms can be responsible for the potentiation observed.
In theory, local anesthetics may interfere with any step of the neuromuscular transmission, both in pre- and post-synaptic membranes. In the pre-synaptic membrane, local anesthetics can cause depression of the motor nerve conduction, decrease the quantal content of acetylcholine or the number of quanta released at rest or after nervous stimulation [11-13]. Their post-synaptic effects include binding to specific areas of nicotinic receptors different from acetylcholine, promoting a loss of sensitization of such receptors [14,15] , and directly interfering with the muscle fibers by blocking the sodium channels (procaine) [16] or the sodium and potassium channels (lidocaine) [17].
Matsuo et al. [5] reported in rat phrenic nerve - hemidiaphragm preparation, the action of d-tubocurarine association to lidocaine. They observed dose response curve of neuromuscular blocking agent deviating to the left, reflecting potentiation of these drugs. Local anesthetics also had reduced ED50 for the neuromuscular blocking agent. The authors concluded that the interaction of neuromuscular blocking agents - local anesthetics can be consequent to the true potentied.
Wang et al. [18] stated that lidocaine and procaine significantly increased the inhibitory effects of vecuronium and cisatracurium on adult mouse muscle type nicotinic acetylcholine receptor. They suggested that this was the result of local anesthetics acting on the regulatory site of nicotinic acetylcholine receptors and increasing the affinity for the receptor of non-depolarizing muscle relaxant.
In studies with cats, the neuromuscular blockade, induced by the lidocaine-pancuronium association, was 20% greater than that of the pancuronium alone, which was statistically significant [19].
Also, Carvalho et al. [20] demonstrated the lidocaine potentiated neuromuscular blockade of rocuronium in the neuromuscular junction in the phrenic nerve-diaphragm preparation of rats. Harrah et al. [21] reported that lidocaine only exerted a measurable effect on the neuromuscular function in the presence of partial neuromuscular blockade. The interaction between local anesthetics and neuromuscular blockers observed in vitro studies has also been reported in clinical practices, resulting in the potentiation of the effects of neuromuscular blockers by local anesthetics administered by different routes. Lee and Chung [22] have stated that the onset time for vecuronium 0.1 mg/kg was reduced after administering 1.5 mg/kg lidocaine.
In a different study, intravenous lidocaine administered before roucuronium during anesthetic induction was unable to shorten its onset, but prolonged its pharmacological duration without prolonging total neuromuscular function recovery [23].
Synergistic effects of lidocaine-neuromuscular blocker can vary depending on the sensitivity of neuromuscular blocker for lidocaine and the dominant action mechanism of lidocaine and the neuromuscular blocker in the neuromuscular junction. However, the reports of clinical studies on the interaction between rocuronium and lidocaine are rare. Although it has been reported that lidocaine and its derivatives act specifically at the neuromuscular junction, within the concentration range of 0.05 to 2.0 Mm in an animal experimental study [24], it is hard to extrapolate lidocaine concentration for human from an experimental concentration because the potency of lidocaine varies based on various factors, such as species and experimental environment [20]. Furthermore, almost all clinical studies that have demonstrated the synergistic effect of lidocaine with roucuronium were conducted with 1.5 mg/kg lidocaine, which is known to be the dose that blunts the hemodynamic response due to endotracheal intubation.
Therefore, we aimed to evaluate the effects of lidocaine on the neuromuscular blockade of rocuronium in popular and practical clinical technique, mixing of 40 mg lidocaine to prevent injection pain of propofol. Our study showed that the mixing 40mg of lidocaine with propofol does not have an effect on the neuromuscular blockade of rocuronium.
In this study, as 40 mg of lidocaine would be measured as only 0.63 mg/kg considering the patients' weights (64.9 ± 11.7; 64.4 ± 10.6), as expected, lidocaine did not have an effect on hemodynamic changes of endotracheal intubation. Such results are consistent with the finding of Abou-Madi et al. [25], who reported that 0.75 mg/kg lidocaine does not affect hypertension or tachycardia related laryngoscopy.
In conclusion, 40 mg of lidocaine mixed with propofol to prevent injection pain did not affect the onset time of rocuronium, intubating conditions and intubation related hemodynamic changes.
References
- 1.Picard P, Tramer MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90:963–969. doi: 10.1097/00000539-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998;53:468–476. doi: 10.1046/j.1365-2044.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 3.Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43:492–494. doi: 10.1111/j.1365-2044.1988.tb06641.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RA, Harper NJ, Chadwick S, Vohra A. Pain on injection of propofol. Methods of alleviation. Anaesthesia. 1990;45:439–442. doi: 10.1111/j.1365-2044.1990.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo S, Rao DB, Chaudry I, Foldes FF. Interaction of muscle relaxants and local anesthetics at the neuromuscular junction. Anesth Analg. 1978;57:580–587. doi: 10.1213/00000539-197809000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Katz RL, Gissen AJ. Effects of intravenous and intra-arterial procaine and lidocaine on neuromuscular transmission in man. Acta Anaesthesiol Scand Suppl. 1969;36:103–113. doi: 10.1111/j.1399-6576.1969.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 7.Loyola YC, Braga Ade F, Poterio GM, Sousa SR, Fernandes SC, Braga FS. Influence of lidocaine on the neuromuscular block produced by rocuronium: study in rat phrenic-diaphragmatic nerve preparation. Rev Bras Anestesiol. 2006;56:147–156. doi: 10.1590/s0034-70942006000200006. [DOI] [PubMed] [Google Scholar]
- 8.Braga Ade F, Carvalho VH, Braga FS, Rodrigues-Simioni L, Loyola YC, Poterio GB. Influence of local anesthetics on the neuromuscular blockade produced by rocuronium: effects of lidocaine and 50% enantiomeric excess bupivacaine on the neuromuscular junction. Rev Bras Anestesiol. 2009;59:725–734. doi: 10.1016/s0034-7094(09)70097-2. [DOI] [PubMed] [Google Scholar]
- 9.Yorukoglu D, Asik Y, Okten F. Rocuronium combined with i.v. lidocaine for rapid tracheal intubation. Acta Anaesthesiol Scand. 2003;47:583–587. doi: 10.1034/j.1399-6576.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 10.Debaene B, Beaussier M, Meistelman C, Donati F, Lienhart A. Monitoring the onset of neuromuscular block at the orbicularis oculi can predict good intubating conditions during atracurium-induced neuromuscular block. Anesth Analg. 1995;80:360–363. doi: 10.1097/00000539-199502000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Usubiaga JE, Standaert F. The effects of local anesthetics on motor nerve terminals. J Pharmacol Exp Ther. 1968;159:353–361. [PubMed] [Google Scholar]
- 12.Hirst GD, Wood DR. On the neuromuscular paralysis produced by procaine. Br J Pharmacol. 1971;41:94–104. doi: 10.1111/j.1476-5381.1971.tb09939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews EK, Quilliam JP. Effects of central depressant drugs upon acetylcholine release. Br J Pharmacol Chemother. 1964;22:415–440. doi: 10.1111/j.1476-5381.1964.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J, Boyd N, Shera N. Interactions of anesthetics with nicotinic postsynaptic membranes isolated from Torpedo electric tissue, em: Fink BR-molecular mechanism of anesthesia. Progress in Anesthesiology, 2. New York: Reaven Press; 1980. pp. 165–174. [Google Scholar]
- 15.Sine SM, Taylor P. Local anesthetics and histrionicotoxin are allosteric inhibitors of the acetylcholine receptor. Studies of clonal muscle cells. J Biol Chem. 1982;257:8106–8114. [PubMed] [Google Scholar]
- 16.Straub R. Effect of local anesthetics on ion-determined resting potential changes of myelinated nerve fibers in frogs. Arch Int Pharmacodyn Ther. 1956;107:414–430. [PubMed] [Google Scholar]
- 17.Maeno T, Edwards C, Hashimura S. Difference in effects of end-plate potentials between procaine and lidocaine as revealed by voltage-clamp experiments. J Neurophysiol. 1971;34:32–46. doi: 10.1152/jn.1971.34.1.32. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Zhang Y, Li ST. The effect of local anesthetics on the inhibition of adult muscle-type nicotinic acetylcholine receptors by nondepolarizing muscle relaxants. Eur J Pharmacol. 2010;630:29–33. doi: 10.1016/j.ejphar.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter RL, Mulroy MF. Edrophonium antagonizes combined lidocaine-pancuronium and verapamil-pancuronium neuromuscular blockade in cats. Anesthesiology. 1986;65:506–510. doi: 10.1097/00000542-198611000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho VH, Braga Ade F, Braga FS, Loyola YC, de Araujo DR, Mantovani M. The influence of lidocaine and racemic bupivacaine on neuromuscular blockade produced by rocuronium. A study in rat phrenic nerve-diaphragm preparation. Acta Cir Bras. 2009;24:211–215. doi: 10.1590/s0102-86502009000300009. [DOI] [PubMed] [Google Scholar]
- 21.Harrah MD, Way WL, Katzung BG. The interaction of d-tubocurarine with antiarrhythmic drugs. Anesthesiology. 1970;33:406–410. doi: 10.1097/00000542-197010000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Chung C. The effect of lidocaine on the onset of vecuronium-induced neuromuscular block. Korean J Anesthesiol. 1996;30:595–603. [Google Scholar]
- 23.Cardoso LS, Martins CR, Tardelli MA. Effects of intravenous lidocaine on the pharmacodynamics of rocuronium. Rev Bras Anestesiol. 2005;55:371–380. doi: 10.1590/s0034-70942005000400001. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach AB. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J Gen Physiol. 1968;52:144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou-Madi MN, Keszler H, Yacoub JM. Cardiovascular reactions to laryngoscopy and tracheal intubation following small and large intravenous doses of lidocaine. Can Anaesth Soc J. 1977;24:12–19. doi: 10.1007/BF03006808. [DOI] [PubMed] [Google Scholar]


