Abstract
Background
This study evaluated the relationship between skin intrinsic fluorescence (SIF) and long-term mean hemoglobin A1c (HbA1c) in individuals with type 1 diabetes.
Subjects and Methods
We undertook a cross-sectional analysis of 172 individuals with type 1 diabetes followed longitudinally with HbA1c data available over an average of 16.6 years. SIF was evaluated cross-sectionally using the SCOUT DS® device (VeraLight Inc., Albuquerque, NM) and correlated with most recent HbA1c and long-term mean HbA1c. Potential determinants of this relationship, including age, gender, smoking status, duration of diabetes, and renal function, were also evaluated.
Results
Age-adjusted skin intrinsic fluorescence significantly correlated with long-term mean HbA1c (R=0.44, P<0.0001). In contrast, there was no significant relationship between SIF and most recent HbA1c (R=0.14, P=0.075). The best-fit model describing the relationship between SIF and mean HbA1c controlled for factors of age, duration of disease, renal function, and site of study conduct. Controlling for these factors was also important in understanding the relationship between most recent HbA1c and SIF. Evaluating longer-term HbA1c data also strengthened the relationship between SIF and mean HbA1c. In the presence of renal dysfunction or damage, as indicated by an estimated glomerular filtration rate of <60 mL/min/1.73 m2 or presence of gross proteinuria, there was no significant correlation between SIF and mean HbA1c.
Conclusions
Noninvasive detection of SIF significantly correlates with long-term mean HbA1c, providing insight into long-term glycemic exposure. Age, duration of diabetes, and renal function are potential contributors to this relationship.
Introduction
Chronic exposure to hyperglycemia contributes to the pathogenesis of diabetes-related complications. Long-term prospective studies such as the Diabetes Control and Complications Trial (DCCT)1 and the United Kingdom Prospective Diabetes Study (UKPDS)2 have demonstrated the utility of assessing glycemic exposure, for example, by mean hemoglobin A1c (HbA1c) over time,2 in determining risk of diabetes-related complications. In the DCCT, there was a continuously increasing risk of progression of retinopathy with increasing mean glycosylated hemoglobin values.1 In the UKPDS, every percentage point drop in mean HbA1c over time was associated with a reduction in microvascular complications by 37% and a reduction in the risk of any end point or death related to diabetes by 21%.2
Few medical clinics have complete records of measured glycated hemoglobin over many years. They instead rely on single recent measurements of HbA1c, which only provide information about glycemic exposure over the preceding 2–3 months.
An alternative method of understanding cumulative historical glycemic exposure is based on the role of advanced glycation end products (AGEs). AGEs accumulate naturally with aging but accumulate faster in individuals with abnormal glucose regulation.3 With increased glucose exposure, glycated and glycoxidated proteins form early and intermediate glycation products such as HbA1c as well as stable AGEs that accumulate in skin and in tissues affected by diabetes. Conveniently, AGEs are also stably bound to skin collagen, which has an estimated half-life of 15–20 years.4 Therefore, skin levels of AGEs are able to provide historical information about chronic exposure to hyperglycemia and may predict future progression of diabetes-related complications. Indeed, several studies, including the DCCT and the Epidemiology of Diabetes Interventions and Complications Study, have shown that elevated levels of skin AGEs are predictive of future development and progression of diabetes-related complications.5,6
Skin AGEs can be assessed noninvasively by exciting and measuring fluorescence produced by the AGEs in vivo. Using noninvasive technology, another study following over 800 participants with type 2 diabetes for 3.1 years demonstrated a correlation between baseline noninvasive skin autofluorescence and diabetes-related microvascular complications, including neuropathy or microalbuminuria.7
In this study, we cross-sectionally evaluated the skin intrinsic fluorescence (SIF) of participants with type 1 diabetes with HbA1c data available over a mean duration of 16.6 years. We hypothesized that noninvasive SIF would be correlated with mean HbA1c over time and further sought to explore the potential role of factors such as age, gender, smoking status, duration of diabetes, and renal function on this putative relationship.
Research Design and Methods
Participants were enrolled in this cross-sectional evaluation from two sites: the University of Pittsburgh (Pittsburgh, PA) and MedStar Health Research Institute (MHRI) (Hyattsville, MD). Participants from the University of Pittsburgh were included from the longitudinal observational Pittsburgh Epidemiology of Diabetes Complications (EDC) cohort,8,9 where skin fluorescence was assessed in their 20th year of follow-up, 2 years after routine clinical and laboratory assessment, and participants from MHRI were recruited and enrolled from patients receiving their diabetes care from MHRI's investigator (R.E.R.) or immediate colleagues. Participants were excluded for any wounds, injuries, or rashes on the volar forearm that could potentially interfere with skin fluorescence assessment. Other exclusions included current receipt of other investigational treatments, current receipt of medications that may alter skin fluorescence or photosensitivity, pregnancy, or inability to follow study procedures. No specific intervention was directed at the participants prior to evaluation of skin fluorescence, but rather participants were evaluated in the context of their usual care and follow-up. All procedures for this cross-sectional evaluation were reviewed and approved by the Institutional Review Board of the University of Pittsburgh and the MHRI Institutional Review Board, respectively. All participants provided written informed consent prior to any study procedures. Medical history and physical examination were performed. Criteria for inclusion in the analysis included availability of historical HbA1c data for a minimum of 4 years prior to skin fluorescence measurement.
Blood samples were assayed for HbA1c and serum creatinine. DCCT-aligned HbA1c values were used to calculate the mean HbA1c. For the EDC investigative population, the level of stable glycated hemoglobin A1 (HbA1) was originally measured in saline-incubated samples by microcolumn cation-exchange chromatography (Isolab, Akron, OH). On October 26, 1987, the method was changed to high-performance liquid chromatography (Diamat®; Bio-Rad Laboratories, Hercules, CA). The two methods were highly correlated (r=0.95; Diamat HbA1=0.18±1.00 Isolab HbA1). Beginning in 1998, HbA1c was measured using the DCA2000 analyzer (Bayer Diagnostics, Tarrytown, NY). Original HbA1 (1986–1998) and HbA1c (1998–2004) were converted to DCCT-aligned HbA1c values using regression formulas derived from duplicate analyses: DCCT HbA1c=(0.83×EDC HbA1)+0.14 or DCCT HbA1c=(EDC HbA1c – 1.13)/0.81.8 For the MHRI diabetes clinic, HbA1c was obtained with DCCT-aligned DCA 2000/DCA 2000+instruments or by DCCT-aligned laboratory values from local laboratories, and historical HbA1c values were captured through detailed chart review.
Exposure to glycemia was determined in a manner similar to that used in the UKPDS,2 with exposure to glycemia over time measured as an updated mean of historical measurements of HbA1c, referred to here as mean HbA1c. This was calculated by taking the sum of an individual's HbA1c values over the duration of follow-up and dividing by the number of HbA1c measurements taken for that individual. Another indicator of cumulative glycemic exposure, A1 months, was calculated by multiplying the number of HbA1 units above normal at each cycle by the number of months between the midpoints of the preceding and succeeding cycle intervals.10 Level of albuminuria was determined by timed urine collections (EDC) or spot urine collections (MHRI). Gross proteinuria was defined as two or more timed urine collections with albumin excretion rate of >300 μg/min (EDC) or spot urine albumin:creatinine ratio of >300 mg of albumin/g of creatinine (MHRI). Glomerular filtration rate was determined by the Chronic Kidney Disease Epidemiology Collaboration CKD-EPI formula.11
SIF was determined noninvasively on the left volar forearm of all participants using the investigational SCOUT DS® skin fluorescence spectrometer (VeraLight, Inc., Albuquerque, NM). Two measurements were repeated consecutively approximately 1 min apart. All measurements were done without regard to the timing of meals. A calibration check was performed before each SCOUT DS subject measurement. Skin fluorescence was excited with a light-emitting diode centered at 405 nm and was detected over the range of 441–482 nm. The skin reflectance was measured over both the excitation and emission regions and was used to compensate for absorbance caused by melanin and hemoglobin.9,12 The intrinsic fluorescence correction is expressed in Eq. 1:
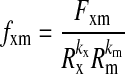 |
(1) |
where measured fluorescence, Fxm, is divided by reflectance values at the excitation and emission wavelengths, Rx and Rm, respectively.12 The reflectance values are adjusted by the respective dimensionless exponents, kx and km. As in previous reports regarding associations of SIF with neuropathy and coronary artery disease in this cohort, kx was set to 0.9, km was set to 0.0, and the resulting intrinsic fluorescence, fxm, was integrated over the 441–482-nm spectral region to give the SIF (in arbitrary units).9,13 The Hoorn coefficient of variation of SIF, quantifying the intrasubject variance, was previously reported at 4%.14
Statistical analysis
Baseline characteristics of the study population were calculated both overall and by site. Student's t tests and the χ2 test were used to compare characteristics between study sites. SIF was log normally distributed and therefore transformed by natural logarithm for all subsequent statistical analyses. Multiple linear regression analyses were used to determine the association between both mean HbA1c and the most recent HbA1c to SIF. Variables factored into the regression analyses included age, duration of disease, follow-up length, serum creatinine, evidence of proteinuria, site of study, gender, race, and smoking exposure. The β coefficients were expressed with 95% confidence intervals to illustrate both effect size and statistical significance. We tested for interactions with age, duration of disease, and follow-up length. Variance inflation factors were calculated to check for multicollinearity. Model discrimination was assessed with both the use of R2 values and Akaike Information Criteria. Period intervals of HbA1c values (0–5 years, 0–10 years, 0–15 years, 0–20 years) were assessed into the best-fit model to evaluate the relationship between SIF and HbA1c over time. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC), and scatterplot/regression lines were created using STATA version 10.1 (STATA Corp., College Station, TX).
Results
Population characteristics
Baseline characteristics of the studied population are presented in Table 1. In total, 172 participants with type 1 diabetes were evaluated. Mean age was 48.9 years, and mean duration of diabetes was 36.1 years. Average length of follow-up was 16.6 years. Mean HbA1c was higher than most recent HbA1c at 8.4% versus 7.7%, respectively (P<0.0001). When evaluated by site, participants enrolled at Pittsburgh from the EDC population demonstrated a longer duration of disease (40.1 vs. 28.6 years, P<0.0001) and were followed up for a longer length of time (20 years vs. 10.27 years, P<0.0001) compared with participants enrolled in the MHRI diabetes clinic. Similarly, participants enrolled at Pittsburgh from the EDC population demonstrated a higher mean HbA1c value (8.77% vs. 7.83%, P<0.0001) and higher SIF measurement than participants enrolled in the MHRI diabetes clinic.
Table 1.
Characteristics of Study Participants at Time of Skin Fluorescence Assessment
| Baseline characteristic | Total population | MHRI | Pittsburgh | P value (between sites) |
|---|---|---|---|---|
| Participants (n)a | 172 | 60 | 112 | NA |
| Age (years) | 48.9 (8.62) | 49.4 (8.99) | 48.7 (7.38) | 0.62 |
| Female [n (%)] | 93 (54.1) | 30 (50.0) | 63 (56.3) | 0.43 |
| Black or African-American [n (%)] | 6 (3.5) | 2 (3.3) | 4 (3.6) | 1.00 |
| History of smoking [n (%)] | 43 (28.5) (n=151) | 10 (23.3) (n=43) | 36 (33.3) (n=108) | 0.22 |
| Gross proteinuria [n (%)] | 35 (20.7) (n=169) | 5 (8.6) (n=58) | 30 (27.0) (n=111) | 0.01 |
| Duration of disease (years) | 36.1 (10.54) | 28.6 (11.88) | 40.1 (7.08) | <0.0001 |
| Average length of follow-up (years) | 16.6 (5.45; 3.74–23.68) | 10.27 (4.84; 3.74–23.68) | 20 (0; 20–20) | <0.0001 |
| Mean HbA1c | 8.4 (1.10) | 7.83 (0.95) | 8.77 (1.04) | <0.0001 |
| Most recent HbA1c | 7.7 (1.38) | 7.8 (1.05) | 7.6 (1.53) | 0.34 |
| Recent serum creatinine | 1.1 (0.70) | 1.0 (0.41) | 1.2 (0.80) | 0.07 |
| eGFR | 80.3 (27.29) (n=171) | 83.0 (23.97) (n=59) | 78.2 (28.88) (n=112) | 0.34 |
| Log (SIF×1,000) | 2.1 (0.30) | 1.9 (0.29) | 2.2 (0.26) | <0.0001 |
Data are mean (SD) or mean (SD; minimum–maximum) values unless indicated otherwise, presented by overall population and by site of study.
For participants with a hemoglobin A1c (HbA1c) value within 5 years.
eGFR, estimated glomerular filtration rate; MHRI, MedStar Health Research Institute; NA, not applicable; SIF, skin intrinsic fluorescence.
Correlation of SIF with HbA1c
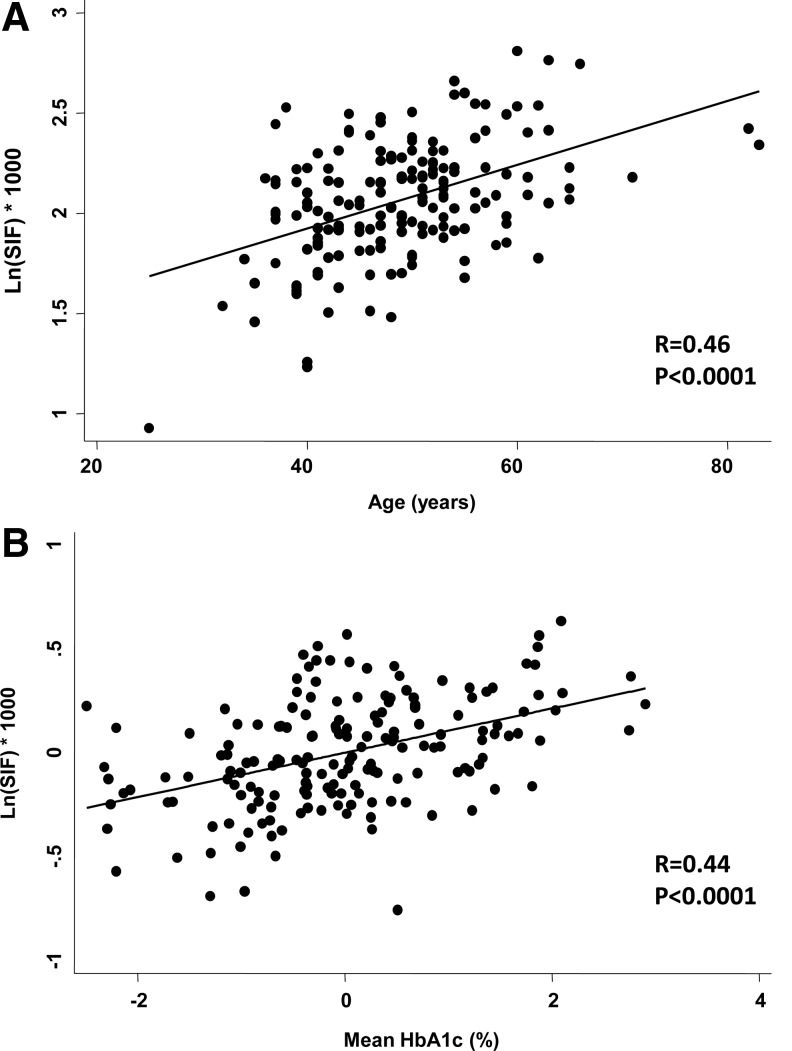
As shown in Figure 1A, log SIF significantly correlated with age (R=0.46, P<0.0001). As shown in Figure 1B, log SIF adjusted for age significantly correlated with mean HbA1c (R=0.44, P<0.0001). Likewise, A1 months, another indicator of chronic glycemic exposure,10 was significantly correlated with log SIF (R=0.31, P<0.0001). In contrast, a single, most recent measurement of HbA1c, as an indicator of glycemic exposure in a limited period of the preceding 2–3 months, did not correlate with log SIF (R=0.14, P=0.075).
FIG. 1.
Relationship between skin intrinsic fluorescence (SIF) and (A) age and (B) mean hemoglobin A1c (HbA1c), adjusted for age. SIF was naturally logarithmically transformed prior to analysis.
Potential determinants of relationship between SIF and mean HbA1c
To explore potential contributions to the relationship between SIF and HbA1c, several models were assessed. As shown in Table 2A, the lowest Akaike Information Criteria value, indicating the best-fit model between mean HbA1c and SIF, was seen when we controlled for age, duration of disease, creatinine, and site. Race and smoking exposure had nonsignificant contributions to the model (data not shown). Gross proteinuria and creatinine demonstrated high collinearity; thus creatinine was used in the final model. Unlike with mean HbA1c, most recent HbA1c demonstrated no significant relationship with SIF. However, controlling for factors of age, duration of disease, creatinine, and site, as seen in the best-fit model (Table 2B), demonstrated a significant relationship between recent HbA1c and SIF.
Table 2.
Potential Determinants of Relationship Between Skin Intrinsic Fluorescence and (A) Mean HbA1c, and (B) Most Recent HbA1c
|
A | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Model 1 (n = 172) | Model 2 (n = 172) | Model 3 (n = 172) | Model 4 (n = 172) | Model 5 (n = 171) | Model 6 (n = 171) | Best Model |
| Mean HbA1c | 0.096* (0.058–0.135) | 0.107* (0.074–0.056) | 0.081* (0.139–0.816) | 0.069* (0.036–0.103) | 0.067* (0.034–0.100) | 0.064* (0.032–0.097) | 0.065* (0.033–0.097) |
| Age | 0.017* (0.013–0.021) | 0.011* (0.007–0.016) | 0.011* (0.007–0.016) | 0.011* (0.007–0.015) | 0.013* (0.008–0.018) | 0.013* (0.008–0.017) | |
| Duration of Disease | 0.010* (0.006–0.014) | 0.008* (0.004–0.012) | 0.008* (0.004–0.012) | 0.006^ (0.002–0.011) | 0.006^ (0.002–0.011) | ||
| Follow-Up Length | 0.008^ (0.001–0.015) | 0.008^ (0.001–0.015) | −0.001 (–0.0134 – 0.011) | ||||
| Most Recent Creatinine | 0.084^ (0.037–0.131) | 0.083^ (0.036–0.129) | 0.081^ (0.035–0.127) | ||||
| Site (Pittsburgh) | 0.138 (–0.015–0.291) | 0.123^ (0.035–0.211) | |||||
| Gender (Male) | −0.018 (–0.084–0.048) | ||||||
| R-Squared | 0.13 | 0.37 | 0.46 | 0.47 | 0.51 | 0.52 | 0.52 |
| AIC | −436.45 | −489.55 | −514.48 | −517.48 | −524.16 | −523.55 | −527.24 |
|
B | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Model 1 (n = 167) | Model 2 (n = 167) | Model 3 (n = 167) | Model 4 (n = 167) | Model 5 (n = 166) | Model 6 (n = 166) | Best Model (n = 166) |
| Most Recent HbA1c | 0.026 (–0.007–0.059) | 0.027 (–0.003–0.056) | 0.026 (–0.000–0.052) | 0.027^ (0.002–0.053) | 0.035^ (0.010–0.059) | 0.037^ (0.012–0.061) | 0.038^ (0.014–0.062) |
| Age | 0.016* (0.011–0.020) | 0.009^ (0.004–0.014) | 0.0099* (0.005–0.014) | 0.009* (0.005–0.014) | 0.012* (0.007–0.017) | 0.012* (0.007–0.017) | |
| Duration of Disease | 0.013* (0.009–0.016) | 0.0092* (0.005–0.013) | 0.009* (0.005–0.013) | 0.006^ (0.002–0.011) | 0.006^ (0.002–0.011) | ||
| Follow-Up Length | 0.013^ (0.006–0.020) | 0.012^ (0.005–0.019) | 0.00 (–0.013–0.012) | ||||
| Most Recent Creatinine | 0.103* (0.055–0.152) | 0.102* (0.054 – 0.150) | 0.100* (0.052–0.148) | ||||
| Site (Pittsburgh) | 0.184^ (0.028–0.339) | 0.180* (0.093–0.266) | |||||
| Gender (Male) | −0.023 (–0.092–0.045) | ||||||
| R-Squared | 0.01 | 0.23 | 0.39 | 0.43 | 0.48 | 0.50 | 0.50 |
| AIC | −405.14 | −443.72 | −479.95 | −490.11 | −501.64 | −503.45 | −506.97 |
Results are expressed as β coefficients with 95% confidence intervals. SIF was naturally logarithmically transformed prior to analysis. *P ≤ 0.0001; ^P < 0.05. AIC, Akaike Information Criteria; HbA1c, hemoglobin A1c.
To evaluate the duration of HbA1c-related glycemic exposure captured by SIF, we then modeled the relationship between mean HbA1c and SIF while controlling for mean HbA1c measurements available for prespecified time intervals (0–5 years, 0–10 years, 0–15 years, 0–20 years) in a subcohort of participants followed up for the full 20 years (n=103). We found the strongest magnitude of effect when HbA1c values were factored in over a 20-year period (β coefficient 0.089, 95% confidence interval 0.046–0.132), with the weakest magnitude seen between mean HbA1c and SIF when HbA1c values were only included 0–5 years prior to SIF measurement (β coefficient 0.066, 95% confidence interval 0.028–0.103). These findings were corroborated with a univariate lag analysis using 5-, 10-, 15-, and 20-year time points (data not shown).
Impact of renal function and stages of renal disease on relationship between SIF and chronic glycemic exposure
As serum AGEs are cleared by the kidneys, the effect of renal dysfunction and renal damage on the relationship between SIF and long-term mean HbA1c was evaluated. Results are tabulated in Table 3. In the presence of advanced stages of renal damage, as evidenced by gross proteinuria or declining renal function by estimated glomerular filtration rate (eGFR), there is no significant correlation between log SIF and mean HbA1c. Log SIF significantly correlates with mean HbA1c in the absence of gross proteinuria (R=0.46, P<0.0001, n=134) but does not in the presence of gross proteinuria (R=0.15, P=0.39, n=35). Similarly, in individuals with eGFR<60 mL/min/1.73 m2, there is no significant correlation between log SIF and mean HbA1c (R=0.25, P=0.11, n=43), whereas there is a significant relationship in those with an eGFR>60 mL/min/1.73 m2 (R=0.48, P<0.0001, n=128).
Table 3.
Relationship Between Mean Hemoglobin A1c and Skin Intrinsic Fluorescence in the Presence or Absence of Renal Disease
| Renal status, presence or absence | R value (Pearson) | P value |
|---|---|---|
| History or presence of microalbuminuria | ||
| Yes (n=45) | 0.53 | 0.0002 |
| No (n=89) | 0.35 | 0.0010 |
| History or presence of gross proteinuria | ||
| Yes (n=35) | 0.15 | 0.39 |
| No (n=134) | 0.46 | <0.0001 |
| Renal function (eGFR<60 mL/min/1.73 m2) | ||
| Yes (n=43) | 0.25 | 0.11 |
| No (n=128) | 0.48 | <0.0001 |
Skin intrinsic fluorescence was naturally logarithmically transformed prior to analysis.
eGFR, estimated glomerular filtration rate.
Discussion
Chronic exposure to hyperglycemia contributes to the accumulation of early and intermediate glycation products, including HbA1c, and to AGEs, which may be related to the development of diabetes-related complications.15,16 In this cross-sectional analysis, we demonstrated that SIF, a marker of AGE accumulation, is significantly and positively associated with long-term mean HbA1c. The strongest magnitude of a relationship was seen when factors of age, duration of diabetes, renal function, and site of study were controlled for in the statistical models. Modeling also indicated that availability of longer-term HbA1c data contributed to a greater magnitude of relationship between SIF and mean HbA1c.
These observations are consistent with the underlying physiology of glycation and AGE accumulation.5,17–20 AGEs are formed from nonenzymatic glycation of tissue proteins and are found in a variety of tissues, including skin, tracheal cartilage, tendons, ligaments, cortical bone, aorta, cardiac muscle, lung, liver, kidney, lens, red blood cells, and blood proteins.17 In skin biopsy studies, Dyer21 previously showed that concentrations of AGEs such as N'-(carboxymethyl)lysine and pentosidine, as well as AGE-associated skin fluorescence, increase in human skin collagen with age and that the rate of their increase is accelerated in diabetes. This author also reported a strong correlation (R=0.89, P<0.0001) between skin collagen glycation content obtained by skin biopsy in type 1 diabetes and mean percentage glycation of hemoglobin, reflective of glycemic control over the 6 years before skin biopsy.21 Here, too, we report a relationship between integrated glycemic exposure (mean HbA1c) and noninvasive measurement of SIF, with duration of diabetes contributing positively to this relationship.
Another study using the same technology in children with type 1 diabetes likewise demonstrated an association between SIF and mean HbA1c, after controlling for age, race, sex, z-score for body mass index, and duration of diabetes. In children with type 1 diabetes, just as in our adult population, SIF increased with age and correlated with mean HbA1c. It is interesting that in children there was also an influence of gender on SIF, with higher levels in females at all ages than males, which we did not find in our adult cohort. In this study by Felipe et al.,20 which had 29% black or African American participants, race was also an influence on SIF, with SIF levels being higher in blacks than in whites. In our model, ethnicity was not a significant contributor, although our population was predominantly white. The study by Felipe et al.20 found no significant association between mean blood glucose or mean blood glucose over time with SIF, suggesting factors other than mean blood glucose might influence nonenzymatic glycation and formation of AGEs.
Our findings of an absent relationship between log SIF and mean HbA1c at low eGFR or in the context of gross proteinuria are also consistent with AGE physiology. Increased serum and tissue levels of AGEs are seen in the context of end-stage renal failure, because of reduced removal by the kidneys.22 Thus, in the context of advanced kidney disease, the accumulation of AGEs with impaired clearance may limit the ability of SIF to discern the level of historical glycemic control. These findings and understanding the relationship between SIF and nephropathy warrant further exploration. Specific use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was not directly ascertained, which is a limitation of the analysis.
The ability to noninvasively measure AGE-associated fluorescence as a correlate, or indicator, of cumulative glycemic exposure provides a plausible approach to assess risk of diabetes-related complications. Several studies have already demonstrated the ability of skin fluorescence to detect and predict diabetes-related complications. Chabroux et al.23 published an independent association between skin fluorescence levels and diabetic nephropathy and neuropathy in type 1 diabetes, although this was not seen with retinopathy. In a prospective study of patients with well-controlled type 2 diabetes, after a mean follow-up of 3.1 years, patients with a higher baseline skin fluorescence reading had a higher likelihood of developing microalbuminuria or neuropathy, but again not retinopathy.7 Conway et al.9 demonstrated that SIF was even more strongly associated with the presence of autonomic neuropathy and confirmed distal symmetrical polyneuropathy than mean HbA1c.
There also appears to be an association between skin fluorescence and risk of macrovascular complications. In a prospective study, baseline skin fluorescence was an additive predictor to the UKPDS risk engine in determining risk of subsequent cardiovascular events in patients with type 2 diabetes.24 An association between coronary artery calcification and noninvasive SIF measurement has also been reported in participants with type 1 diabetes from the EDC study.14
There are several potential limitations to this study. One potential limitation to this study is differences in the participant populations recruited from the two collaborative sites for this evaluation. When compared directly, gender, recent HbA1c, and creatinine did not differ between the two sites, whereas duration of diabetes and length of follow-up did. To minimize any bias from the differences in study settings, only data that were similarly obtained by both populations (e.g., SIF measurements) or able to be standardized (e.g., clinical assessment, HbA1c, renal function) were decided upon prior to the pooled analysis. Potential differences in the populations recruited at each site were also accounted for in the statistical models, controlling for factors such as age, gender, ethnicity, smoking exposure, duration of diabetes, and renal function, as well as site of study. Additional potential limitations include the use of single spot measurements of the albumin:creatinine ratio as an indicator of proteinuria (MRHI), although there is precedent of doing so in large multicenter trials,25 and limited historical data are available on the use of angiotensin converting enzyme inhibitors to better understand the relationship between SIF and mean HbA1c in the context of renal dysfunction. Finally, this study focused on long-term mean HbA1c as an indicator of glycemia, comparable to that used in the UKPDS.2 However, other variables of interest to evaluate chronic glycemic exposure may have included mean blood glucose,20 which was not available for this study.
Our study uniquely evaluated and confirmed a significant relationship between SIF and long-term mean HbA1c in patients with type 1 diabetes with confirmatory HbA1c data of up to 20 years in duration. Mean HbA1c, as an indicator of long-term glycemic exposure,2 is often not available or feasible in the office setting, particularly when patients are not followed up regularly by a single provider/system. As an alternative to mean HbA1c, which may not be available, noninvasive assessment of SIF may provide insight to historical glycemic exposure. As an area for additional investigation, understanding cumulative glycemic exposure in this manner carries potential to further inform glycemia management and prevention of diabetes-related complications.
Acknowledgments
This work was supported by grant DK 34818 from the National Institutes of Health (to T.J.O.) and was in part conducted through the Georgetown–Howard Universities Center for Clinical and Translational Science and supported by the National Institutes of Health National Center for Research Resources, grant UL1RR031975 (to R.E.R. and V.R.A.). This project was funded in part by VeraLight, Inc. We would like to acknowledge the dedication and contributions of our participants and research staff.
Author Disclosure Statement
V.R.A. and R.E.R. have received research grant funding from VeraLight Inc. and have served as consultants for VeraLight Inc. T.J.O. has received research grant funding from VeraLight Inc. N.I.M. and J.D.M. are employees and stock option holders of VeraLight Inc. B.N.C. and S.J.F. declare no competing financial interests exist. V.R.A. researched data, contributed to discussion, wrote/reviewed/edited the manuscript, and takes responsibility for the content of this article. B.N.C., S.J.F., T.J.O., and R.E.R researched data, contributed to discussion, and reviewed/edited the manuscript. N.I.M. and J.D.M. contributed to discussion and reviewed/edited the manuscript.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM. Adler AI. Neil HA. Matthews DR. Manley SE. Cull CA. Hadden D. Turner RC. Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–441. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1991;196:17–29. doi: 10.3181/00379727-196-43158c. [DOI] [PubMed] [Google Scholar]
- 4.Verzijl N. DeGroot J. Thorpe SR. Bank TA. Shaw JN. Lyons TJ. Bijlsma JW. Lafeber FP. Baynes JW. TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;50:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 5.Monnier VM. Bautista O. Kenny D. Sell DR. Fogarty J. Dahms W. Cleary PA. Lachin J. Genuth S. Skin collagen glycation, glycoxidation, crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48:870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genuth S. Sun W. Cleary P. Sell DR. Dahms W. Malone J. Sivitz W. Monnier VM. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerrits EG. Lutgers HL. Kleefstra N. Graaff R. Groenier KH. Smit AJ. Gans RO. Bilo HJ. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31:517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 8.Pambianco G. Costacou T. Ellis D. Becker DJ. Klein R. Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 9.Conway BN. Aroda VR. Maynard JD. Matter N. Fernandez S. Ratner RE. Orchard TJ. Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes Care. 2011;34:1000–1005. doi: 10.2337/dc10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orchard TJ. Forrest KY. Ellis D. Becker DJ. Cumulative glycemic exposure, microvascular complications in insulin-dependent diabetes mellitus. The glycemic threshold revisited. Arch Intern Med. 1997;157:1851–1856. [PubMed] [Google Scholar]
- 11.Levey AS. Stevens LA. Schmid CH. Zhang YL. Castro AF., 3rd Feldman HI. Kusek JW. Eggers P. Van Lente F. Greene T. Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hull E. Ediger M. Unione A. Deemer E. Stroman M. Baynes J. Noninvasive, optical detection of diabetes: model studies with porcine skin. Opt Express. 2004;12:4496–4510. doi: 10.1364/opex.12.004496. [DOI] [PubMed] [Google Scholar]
- 13.Conway BN. Aroda VR. Maynard JD. Matter N. Fernandez S. Ratner RE. Orchard TJ. Skin intrinsic fluorescence is associated with coronary artery disease individuals with long duration of type 1 diabetes. Diabetes Care. 2012;35:2331–2336. doi: 10.2337/dc12-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conway B. Edmundowicz D. Matter N. Maynard J. Orchard T. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther. 2010;12:339–345. doi: 10.1089/dia.2009.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brownlee M. Negative consequences of glycation. Metabolism. 2000;49:9–13. doi: 10.1016/s0026-0495(00)80078-5. [DOI] [PubMed] [Google Scholar]
- 16.King GL. Brownlee M. The cellular and molecular mechanisms of diabetes complications. Endocrinol Metab Clin North Am. 1996;25:255–270. doi: 10.1016/s0889-8529(05)70324-8. [DOI] [PubMed] [Google Scholar]
- 17.Sell DR. Nagaraj RH. Grandhee SK. Odetti P. Lapolla A. Fogarty J. Monnier VM. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab Rev. 1991;7:239–251. doi: 10.1002/dmr.5610070404. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed N. Babae-Jadidi R. Howell SK. Thornalley PJ. Beisswenger PJ. Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetes. Diabetes Care. 2005;28:2465–2471. doi: 10.2337/diacare.28.10.2465. [DOI] [PubMed] [Google Scholar]
- 20.Felipe DL. Hempe JM. Liu S. Matter N. Maynard J. Linares C. Chalew SA. Skin intrinsic fluorescence is associated with hemoglobin A1c and hemoglobin glycation index but not mean blood glucose in children with type 1 diabetes. Diabetes Care. 2011;34:1816–1820. doi: 10.2337/dc11-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer DG. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wautier JL. Guillausseau PJ. Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab. 2001;27:535–542. [PubMed] [Google Scholar]
- 23.Chabroux S. Canouï-Poitrine F. Reffet S. Mills-Joncour G. Morelon E. Colin C. Thivolet C. Advanced glycation end products assessed by skin autofluorescence in type 1 diabetics are associated with nephropathy, but not retinopathy. Diabetes Metab. 2010;36:152–157. doi: 10.1016/j.diabet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Lutgers HL. Gerrits EG. Graaff R. Links TP. Sluiter WJ. Gans RO. Bilo HJ. Smit AJ. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia. 2009;52:789–797. doi: 10.1007/s00125-009-1308-9. [DOI] [PubMed] [Google Scholar]
- 25.Friedman AN. Marrero D. Ma Y. Ackermann R. Narayan KM. Barrett-Connor E. Watson K. Knowler WC. Horton ES Diabetes Prevention Program Research Group. Value of urinary albumin-to-creatinine ratio as a predictor of type 2 diabetes in pre-diabetic individuals. Diabetes Care. 2008;31:2344–2348. doi: 10.2337/dc08-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]