Abstract
Background
Concerning continuous subcutaneous insulin infusion (CSII), there are controversial results related to changes in glycemic response according to the meal composition and bolus design. Our aim is to determine whether the presence of protein and fat in a meal could involve a different postprandial glycemic response than that obtained with only carbohydrates (CHs).
Subjects and Methods
This was a crossover, randomized clinical trial. Seventeen type 1 diabetes (T1D) patients on CSII wore a blinded continuous glucose monitoring system sensor for 3 days. They ingested two meals (meal 1 vs. meal 2) with the same CH content (50 g) but different fat (8.9 g vs. 37.4 g) and protein (3.3 g vs. 28.9 g) contents. A single-wave insulin bolus was used, and the interstitial glucose values were measured every 30 min for 3 h. We evaluated the different postprandial glycemic response between meal 1 and meal 2 by using mixed-effects models.
Results
The postmeal glucose increase was 22 mg/dL for meal 1 and 31 mg/dL for meal 2. In univariate analysis, at different times not statistically significant differences in glucose levels between meals occurred. In mixed-model analysis, a time×meal interaction was found, indicating a different response between treatments along the time. However, most of the patients remained in the normoglycemic range (70–180 mg/dL) during the 3-h postmeal period (84.4% for meal 1 and 93.1% for meal 2).
Conclusions
The presence of balanced amounts of protein and fat determined a different glycemic response from that obtained with only CH up to 3 h after eating. The clinical relevance of this finding remains to be elucidated.
Introduction
According to the recommendations of the American Diabetes Association, fasting plasma glucose should be 70–130 mg/dL, and postprandial glycemia should be lower than 180 mg/dL. The majority of patients with type 1 diabetes (T1D) are still far from achieving these values, mainly because intensive insulin therapy precipitates hypoglycemia. Therefore, new technologies that effectively improve metabolic control in these patients are needed. Postprandial hyperglycemia is an important risk factor for diabetes complications and macrovascular disease,1–7 so managing postprandial glucose levels is of utmost importance for individuals with diabetes.
Continuous subcutaneous insulin infusion (CSII) therapy allows patients to achieve different basal insulin rates and deliver different types of boluses: in standard boluses, insulin is delivered rapidly as a shot, whereas square- and dual-wave (Dw) boluses deliver insulin for an extended period of time.8
There is convincing evidence that increasing the amount of carbohydrate (CH) in a meal increases the glycemic response and thus the amount of insulin necessary to restore euglycemia.9–12 Therefore, boluses are traditionally calculated based on CH content. However, there is little and controversial information concerning glucose responses to protein- and fat-added meals.13–15
Most evidence suggests that fat and protein ingested in a meal should be covered by insulin delivered over an extended time in a Dw bolus to achieve the best postprandial metabolic control. However, the time (1–8 h) needed to correct postprandial glycemia and the amount of insulin (30–70% of total bolus insulin dose) have not been well established.16–21 Additionally, the presence of a fatty meal with CHs attenuates the postprandial glycemic response induced by the fat and CHs.22 Therefore, previous studies have used meals higher in fat/protein and CH in comparison with typical meals. Different meals that are more balanced and recommended for diabetes patients have never been investigated.
The aim of this study was to examine in T1D patients whether the presence of fat and protein in a meal could induce a different postprandial glycemic response than that obtained with only CH.
Subjects and Methods
This was a randomized crossover clinical trial conducted in a single center: Hospital Clínico Universitario de Santiago de Compostela in Northwestern Spain.
Patients
Seventeen patients with T1D mellitus were enrolled in this short-term study. The characteristics of the study group are presented in Table 1. All subjects had had T1D for at least 2 years, were >18 years old, and had at least 6 months of experience with CSII therapy. The pumps used were Paradigm 712 and 722 from Medtronic (Northridge, CA), Accu-Chek® Spirit from Roche (Burgdorf, Switzerland), or Animas 2020 from Johnson & Johnson (West Chester, PA). Fast-acting insulin analogs (lispro, glulisine, and aspart) were used.
Table 1.
Demographic Data of Type 1 Diabetes Patients
| Demographic | Value |
|---|---|
| Men/women (n) | 4/13 |
| Age (years) | 35.8±8.4 |
| Weight (kg) | 70.6±14.0 |
| BMI (kg/m2) | 25.3±4.0 |
| HbA1c (%) | 7.7±0.8 |
| Diabetes duration (years) | 17.7±7.7 |
| Daily insulin requirement (IU/day) | 38.3±11.8 |
BMI, body mass index; HbA1c, glycated hemoglobin.
The exclusion criteria included (1) celiac or any other gastrointestinal disease likely to affect gastrointestinal motility or absorption, major gastrointestinal symptoms, or prior abdominal surgery except appendectomy, (2) any diabetes complication, such as retinopathy, nephropathy, or neuropathy, (3) corticosteroid use or any medication that could modify gastric emptying (except insulin), (4) autonomic dysfunction, (5) symptomatic infection, (6) pregnancy/breastfeeding, (7) females prior to Day 1 of their menstrual cycle, and (8) inability to fulfill the protocol.
Test meals
Subjects ingested two different test meals on two different days in a randomized order. Both meals contained the same amount of CHs but different fat and protein contents. The meal compositions are described in Table 2. To minimize between-batch variation, test meals were prepared under the supervision of a nutritionist. Subjects were allowed to drink 200 mL of water with the meal. Both meals were consumed in 20–30 min in a sitting position. The standard bolus was administered just prior to the meal.
Table 2.
Composition of Test Meals
| Test meal | Total carbohydrate (g) | Total protein (g) | Total fat (g) |
|---|---|---|---|
| Test meal 1 (standard): pasta (60 g of pasta), tomato sauce (50 mL) | 50 | 3.3 | 8.9 |
| Test meal 2 (protein- and fat-added): pasta (60 g of pasta), tomato sauce (50 mL), veal chop (150 g), and olive oil (10 mL) | 50 | 28.9 | 37.4 |
Study procedure
Four visits to our clinic were required. The protocol was as follows. During the first visit (Day 1), patients were informed about the procedure they needed to follow. A blind continuous glucose monitoring system sensor (CGMS®; Medtronic) was inserted subcutaneously in the abdominal periumbilical area. Patients were asked not to inject insulin on the same side as the sensor insertion during the period of monitoring. This allowed subcutaneous interstitial glucose levels to be monitored on an ambulatory basis over a period of 3 consecutive days. Following a 60-min initialization period, subjects recorded a self-monitoring blood glucose value, and continuous glucose tracking began. During the glucose monitoring, alcohol intake and exercise were not allowed. Patients received their usual basal insulin.
At the second visit (Day 2), participants ate a test meal after at least a 3-h fast. A standard single-wave preprandial insulin bolus was delivered based on each subject's CH-to-insulin ratio and carbohydrate count (patients were previously trained in the use of the bolus calculator and CH counting). Subjects needed to be nearly normoglycemic (70–180 mg/dL) at baseline (when ingestion of the meal began) because both hyper- and hypoglycemia can affect the gastric emptying rate and subsequent postprandial glucose response. As no insulin bolus was allowed during the 3 h prior to the test meal, if a subject presented a blood glucose level <70 mg/dL or >180 mg/dL, the test was not performed.
After finishing the meal, patients remained at rest. Neither food ingestion nor smoking was allowed for at least 3 h. During this time, patients performed one self-measurement of capillary blood glucose per hour. Home blood glucose meters (Contour Link®; Bayer, Basel, Switzerland) and a diary were provided for this purpose and for proper calibration. At the third visit (Day 3), participants ate the other test meal. The same protocol as for the second day was followed.
At the last visit (Day 4), the CGMS was removed, and its data were downloaded into a computer for evaluation. Data of continuous glucose measurements during the 72-h period were finally obtained in 17 patients with T1D.
We measured interstitial glucose values at 30-min intervals until 3 h after both meals. We also analyzed the postmeal glucose increase (measured from the glucose value at 0 min to the peak value after the meal) and the percentage of glucose values below 70 mg/dL and above 180 mg/dL.23
In this two-treatment crossover study, to detect a difference in glucose levels of 30 mg/dL, with a two-sided 5% significance level and a power of 80%, a sample size of 16 patients was necessary, based on our assumption that the SD of the difference is 40 mg/dL.
The study was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All subjects provided written informed consent.
Statistical analysis
Comparisons between test meals (meal 1 vs. meal 2) for the above-mentioned variables were performed using t test for paired data. Mixed-effects models, with each individual as a random effect, were used to assess the effect of both meals on serum glucose.24 We used these models because they take into account the correlations among serial measures obtained from the same individual. In the models, glucose concentrations were the dependent variables, and baseline glucose concentrations, type of meal (meal 1 vs. meal 2), and time were the independent variables. Continuous variables were assessed linearly and curvilinearly with the addition of polynomial terms.
The interaction term meal×time was included to evaluate whether differences in meals varied over time. The most appropriate variance–covariance structure for each model was determined using a combination of Bayesian information criterion scores and plots of fitted values versus residuals based on a full-model specification using restricted maximum likelihood. All of the analyses were performed using the nlme package in the R statistical and programming environment.24,25 P values<0.05 were considered statistically significant.
Results
We initially selected 25 patients who fulfilled the inclusion criteria. Seventeen adults with T1D volunteered to participate and completed the study. Their clinical characteristics are shown in Table 1. The basal glucose levels were 111±28 mg/dL before test meal 1 and 112±28 mg/dL before test meal 2.
The glucose values at 0, 30, 60, 90, 120, 150, and 180 min are provided for both meals in Table 3. In univariate analysis, glucose levels at different times were not statistically significantly different between meals.
Table 3.
Mean Glucose Values at 30-Min Intervals in Type 1 Diabetes Patients After Both Meals
| |
Glucose value (mg/dL) at time (min) |
||||||
|---|---|---|---|---|---|---|---|
| Meal | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
| Meal 1 | 111.5±28.3 | 116.4±29.0 | 129.3±38.6 | 119.4±36.1 | 111.7±41.9 | 105.7±37.0 | 105.6±39.0 |
| Meal 2 | 112.1±28.2 | 111.8±30.5 | 124.9±36.4 | 131.2±35.2 | 123.6±32.9 | 123.6±28.0 | 126.3±37.5 |
| P valuea | 0.653 | 0.871 | 0.822 | 0.258 | 0.158 | 0.135 | 0.078 |
Data are mean±SD values.
By t test for paired data (not corrected for multiple comparisons).
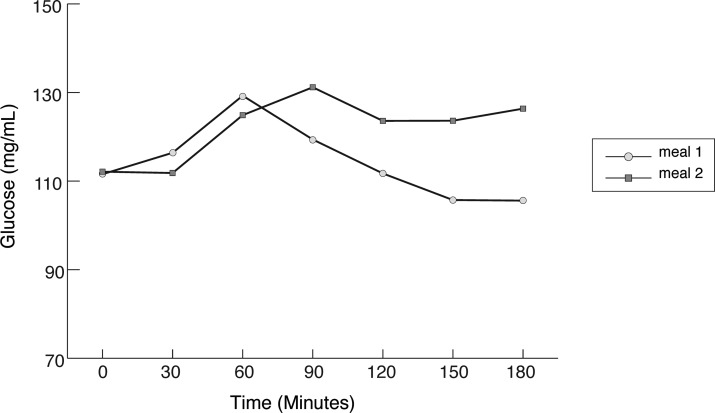
For most of the 3-h postmeal period, patients remained in the normoglycemic range (70–180 mg/dL) (Fig. 1), with no significant differences between test meals (mean 84.4% of the time with meal 1 vs. mean 93.1% of the time with meal 2).
FIG. 1.
Mean glucose values at 30, 60, 90, 120, 150, and 180 min in type 1 diabetes patients after both meals. By mixed-model analysis, P<0.001 for time, P>0.05 for meal, and P<0.05 for meal×time interaction.
As shown in Figure 1, both meals were followed by a significant increase in serum glucose. The maximum glucose value was reached at 60 (meal 1) or 90 min (meal 2), and then the glucose concentrations fell differently depending on the meal. After meal 1, glucose reached the preprandial level at 3 h, whereas after meal 2, glucose remained high during the whole 3-h period. In mixed model analysis, there were significant differences in glucose levels at different times after meal (P<0.001), and there was a significant interaction time by meal (P<0.05), indicating different profiles in glucose levels for each meal with time after the meal.
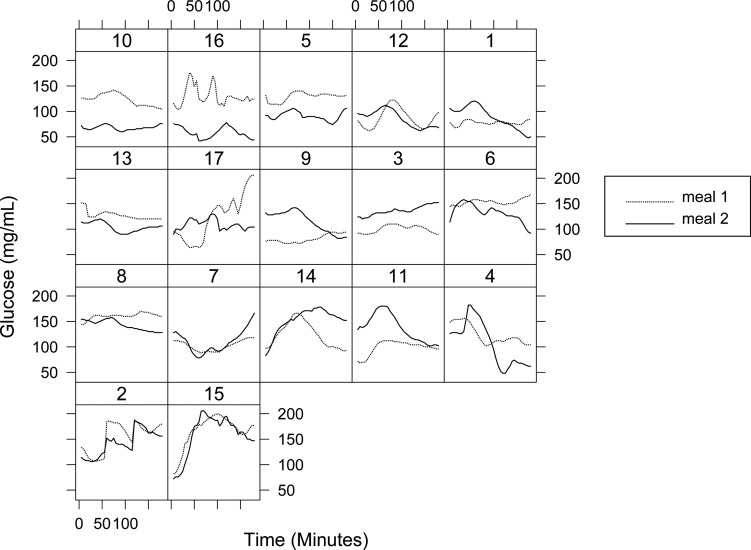
No patient had severe hypoglycemia during the test. Five patients had glucose values lower than 70 mg/dL after both meals but without hypoglycemic symptoms. Two patients after meal 1 had glucose values under 50 mg/dL but did not require any medical help. The results obtained from each individual patient after the intake of both meals are shown in Figure 2.
FIG. 2.
Individual glucose values in type 1 diabetes patients after both meals until 180 min.
Discussion
Our results show that the intake of a high-fat meal involved a different glycemic response compared with a low-fat meal (with the same amount of CH in both meals). We also observed that as fat was added to meal, the glucose level remained higher longer. The presence of fat therefore promoted a prolonged, late relative hyperglycemia. Using a single bolus, most of the patients (84.4% for meal 1 and 93.1% for meal 2) remained within the normoglycemic range (70–180 mg/dL), regardless of the addition of fat to the CHs.
Lindholm-Olinder et al.20 observed no overall differences in glucose excursions between different bolus methods after a pasta meal (36% fat). In their study the glucose values were also followed for 3 h after the meal.
In our study, after meal 2 (high fat), we noted a higher and slightly flattened postprandial glycemic response between 90 and 180 min. Similar results were observed by Lodefalk et al.,22 who found that a meal high in fat reduced the initial (2-h) postprandial glycemic response, even though the CH content of the meals was the same. One of the most important effects of fats on glycemia comes from the delayed gastric emptying, which promotes a decrease in the immediate postprandial glycemia and may result in a late hyperglycemia.26
A few more studies have been carried out on this topic, although most of them had pizza as the test meal. Jones et al.16 concluded that less postprandial hyperglycemia was observed in the late postprandial period (8–12 h) after using a Dw bolus (50/50%) extended over 8 h. Likewise, Chase et al.17 demonstrated that the Dw therapy achieved a significantly lower postprandial hyperglycemia at 4 h in comparison with a single-wave bolus after a meal high in CHs and fat. However, with both single-wave and Dw boluses, all glucose values were within the normoglycemic range. It must be taken into account that their sample could be inadequate (nine patients), and the baseline values suggest that some subjects had high preprandial glycemia, which could have skewed their results.
Another study18 concluded that the Dw bolus (70/30% over a 5-h period) better controls the postprandial glycemic excursion after a high-fat meal from 5 h through 14 h. There were no significant differences in the 3 h immediately following the meal in comparison with the single-wave bolus. It should be noted that the number of participants was lower (n=10) and the type of food studied was atypical for our culture (burritos and cheese pizza).
De Palma et al.27 observed that a simple bolus injected 15 min prior to the meal (margherita pizza) seemed to be the best option to control the glycemic rise. The observation period was 6 h, and they compared this bolus type with dual and square types. The limitation to their study was that there was a large dispersion in the baseline values, and there were no data about the number of mild hypoglycemias.
Finally, Pańkowska et al.21 focused on the best method of insulin bolus delivery following a mixed meal. The average glucose values between 2 and 6 h after the test meal were lower in the group treated with a Dw bolus. The main limitations were the use of additional insulin and four hypoglycemic episodes (<50 mg/dL) in the Dw bolus group. Furthermore, using the American Diabetes Association criterion (hypoglycemia <70 mg/dL) would increase their number of hypoglycemia episodes, and this could lead to a bias when analyzing results. In addition to being familiar with the CH counting system, the patients needed to use the fat-protein unit counting system for this type of Dw bolus. This makes calculation more difficult for patients because it is rather technical and complex.26
According to our results, there was a different glycemic response after fatty meals. However, the glucose levels remained within the postprandial targets after both meals. In previous studies, the differences between meals appeared later than 3 h.
The strengths of this study are the homogeneity of glucose levels at baseline (which eliminates some bias from the results), the rarity of hypoglycemia episodes, and the use of balanced and typical test meals.
Despite all these advantages, there are also several potential limitations. First, we had a sample adequate to detect moderate differences (30 mg/dL). However, most of the previous published studies were also performed in groups containing 10–24 patients. Additionally, and in contrast to most of the current literature, we did not use different types of insulin boluses. This is because our aim was not to compare boluses but to determine if the traditional simplest bolus could be useful in achieving an optimum postprandial glycemic response. Finally, the individual subject analysis showed great heterogeneity despite the strict adherence to the protocol design and execution.
In conclusion, this study has shown that the presence of balanced amounts of protein and fat determined a different glycemic response from that obtained with only CHs up to 3 h after eating. The clinical relevance of this finding remains to be elucidated.
Acknowledgments
This work was performed with funding grants from the Spanish Diabetes Society and the Instituto de Salud Carlos-III (Spanish Ministry of Science and Technology, PI11/02219).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ceriello A. The emerging role of postprandial hyperglycaemic spikes in the pathogenesis of diabetic complications. Diabet Med. 1998;15:188–193. doi: 10.1002/(SICI)1096-9136(199803)15:3<188::AID-DIA545>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Temelkova-Kurktschiev TS. Koehler C. Henkel E. Leonhardt W. Fuecker K. Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 3.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 4.Stamler J. Vaccaro O. Neaton J. Wentworth D Multiple Risk Factor Intervention Trial Research Group. Diabetes, other risk factors and 12-year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 5.Balkau B. Shipley M. Jarrett RJ. Pyorala K. Pyorala M. Forhan A. Eschwège E. High blood glucose concentration is a risk factor for mortality in middle-aged non diabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360–367. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- 6.The DECODE Study Group: Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 7.Barrett-Connor E. Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. Diabetes Care. 1998;21:1236–1239. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 8.Pánkowska E. Szypowska A. Lipka M. Szpotanska M. Blazik M. Groele L. Application of novel dual wave meal bolus and its impact on glycated hemoglobin A1c level in children with type 1 diabetes. Pediatr Diabetes. 2009;10:298–303. doi: 10.1111/j.1399-5448.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 9.Pickup J. Mattock M. Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: metaanalysis of randomised controlled trials. BMJ. 2002;324:705. doi: 10.1136/bmj.324.7339.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slama G. Klein IC. Delage A. Ardila E. Lemaignen H. Papoz L. Tchobroutsky G. Correlation between the nature and amount of carbohydrate in meal intake and insulin delivery by the artificial pancreas in 24 insulin-dependent diabetics. Diabetes. 1981;30:101–105. doi: 10.2337/diab.30.2.101. [DOI] [PubMed] [Google Scholar]
- 11.Service FJ. Hall LD. Westland RE. O'Brien PC. Go VL. Haymond MW. Rizza RA. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia. 1983;21:316–321. doi: 10.1007/BF00253193. [DOI] [PubMed] [Google Scholar]
- 12.Service Fl. Rizza RA. Hall LD. Westland RE. O'Brien PC. Clemens AH. Haymond MW. Gerich JE. Prandial insulin requirements in insulin-dependent diabetics: effects of size, time of day and sequence of meals. J Clin Endocrinol Metab. 1983;57:931–936. doi: 10.1210/jcem-57-5-931. [DOI] [PubMed] [Google Scholar]
- 13.Conn JW. Newburgh LH. The glycemic response to isoglucogenic quantities of protein and carbohydrate. J Clin Invest. 1936;15:665–671. doi: 10.1172/JCI100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinowitz D. Maffezzoli R. Merimee TI. Burgess JA. Patterns of hormonal release after glucose, protein and glucose plus protein. Lancet. 1966;2:454–456. doi: 10.1016/s0140-6736(66)92767-x. [DOI] [PubMed] [Google Scholar]
- 15.Nuttall FQ. Gannon MC. Wald IL. Ahmed M. Plasma glucose and insulin profiles in normal subjects ingesting diets of varying carbohydrate, fat and protein content. J Am Coll Nutr. 1985;4:437–450. doi: 10.1080/07315724.1985.10720086. [DOI] [PubMed] [Google Scholar]
- 16.Jones SM. Quarry JL. Caldwell-McMillan M. Mauger DT. Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther. 2005;7:233–240. doi: 10.1089/dia.2005.7.233. [DOI] [PubMed] [Google Scholar]
- 17.Chase HP. Saib SZ. MacKenzie T. Hansen MM. Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19:317–321. doi: 10.1046/j.1464-5491.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee Sw. Cao M. Sajid S. Hayes M. Choi L. Rother C. de León R. The dual-wave bolus feature in continuous subcutaneous insulin infusion pumps controls prolongued postprandial hyperglycaemia better than standard bolus in type 1 diabetes. Diabetes Nutr Metab. 2004;17:211–216. [PubMed] [Google Scholar]
- 19.Heinemann L. Insulin pump therapy: what is the evidence for using different types of boluses for coverage of prandial insulin requirements? J Diabetes Sci Technol. 2009;3:1490–1500. doi: 10.1177/193229680900300631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindholm-Olinder A. Runefors J. Smide B. Kernell A. Post-prandial glucose levels following three methods of insulin bolusing: a study in adolescent girls and in comparison with girls without diabetes. Pract Diabetes Int. 2009;26:2110–2115. [Google Scholar]
- 21.Pańkowska E. Blazik M. Groele L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther. 2012;14:16–22. doi: 10.1089/dia.2011.0083. [DOI] [PubMed] [Google Scholar]
- 22.Lodefalk M. Aman J. Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with type 1 diabetes. Diabet Med. 2008;25:1030–1035. doi: 10.1111/j.1464-5491.2008.02530.x. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association: Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbeke G. Molenberghs G. Springer Series in Statistics: Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 25.Pinheiro J. Bates D. Debroy Sarhar D. R Package Version 3.1-100. Vienna: R Foundation for Statistical Computing; 2011. Linear, Nonlinear Mixed Effects Models. [Google Scholar]
- 26.Maahs D. Higgins J. Is carbohydrate counting enough? Towards perfection or unwanted complexity? Diabetes Technol Ther. 2012;14:3–5. doi: 10.1089/dia.2011.0234. [DOI] [PubMed] [Google Scholar]
- 27.De Palma A. Giani E. Iafusco D. Bosetti A. Macedoni M. Gazzarri A. Spiri D. Scaramuzza A. Zuccotti GV. Lowering postprandial glycemia in children with type 1 diabetes after Italian pizza “margherita” (TyBoDi2 Study) Diabetes Technol Ther. 2011;13:483–487. doi: 10.1089/dia.2010.0163. [DOI] [PubMed] [Google Scholar]