Abstract
Cell adhesion and spreading depend on activation of mitogen-activated kinase, which in turn is regulated both by growth factor and integrin signaling. Growth factors, such as epidermal growth factor, are capable of activating Ras and Raf, but integrin signaling is required to couple Raf to MEK and MEK to extracellular signal-regulated protein kinase (ERK). It was previously shown that Rac-p21-activated kinase (PAK) signaling regulated the physical association of MEK1 with ERK2 through phosphorylation sites in the proline-rich sequence (PRS) of MEK1. It was also shown that activation of MEK1 and ERK by integrins depends on PAK phosphorylation of S298 in the PRS. Here we report a novel MEK1-specific regulatory feedback mechanism that provides a means by which activated ERK can terminate continued PAK phosphorylation of MEK1. Activated ERK can phosphorylate T292 in the PRS, and this blocks the ability of PAK to phosphorylate S298 and of Rac-PAK signaling to enhance MEK1-ERK complex formation. Preventing ERK feedback phosphorylation on T292 during cellular adhesion prolonged phosphorylation of S298 by PAK and phosphorylation of S218 and S222, the MEK1 activating sites. We propose that activation of ERK during adhesion creates a feedback system in which ERK phosphorylates MEK1 on T292, and this in turn blocks additional S298 phosphorylation in response to integrin signaling.
The extracellular signal-regulated protein kinases (ERKs) are ubiquitous protein kinases that function downstream of the ras oncogene and are involved in many cellular responses, including adhesion and migration (43). Ras is activated in response to multiple extracellular stimuli and in turn regulates multiple downstream signaling pathways (7), including the Raf protein kinases (58, 60). Raf proteins phosphorylate and activate MEKs (mitogen-activated protein [MAP] kinase or ERKs) (16, 30, 37, 62), which phosphorylate and activate ERK1 and ERK2 on a TEY sequence in the ERK catalytic domain (27, 44).
Propagation of the Ras/Raf/MEK/ERK signal is modulated by a variety of inputs that must be integrated to achieve a full signaling response. Cell adhesion is required to couple Ras activation to MEK and ERK activation, as stimulation of suspended cells with growth factors leads to Ras and possibly Raf activation; however, activation of MEK and ERK fails to occur (38, 47). Signal transduction through the Ras-ERK pathway can be enhanced by the Rho family GTPases Rac and Cdc42 through their effector p21-activated kinase (PAK) (12, 23, 24), and Rac and Raf can synergize to promote cellular transformation (31). Expression of constitutively active Rac or Cdc42 activates the Jun N-terminal kinases (JNKs) and p38 kinases but not the ERKs (14). However, Rac and Cdc42 are able to synergize with Raf to stimulate ERK activation through mechanisms involving PAK1 phosphorylation of the MEK1 proline-rich sequence (PRS) (12, 23, 54) and PAK3 phosphorylation of Raf-1 (11, 32). PAK1 has been reported to enhance the phosphorylation of T292 and S298 of MEK1 in cells, although only S298 is phosphorylated by PAK in vitro (12, 23). Combined mutation of both of these sites inhibits Raf binding (23) and the ability of Raf to activate MEK (12, 54).
We recently reported that Rac-PAK signaling modulates the ERK pathway, at least in part by enhancing the association of MEK1 with ERK. We also showed that mutation of MEK1 at both T292 and S298 to alanine inhibited this effect (18). In addition, we demonstrated a requirement for PAK activity in the formation of MEK1-ERK complexes and ERK activation in newly adhering cells (18). Moreover, cellular adhesion to fibronectin was shown to induce PAK phosphorylation of MEK1 on S298, an event required for MEK1 activation upon adhesion (54).
In the present study we demonstrate that PAK phosphorylation of MEK1 at S298 is required for the Rac-induced enhancement of the MEK1-ERK interaction, while T292 phosphorylation is inhibitory. The inhibitory effect of T292 phosphorylation is explained by the observation that ERK feedback phosphorylation of MEK1 on T292 inhibits the ability of PAK to phosphorylate S298. We show that ERK phosphorylation of T292, like PAK phosphorylation of S298 (54), is stimulated by adhesion. Because PAK phosphorylation of MEK1 on S298 is required for MEK1 activation upon cellular adhesion (54), preventing ERK feedback phosphorylation on T292 enhanced MEK1 S298 phosphorylation and prolonged adhesion-induced MEK1 phosphorylation on its activating sites. These data demonstrate that opposing phosphorylation events in the MEK1 PRS regulate MEK1 adhesion-dependent complex formation and activation.
MATERIALS AND METHOD
Cell culture and reagents
COS-1 cells were grown in 10% fetal bovine serum in Dulbecco's modified Eagle medium (DMEM; Gibco, Frederick, Md.) at 37°C with 5% CO2. FLAG-ERK2, Rac1 L61, and MEK1 constructs have been described previously (10, 17, 18). Antibodies were obtained from the following sources: anti-phospho T292 MEK1, BioSource, Camarillo, Calif., and Upstate Biotechnology, Charlottesville, Va.; anti-phospho-ERK and anti-FLAG M2 antibody, Sigma, St. Louis, Mo. Anti-phospho-S298 MEK1 antiserum was obtained from Mark Marshall (Lilly Research Laboratories, Indianapolis, Ind.).
Coimmunoprecipitations
COS-1 cells in 10-cm-diameter dishes were transfected with 0.8 μg of ERK2 plasmid along with 3.2 μg of the indicated MEK plasmid and either 0.25 μg of RacL61 or empty vector. The cells were serum starved the following day for 4 h prior to harvest in hypotonic buffer (50 mM HEPES [pH 7.4], 2 mM MgCl2, 2 mM EGTA, and aprotinin). MEK-ERK complexes were immunoprecipitated as described previously (9, 17, 18).
For MEK1 immunoprecipitation experiments on adherent cells, COS-1 cells were plated onto 6-cm-diameter dishes and were transfected with 0.3 μg of hemagglutinin (HA)-MEK, 0.25 μg of FLAG-ERK, and empty vector for a total of 2 μg of DNA. After treatment the cells were harvested in FLAG lysis buffer (17) containing sodium fluoride (50 mM), sodium pyrophosphate (5 mM), sodium orthovanadate (0.1 mM), and phenylmethylsulfonyl fluoride (2 mM). Lysates were cleared by centrifugation, and immunoprecipitations were performed with anti-HA antibody. For MEK1 immunoprecipitation experiments on suspended and replated cells, 15-cm-diameter dishes were transfected with 0.3 μg of HA-MEK1, 0.3 μg of FLAG-ERK2, and empty vector for a total of 12.5 μg of DNA. The day following transfection the cells were trypsinized by using trypsin-EDTA solution (Gibco), washed with 1 mg of soybean trypsin inhibitor (Sigma)/ml, and suspended in serum-free DMEM for 90 min at 37°C and 5% CO2. When indicated, U0126 (Calbiochem, La Jolla, Calif.) was added at a final concentration of 10 μM. Cells were either left in suspension or allowed to adhere to bovine fibronectin-coated dishes (10 μg/ml in phosphate-buffered saline, left overnight at 4°C; Sigma) for the indicated times at 37°C and 5% CO2 prior to lysis in FLAG lysis buffer. MEK1 proteins were immunoprecipitated with anti-HA antibody 12CA5.
In vitro MEK1 phosphorylation
Wild-type or mutant GST-MEK1 protein (1 μg), purified as described previously (17), was incubated with or without purified, activated ERK2 (specific activity, 1.7 μmol/min/mg with myelin basic protein as substrate) in kinase buffer (25 mM HEPES-NaOH [pH 7.5], 10 mM MgCl2, 0.5 mM ATP, 1 mM dithiothreitol) for 40 min at 30°C. Control experiments indicated that MEK1 was phosphorylated to a stoichiometry of ∼1 mol/mol under these conditions. Purified recombinant PAK3 (0.675 or 6.75 ng per reaction; a gift from Mark Marshall) or buffer control was added and reactions continued at 30°C for an additional 10 min. Reactions were terminated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and were resolved by electrophoresis on a 10% polyacrylamide gel. After transfer to Immobilon P membrane, phosphorylation of T292 and S298 was assessed by blotting with appropriate phospho-MEK1-specific antisera. Coomassie staining confirmed equivalent loading of all glutathione S-transferase (GST)-MEK1 proteins.
RESULT
Phosphorylation of MEK1 on T292 and S298 differentially regulates Rac-induced MEK1-ERK association
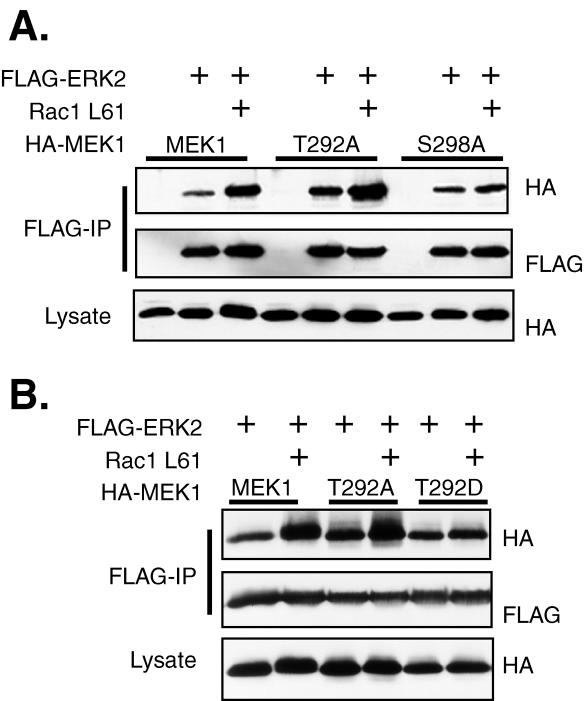
We have previously shown that Rac-PAK signaling enhances the association of MEK1 with ERK2 in adherent and newly adhering COS-1 cells and that expression of activated Rac can serve as a surrogate for integrin signaling in this process. Mutation of both T292 and S298 of MEK1 to alanine abrogated the effects of Rac on the MEK1-ERK2 interaction but did not affect the inherent association of MEK1 and ERK2 in adherent cells (18). To further investigate the role of these MEK1 phosphorylation sites in the regulation of the MEK1-ERK interaction by Rac-PAK signaling, we examined the role of each individual site in the ability of Rac to enhance this interaction. COS-1 cells were transfected with plasmids encoding FLAG-ERK2, Rac1 L61, and either HA-MEK1, HA-MEK1 T292A, or HA-MEK1 S298A. As was previously reported (18), Rac1-L61 increased the association of MEK1 with ERK2 (Fig. 1). Mutation of MEK1 at S298 to alanine had no effect on the basal association of MEK1 with ERK2. However, no induction of association between MEK1 S298A and ERK2 was detected upon cotransfection of activated Rac. In contrast, MEK1 T292A had a consistently higher basal level of association with ERK2, and cotransfection with Rac1-L61 further increased this association. The data indicate that these two potential phosphorylation sites on MEK1 differentially regulate the basal and Rac-induced association of MEK1 and ERK. Phosphorylation of T292 may negatively regulate the basal MEK1-ERK association, but it was not required for the enhancement of the interaction by Rac. Conversely, phosphorylation of S298 may not be involved in the basal interaction but was absolutely required for the enhancement of ERK binding in the presence of Rac.
FIG. 1.
Rac stimulation of the MEK1-ERK2 interaction requires S298 of MEK1. (A) COS-1 cells were transfected with plasmids for FLAG-ERK2, RacL61, and MEK1 or a MEK1 mutant as indicated. The following day the cells were serum deprived for 4 h before harvest in hypotonic buffer. The cells were lysed by centrifugation at 13,000 × g for 20 min. FLAG immunoprecipitations were performed and eluted from the antibody overnight at 4°C with FLAG peptide. Eluted proteins were resolved on SDS-PAGE and were immunoblotted with anti-HA and anti-FLAG antibodies. (B) Mutation of T292 to aspartate inhibits the Rac effect. COS-1 cells were transfected with plasmids for FLAG-ERK2, RacL61, and MEK1 or a MEK1 292 mutant. Cells were treated and immunoprecipitations (IP) and immunoblots were performed as described for panel A.
We further investigated the potential negative regulatory role of T292 phosphorylation in the MEK1-ERK interaction by mutating this residue to aspartate to mimic phosphorylation and testing its effect on MEK1-ERK2 basal association or Rac1-induced association. MEK1 T292D had a level of basal binding to ERK2 similar to that of wild-type MEK1 (Fig. 1B). However, binding of MEK1 T292D to ERK2 was not enhanced in the presence of active Rac, similar to the results obtained with MEK1 S298A. These data suggested that phosphorylation of MEK1 at T292 may inhibit the positive effect of Rac-PAK signaling on the association of MEK1 and ERK. Since the Rac-induced increase in MEK1-ERK2 association required phosphorylation of MEK1 at S298, we hypothesized that phosphorylation of MEK1 at T292 negatively regulated the phosphorylation of MEK1 at S298.
ERK phosphorylation of T292 inhibits S298 phosphorylation by PAK
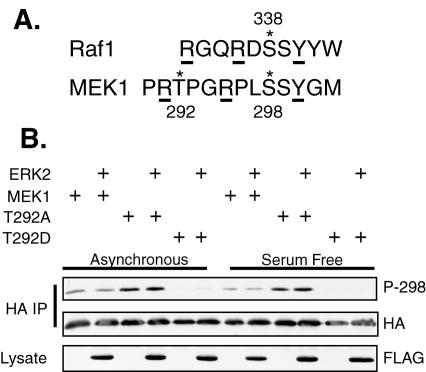
PAK1 phosphorylates MEK1 at S298 (12, 23, 54). Transfection of constitutively active PAK into cells increased not only S298 phosphorylation but also T292 phosphorylation (12), although only S298 was phosphorylated by PAK in vitro (12, 23). Investigation of the PAK phosphorylation of Raf-1 on S338 revealed a consensus sequence containing a tyrosine at the +2 position and arginine residues at the −2 and −5 positions (33). The sequence surrounding S298 of MEK1 loosely conforms to this consensus, with a tyrosine at the +2 position and arginines at the −3 and −7 positions. T292, a reported ERK phosphorylation site (5, 40), lies directly C terminal to the −7 arginine (Fig. 2A). We hypothesized that the negative charge generated by phosphorylation of T292 might neutralize the positive charge at arginine 291, disrupting the PAK consensus and inhibiting the ability of PAK to phosphorylate MEK1 at S298.
FIG. 2.
Mutation of T292 to alanine increases S298 phosphorylation while mutation to aspartate inhibits it. (A) Amino acid sequence comparison between Raf1 and MEK1 indicating the phosphorylation sites (*). Residues critical for PAK phosphorylation of Raf1 and analogous sites in MEK1 are underlined. (B) COS-1 cells were transfected with plasmids for HA-MEK1 or an HA-MEK1 T292 mutant and either FLAG-ERK2 or empty vector. The cells were incubated in 10% serum (Asynchronous) or in serum-free media (Serum Free) overnight before harvest at 24 h posttransfection. HA-MEKs were immunoprecipitated (IP) with anti-HA antibody, resolved on SDS-PAGE, and immunoblotted with anti-HA and anti-phospho S298 antibodies. In addition, equal amounts of cellular lysate were resolved and immunoblotted with anti-FLAG antibodies.
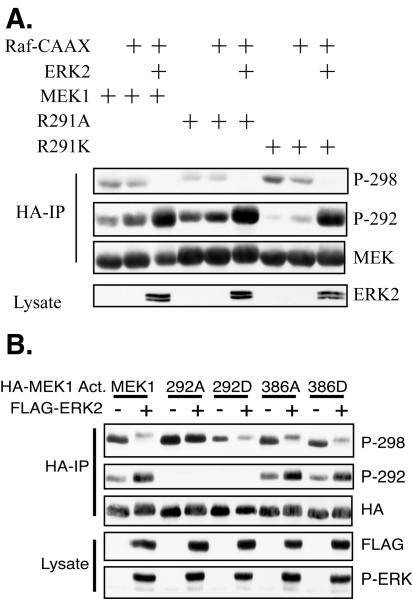
T292 lies within a consensus phosphorylation site for ERK. We therefore tested whether ERK phosphorylation of T292 affects S298 phosphorylation by PAK. For these experiments we utilized a polyclonal antibody that specifically recognizes MEK1 phosphorylated at S298 (54). COS-1 cells transfected with ERK2 and either MEK1, MEK1 T292A, or MEK1 T292D were grown in 10% serum or were serum starved. Wild-type MEK1 had the same level of S298 phosphorylation in cells grown in serum or that were serum deprived (Fig. 2B). This result is in agreement with previous findings that S298 phosphorylation is relatively constant in adherent cells and is unaffected by growth factor addition (10). Importantly, MEK1 T292A had a consistently higher level of S298 phosphorylation than wild-type MEK1 in both serum-starved and asynchronous cells. Conversely, the phosphomimicking mutant MEK1 T292D had a greatly reduced level of S298 phosphorylation under both growth conditions. These data suggest that phosphorylation of T292 might negatively regulate phosphorylation of MEK1 on S298. In both growth conditions the presence of ERK2 did not affect S298 phosphorylation; however, ERK was largely in the inactive form, as indicated by the absence of a mobility shift on SDS-PAGE of total FLAG-ERK (Fig. 2B) and the absence of a signal when immunoblotting with a phospho-ERK antibody (data not shown).
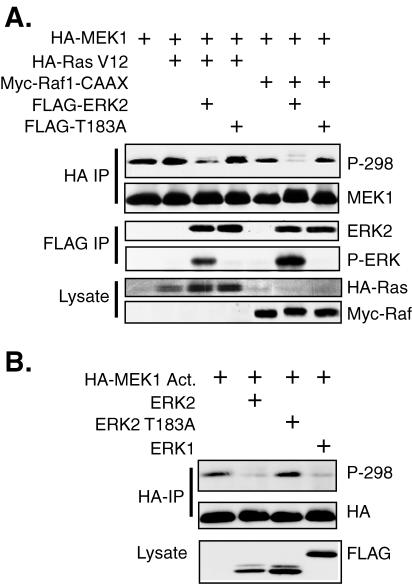
We then examined whether active ERK could affect phosphorylation of MEK1 on S298. COS-1 cells were transfected with expression plasmids for MEK1 and either wild-type ERK2 or ERK2 T183A, a phosphorylation site mutant that cannot be activated by MEK. In addition, mutationally activated Ras (Ras V12) or Raf (Raf-CAAX) was cotransfected to stimulate ERK2 activity. Transfection of Ras V12 alone, which would stimulate the ERK pathway as well as potentially activate the Rac-PAK pathway via activation of phosphatidylinositol 3 kinase (48), had little effect on S298 phosphorylation of MEK1 (Fig. 3A). However, cotransfection of Ras V12 with ERK2, but not ERK2-T183A, greatly inhibited phosphorylation of MEK1 at S298. Cotransfection of Raf-CAAX, which would primarily stimulate only the ERK pathway, inhibited S298 phosphorylation somewhat more than the empty vector. However, cotransfection of Raf-CAAX with ERK2, but not ERK2 T183A, strongly inhibited MEK1 S298 phosphorylation. The greater inhibition of S298 phosphorylation by Raf-CAAX and ERK2 compared to that of Ras-V12 and ERK2 correlated with a greater activation of ERK2 by Raf-CAAX (Fig. 3A). A similar inhibition of S298 phosphorylation was observed following cotransfection of ERK1 or ERK2 and a mutationally activated form of MEK1, MEK1 S218/222D (designated MEK1 Act.), in which the activating phosphorylation sites (62) were mutated to aspartate to mimic phosphorylation (Fig. 3B). These data demonstrate that both activated ERK1 and ERK2 were able to inhibit the phosphorylation of MEK1 at S298.
FIG. 3.
ERK activation inhibits phosphorylation of MEK1 on S298. (A) COS-1 cells were transfected with plasmids for HA-MEK1, FLAG-ERK2, or kinase-deficient ERK2 T183A and mutationally activated Ras V12 or Raf-1 CAAX. The cells were incubated in serum-free media after transfection and were harvested at 24 h. HA-MEK1 was immunoprecipitated (IP) with anti-HA antibodies, resolved on a gel, and immunoblotted with anti-HA and anti-phospho-S298 antibodies. In addition, FLAG-ERKs were also immunoprecipitated and immunoblotted with antibodies to FLAG and phospho-ERK. Equal amounts of cell lysate were immunoblotted for HA-Ras and Myc-Raf. (B) COS-1 cells were transfected with mutationally activated HA-MEK1 S218/222D (MEK1 Act.) and either FLAG-ERK1, ERK2, or ERK2 T183A. The cells were serum starved, and immunoprecipitations and immunoblots were performed as described for panel A.
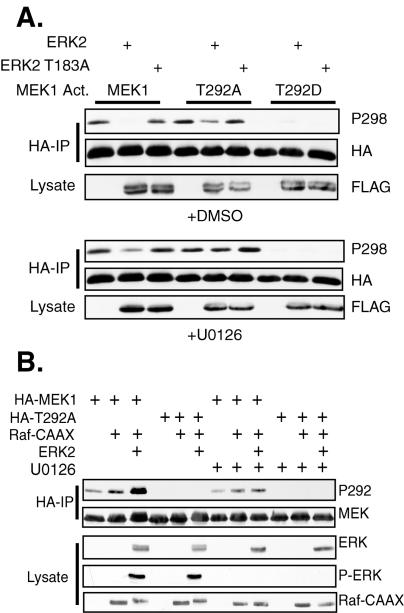
We next determined the potential role of T292 of MEK1 in the ability of ERK to inhibit PAK phosphorylation of S298. These studies utilized MEK1 Act. proteins that contained additional mutations of T292 to either alanine or aspartate to prevent or mimic phosphorylation, respectively. MEK1 Act. was phosphorylated on S298, and this phosphorylation could be inhibited by cotransfection of ERK2 but not ERK2 T183A (Fig. 4A, top panel). Treatment of the cells for the last 5 h before harvest with U0126, a MEK inhibitor that inhibits MEK activation of ERK (21), partially restored S298 phosphorylation in the presence of exogenous ERK2 (Fig. 4A, bottom panel), further suggesting that ERK activity was required for the inhibition of S298 phosphorylation. MEK1 Act. T292A was also phosphorylated on S298; however, its phosphorylation was only slightly inhibited by cotransfection of ERK2 (Fig. 4A, top panel). This suggests that T292 of MEK1 is required for ERK2 to inhibit S298 phosphorylation. Treatment of the cells with U0126 completely restored S298 phosphorylation of MEK1 Act. T292A (Fig. 4A, bottom panel). As with MEK1 T292D, mimicking constitutive phosphorylation of T292 with an aspartic acid mutation prevented S298 phosphorylation of MEK1 Act. T292D (Fig. 4A, top panel), and U0126 treatment could not restore S298 phosphorylation of this mutant (Fig. 4A, bottom panel). These data strongly suggest that ERK2 inhibits PAK phosphorylation of MEK1 on S298, at least in part, through phosphorylation of T292.
FIG. 4.
ERK feedback inhibition of S298 phosphorylation requires T292. (A) COS-1 cells were transfected with mutant T292 forms of MEK1 Act. and either FLAG-ERK2 or ERK2 T183A. The cells were serum deprived after the transfection and were treated with either DMSO or 10 mM U0126 for the last 5 h before harvest. HA-MEK immunoprecipitates (IP) were immunoblotted with anti-HA and anti-phospho-S298 antibodies. (B) ERK phosphorylates MEK on T292. MEK1 or T292A were transfected with FLAG-Raf-CAAX and FLAG-ERK. The cells were serum deprived and treated with DMSO or U0126 as described for panel A. HA-MEK1 immunoprecipitates were immunoblotted with anti-HA and anti-phospho-MEK1 T292 antibodies.
To demonstrate that ERK2 actually phosphorylated MEK1 on T292, cells were transfected with MEK1 or MEK1 T292A, along with Raf-CAAX and ERK2. Phosphorylation of T292 of MEK1, but not MEK1 T292A, was stimulated by Raf-CAAX, as determined by immunoblotting HA-MEK immunoprecipitates with an anti-phospho-T292 MEK1 antibody (Fig. 4B). Cotransfection of ERK2 further stimulated MEK1 T292 phosphorylation, demonstrating that ERK stimulates phosphorylation of this site. Treatment of the cells for the last 5 h of the experiment with U0126 significantly inhibited the induction of T292 phosphorylation, although some phosphorylation of T292 was still detected. This could result from a slow turnover of phosphorylation of this site that would not be affected by the 5 h of U0126 treatment. Alternatively, other kinases besides ERK could contribute to T292 phosphorylation.
In vitro experiments were performed to further test the hypothesis that ERK phosphorylation of MEK1 at T292 inhibited PAK phosphorylation of S298. Recombinant wild-type MEK1, MEK1 T292A, or MEK1 T292D were incubated in kinase buffer containing ATP in the presence or absence of recombinant, active ERK for 40 min. Recombinant PAK3 (1 or 10 U; 1 U = 6.75 ng) or buffer control was then added, and reactions were continued for 10 min. MEK1 proteins were then blotted to assess phosphorylation of S298 by PAK3 and phosphorylation of T292 by ERK (Fig. 5A). Wild-type MEK1 and MEK1 T292A were robustly phosphorylated on S298 in a dose-dependent manner by PAK3 (Fig. 5A, lanes 1 to 3). In contrast, MEK1 T292D was phosphorylated poorly on S298 (lanes 7 to 9), suggesting that a negative charge at position 292 inhibits PAK phosphorylation at S298. Importantly, prior phosphorylation of MEK1 on T292 by ERK greatly inhibited subsequent PAK3 phosphorylation of MEK1 S298 (lanes 10 to 12). These data suggest that prior T292 phosphorylation or substitution with a phosphomimetic residue inhibits subsequent PAK phosphorylation of MEK1.
FIG. 5.
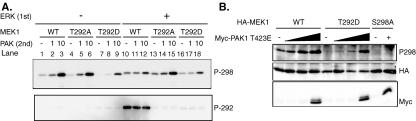
ERK phosphorylation of MEK1 inhibits PAK1 phosphorylation of S298 in vitro. (A) Purified GST-MEK1 was preincubated for 40 min with or without recombinant active ERK2. Aliquots were subsequently incubated with limiting concentrations of purified PAK3 for 10 min. Phosphorylation of MEK1 on S298 and T292 was determined by immunoblotting with the appropriate phospho-MEK1-specific antibody. (B) COS-1 cells were transfected with plasmids encoding HA-MEK1, HA-MEK1 T292D, and HA-MEK1 S298A and increasing amounts of mutationally activated PAK1 423E. For MEK1 S298A, the highest concentration on PAK1 423E was used in the lane marked with a plus sign. Cells were suspended in serum-free media for 90 min before lysis. Anti-HA immunoprecipitates were run on a gel and immunoblotted with anti-HA and anti-phospho-S298 MEK1 antibodies. In addition, an equal amount of cell lysate was run on a gel and immunoblotted with anti-Myc antibody to detect myc-tagged PAK1 T423E. WT, wild type.
To further test the necessity of T292 phosphorylation, we asked whether ERK could inhibit PAK3 phosphorylation of MEK1 T292A. Importantly, PAK3 phosphorylation of MEK1 T292A was unaffected by prior incubation with ERK (compare lanes 1 to 3 and 10 to 12 with 4 to 6 and 13 to 15). These data strongly suggest that phosphorylation of T292 is required for ERK to inhibit PAK3 phosphorylation of S298. These data also demonstrate that ERK does not directly inactivate PAK3 under these conditions.
Our results suggest that ERK phosphorylation of T292 is necessary to inhibit PAK3 phosphorylation of MEK1 S298. They further suggest that any other MEK1 sites phosphorylated by ERK in these assays (e.g., T386) alone cannot account for ERK-mediated inhibition of PAK phosphorylation. To further test this contention, we asked whether S298 phosphorylation of MEK1 T292D could be further inhibited by prior incubation with ERK. Our data (lanes 7 to 9 and 16 to 18) indicate that prior incubation of MEK1 T292D with ERK does not change its sensitivity to subsequent PAK3 phosphorylation. Together these data indicate that phosphorylation of T292 is necessary and sufficient for ERK to inhibit PAK3 phosphorylation of MEK1 S298 in vitro. These data are in good agreement with the experiments performed in cultured cells. Note that the slight MEK1 S298 phosphorylation stimulated by ERK alone (lanes 10, 13, and 16) has been reported previously as an in vitro artifact (12).
We hypothesized that excess PAK might overcome the inhibition of S298 phosphorylation generated by ERK phosphorylation of T292. We therefore determined if excess PAK activity in cells could overcome the inhibition of S298 phosphorylation caused by a negative charge at T292. COS-1 cells were transfected with expression plasmids for MEK1, MEK1 T292D, or MEK1 S298A, along with increasing amounts of mutationally activated PAK1 (PAK1 T423E). Cells were placed in suspension to reduce endogenous PAK activity, and phosphorylation of exogenous MEK1 proteins was assessed following immunoprecipitation. PAK1 T423E increased the level of S298 phosphorylation of MEK1 in suspended cells in a dose-dependent manner (Fig. 5B). MEK1 T292D was resistant to S298 phosphorylation with low levels of activated PAK1. However, S298 phosphorylation of this mutant was observed to levels similar to those in wild-type MEK1 upon cotransfection of the highest concentrations of activated PAK1. Similarly, excess activated PAK3 resulted in efficient phosphorylation of ERK-phosphorylated MEK1 in vitro (data not shown). Collectively, these data clearly demonstrate that a negative charge at T292 of MEK1 inhibits the ability of PAK to phosphorylate S298, but that this inhibition can be overcome, in vitro and in vivo, with high concentrations of active PAK. Importantly, these data also demonstrate that the phospho-MEK1 S298 antibody recognizes MEK1 that was either phosphorylated or had an acidic residue at T292. This is further verified by our finding that the phospho-S298 MEK1 antibody efficiently immunoprecipitates MEK1 doubly phosphorylated on both S298 and T292 (data not shown).
To directly test whether interference with the positive charge at MEK1 R291 affects the ability of PAK1 to phosphorylate S298, we generated mutants of MEK1 at position 291 to either neutralize (alanine mutation) or mimic (lysine mutation) the positive charge of the arginine. While mutation to lysine resulted in S298 phosphorylation equal to that of wild-type MEK1, mutation to alanine inhibited the phosphorylation of MEK1 on S298 (Fig. 6A). These results demonstrate that the positive charge at R291 contributes to PAK phosphorylation of S298 and that neutralization of this charge by ERK phosphorylation of T292 may be one mechanism whereby ERK feedback phosphorylation inhibits the ability of PAK to phosphorylate MEK. Interestingly, cotransfection of ERK2 further inhibited S298 phosphorylation of the MEK1 R291A mutant, suggesting that there may be an additional mechanism by which ERK inhibits S298 phosphorylation (see Discussion).
FIG. 6.
MEK1 R291, but not T386, modulates S298 phosphorylation. (A) A charge-neutralizing mutation of arginine 291 inhibits PAK phosphorylation of S298. COS-1 cells were transfected with Raf-CAAX, ERK2, and HA-MEK1 or a MEK1 R291 mutant. The cells were serum starved following transfection, and HA immunoprecipitates (IP) were immunoblotted as indicated. (B) MEK1 T386 phosphorylation has little effect on S298 phosphorylation. COS-1 cells were transfected with MEK1 Act. or MEK1 Act. containing a mutation at T292 or T386. The cells were incubated in serum-free media after the transfection, and HA immunoprecipitates were immunoblotted as indicated.
MEK1 was also reported to be phosphorylated by ERK2 at T386 (40). We therefore determined if neutralizing or acidic mutations of MEK1 at T386 could affect S298 phosphorylation. In this experiment, mutation of T292 to alanine completely restored S298 phosphorylation in the presence of active ERK. However, mutation of T386 to alanine resulted in only a marginal increase in S298 phosphorylation (Fig. 6B), while an aspartate mutation at T386 to mimic phosphorylation had no effect. These data suggest that ERK feedback phosphorylation of T386 may only have a minor role, if any, in the inhibition of S298 phosphorylation by ERK.
Regulation of MEK1 T292 phosphorylation by cellular adhesion
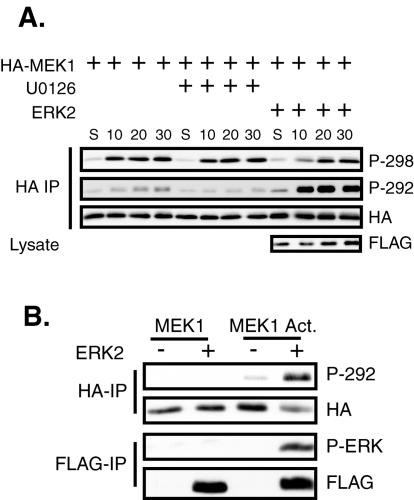
It was previously reported that MEK1 S298 and T292 were unaffected by growth factor treatment of adherent CCL39 cells (10). Recent results show that cell adhesion stimulates PAK phosphorylation of MEK1 on S298 and subsequent MEK activation (54). Therefore, we examined whether phosphorylation of T292 is regulated by cellular adhesion. In addition, we determined if phosphorylation of T292 could affect MEK1 S298 phosphorylation induced by adhesion to fibronectin. Transfected COS-1 cells were placed in suspension (S) in serum-free media with either the MEK inhibitor U0126 or dimethylsulfoxide (DMSO) (Fig. 7A). The cells were then either harvested or replated onto fibronectin-coated dishes for the indicated time. Immunoblotting revealed that T292 of MEK1 was phosphorylated at a low level in cells in suspension, and phosphorylation of this site gradually increased after cellular adhesion to a fibronectin matrix, not becoming maximal until 30 min after replating. U0126 treatment prevented the induction of T292 phosphorylation upon cellular adhesion. Cotransfection of ERK2 slightly enhanced T292 phosphorylation in suspended cells but greatly stimulated this phosphorylation upon cellular adhesion. As demonstrated previously (54), immunoblotting these immunoprecipitates with the phosphospecific MEK1 S298 antibody showed that phosphorylation of this site was also low in suspended cells but became maximally induced within 10 min of adhesion to fibronectin and remained constant throughout the time course. Treatment of the cells with U0126 did not affect S298 phosphorylation, while cotransfection of ERK2 slightly delayed the kinetics of S298 phosphorylation.
FIG. 7.
Feedback phosphorylation of T292 upon cellular adhesion inhibits S298 phosphorylation. (A) COS-1 cells were transfected with HA-MEK1 and FLAG-ERK2. The following day the cells were trypsinized and put in suspension for 90 min in serum-free medium containing either 10 mM U0126 or DMSO. The cells were then either harvested (S) or replated onto fibronectin-coated dishes for the indicated time, still in the presence of U0126 or DMSO. HA immunoprecipitates (IP) were immunoblotted with antibodies for HA, phospho-T292, and phospho-S298 MEK1. (B) Active ERK can stimulate T292 phosphorylation in suspended cells. COS-1 cells were transfected with either HA-MEK1 or HA-MEK1 Act. and either FLAG-ERK2 or empty vector. The cells were trypsinized and suspended in serum-free media for 90 min before harvest. HA-MEKs and FLAG-ERKs were immunoprecipitated and immunoblotted as indicated.
ERK regulates the duration of MEK1 activation during adhesion
The above data suggest that phosphorylation of MEK1 on S298 by PAK precedes phosphorylation on T292 by ERK. These kinetics are consistent with T292 phosphorylation playing a feedback role during adhesion to fibronectin. To test this hypothesis, we determined whether prephosphorylation of MEK1 on T292 by ERK2 in suspended cells could inhibit the phosphorylation of MEK1 on S298 by PAK upon cell adhesion. We first tested the ability of MEK1 Act. to stimulate ERK activation and T292 feedback phosphorylation in suspended cells. MEK1 Act., but not wild-type MEK1, activated ERK2 in suspended cells (Fig. 7B), as had been demonstrated previously (47). MEK1 was not phosphorylated on T292 in suspended cells, even in the presence of exogenous ERK2. Conversely, MEK1 Act. was slightly phosphorylated on T292 in suspended cells, and this was significantly increased by cotransfection of ERK. Therefore, active MEK1 signaled to ERK in suspended cells and the activated ERK then phosphorylated MEK1 Act. on T292.
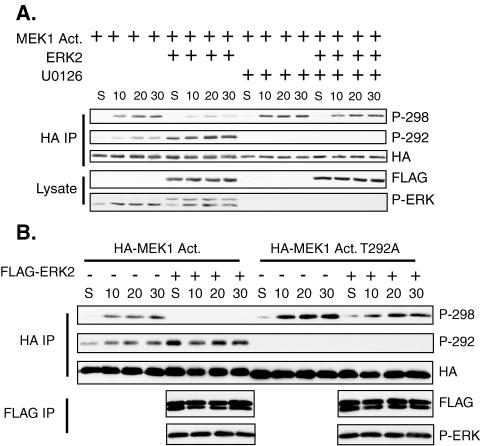
We next determined if MEK1 prephosphorylated on T292 would prevent phosphorylation of S298 by PAK upon cellular adhesion. MEK1 Act. transfected alone displayed no T292 or S298 phosphorylation in suspended cells, but phosphorylation of both sites was induced upon cell adhesion (Fig. 8A). Cotransfection of ERK2 enhanced T292 phosphorylation in suspended and newly adherent cells, correlating with an inhibition of S298 phosphorylation. Treatment of the cells with U0126 when the cells were suspended and replated prevented T292 phosphorylation and slightly enhanced S298 phosphorylation upon adhesion. In the presence of U0126, cotransfection with ERK2 did not enhance T292 phosphorylation or inhibit S298 phosphorylation. These data demonstrate that ERK activation in newly adherent cells controls T292 phosphorylation of MEK1, and this in turn regulates the phosphorylation of MEK1 S298 by PAK.
FIG. 8.
Prephosphorylation of MEK1 on T292 inhibits adhesion-induced phosphorylation of S298. (A) COS-1 cells were transfected with MEK1 Act. and either FLAG-ERK2 or empty vector. The cells were trypsinized and suspended for 90 min in the presence of either 10 mM U0126 or DMSO. The cells were then either harvested (S) or replated onto fibronectin-coated dishes for the indicated time, still in the presence of U0126 or DMSO. HA immunoprecipitates (IP) were immunoblotted with antibodies for HA, phospho-MEK1 T292, and phospho-MEK1 S298. (B) Inhibition of S298 phosphorylation upon adhesion requires T292. COS-1 cells were transfected with MEK1 Act. or MEK1 Act. T292A and FLAG-ERK2 or empty vector. The cells were trypsinized and placed in suspension in serum-free media. Suspended cells were either harvested (S) or replated onto fibronectin for the indicated time. HA immunoprecipitates were immunoblotted as described for panel A. FLAG immunoprecipitates were immunoblotted for FLAG and phospho-ERK.
To determine if phosphorylation of T292 was responsible for the decrease in S298 phosphorylation, the experiment was repeated by using either MEK1 Act. or MEK1 Act. T292A (Fig. 8B). Phosphorylation of MEK1 Act. T292A on S298 was enhanced compared to that of MEK1 Act. upon adhesion. While ERK2 cotransfection inhibited MEK1 Act. S298 phosphorylation, phosphorylation of MEK1 Act. T292A on S298 was only slightly inhibited by ERK2. These data demonstrate that inhibition of S298 phosphorylation by ERK upon adhesion requires T292 phosphorylation.
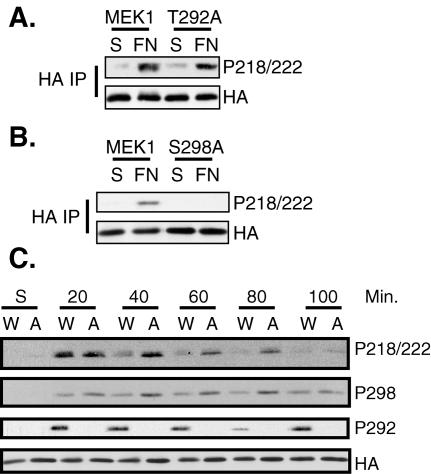
PAK phosphorylation of MEK1 on S298 is required for MEK1 activation during cellular adhesion to fibronectin (54). Therefore, we determined whether T292 phosphorylation also affected adhesion-stimulated MEK1 activation. Transfected cells were trypsinized and suspended in serum-free media for 90 min and either harvested or replated onto fibronectin-coated dishes for 20 min. Immunoblotting HA immunoprecipitates with a phosphospecific antibody that recognizes the activating phosphorylation sites on MEK (phospho-S218/222) demonstrated MEK1 activation induced by cellular adhesion to fibronectin (Fig. 9A and B). Mutation of T292 to alanine did not inhibit MEK1 activation after 20 min of adhesion (Fig. 9A). Consistent with previous observations, mutation of S298 to alanine inhibited MEK1 activation under these conditions (Fig. 9B) (54), demonstrating that PAK phosphorylation of S298 was required for adhesion-induced MEK1 activation by Raf.
FIG. 9.
T292 and S298 differentially regulate MEK1 activation upon cellular adhesion. (A and B) COS-1 cells were transfected with HA-MEK1, HA-MEK1 S298A, or HA-MEK1 T292A. The cells were trypsinized and placed in suspension (S) for 90 min (S) or suspended and then replated on fibronectin (FN) for 20 min. HA immunoprecipitates (IP) were run on a gel and were immunoblotted with antibodies for HA and phospho-218/222 MEK1. (C) Inhibiting feedback phosphorylation by ERK prolongs MEK1 activation upon adhesion. COS-1 cells were transfected with HA-MEK1 or HA-MEK1 T292. The cells were trypsinized and placed in suspension (S) for 90 min or were suspended and then replated on fibronectin (FN) for the indicated time. The cells were harvested and HA immunoprecipitates were immunoblotted with antibodies for HA, phospho-218/222, phospho-S298, and phospho-T292. W, wild-type MEK1; A, MEK1 T292A.
Because phosphorylation of MEK1 on S298 was required for MEK1 activation upon cellular adhesion and T292 phosphorylation by ERK inhibits S298 phosphorylation, we determined if feedback phosphorylation by ERK2 on T292 of MEK1 affects the duration of MEK1 activation as cells adhere and spread. Therefore, we performed a time course of activation of MEK1 or MEK1 T292A upon adhesion of cells to fibronectin (Fig. 9C). As shown in Fig. 9A, neither MEK1 nor MEK1 T292A from suspended cells was active. Both MEK1 and MEK1 T292A were activated to equal levels 20 min after cells were replated onto fibronectin. However, while activation of MEK1 was reduced to near basal levels at 40 min, activation of MEK1 T292A was sustained for up to 80 min. Similarly, MEK1 T292A sustained an enhanced level of phospho-S298 at later time points than did MEK1, and this correlated with the sustained level of MEK1 T292A activation. As expected, only MEK1 was phosphorylated on T292 after cellular adhesion. These data strongly suggest that ERK2 feedback phosphorylation of MEK1 at T292 acts to turn off MEK1 activation in newly adhering cells by preventing further enhancement of S298 phosphorylation by PAK, which initially was required for MEK1 activation (Fig. 9B) (54).
DISCUSSION
MAP kinase signaling is essential for normal cell adhesion and spreading, and this signaling system is regulated by the combined activity of growth factors and of integrins. Integrin signaling is mediated by Rac through PAK, and it potentiates MAP kinase activation by phosphorylation of both Raf1 and MEK1 (23, 32, 54). In particular, PAK phosphorylates MEK1 on S298, increasing the ability of Raf-1 to activate MEK1 (12, 23, 54). We previously reported that Rac-PAK signaling enhanced the MEK1-ERK interaction and that this enhancement required T292 and/or S298 of MEK1 (18). We have now demonstrated that these two sites differentially regulate the Rac-PAK effect on MEK1-ERK complex formation. MEK1 S298 phosphorylation promoted, while T292 phosphorylation inhibited, MEK1-ERK complex formation stimulated by Rac.
Each level of the MAP kinase pathway can be regulated by feedback phosphorylation. MEK-dependent phosphorylation of SOS downregulates Ras activation in mammalian cells in response to growth factor stimulation (28). Degradation of the Saccharomyces cerevisiae MEK activator Ste11 is induced through a MAP kinase feedback mechanism (20), and mammalian Raf-1 is also a substrate for ERK, although the importance of this phosphorylation is unknown (3). In addition, ERKs phosphorylate MEK1 on T292 and T386 (5, 40). These phosphorylations were reported to have no effect on MEK1 activity in vitro (40), although mutation of these sites to alanine enhanced the amplitude of MEK1 activity after serum stimulation of cells (5). We propose that T292 phosphorylation by ERK is a feedback mechanism to limit the extent to which integrin signaling enhances MAP kinase signaling.
PAK phosphorylation of S298 is regulated by cellular adhesion and is required for MEK1 activation upon adhesion (54). ERK feedback phosphorylation of MEK1 on T292 inhibited the ability of PAK to phosphorylate S298 in vitro and in vivo and negatively regulated adhesion-stimulated MEK1 activation. Therefore, the MEK1 PRS serves as an integration point for positive and negative phosphorylation signals that regulate the formation of MEK1 complexes and activation during cellular adhesion to the extracellular matrix.
Opposing phosphorylation events regulate MEK1-ERK complex formation
Frost et al. (23) observed that mutation of MEK1 at both T292 and S298 inhibited the basal association of MEK1 and Raf-1, but they were unable to detect an induction of MEK1-Raf-1 complexes upon cotransfection of activated Rac. We recently demonstrated that activated Rac could stimulate the formation of stable MEK1-ERK2 complexes in serum-deprived cells and that this stimulation of complexes was inhibited by alanine mutations of MEK1 at both T292 and S298 (18). These observations are consistent with previous reports that the MEK1 PRS is important for functional MEK1-ERK signaling (10, 15). Neither phosphorylation site on MEK1 was required for basal association with ERK2 in adherent cells; however, phosphorylation of S298, but not T292, was required for the enhancement of MEK1-ERK association by Rac. MEK1 S298 phosphorylation could act to stimulate the MEK1-ERK association in several ways. First, S298 phosphorylation might stimulate a conformational change in MEK1 that promotes its interaction with ERK. Our attempts to test this possibility in vitro have been inconclusive (data not shown). Second, the S298 phosphorylation may allow MEK1 to interact with an ERK-binding scaffold protein. We previously demonstrated that the MEK1-ERK association is enhanced by the small scaffold protein MP1, which binds to the MEK1 PRS (51). However, MP1 is an unlikely candidate scaffold, because the MEK1-MP1 interaction does not require S298 phosphorylation (data not shown). Third, S298 phosphorylation may allow the formation of MEK1-ERK complexes upon cytoskeletal structures induced by active Rac or Cdc42. Interestingly, mutation of S298 to aspartate did not detectably enhance binding to ERK2 (data not shown), suggesting that it is either the actual phosphoserine 298 form of MEK1 that is required for the enhanced interaction or a combination of the phosphoserine and Rac downstream signals.
In contrast to MEK1 S298, T292 phosphorylation negatively regulates Rac-enhanced MEK1-ERK association. Mutation of T292 to alanine enhanced both basal and Rac-inducible ERK association, while mutation to aspartate prevented the ability of Rac to enhance ERK association over the basal level. Together, these data suggest that phosphorylation of T292 might inhibit PAK phosphorylation of MEK1 on S298.
ERK regulates PAK phosphorylation of MEK1
One potential mechanism for ERK regulation of PAK phosphorylation of MEK1 is revealed by an analysis of the putative PAK consensus sequence. PAK phosphorylates Raf-1 on S338 (32). Marshall and coworkers (33) observed that arginines N terminal to S338 in Raf-1 are important for PAK phosphorylation of this site. MEK1 contains arginines at similar positions relative to the S298 phosphorylation site. Indeed, we demonstrate that a positive charge at R291 is necessary for efficient S298 phosphorylation by PAK. Phosphorylation of T292 may act to neutralize the positive charge at R291, interfering with PAK recognition of S298. Support for this charge neutralization hypothesis comes from the observation that aspartate mutation at T292 completely inhibited MEK1 S298 phosphorylation in cells, except in the presence of excess activated PAK. Additionally, prevention of T292 phosphorylation by alanine mutation enhanced S298 phosphorylation. MAP kinase has been shown to phosphorylate MEK1 on both T292 and T386, although the functional consequences of these phosphorylations have been unclear (5, 40). Both our in vivo and in vitro data support a major role for ERK phosphorylation of T292, but little or no role for T386, in the regulation of PAK phosphorylation of MEK1. Either expression of active ERK in cells or prior phosphorylation of MEK1 by ERK in vitro inhibited PAK phosphorylation of MEK1. Interestingly, cotransfection of ERK in cells further inhibited PAK phosphorylation of both MEK1 R291A and MEK1 T292A, suggesting that there is an additional mechanism by which ERK inhibits PAK phosphorylation of S298. This other mechanism could involve inhibition of PAK activity, sequestration of MEK1, or steric interference with the PAK-MEK1 interaction.
Adhesion-regulated phosphorylation and activation of MEK1
Feedback phosphorylations by MAP kinases on upstream members of their activation pathway generally act to downregulate pathway activation. Mansour et al. (40) reported that while ERK phosphorylated MEK1 on T292 and T386, these phosphorylations had no effect on MEK1 kinase activity in vitro. However, Brunet et al. (5) reported that mutation of these phosphorylation sites enhanced the activity, but not the duration, of MEK1 activity in response to serum stimulation through an unknown mechanism. Our results demonstrate that MEK1 T292 phosphorylation, like S298 phosphorylation (this work and reference 54), is regulated by cellular adhesion. The requirement for MEK1 S298 phosphorylation in MEK1 activation (54) suggests that this phosphorylation precedes MEK1 and ERK activation as well as T292 phosphorylation by ERK. Indeed, T292 phosphorylation upon cellular adhesion was delayed relative to S298 phosphorylation. This suggests a sequential phosphorylation sequence upon cellular adhesion whereby PAK phosphorylates MEK1, MEK1 becomes phosphorylated on its activating sites, MEK1 activates ERK, and ERK phosphorylates MEK1 on T292, preventing further phosphorylation of MEK1 by PAK. In agreement with this, prephosphorylation of T292 in suspended cells with activated ERK inhibited S298 phosphorylation by PAK following adhesion to fibronectin.
We demonstrate that one level of regulation of MAP kinase pathway activation during adhesion is through ERK feedback phosphorylation. The importance of T292 phosphorylation in limiting MEK1 activation was demonstrated by the prolonged activation of the MEK1 T292A mutant compared to wild-type MEK1. One explanation for this observation is that the elevated level of S298 phosphorylation observed for the MEK1 T292 mutant enhances coupling to Raf. Thus, under normal conditions of cellular adhesion, ERK feedback phosphorylation of T292 prevents increasing levels of S298 phosphorylation by PAK, potentially inhibiting the duration of MEK1 activation by Raf. Our observations suggest a plausible mechanism for the transient activation of MEK1 and ERK during adhesion to the extracellular matrix and provide a biological context for previous observations regarding the integration of Rho family GTPases into the MAP kinase pathway. The data presented here and elsewhere (12, 23, 54) suggest that adhesion-regulated assembly and disassembly of signaling complexes around MEK1 and subsequent activation of MEK1 and ERK are controlled by the integration of phosphorylation signals within the MEK1 PRS.
In addition to being regulated by cellular adhesion and spreading, both PAK and ERK have been shown to regulate cell spreading and migration (1, 34, 35, 52). While the mechanisms and pathways that regulate these processes are poorly understood, both PAK and ERK regulate cell spreading, possibly through the regulation of actin/myosin cytoskeletal dynamics and focal adhesion turnover (22, 50, 53, 57). Preliminary results suggest that transfection of MEK1 T292A into cells does not affect cell adhesion or spreading (data not shown). However, feedback inhibition of MEK1 activation by ERK may act to regulate the localized activation of ERK during adhesion and spreading. Active ERK localizes to the periphery of newly adherent cells (22). In addition, ERK phosphorylates paxillin (36, 39), a mutlifunctional focal adhesion docking protein, and phosphorylates and activates the protease M-calpain (25, 26), which cleaves several focal adhesion proteins, such as FAK, ezrin, talin, and integrin β1 and β3 cytoplasmic tails (8, 13, 42, 45, 46, 55). Thus, the coordinated regulation of ERK activity may help control focal adhesion dynamics and turnover. PAK has been reported to regulate actin dynamics through phosphorylation of LIM kinase and its subsequent effects on cofilin (19, 61) as well as through regulation of myosin light-chain kinase (50). The ability of ERK and PAK to regulate cell adhesion dynamics has implications not only for cell migration but also for tumorigenesis and progression. Activated PAK has been reported to enhance tumor growth, invasion, and metastasis in model systems (2, 4, 41, 56, 59). Furthermore, its elevated expression and activity in tumors (6, 49, 56) and its ability to stimulate adhesion-independent activation of ERK (29) suggest that activated PAK may be able to enhance MEK1 activation either directly or to overcome the negative regulation of MEK1 activation by ERK, potentially promoting cell proliferation, migration, and oncogenesis.
Acknowledgments
We thank members of the Weber laboratory and the Parsons Weber Parsons group for helpful discussions and Evangeline McKinnon for technical assistance.
This work was supported by NIH grants CA39076 and CA40042 (M.J.W.) and also GM68111 (A.D.C.).
REFERENCE
- 1.Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273:28238-28246. [DOI] [PubMed] [Google Scholar]
- 2.Adam, L., R. Vadlamudi, M. Mandal, J. Chernoff, and R. Kumar. 2000. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J. Biol. Chem. 275:12041-12050. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, N. G., L. I. Ping, L. A. Marsden, N. Williams, T. M. Roberts, and T. W. Sturgill. 1991. Raf-1 is a potential substrate for mitogen-activated protein-kinase in vivo. Biochem. J. 277:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagheri-Yarmand, R., M. Mandal, A. H. Taludker, R. A. Wang, R. K. Vadlamudi, H. J. Kung, and R. Kumar. 2001. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J. Biol. Chem. 276:29403-29409. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, A., G. Pages, and J. Pouyssegur. 1994. Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1). FEBS Lett. 346:299-303. [DOI] [PubMed] [Google Scholar]
- 6.Callow, M. G., F. Clairvoyant, S. Zhu, B. Schryver, D. B. Whyte, J. R. Bischoff, B. Jallal, and T. Smeal. 2002. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277:550-558. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 8.Carragher, N. O., B. Levkau, R. Ross, and E. W. Raines. 1999. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J. Cell Biol. 147:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catling, A. D., S. T. Eblen, H. J. Schaeffer, and M. J. Weber. 2001. Scaffold protein regulation of the MAP kinase cascade. Methods Enzymol. 332:368-387. [DOI] [PubMed] [Google Scholar]
- 10.Catling, A. D., H. J. Schaeffer, C. W. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhary, A., W. G. King, M. D. Mattaliano, J. A. Frost, B. Diaz, D. K. Morrison, M. H. Cobb, M. S. Marshall, and J. S. Brugge. 2000. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 10:551-554. [DOI] [PubMed] [Google Scholar]
- 12.Coles, L. C., and P. E. Shaw. 2002. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene 21:2236-2244. [DOI] [PubMed] [Google Scholar]
- 13.Cooray, P., Y. Yuan, S. M. Schoenwaelder, C. A. Mitchell, H. H. Salem, and S. P. Jackson. 1996. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 318:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 15.Dang, A., J. A. Frost, and M. H. Cobb. 1998. The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J. Biol. Chem. 273:19909-19913. [DOI] [PubMed] [Google Scholar]
- 16.Dent, P., W. Haser, T. A. J. Haystead, L. A. Vincent, T. M. Roberts, and T. W. Sturgill. 1992. Activation of mitogen-activated protein-kinase kinase by V-Raf in NIH 3T3 cells and in vitro. Science 257:1404-1407. [DOI] [PubMed] [Google Scholar]
- 17.Eblen, S. T., A. D. Catling, M. C. Assanah, and M. J. Weber. 2001. Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol. Cell. Biol. 21:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eblen, S. T., J. K. Slack, M. J. Weber, and A. D. Catling. 2002. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22:6023-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards, D. C., L. C. Sanders, G. M. Bokoch, and G. N. Gill. 1999. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1:253-259. [DOI] [PubMed] [Google Scholar]
- 20.Esch, R. K., and B. Errede. 2002. Pheromone induction promotes Ste11 degradation through a MAPK feedback and ubiquitin-dependent mechanism. Proc. Natl. Acad. Sci. USA 99:9160-9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 22.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 19:2911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost, J. A., H. Steen, P. Shapiro, T. Lewis, N. Ahn, P. E. Shaw, and M. H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16:6426-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost, J. A., S. Xu, M. R. Hutchison, S. Marcus, and M. H. Cobb. 1996. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol. Cell. Biol. 16:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glading, A., P. Chang, D. A. Lauffenburger, and A. Wells. 2000. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem. 275:2390-2398. [DOI] [PubMed] [Google Scholar]
- 26.Glading, A., F. Uberall, S. M. Keyse, D. A. Lauffenburger, and A. Wells. 2001. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J. Biol. Chem. 276:23341-23348. [DOI] [PubMed] [Google Scholar]
- 27.Her, J. H., S. Lakhani, K. Zu, J. Vila, P. Dent, T. W. Sturgill, and M. J. Weber. 1993. Dual phosphorylation and autophosphorylation in mitogen-activated protein (MAP) kinase activation. Biochem. J. 296:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt, K. H., B. G. Kasson, and J. E. Pessin. 1996. Insulin stimulation of a MEK-dependent but ERK-independent SOS protein kinase. Mol. Cell. Biol. 16:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe, A. K., and R. L. Juliano. 2000. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat. Cell Biol. 2:593-600. [DOI] [PubMed] [Google Scholar]
- 30.Howe, L. R., S. J. Leevers, N. Gomez, S. Nakielny, P. Cohen, and C. J. Marshall. 1992. Activation of the map kinase pathway by the protein-kinase Raf. Cell 71:335-342. [DOI] [PubMed] [Google Scholar]
- 31.Khosravi-Far, R., P. A. Solski, G. J. Clark, M. S. Kinch, and C. J. Der. 1995. Activation of Rac1, Rhoa, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 15:6443-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-183. [DOI] [PubMed] [Google Scholar]
- 33.King, A. J., R. S. Wireman, M. Hamilton, and M. S. Marshall. 2001. Phosphorylation site specificity of the Pak-mediated regulation of Raf-1 and cooperativity with Src. FEBS Lett. 497:6-14. [DOI] [PubMed] [Google Scholar]
- 34.Kiosses, W. B., R. H. Daniels, C. Otey, G. M. Bokoch, and M. A. Schwartz. 1999. A role for p21-activated kinase in endothelial cell migration. J. Cell Biol. 147:831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klemke, R. L., S. Cai, A. L. Giannini, P. J. Gallagher, P. de Lanerolle, and D. A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ku, H., and K. E. Meier. 2000. Phosphorylation of paxillin via the ERK mitogen-activated protein kinase cascade in EL4 thymoma cells. J. Biol. Chem. 275:11333-11340. [DOI] [PubMed] [Google Scholar]
- 37.Kyriakis, J. M., H. App, X. F. Zhang, P. Banerjee, D. L. Brautigan, U. R. Rapp, and J. Avruch. 1992. Raf-1 activates MAP kinase-kinase. Nature 358:417-421. [DOI] [PubMed] [Google Scholar]
- 38.Lin, T. H., Q. Chen, A. Howe, and R. L. Juliano. 1997. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J. Biol. Chem. 272:8849-8852. [PubMed] [Google Scholar]
- 39.Liu, Z. X., C. F. Yu, C. Nickel, S. Thomas, and L. G. Cantley. 2002. Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin-focal adhesion kinase association. J. Biol. Chem. 277:10452-10458. [DOI] [PubMed] [Google Scholar]
- 40.Mansour, S. J., K. A. Resing, J. M. Candi, A. S. Hermann, J. W. Gloor, K. R. Herskind, M. Wartmann, R. J. Davis, and N. G. Ahn. 1994. Mitogen-activated protein (MAP) kinase phosphorylation of MAP kinase kinase: determination of phosphorylation sites by mass spectrometry and site-directed mutagenesis. J. Biochem. (Tokyo) 116:304-314. [DOI] [PubMed] [Google Scholar]
- 41.Mira, J. P., V. Benard, J. Groffen, L. C. Sanders, and U. G. Knaus. 2000. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc. Natl. Acad. Sci. USA 97:185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muguruma, M., S. Nishimuta, Y. Tomisaka, T. Ito, and S. Matsumura. 1995. Organization of the functional domains in membrane cytoskeletal protein talin. J. Biochem. (Tokyo) 117:1036-1042. [DOI] [PubMed] [Google Scholar]
- 43.Nishida, E., and Y. Gotoh. 1993. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18:128-131. [DOI] [PubMed] [Google Scholar]
- 44.Payne, D. M., A. J. Rossomando, P. Martino, A. K. Erickson, J. H. Her, J. Shabanowitz, D. F. Hunt, M. J. Weber, and T. W. Sturgill. 1991. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 10:885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaff, M., X. Du, and M. H. Ginsberg. 1999. Calpain cleavage of integrin beta cytoplasmic domains. FEBS Lett. 460:17-22. [DOI] [PubMed] [Google Scholar]
- 46.Potter, D. A., J. S. Tirnauer, R. Janssen, D. E. Croall, C. N. Hughes, K. A. Fiacco, J. W. Mier, M. Maki, and I. M. Herman. 1998. Calpain regulates actin remodeling during cell spreading. J. Cell Biol. 141:647-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renshaw, M. W., X. D. Ren, and M. A. Schwartz. 1997. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 16:5592-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Viciana, P., P. H. Warne, A. Khwaja, B. M. Marte, D. Pappin, P. Das, M. D. Waterfield, A. Ridley, and J. Downward. 1997. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89:457-467. [DOI] [PubMed] [Google Scholar]
- 49.Salh, B., A. Marotta, R. Wagey, M. Sayed, and S. Pelech. 2002. Dysregulation of phosphatidylinositol 3-kinase and downstream effectors in human breast cancer. Int. J. Cancer 98:148-154. [DOI] [PubMed] [Google Scholar]
- 50.Sanders, L. C., F. Matsumura, G. M. Bokoch, and P. de Lanerolle. 1999. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283:2083-2085. [DOI] [PubMed] [Google Scholar]
- 51.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1—a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 52.Sells, M. A., J. T. Boyd, and J. Chernoff. 1999. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 145:837-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Hum. p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 54.Slack-Davis, J. K., S. T. Eblen, M. Zecevic, S. A. Boerner, A. Tarcsafalvi, H. B. Diaz, M. S. Marshall, M. J. Weber, J. T. Parsons, and A. D. Catling. 2003. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 162:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tranqui, L., and M. R. Block. 1995. Intracellular processing of talin occurs within focal adhesions. Exp. Cell Res. 217:149-156. [DOI] [PubMed] [Google Scholar]
- 56.Vadlamudi, R. K., L. Adam, R. A. Wang, M. Mandal, D. Nguyen, A. Sahin, J. Chernoff, M. C. Hung, and R. Kumar. 2000. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J. Biol. Chem. 275:36238-36244. [DOI] [PubMed] [Google Scholar]
- 57.van Leeuwen, F. N., S. van Delft, H. E. Kain, R. A. van der Kammen, and J. G. Collard. 1999. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1:242-248. [DOI] [PubMed] [Google Scholar]
- 58.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with the serine threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 59.Wang, R. A., A. Mazumdar, R. K. Vadlamudi, and R. Kumar. 2002. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 21:5437-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warne, P. H., P. R. Viciana, and J. Downward. 1993. Direct interaction of Ras and the amino-terminal region of Raf-1 in-vitro. Nature 364:352-355. [DOI] [PubMed] [Google Scholar]
- 61.Zebda, N., O. Bernard, M. Bailly, S. Welti, D. S. Lawrence, and J. S. Condeelis. 2000. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J. Cell Biol. 151:1119-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng, C. F., and K. L. Guan. 1994. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 13:1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]