Abstract
Objective:
The objective of this study is to compare all-cause in-hospital mortality in preterm infants with respiratory distress syndrome (RDS) treated with poractant alfa, calfactant or beractant.
Study Design:
A retrospective cohort study of 14 173 preterm infants with RDS, treated with one of three surfactants between 2005 and 2009, using the Premier Database was done. Multilevel, multivariable logistic regression modeling, adjusting for patient- and hospital-level factors was performed.
Result:
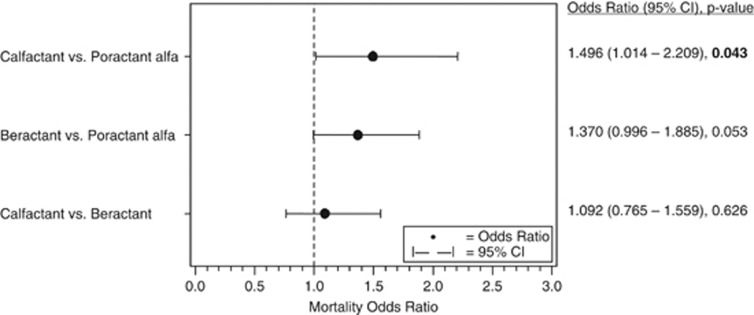
Calfactant treatment was associated with a 49.6% greater likelihood of death than poractant alfa (odds ratio (OR): 1.496, 95% confidence interval (CI): 1.014–2.209, P=0.043). Beractant treatment was associated with a non-significant 37% increase in mortality, compared with poractant alfa (OR: 1.370, 95% CI: 0.996–1.885, P=0.053). No differences in mortality were observed between calfactant and beractant treatment (OR: 1.092, 95% CI: 0.765–1.559, P=0.626).
Conclusion:
Poractant alfa treatment for RDS was associated with a significantly reduced likelihood of death when compared with calfactant and a trend toward reduced mortality when compared with beractant.
Keywords: surfactant, mortality, respiratory distress syndrome, poractant alfa, calfactant, beractant
Introduction
Preterm births continue to increase in spite of major advances in perinatal care, especially in developed countries.1 Prematurity and low birth weight (LBW, < 2500 g) accounted for 16.5% of all infant deaths in 2005 and was the second leading cause of infant mortality.2 Respiratory distress syndrome (RDS) is the most common cause of respiratory distress in preterm infants and occurs in nearly 50% of preterm infants born at less than 30 weeks of gestation.3
Treatment with surfactant for RDS has been shown to significantly decrease pneumothorax, and neonatal and infant mortality.3, 4, 5, 6, 7, 8 The animal-derived surfactants, poractant alfa (Curosurf, Chiesi Farmaceutici SpA, Parma, Italy), calfactant (Infasurf, Ony, St Louis, MO, USA) and beractant (Survanta, Abbott Nutrition, Columbus, OH, USA) have been shown to be associated with greater early improvement in the requirement for ventilatory support, fewer pneumothoraces and reduced mortality when compared with treatment with first-generation synthetic surfactants.9, 10
Nine randomized, controlled clinical trials (RCTs)11, 12, 13, 14, 15, 16, 17 and one retrospective study18 comparing these surfactant preparations in the setting of RDS treatment have now been published. No significant differences in mortality were found in the four trials that compared beractant with calfactant.11, 12 Among the five trials that compared beractant with poractant alfa,13, 14, 15, 16 one reported significantly lower mortality with poractant alfa in infants ⩽32 weeks gestational age.15 Furthermore, in a meta-analysis of comparative trials, mortality was significantly lower (relative risk: 0.57, 95% CI: 0.34–0.96, P<0.05) with poractant alfa compared with beractant.3 In the only retrospective study published to date comparing surfactants, Clark et al.18 found no significant differences in all-cause mortality between beractant- and calfactant-treated patients overall or in any birth weight (BW) subgroups.
There are no published studies comparing mortality in preterm infants treated with the three animal-derived surfactants available in the US. RCTs using mortality as a primary outcome require large sample size, are expensive, and may take several years to complete. The difficulties with conducting comparative RCTs in premature infants with RDS are evidenced by the premature interruption of studies aimed at comparing different surfactant preparations in terms of mortality or bronchopulmonary dysplasia due to insufficient enrollment.12 To overcome these hurdles, we used data from a large national hospital database to assess whether there were differences in all-cause mortality among preterm infants treated with poractant alfa, calfactant or beractant.
Patients and methods
A retrospective observational cohort analysis was conducted using the US hospital administrative data from the Premier Database.19, 20 The Premier Database is a large US hospital-based database, containing information on approximately 5.5 million annual hospital discharges (approximately one- fifth of all acute care hospitalizations in the US) with day-by-day service level detail. The Premier Data included hospitalizations from more than 600 hospitals, approximately 30 of which were children's hospitals or had children's hospital facilities. These hospitals utilize the database for quality and utilization benchmarking. Hospitals submit data to the database, which undergo quality checks and validation. As well, the data are also used by the US government agencies such as the Food and Drug Administration and Centers for Medicare and Medicaid Services.19, 20
The analyses were conducted using de-identified data in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). No institutional review board approval for the study was sought, as, in addition to being HIPAA compliant, the de-identified nature of the database would preclude the researchers from identifying any hospital sites or patients. The study was designed to compare all-cause in-hospital mortality, defined by the discharge status of ‘expired', in preterm infants treated with poractant alfa, calfactant or beractant.
Study population
Infants were included in the study if they were discharged as an inpatient from a Premier Database hospital from 1 January 2005, through 31 December 2009. Inclusion criteria for the study included having gestational age of 25–32 weeks, BW 500–1999 g, diagnosis of RDS, age ⩽ 2 calendar days, when they received the first dose of surfactant, and having received only one of the three study surfactants.
Patients were excluded from the study in case of missing values for any of the variables planned a priori to be included in the logistic regression model, if they received more than one surfactant during their hospitalization, or if there was evidence of congenital abnormalities such as trisomy 13 or 18, anencephaly or dwarfism. Information on diagnosis of RDS, gestational age at birth, BW and congenital anomalies was obtained from International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) codes.
Statistical analysis
Patient demographics and hospital characteristics were compared between treatment groups using the χ2-test for categorical variables. The comparison between surfactants in terms of mortality was based on a mixed multilevel, multivariable logistic regression model. The multilevel structure accounted for clustering of infants within hospitals, including a random center effect in the model.21 Other than the type of surfactant, the following patient-level factors were included in the model to control for potentially confounding variables: gestational age (categorized into 2-week groups, from 25–26 weeks to 31–32 weeks), BW (categorized into 250-g groups, from 500–749 g to 1750–1999 g), gender, race, 3M All Patient Refined Diagnosis Related Group severity of illness category and risk of mortality category.22, 23 Furthermore, the following hospital-level factors were included as covariates: US Census region, population served (urban/rural), teaching status (teaching/non-teaching) and hospital size (categorization based on the number of beds).
To assess the sensitivity of the results, three alternative models were estimated. In the first of these, gestational age at birth was excluded from the factors due to the potential inaccuracy in gestational dating.24 In the second model, the covariates were submitted to a backward selection procedure (removal from the model if P>0.1) to reduce the number of parameters and to obtain more stable estimates of the effects of the surfactants. In the third sensitivity model, the year during which the hospital discharge occurred was added as a covariate to account for potential trends in mortality over time. All statistical analyses were performed using SAS 9.1.3 (SAS Institute Cary, NC, USA).
Results
A total of 14 173 infants discharged from 236 hospitals were included in the study population. Patient demographics and hospital characteristics of the study population are shown in Table 1.
Table 1. Patient demographics and hospital characteristics by surfactant treatment.
|
Poractant alfa |
Calfactant |
Beractant |
All |
P vs C | P vs B | C vs B | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||||
| Discharges | 5097 | (100.0) | 3378 | (100.0) | 5698 | (100.0) | 14 173 | (100.0) | |||
| Calendar year of discharge | <0.001 | <0.001 | <0.001 | ||||||||
| 2005 | 653 | (12.8) | 832 | (24.3) | 1196 | (21.0) | 2681 | (18.9) | |||
| 2006 | 918 | (18.0) | 802 | (23.7) | 1363 | (23.9) | 3083 | (21.8) | |||
| 2007 | 1022 | (20.1) | 578 | (17.1) | 1135 | (19.9) | 2735 | (19.3) | |||
| 2008 | 1252 | (24.6) | 558 | (16.5) | 991 | (17.4) | 2801 | (19.8) | |||
| 2009 | 1252 | (24.6) | 608 | (18.0) | 1013 | (17.8) | 2873 | (20.3) | |||
| Gestational age | <0.001 | <0.001 | 0.629 | ||||||||
| 25–26 weeks | 1016 | (19.9) | 754 | (22.3) | 1272 | (22.3) | 3042 | (21.5) | |||
| 27–28 weeks | 1309 | (25.7) | 936 | (27.7) | 1544 | (27.1) | 3789 | (26.7) | |||
| 29–30 weeks | 1427 | (28.0) | 909 | (26.9) | 1602 | (28.1) | 3938 | (27.8) | |||
| 31–32 weeks | 1345 | (26.4) | 779 | (23.1) | 1280 | (22.5) | 3404 | (24.0) | |||
| Birth weight | 0.451 | 0.002 | 0.437 | ||||||||
| 500–749 g | 495 | (9.7) | 329 | (9.7) | 506 | (8.9) | 1330 | (9.4) | |||
| 750–999 g | 1164 | (22.8) | 806 | (23.9) | 1419 | (24.9) | 3389 | (23.9) | |||
| 1000–1249 g | 1160 | (22.8) | 770 | (22.8) | 1358 | (23.8) | 3288 | (23.2) | |||
| 1250–1499 g | 959 | (18.8) | 664 | (19.7) | 1105 | (19.4) | 2728 | (19.2) | |||
| 1500–1749 g | 806 | (15.8) | 498 | (14.7) | 822 | (14.4) | 2126 | (15.0) | |||
| 1750–1999 g | 513 | (10.1) | 311 | (9.2) | 488 | (8.6) | 1312 | (9.3) | |||
| Gender | 0.475 | 0.489 | 0.179 | ||||||||
| Female | 2308 | (45.3) | 1503 | (44.5) | 2618 | (45.9) | 6429 | (45.4) | |||
| Male | 2789 | (54.7) | 1875 | (55.5) | 3080 | (54.1) | 7744 | (54.6) | |||
| Race | <0.001 | <0.001 | <0.001 | ||||||||
| White | 2920 | (57.3) | 2185 | (64.7) | 2763 | (48.5) | 7868 | (55.5) | |||
| Black | 1343 | (26.3) | 866 | (25.6) | 2100 | (36.9) | 4309 | (30.4) | |||
| Hispanic | 564 | (11.1) | 251 | (7.4) | 698 | (12.2) | 1513 | (10.7) | |||
| Other | 270 | (5.3) | 76 | (2.2) | 137 | (2.4) | 483 | (3.4) | |||
| 3M APR-DRG severity of illness | <0.001 | <0.001 | <0.001 | ||||||||
| 1=minor | 47 | (0.9) | 33 | (1.0) | 46 | (0.8) | 126 | (0.9) | |||
| 2=moderate | 561 | (11.0) | 268 | (7.9) | 418 | (7.3) | 1247 | (8.8) | |||
| 3=major | 2436 | (47.8) | 1395 | (41.3) | 2670 | (46.9) | 6501 | (45.9) | |||
| 4=extreme | 2053 | (40.3) | 1682 | (49.8) | 2564 | (45.0) | 6299 | (44.4) | |||
| 3M APR-DRG risk of mortality | <0.001 | 0.008 | <0.001 | ||||||||
| 1=minor | 1401 | (27.5) | 725 | (21.5) | 1422 | (25.0) | 3548 | (25.0) | |||
| 2=moderate | 1907 | (37.4) | 1184 | (35.1) | 2128 | (37.3) | 5219 | (36.8) | |||
| 3=major | 1472 | (28.9) | 1218 | (36.1) | 1783 | (31.3) | 4473 | (31.6) | |||
| 4=extreme | 317 | (6.2) | 251 | (7.4) | 365 | (6.4) | 933 | (6.6) | |||
| US census region of treating hospital | <0.001 | <0.001 | <0.001 | ||||||||
| Northeast | 371 | (7.3) | 648 | (19.2) | 450 | (7.9) | 1469 | (10.4) | |||
| Midwest | 808 | (15.9) | 173 | (5.1) | 899 | (15.8) | 1880 | (13.3) | |||
| South | 2715 | (53.3) | 2466 | (73.0) | 3988 | (70.0) | 9169 | (64.7) | |||
| West | 1203 | (23.6) | 91 | (2.7) | 361 | (6.3) | 1655 | (11.7) | |||
| Population served by treating hospital | <0.001 | <0.001 | <0.001 | ||||||||
| Urban | 4758 | (93.3) | 3313 | (98.1) | 5219 | (91.6) | 13290 | (93.8) | |||
| Rural | 339 | (6.7) | 65 | (1.9) | 479 | (8.4) | 883 | (6.2) | |||
| Teaching status of treating hospital | <0.001 | <0.001 | <0.001 | ||||||||
| Teaching | 3199 | (62.8) | 2281 | (67.5) | 3325 | (58.4) | 8805 | (62.1) | |||
| Non-teaching | 1898 | (37.2) | 1097 | (32.5) | 2373 | (41.6) | 5368 | (37.9) | |||
| Size (no. of beds) of treating hospital | <0.001 | <0.001 | <0.001 | ||||||||
| <100 | 1 | (0.02) | 87 | (2.6) | 55 | (1.0) | 143 | (1.0) | |||
| 100–299 | 698 | (13.7) | 441 | (13.1) | 824 | (14.5) | 1963 | (13.9) | |||
| 300–499 | 2325 | (45.6) | 736 | (21.8) | 1760 | (30.9) | 4821 | (34.0) | |||
| 500+ | 2073 | (40.7) | 2114 | (62.6) | 3059 | (53.7) | 7246 | (51.1) | |||
Abbreviations: 3M APR-DRG, 3M All Patient Refined Diagnosis Related Group; B, beractant; C, calfactant; P, poractant alfa.
P-values are based on the χ2-test.
Bold values are statistically significant.
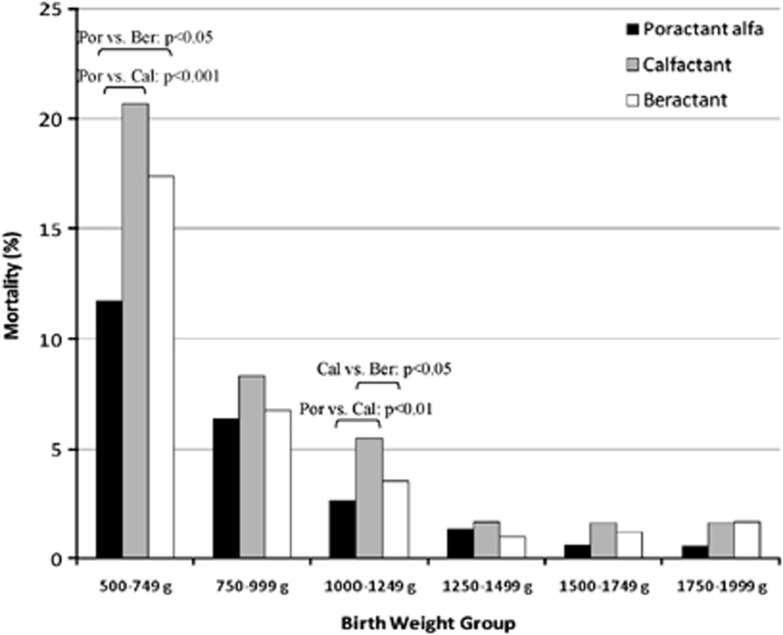
Overall, the unadjusted all-cause in-hospital mortality rates were 3.61% (n=184) in the poractant alfa group, 5.95% (n=201) in the calfactant group, and 4.58% (n=261) in the beractant group. When stratified by BW, as shown in Figure 1, the lowest mortality rate was always observed in the poractant alfa group, except for the category 1250–1499 g, where beractant-treated infants had the lowest mortality. Mortality was significantly lower for infants 500–749 g, who received poractant alfa (11.72%) than for those who received calfactant (20.67%, P<0.001) or beractant (17.39%, P=0.011). In the 1000–1249 g BW category, mortality was significantly higher in the calfactant group (5.46%) than in the poractant alfa (2.67%, P=0.002) and beractant (3.54%, P=0.035) groups.
Figure 1.
Unadjusted mortality rates by BW among the three surfactant-treated groups.
The results of the multilevel, multivariable logistic regression model for all-cause in-hospital mortality are shown in Figure 2. Calfactant was found to be associated with a 49.6% greater likelihood of death than poractant alfa (odds ratio (OR): 1.496, 95% confidence intervals (CI): 1.014–2.209, P=0.043). Beractant treatment was associated with a 37.0% increased mortality compared with poractant alfa, but the difference did not reach statistical significance (OR: 1.370, 95% CI: 0.996–1.885, P=0.053). No differences in mortality were observed between calfactant- and beractant-treated infants (OR: 1.092, 95% CI: 0.765–1.559, P=0.626).
Figure 2.
Comparison of mortality among the three surfactant-treated groups.
These results were supported by the sensitivity analyses performed. The increase in the likelihood of death with calfactant compared with poractant alfa was significant in the first two alternative models (51.9% increase, P=0.036 in the model excluding gestational age and 56.3%, P=0.016 in the backward selection model) and non-significant in the third model adding discharge year (35.0% increase, P=0.134). The trend towards an increased mortality with beractant compared with poractant alfa that was observed in the main model reached statistical significance in the first two alternative models (38.2% increase, P=0.048 and 37.7%, P=0.040, respectively), but did not reach significance in the third model (24.7% increase, P=0.179). In all alternative models, no differences in mortality were observed between calfactant and beractant.
Discussion
The present study retrospectively investigated, for the first time, all-cause mortality among preterm infants with RDS, treated with the three animal-derived surfactants available in the US, namely, poractant alfa, calfactant or beractant.
To overcome the difference in the demographic characteristics of the population investigated, which can be an intrinsic limitation of retrospective studies, a logistic regression model adjusting for patient and hospital factors was applied. Furthermore, the clustering of infants within hospitals was accounted for by the inclusion of the center effect in the model.
This model found calfactant to be associated with a significantly greater likelihood of death than poractant alfa. Beractant was associated with a non-significant increase in mortality, compared with poractant alfa, and no differences were observed between calfactant and beractant. The results obtained in the full model were also supported by the sensitivity analyses. The alternative models showed a statistically significant reduction of the likelihood death with poractant alfa compared with both calfactant and beractant, except the analysis including discharge year, where the mortality reduction with poractant alfa did not reach statistical significance. In particular, the model which excluded gestational age was performed similar to the approach followed by Clark et al.18 in the only retrospective comparison of calfactant and beractant published in the literature, which used BW, but not gestational age, as a key covariate. Some evidence suggests that, despite the fact that gestational age is a key factor in determining the outcome in preterm infants, the methods to calculate it are not precise unless an early first trimester fetal ultrasound was used for estimating the gestational age.24
The unadjusted results were consistent with the adjusted model. Overall, the unadjusted mortality rates found in this study were 3.61% for poractant alfa, 4.58% for beractant and 5.95% for calfactant.
The results of this large retrospective study should be interpreted and validated in the context of other evidence from the medical literature, which report comparisons between animal-derived surfactants. Focusing on poractant alfa and beractant, in a pilot study of 75 preterm infants with RDS by Speer et al.,13 mortality at 28 days was 3% in the poractant alfa 200 mg kg−1 group and 12.5% in the beractant 100 mg kg−1 group; however, this difference did not reach significance (adjusted OR: 0.23, 95% CI: 0.02–2.54, P=0.23). In a prospective study of 58 RDS infants, Malloy et al.16 found no significant difference in mortality at 40 weeks between infants receiving poractant alfa and beractant (0 vs 10%, respectively P=0.08). A larger study by Ramanathan et al.15 in 293 RDS infants, found in those who were no more than 32 weeks gestational age (n=270), a 3% mortality at 36 weeks post-menstrual age in the poractant alfa (200 mg kg−1)-treated infants versus 11% for beractant (100 mg kg−1)- or poractant alfa (100 mg kg−1)-treated patients (P=0.034 and P=0.046, respectively). In another RCT in 52 RDS patients, Fujii et al.17 reported that mortality was 8% in the poractant alfa 200 mg kg−1 group versus 19% in the beractant 100 mg kg−1 group (P=0.27). All together, these randomized, controlled studies consistently showed a survival advantage with poractant alfa over beractant, although this reduction in mortality did not reach significance in most trials due to small sample size. Therefore, the trend towards increased mortality with beractant compared with poractant alfa found in the present retrospective study confirmed the findings of the smaller RCTs performed between these two surfactants.
As far as beractant and calfactant comparisons, both RCTs and retrospective evaluations have shown no mortality difference. The first RCT comparing beractant and calfactant in 1997 showed no difference in mortality between these two surfactants in the overall population.11 Additionally, Bloom et al.12 found 10% and 11% mortality rates at 36 weeks post menstrual age for beractant- and calfactant-treated patients, respectively (p⩾0.05). Finally, in the retrospective study by Clark et al.18 on 5169 infants, no differences were found in mortality rates before 28 days of age between calfactant and beractant (OR: 1, 95% CI: 0.8–1.3). Our study confirmed the absence of differences in mortality between beractant and calfactant in prospective as well as retrospective studies published to date.
Lastly, no study has been published comparing mortality between poractant alfa and calfactant. This is therefore the first direct comparison available between these two surfactants, showing a significant greater likelihood of death with calfactant than poractant alfa.
Our study has certain limitations due to the retrospective nature of the database used. Among the restrictions of the database, information on the precise cause of death is unavailable and the number of surfactant doses is not reliably calculable. The database also lacked reliable antenatal steroid use data, partly because antenatal steroids may have been given to the mother before entry into the hospital for delivery, and the Premier Database focused on hospital data by design. It was not possible, therefore, to adjust the model for this factor as a covariate, despite the importance that antenatal steroid use has for improving lung function and reducing RDS severity and its related mortality risk. However, in the Clark et al.18 study where data on antenatal steroids were available and the comparison between surfactants was adjusted for this covariate, the finding of no difference in the outcome between calfactant and beractant was coherent with our study results.
We acknowledge that the value of retrospective studies, despite their limitations, lies in the possibility to study large patient sample size, contributing to increased study power. This is particularly important in the field of clinical investigation on surfactants, where the known efficacy of treatment on mortality outcomes implies the need for large sample size to detect even small, but significant difference, making RCTs often unaffordable in terms of costs and recruitment. As an example, the treatment trial published by Bloom et al.12 required a sample size of 2080 infants to detect a 6% difference in infants alive without bronchopulmonary dysplasia between calfactant and beractant. However, the study was terminated prematurely after enrollment of 1361 infants (65.4% of the target). Finally, emerging evidence shows that findings from retrospective studies may provide the medical community with information on drug effectiveness in real-world settings.25
The lower mortality observed in poractant alfa-treated infants compared with calfactant or beractant prompts one to look for a possible explanation for such different outcomes for poractant alfa over the other two surfactants. The most likely explanation may be due to different surfactant doses administered to the infants included in the database, according to their US-prescribing information: 200 mg kg−1 for poractant alfa, 100 mg kg−1 for beractant and 105 mg kg−1 for calfactant. Poractant alfa is the only surfactant that has been studied using 200 mg kg−1 for the initial dose, and has been associated with faster weaning of oxygen and peak inspiratory pressure, fewer doses and lower mortality. Evidence from a RCT has shown that poractant alfa 200 mg kg−1 is better than poractant alfa 100 mg kg−1 in reducing mortality, whereas when poractant alfa and beractant are used at the same dose of 100 mg kg−1 for the initial dose, no difference in mortality was observed, despite the faster onset of action with poractant alfa.15 Compared with poractant alfa 100 mg kg−1, poractant alfa 200 mg kg−1 has also been shown to result in longer surfactant half-life, fewer retreatments and improved oxygenation.26 Poractant alfa is the surfactant preparation that closely resembles phosphatidylcholine molecular species composition of human surfactant and contains27, 28 the highest amount of polyunsaturated fatty acid-phospholipids and plasmalogens amongst other surfactant preparations, when normalized for phospholipid amounts.29 These components are important for reducing viscosity and interacting with surfactant protein B to regulate the adsorption and spreading properties of the phospholipids.30
In conclusion, this large retrospective study of preterm infants with RDS found lower mortality among infants who received poractant alfa, compared with infants who received either calfactant or beractant, even after adjusting for patient characteristics such as gestational age and BW, and after accounting for hospital characteristics and center effects. These results in real-world settings are consistent with prior RCTs, but provide additional significant findings that most RCTs have not been able to provide due to their relatively small sample size.
Acknowledgments
We would like to thank Stefano Vezzoli for his extensive statistical support. The authors also thank Raffaella Monno and Carmen Dell'Anna for their scientific contribution and assistance with the manuscript, as well as Teresa Davis for her database programming support.
This study was sponsored by Chiesi Farmaceutici SpA, the manufacturer of poractant alfa. Frank R Ernst is an employee of Premier, which contracted with Chiesi Farmaceutici SpA to conduct the study. Rangasamy Ramanathan, Kris Sekar and Jatinder Bhatia have served as consultants to Chiesi Farmaceutici SpA.
References
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121 (4:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- Kung HC, Hoyert DL, Xu JQ, Murphy SL.Deaths: Final data for 2005. National Vital Statistics Reports 56(10)National Center for Health Statistics: Hyattsville, MD; . http://www.cdc.gov/nchs/data/n vsr/nvsr56/nvsr56_10.pdf , 2008 (accessed on 21 December 2010). [PubMed] [Google Scholar]
- Halliday HL. History of surfactant from 1980. Biol Neonate. 2005;87:317–322. doi: 10.1159/000084879. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Merrill JD, Godinez MH, Troug WE, Ballard RA. Surfactant protein profile of pulmonary surfactant in premature infants. Am J Respir Crit Care Med. 2003;168:1123–1128. doi: 10.1164/rccm.200304-479OC. [DOI] [PubMed] [Google Scholar]
- Minoo P, Segura L, Coalson JJ, King RJ, DeLemos RA. Alterations in surfactant protein gene expression associated with premature birth and exposure to hyperoxia. Am J Physiol. 1991;261:L386–L392. doi: 10.1152/ajplung.1991.261.6.L386. [DOI] [PubMed] [Google Scholar]
- Merrill JD, Ballard RA, Cnaan A, Hibbs AM, Godinez RI, Godinez MH, et al. Dysfunction of pulmonary surfactant in chronically ventilated premature infants. Pediatr Res. 2004;56:918–926. doi: 10.1203/01.PDR.0000145565.45490.D9. [DOI] [PubMed] [Google Scholar]
- Horbar JD, Wright EC, Onstad L, Members of the National Institute of Child health and Human Development Neonatal Research Network Decreasing mortality associated with the introduction of surfactant therapy: an observational study of neonates weighing 601–1300 grams at birth. Pediatrics. 1993;92:191–196. [PubMed] [Google Scholar]
- Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001;2:CD0014444. doi: 10.1002/14651858.CD000144. [DOI] [PubMed] [Google Scholar]
- Halliday HL. Surfactants: past, present and future. J Perinatol. 2008;28:S47–S56. doi: 10.1038/jp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR, et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics. 1997;100:31–38. doi: 10.1542/peds.100.1.31. [DOI] [PubMed] [Google Scholar]
- Bloom BT, Clark RH. Comparison of Infasurf (calfactant) and Survanta (beractant) in the prevention and treatment of respiratory distress syndrome. Pediatrics. 2005;116:392–399. doi: 10.1542/peds.2004-2783. [DOI] [PubMed] [Google Scholar]
- Speer CP, Gefeller O, Groneck P, Laufkötter E, Roll C, Hanssler L, et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1995;72:F8–13. doi: 10.1136/fn.72.1.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroutis G, Kaleyias J, Liarou T, Papathoma E, Hatzistamatiou Z, Costalos C. Comparison of three treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Eur J Pediatr. 2003;162:476–480. doi: 10.1007/s00431-002-1144-0. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K, North American Study Group A randomized, multicenter masked comparison trial of poractant alfa (curosurf) versus beractant (survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. 2004;21:109–119. doi: 10.1055/s-2004-823779. [DOI] [PubMed] [Google Scholar]
- Malloy CA, Nicoski P, Muraskas JK. A randomized trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatr. 2005;94:779–784. doi: 10.1111/j.1651-2227.2005.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Fujii AM, Patel SM, Allen R, Doros G, Guo CY, Testa S. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J Perinatol. 2010;30:665–670. doi: 10.1038/jp.2010.20. [DOI] [PubMed] [Google Scholar]
- Clark RH, Auten RL, Peabody J. A comparison of the outcomes of neonates treated with two different natural surfactants. J Pediatr. 2001;139:828–831. doi: 10.1067/mpd.2001.119624. [DOI] [PubMed] [Google Scholar]
- Premier Database Premier healthcare alliance Premier: Charlotte, NC; . http://premierinc.com/quality-safety /tools-services/prs/data/perspective.jsp(accessed on 12 December 2010). [Google Scholar]
- Premier Research Services Premier healthcare allianceBibliography of Peer-Reviewed publications: Charlotte, NC. . http://www.premierinc.com/qual ity-safety/tools-services/prs/published-research/index.jsp (accessed on 10 February 2011).
- Clarke P. When can group level clustering be ignored? Multilevel models versus single-level models with sparse data. J Epidemiol Community Health. 2008;62:752–758. doi: 10.1136/jech.2007.060798. [DOI] [PubMed] [Google Scholar]
- Averill R, Goldfield N, Hughes J, Muldoon J, Gay J, Mc Cullough E, et al. What are APR-DRGs? An introduction to severity of illness and risk of mortality adjustment methodology (White Paper) 3M Health Information Systems: Saltlake City, UT; 2003 , http://solutions.3m.com/3MContentRetrievalAPI/BlobServlet?locale=it_IT&lmd= 1218718280000& assetId=1180603360910&assetType=MMM_Image&blobAttribute=ImageFile (accessed on 10 February 2011). [Google Scholar]
- Muldoon JH. Structure and performance of different DRG classification systems for neonatal medicine. Pediatrics. 1999;103 (1 Suppl E:302–318. [PubMed] [Google Scholar]
- Savitz DA, Terry JW, Jr, Dole N, Thorp JM, Jr, Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med. 2009;361:325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- Cogo PE, Facco M, Simonato M, Verlato G, Rondina C, Baritussio A, et al. Dosing of porcine surfactant: effect on kinetics and gas exchange in respiratory distress syndrome. Pediatrics. 2009;124:e950–e957. doi: 10.1542/peds.2009-0126. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Mottaghian J, Gebert A, Gunnar AR, von der Hardt H, Poets CF. Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium. Am J Respir Crit Care Med. 2000;162:1524–1533. doi: 10.1164/ajrccm.162.4.9908104. [DOI] [PubMed] [Google Scholar]
- Blanco O, Pérez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat respiratory distress syndrome: role of the different components in an efficient pulmonary surfactant. Eur J Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Rüdiger M, Tölle A, Meier W, Rüstow B. Naturally derived commercial surfactants differ in composition of surfactant lipids and in surface viscosity. Am J Physiol Lung Cell Mol Physiol. 2005;288:L379–L383. doi: 10.1152/ajplung.00176.2004. [DOI] [PubMed] [Google Scholar]
- Tölle A, Meier W, Greune G, Rüdiger M, Hofmann P, Rüstow B. Plasmalogens reduce the viscosity of a surfactant-like phospholipid monolayer. Chem Phys Lipids. 1999;100:81–87. [Google Scholar]