Abstract
Background
The aim of the present study was to evaluate how torsion is influenced by left ventricular (LV) remodeling associated with age, gender and hypertension in a large community-based population.
Methods and Results
Myocardial shortening and torsion were assessed by tagged cardiac magnetic resonance (CMR) in 1478 participants without clinically apparent cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Torsion was defined as the difference between apical and basal rotation, divided by slice distance. In multivariable linear regression models, older age was associated with lower stroke volume (−3.6 ml/decade, p<0.001) and higher LV mass –to-volume ratio (0.03 g/ml/decade, p<0.001) along with lower circumferential shortening (−0.17%/decade, p<0.05). Torsion, however, was greater at older ages (0.14 °/decade, p<0.001) and in women (0.37°/cm vs. men, p<0.001). Hypertensive participants had higher LV mass and LV mass –to-volume ratio (15.5g and 0.07 g/ml, respectively, p<0.001 for both). Circumferential shortening was lower in hypertensive (−0.42%, p<0.01), whereas torsion was higher after adjustment for age and gender (0.17°/cm, p<0.05).
Conclusions
Older age is associated with lower LV volumes and greater relative wall thickness, and accompanied by lower circumferential myocardial shortening, whereas torsion is greater with older age. Hypertensive individuals have greater LV volumes and relative wall thickness and lower circumferential shortening. Torsion, however, is greater in hypertension independent of age and gender. Torsion may therefore represent a compensatory mechanism to maintain an adequate stroke volume and cardiac output in the face of progressively reduced LV volumes and myocardial shortening associated with hypertension and aging.
Keywords: Torsion, Hypertension, Age, remodeling, Cardiac Magnetic Resonance
Introduction
Epidemiologic studies report that 40-50% of patients with heart failure have preserved ejection fraction (EF),1, 2 and the prevalence of heart failure with preserved ejection fraction increases with age, especially in women.2 Hypertension increases with age and represents one of the most important risk factors for heart failure, and increases wall thickness in response to elevated blood pressure (BP) as a compensatory mechanism to minimize wall stress.3 However, alterations of left ventricular (LV) structure and function associated with hypertension, independent of aging and gender are not entirely understood. These observations suggest that LV function changes with age and gender and hypertension may predispose to heart failure.
LV systolic deformation is a complex 3-dimentional phenomenon characterized by circumferential and longitudinal shortening, radial thickening as well as ventricular torque, for the purpose of ejecting blood from the left ventricle into the aorta under pressure. Critical to enabling LV systolic deformation, the orientation of myofibers changes smoothly across the LV wall from a left-handed helix in the subepicardium, circumferential orientation in the mid wall, to a right-handed helix in the subendocardium.4-6 During ejection, the subendocardial and subepicardial layers shorten simultaneously resulting in rotation of the apex and base in counterclockwise and clockwise directions respectively, when viewed from the apex.7 LV systolic torsion limits myocardial energy consumption and minimizes transmural fiber stress gradients and oxygen demand, resulting in a more efficient LV contraction.4, 8-10 During isovolumic relaxation, torsional unfolding occurs and contributes to diastolic suction and reduction of LV pressure.5, 7 Thus, torsion and unfolding are considered to be important indicators of cardiac performance, and LV hypertrophy and fibrosis associated with hypertension could result in significant alterations of LV torque. It has previously been suggested that higher LV systolic wringing motion is found in the elderly, 11-14 however, study sample sizes were small. Therefore, torsion has not been previously evaluated in a large cohort study. Also, the assessment of torsional deformation by echocardiography is methodologically challenging, because the distance between the basal and the apical short-axis slices is difficult to assess accurately by echocardiography.
Tagged cardiac magnetic resonance (CMR) provides accurate information on the slice distance and is commonly used as the gold standard method for cardiac deformation assessment.15-17 Since the rotation gradient has been reported to be nearly linear from the LV base to apex, 15, 18 torsion can therefore be accurately expressed as twist normalized by slice distance. The aim of the present study was to evaluate how torsion is influenced by LV remodeling associated with age, gender and hypertension in a large community-based multi-ethnic population.
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective study designed to evaluate mechanisms that underlie development and progression of subclinical cardiovascular diseases among asymptomatic individuals in the general population. Details of the MESA study design have been previously described.19 In brief, between July 2000 and August 2002, 6,814 men and women who identified themselves as Caucasian, African-American, Hispanic, or Chinese and were 45 to 84 years old and free of clinically apparent cardiovascular disease were recruited. CMR was performed in 5004 participants as a part of the baseline examination. In this ancillary study, 1773 consecutive participants underwent tagged CMR studies at enrollment in six centers (Wake Forest University, Columbia University, Johns Hopkins University, University of Minnesota, Northwestern University and University of California). The participants for the tagged CMR study were randomly selected. Of these, results of 295 (16.6%) participants were excluded due to technical issues; poor quality or missing slice acquisition either at the basal or apical level. A total of 1478 participants were thus enrolled in the study. All participants gave informed consent for the study protocol, which was approved by the institutional review boards of all MESA field centers as well as the CMR reading center.
MRI Protocol
Baseline CMR images were acquired using 1.5T MR scanners: Signa LX or CVi (GE Medical Systems, Waukesha, WI, USA), Symphony or Sonata (Siemens Medical Systems, Erlangen, Germany). After acquisition of standard scout images, two- and four-chamber cine MR images were acquired using steady state free precession imaging sequences. Short-axis cine images were then obtained with retrospective gating with a temporal resolution of ≤ 50 ms, from above the mitral valve plane to the LV apex. Three tagged short-axis slices (base to apex) were obtained with an image plane distance of 5-8mm. Parallel striped tags were prescribed in two orthogonal orientations (0° and 90°) using ECG-triggered fast gradient echo sequence with spatial modulation of magnetization. Parameters for tagged images were field of view, 40 cm; slice thickness, 8 to 10 mm; repetition time, 6 ms; echo time, 3.0 ms; flip angle, 10° to 12°; matrix 205×128 with Siemens scanner and 128×64 with GE scanners; temporal resolution, 20 to 41 ms; and tag spacing, 7 mm. The detailed protocol for the tagged CMR studies is previously described.6
MRI data analysis
LV end-systolic volume (LVESV) and end-diastolic volume (LVEDV), stroke volume (SV), LV mass and LVEF were measured using commercially available software (MASS 4.2, MEDIS, Leiden, The Netherlands), as previously described. 20, 21 In short, the endocardial and epicardial myocardial borders were contoured using a semi-automated method. The difference between the epicardial and endocardial areas for all slices was multiplied by slice thickness and section gap and then multiplied by the specific myocardial density (1.04 g/ml) to determine LV mass. LV mass index (LVMI) was defined as LV mass divided by height to the power of 1.7. 22 Mass-to-volume (M/V) ratio was calculated as LV mass divided by LVEDV. LV length at end diastole was calculated as the average distance from epicardial apex to the mitral valve insertion measured from the 2 and 4 chamber views. LV sphericity index at end diastole was calculated as the percentage of the LVEDV relative to the volume of a calculated sphere with the LV length. 23 Higher index represents a more spherical shape of the ventricle.
Rotation and Myocardial Strain Analysis
Rotation and circumferential shortening were assessed in short axis tagged slices using HARP software (Harmonic Phase, Diagnosoft, Palo Alto, CA). Endocardial and epicardial contours were manually traced on the image corresponding to the remaining cardiac phases automatically. Tagged images and rotation, twist and torsion curves were assessed as illustrated in Figure 1 A. Rotation (°) was defined for each short axis slice as average angular displacement in the LV midwall layer. During a normal systole, the basal rotates in a clockwise, and the apex in a counterclockwise direction when viewed from the LV apex. Normal apical rotation is by definition positive; twist (°) was calculated as the net difference between apical and basal rotation angle for each frame during the cardiac cycle using MATLAB ® (The MathWorks, Natick, MA, USA). To normalize twist for slice distance, torsion (°/cm) was calculated by dividing peak systolic twist by the inter-slice distance h (cm). 24 The distance h was calculated as the sum of one image plane thickness and the gap between planes. Circumferential shortening was represented by the absolute peak strain value and determined and averaged from 4 LV segments (anterior, lateral, inferior, and septal) from the LV midwall layer on the mid-ventricular slice. Positive numbers represent more shortening. The ratio between torsion and stroke volume (T/SV) was calculated to estimate the effect of torsion compensation with LV volume change, as showed by the slope during systole in torsion-volume loop as described by Notomi et.al.25 Higher ratio represents higher torsion or compensation for lower SV. Torsion-to-circumferential shortening (T/CS) ratio was calculated to estimate the slope of the relation between torsion and circumferential strain during the ejection period, as described by Lumerns et al.26 This ratio describes subendocardial function, and dysfunction causes the ratio to increase.27
Figure 1.

Definition of rotational deformation and a sample of torsion curve. A. Tagged CMR short axis images and definition of rotational deformation. Torsion (°/cm) was calculated by dividing peak systolic LV twist by the inter-slice distance h (cm). B. Representative rotation, twist and torsion curves. C. The change in rotation by distance from basal to apex was as close to linear in the participants with 3 cm gap.
Risk factor measures
Standardized questionnaires were used to obtain information about smoking history and medication usage, and for history of high BP, high cholesterol, and diabetes. Smoking was defined as current, former, or never. Participant’s height and weight were measured, and resting BP was measured 3 times with participants in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, GE Healthcare, Waukesha, Wisconsin). The average of the last 2 measurements was used. Pulse pressure was calculated as systolic BP minus diastolic BP. Total cholesterol and glucose levels were measured from blood samples obtained after a 12-h fast. Diabetes was defined as fasting glucose ≥126 mg/dl or use of hypoglycemic medication or insulin. Hypertension was classified according to the Seventh Report of the Joint National Committee on the Detection, Evaluation, and treatment of High Blood Pressure.28 Glomerular filtration rate (GFR) was estimated as previously described.29
Statistical analysis
Summary statistics were presented using mean ± standard deviation for continuous variables, and percents for categorical variables. The nonparametric Wilcoxon-type trend tests for ordered groups were used to test the trends of continuously measured clinical characteristics, risk factors and CMR parameters across four age categories (44-54, 55-64, 65-74, and 75-84 years), and the chi-squares tests were used to evaluate the differences across the same age categories for categorical variables. The Wilcoxon rank-sum test was used to test the different distributions of torsion levels between man and woman.
Multivariable linear regression models were used to evaluate the effects of age, gender and hypertension on the CMR measurements. Specifically, different CMR measurements, such as LVESV, LVEDV, among others, were used as dependent variables and the potential covariates include age, gender, ethnicity, heart rate, smoking, systolic BP, total-cholesterol, glucose, GFR, and the use of medication to control hypertension, lipidemia or diabetes, among others. For regression models with torsion and circumferential shortening as dependent variables, LVEDV was included as a covariate in addition to the covariates used for other CMR measurements. In order to evaluate the relationship between LV measurements and gradations of BPs, the regression models included the same covariate adjustments with systolic BP excluded. Standardized values were defined by dividing the differences between the observed values and the sample means by the corresponding standard deviations. Nonparametric regression estimates for the unknown curves were computed using LOWESS smoothing method and the local linear smoothing method. The Epanechnikov kernel and bandwidth=8 were used for the local linear smoothing method. Statistical analyses were performed using the STATA statistical software package (Version 11. College Station, Tex). A two-sided p-value <0.05 was considered statistically significant.
Results
The participants mean age was 65.0±9.7 years and 46.4% were women in the MESA baseline examination. Baseline characteristics according to age categories are displayed in Table 1. Systolic and pulse pressure were greater with increased age. Hypertension was more common among older participants. Of the total population, 14% were diabetic, 48% were hypertensive, and 48 % had none of these conditions.
Table 1.
Baseline characteristics according to age categories
| Age categories n=1478 |
p for trend | ||||
|---|---|---|---|---|---|
| 45-54 years | 55-64 years | 65-74 years | 75-84 years | ||
| n=260 | n=355 | n=573 | n=290 | ||
| Age, years | 49.9 (2.9) | 59.5 (2.8) | 68.8 (2.8) | 78.0 (2.5) | - |
| Ethnicity, n(%) | |||||
| Caucasian | 71 (27.3) | 104 (29.3) | 171 (29.8) | 93 (32.1) | 0.021 |
| Chinese-American | 24 (9.2) | 49 (13.8) | 94 (16.4) | 41 (14.1) | |
| African-American | 96 (36.9) | 94 (26.5) | 151 (26.3) | 68 (23.5) | |
| Hispanic | 69 (26.5) | 108 (30.4) | 157 (27.4) | 88 (30.3) | |
| Women, n(%) | 109 (41.9) | 182 (51.3) | 260 (45.4) | 135 (46.6) | 0.126 |
| Height, cm | 169.2 (9.4) | 165.9 (10.1) | 165.3 (9.9) | 163.7 (10.2) | <0.001 |
| Body mass index, kg/m2 | 28.2(5.0) | 28.2 (4.8) | 27.5 (4.6) | 27.1 (4.6) | 0.006 |
| Systolic BP, mmHg | 117.7 (16.1) | 124.2 (18.6) | 130.5 (21.3) | 136.5 (21.9) | <0.001 |
| Diastolic BP, mmHg | 73.1 (9.8) | 72.9 (10.0) | 71.9 (10.6) | 69.6 (9.6) | <0.001 |
| Pulse pressure, mmHg | 44.5 (10.4) | 51.3 (13.7) | 58.6 (16.8) | 66.9 (18.1) | <0.001 |
| Heart rate, beats/min | 61.9 (8.4) | 63.8 (9.6) | 62.3 (9.7) | 61.8 (9.9) | 0.180 |
| Hypertension, n(%) | 61 (23.5) | 142 (40.0) | 321 (56.0) | 181 (62.4) | <0.001 |
| Diabetes, n(%) | 17 (6.5) | 49 (13.8) | 95 (16.6) | 49 (17.0) | 0.001 |
| Smoker, n(%) | |||||
| Never | 124 (48.1) | 187 (53.0) | 287 (50.3) | 168 (58.3) | <0.001 |
| Former | 88 (34.1) | 108 (30.6) | 238 (41.7) | 104 (36.1) | |
| Current | 46 (17.8) | 58 (16.4) | 46 (8.1) | 16 (5.6) | |
| Total cholesterol, mg/dl | 195.2 (38.5) | 198.7 (35.3) | 193.8 (33.9) | 190.6 (34.6) | 0.025 |
| GFR, ml/min | 88.8 (14.3) | 84.2 (17.3) | 78.8 (17.5) | 72.0 (17.6) | <0.001 |
Values are means (standard deviation).
Torsion by age category and gender
The change in rotation from base to apex was approximately linear (Figure 1C) and the median distance from the basal to the apical slice was 3.0 cm (inter quartile range 3.0cm, 3.01cm). Overall, torsion was 3.9±1.3 °/cm and differed significantly between women and men (4.2±1.3 °/cm vs. 3.5±1.1 °/cm, P<0.001). Torsion was found to differ by gender in all age categories (Figure 2).
Figure 2.
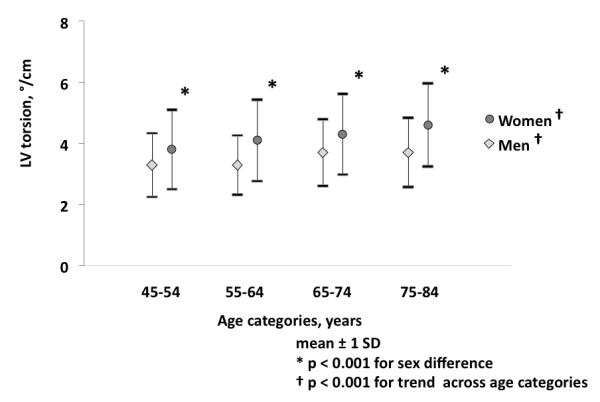
Gender specific torsion with age. Compared with younger ages, older women and men were more likely to have increased torsion (both tests for trends p<0.001). Torsion differed significantly between women and man in each age category (all p <0.001).
LV structure and function with age, gender and hypertension
Associations of LV structure and function with age, gender and hypertension in multivariable linear regression models are displayed in Table 2. Older age was associated with lower LVESV, LVEDV, SV and higher M/V ratio (−2.9, −6.5, −3.6ml/decade and 0.03g/ml respectively, all p<0.001). Circumferential shortening also was significantly lower with older age (−0.17%/decade, p<0.05) and torsion was greater with older age (0.14°/cm/decade p<0.001) after adjusting for traditional risk factors. But the association of torsion with age was no longer significant after adjustment for LVEDV in addition to traditional risk factors. The torsion/stroke volume ratio (T/SV), which can be interpreted as torsion compensation for lower SV, and the torsion/circumferential shortening ratio (T/CS ), interpreted as torsion relative to myocardial dysfunction, were significantly higher with older age.
Table 2.
Association of age, gender and hypertension with LV measurements
| Coefficient (95% confidence interval) | |||
|---|---|---|---|
| Age (decade) | Gender (reference; women) | Hypertension | |
| LVESV, ml | −2.9 (−3.8, −2.0) ‡ | 6.4 (4.1, 8.7) ‡ | 1.7 (0.02, 3.3) * |
| LVEDV, ml | −6.5 (−8.1, −4.9) ‡ | 4.3 (0.2, 8.4) * | 5.9 (3.0, 8.8) ‡ |
| SV, ml | −3.6 (−4.7, −2.6) ‡ | −1.9 (−4.6, 0.8) | 4.2 (2.3, 6.2) ‡ |
| LVEF, % | 0.7 (0.2, 1.1) † | −4.2 (−5.3, −3.1) ‡ | 0.4 (−0.4, 1.1) |
| LV mass, g | −4.2 (−6.0, −2.4) ‡ | 22.7 (18.1, 27.3) ‡ | 15.5 (12.2, 18.8) ‡ |
| M/V ratio, g/ml | 0.03 (0.02, 0.05) ‡ | 0.16 (0.12, 0.20) ‡ | 0.07 (0.05, 0.10) ‡ |
| LV sphericity index, % | −0.03 (−0.4, 0.3) | −3.3 (−4.2, −2.3) ‡ | 0.1 (−0.6, 0.7) |
| Circumferential shortening, % | −0.17 (−0.32, −0.02) * | −1.05 (−1.44 0.66) ‡ | −0.42 (−0.70, −0.14,) † |
| Torsion, °/cm | 0.14 (0.07, 0.21) ‡ | −0.37 (−0.56, −0.19) ‡ | 0.17 (0.04, 0.30) * |
| T/SV ratio, °/cm*L | 4.2 (3.0, 5.4) ‡ | −3.7 (−6.7, −0.7) * | −0.001 (−0.003, 0.001) |
| T /CS ratio, °/cm/% | 0.01 (0.005, 0.014) ‡ | −0.01 (−0.02, 0.002) | 0.02 (0.01, 0.02) ‡ |
Coefficients represent change in the dependent variables per 1 decade increase in age or difference in the dependent variable for men compared to women, or hypertension with adjustments for multiple variables.
Age was adjusted for gender, ethnicity, height, heart rate, smoking, systolic BP, total-cholesterol, glucose, GFR, and the use of medication to control hypertension, lipidemia or diabetes.
Gender was adjusted for age, ethnicity, height, heart rate, smoking, systolic BP, total-cholesterol, glucose, GFR, and the use of medication to control hypertension, lipidemia or diabetes.
Hypertension was adjusted for age, gender, ethnicity, height, heart rate, smoking, total-cholesterol, glucose, GFR, and the use of medication to control lipidemia or diabetes.
p <0.05
p<0.01
p<0.001
LVESV and LVEDV were lower in women (−6.4 ml and −4.3 ml vs. men, respectively, both p<0.05) but SV did not differ between genders. The LV sphericity index as well as circumferential shortening were higher in women (3.3% and 1.05% vs. men, respectively, both p<0.001). Also, torsion was greater in women (0.37°/cm vs. men, p<0.001) and the gender differences remained highly significant after adjustment for LVEDV. There was significantly more torsion compensation (T/SV ratio) with lower SV in women (3.7°/cm*L vs. men, p<0.05) but no differences in myocardial dysfunction (T/CS ratio) were documented.
Hypertensive participants had higher LV mass and M/V ratio (15.5g and 0.07 g/ml, respectively both p<0.001). Circumferential shortening was lower in hypertensive MESA participants (−0.42%, p<0.01), whereas torsion was higher (0.17°/cm, p<0.05) compared to participants without hypertension. In hypertensive participants, greater myocardial dysfunction expressed by a greater T/CS ratio was observed, but there was no increase in the T/SV ratio.
The relation of LV structure and function with gradations of BPs is shown in Table 3. Higher systolic BP was associated with greater LVEDV (1.6ml/10mmHg, p<0.001) and higher LVM (3.2g/10mmHg, P<0.001). Torsion correlated positively with systolic BP (0.06°/cm/10mmHg, p<0.05) as well as pulse pressure (0.11 °/cm/10mmHg, p<0.05) but no correlation was found with diastolic BP. Also the associations of LV remodeling and torsion with increasing BP are displayed in Figure 3.
Table 3.
Association of LV measurements with gradation of BP
| Coefficient (95% confidence interval) | |||
|---|---|---|---|
| Systolic BP, mmHg | Diastolic BP, mmHg | Pulse pressure, mmHg | |
| LVESV, ml | 0.4 (−0.01, 0.8) | 1.2 (0.4, 2.0) † | 0.2 (−0.3, 0.7) |
| LVEDV, ml | 1.6 (0.9, 2.3) ‡ | 1.5 (0.1, 3.0) * | 2.3 (1.4, 3.3) ‡ |
| SV, ml | 1.2 (0.8, 1.7) ‡ | 0.4 (−0.6, 1.3) | 2.1 (1.5, 2.7) ‡ |
| LVEF, % | 0.1 (−0.1, 0.3) | −0.6 (−0.9, −0.2) † | 0.5 (0.2, 0.8) ‡ |
| LV mass, g | 3.2 (2.4, 4.0) ‡ | 4.9 (3.3, 6.6) ‡ | 3.7 (2.6, 4.8) ‡ |
| M/V ratio, g/ml | 0.01 (0.003, 0.02) † | 0.02 (0.01, 0.04) ‡ | 0.01 (−0.003, 0.01) |
| LV sphericity index, % | 0.02 (−0.15, 0.18) | −0.12 (−0.45, 0.21) | 0.008 (−0.14, 0.30) |
| Circumferential shortening, % |
−0.06 (−0.13, 0.00) | −0.29 (−0.43, −0.15) ‡ | 0.01 (−0.08, 0.10) |
| Torsion, °/cm | 0.06 (0.03, 0.10)* | 0.01 (−0.06, 0.07) | 0.11 (0.07, 0.16) * |
| T/SV ratio, °/cm*L | −0.01 (−0.54, 0.51) | −0.67 (−1.74, 0.41) | 0.27 (−0.44, 0.99) |
| T/CS ratio, °/cm/% | 0.004 (0.003, 0.01) ‡ | 0.003 (−0.0005, 0.007) | 0.01 (0.004, 0.01) ‡ |
Coefficients represent change in dependent variables per 10 mmHg increase in BP with adjustments for multiple variables; age, gender, ethnicity, height and heart rate, smoking, total-cholesterol, glucose, GFR, and the use of medication to control hypertension, lipidemia or diabetes.
p <0.05
p<0.01
p<0.001
Figure 3.
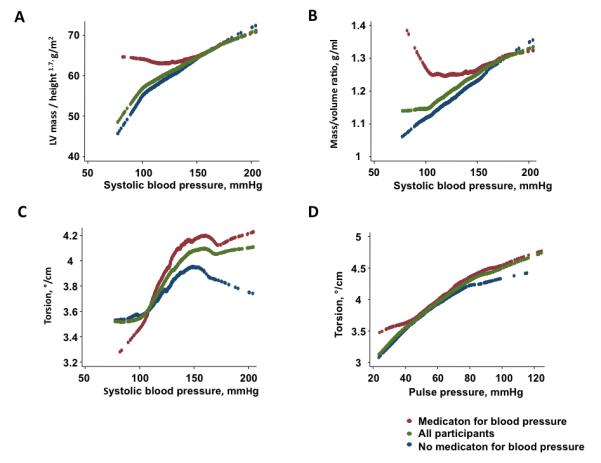
LV remodeling and torsion with gradation of BPs. Systolic BP increased LV mass/height1.7 (A), Mass/volume ratio (B) and torsion (C). Pulse pressure was associated with torsion (D). Red, green and blue dots represents participants with medication for BP, all participants and participants without medication for BP (n=873), respectively.
Reproducibility of LV torsion
To assess inter/ intra observer variability, 30 cases were randomly selected and then independently analyzed by 2 different observers. Inter/ intra-observer class correlation coefficient was 0.94 (95%CI, 0.86-1.03) and 0.91 (95% confident interval; CI, 0.81-1.01), respectively.
Discussion
Torsion is directly related to myocardial fiber orientation, structure and function, and assessment of torsion has been introduced as an important component of LV mechanical behavior. The aim of the present study was to evaluate how LV remodeling associated with age, gender and hypertension influences LV torsion in a large community-based population. In the present study we demonstrate that 1) torsion is higher with increased age and in women; 2) torsion is associated with lower LV volumes and circumferential strain; 3) and that hypertension increases torsion independently of age and gender. Below, we first discuss the effects of age and gender on cardiac remodeling as a necessary pre-requisite for a full understanding of the influence of hypertension on LV mechanical behavior.
Age and torsion
It has been reported that LV twist is higher during infancy compared to adulthood.14 Torsion indexed to LV length, however, decreases until early adulthood because the LV elongates.14 Several smaller studies have suggested higher LV systolic wringing motion in older volunteers, as quantified by the twist magnitude measured by 2D-speckle tracking echocardiography,12 circumferential to longitudinal shear angles measured by 3D-tagged CMR,30, 31 as well as torsion indexed to LV length using Doppler tissue imaging. 14 In the present study we demonstrate that torsion is higher with increased age, and we found that this was related to a gradual increment in apical rather than basal rotation in a community based population of individuals aged 45-84 years and without history of cardiovascular diseases. Ageing has been previously associated with concentric remodeling, 32-34 subendocardial dysfunction,26, 35 and reduced elasticity of restoring forces of the LV,34,36 with consequent impaired LV recoil and untwist.12, 14 The increase in peak systolic torsion appears to parallel age related progressive decline in subendocardial function suggesting that reduced subendocardial function could result in less endocardial opposition to the dominant epicardium, and thus enhanced torsion.12, 37, 38 Beyar et al, using a theoretical mathematical model of LV mechanics, reported a shortening gradient across the LV wall with greater shortening in the subendocardium relative to the subepicardial myocardial layers when torsion was not considered in the model. In that analysis, the introduction of torsion reduced the need for endocardial myofiber shortening, with consequent reduction in myocardial energy demand and oxygen consumption.10 The data suggest that torsion enables efficient LV systolic function without the need to augment endocardial shortening. In our study, the greater torsion seen in older participants could reflect a mechanism to compensate for lower myocardial shortening in order to maintain LVEF and SV. In this regard, according to the lever-arm theory, 39 a greater radius difference between the endocardium and the epicardium (i.e. secondary to greater wall thickness relative to cavity radius) would result in increased torsion, because helical contraction of subendocardial and subepicardial muscle layers would counteract one another. With increasing age, LV volumes become smaller and the M/V ratio is greater with enhanced radius difference between endo- and epicardium, leading to enhanced torsion. However, in the present study, torsion was not positively correlated with M/V ratio, suggesting that reduction of myocardial shortening rather than the pure effect of the lever-arm theory is responsible for the age related increase in torsion. Indeed, previous studies from our group have reported that myofiber shortening is progressively lower in MESA participants with increased LV mass/volume ratio due to the deleterious effects of concentric remodeling on myocardial contraction.33
Gender differences in LV torsion
In the present study, torsion and circumferential shortening were higher in women compared to men. Several studies have shown that women have higher LVEF 20, 40 and greater systolic elastance than men reflecting greater myocardial contractility.34 In the normal ventricle, the helical fiber orientation enables systolic fiber rearrangement, and during contraction, the subendocardial fibers are sheared toward the LV cavity, thus enhancing LV wall thickening. 4, 7 During LV ejection, LV twist has been shown to be linearly and negatively related to LV volume.41 In the present study, we demonstrate that peak torsion is negatively related to both end-systolic and end-diastolic LV volumes. Hearts with smaller ventricles exhibit greater torsion, suggesting that increased torsion is required to maintain an adequate cardiac output in smaller hearts. On the other hand, fiber orientation is dependent on LV shape. 42 In the present study, more spherical hearts were found in women compared to men before and after controlling for age, race, body size and traditional risk factors. A more spherical LV shape entails a more horizontal orientation of fiber angles, thus favoring torsion. Finally, another potential mechanism for gender differences in LV architecture and function was proposed by Redfield et al who reported that arterial stiffening was more pronounced with higher age and more in women than in men. 34 Differences in arterial function might also underlie the observed gender differences in torsion shown in this study. However, further assessment is needed to evaluate such potential mechanisms for the observed gender differences in torsion.
Hypertension and torsion
In the present study, we demonstrated that hypertensive heart has higher LV mass and M/V ratio, and that hypertension is an independent predictor of increased torsion. In pressure overload hypertrophy, increasing systolic wall stress leads to an increasing LV wall thickness as a compensatory mechanism to minimize wall steess.3 This pattern of concentric remodeling might enhance torsion because of the greater radius difference between the endocardium and the epicardium as proposed by the lever-arm theory as we discussed previously (see “age and torsion” section above). We also found that hypertensive individuals had a higher torsion to shortening (T/CS) ratio without differences in the torsion to stroke volume (T/SV) ratio. This suggests that enhanced torsion is not be caused by reduced LV volumes, and that the mechanisms of increased torsion associated with hypertension may thus be at least in part different from that seen in association with aging alone. In hypertension, reduced circumferential shortening would also be expected to reduce torsion. A possible explanation is the increased afterload associated with hypertension. It has been reported that chronic afterload augmentation leads to increased torsion but reduced circumferential shortening in patients with aortic stenosis.43 The apparent contradiction involving increased torsion despite reduced circumferential shortening may be explained by increased endocardial myocardial oxygen consumption, as demonstrated in patients with increased arterial stiffening. 44 Since the oblique fiber structure of the endocardium and epicardium are oriented in different directions, reduced subendocardial function may alter the balance between the opposing rotational forces and thus result in enhanced torsion. This may in fact represent a compensatory mechanism to maintain cardiac function in the face of increased afterload and/or vascular stiffness. Torsion has been found to be lower with increasing extent of transmural ischemia and/or infarction.45, 46 Consequently, increased torsion in hypertensive hypertrophy can function as an early indicator of systolic myocardial dysfunction likely to be predominantly at the subendocardial level. However, as myocardial dysfunction progresses to involve the entire transmural extent of the LV wall, torsion decreases, as documented in advanced decompensated hypertrophic cardiac disease.
Limitations
Approximately 17 % of participants were excluded due to technical issues related to data acquisition and analysis. Even though tagged CMR is currently the reference method for assessment of LV deformation, the limitations of CMR and tagging will benefit from further technical development.
The median distance from the apical to basal slice was only 3 cm. Thus, the slices were relatively close to each other which may lead to underestimation of torsion. Therefore, our measurements of apical rotation may have slightly underestimated true apical rotation. We did not assess longitudinal strain and the spatial resolution of the tagging sequences used is also limited. Despite these technical limitations, this is still the largest myocardial MRI tagging study performed so far, allowing for unique investigation of cardiac mechanics at the population level.
Conclusions
Torsion assessed by tagged CMR is greater with hypertension, older age and in women compared to men in a large cohort of individuals without evidence of prior cardiovascular disease. Age is associated with lower LV volumes and greater relative wall thickness, and accompanied by lower circumferential myocardial shortening, whereas torsion increases with older age. The smaller LV volumes with greater contraction in women drive the greater torsion. Hypertension is associated with greater LV volumes and relative wall thickness as well as with lower circumferential shortening. Torsion, however, is greater in hypertensive individuals independent of age and gender. Torsion may therefore represent a compensatory mechanism that maintains an adequate stroke volume and cardiac output in the face of progressively reduced LV volumes and myocardial shortening associated with ageing and/or hypertension.
Clinical perspective.
Left ventricular (LV) systolic torsion limits myocardial energy consumption and minimizes transmural fiber stress gradients and oxygen demand, resulting in a more efficient contraction in the mathematic model. According to the lever-arm theory, a greater radius difference between the endocardium and the epicardium such as concentric hypertrophy would result in increased torsion. Also, reduced subendocardial function would result in less opposition to the dominant epicardium, and finally result in enhanced torsion, because helical contraction of subendocardial and subepicardial muscle layers would counteract one another. However, the influence of LV remodeling associated with age, gender and hypertension on LV torsion is not well understood. Therefore, we used cardiac MRI to examine LV structure and function among 1478 participants of the Multi-Ethnic Study of Atherosclerosis who had no cardiovascular disease at baseline. In multivariable regression models, older age was associated with lower LV volumes, higher relative wall thickness and a significant fall in stoke volume along with lower myocardial shortening. Torsion however was greater with old age (0.14°/cm/decade, p<0.001). The smaller LV and higher contraction of women heart were accompanied by the greater torsion (0.37°/cm vs. men, p<0.001). Finally, while hypertension is associated with concentric hypertrophy and lower circumferential shortening, torsion is greater in hypertension individuals independent of age and gender (0.17°/cm vs. non hypertension, p<0.05). These findings suggest that torsion represents a compensatory mechanism that maintains an adequate stroke volume and cardiac output in the face of progressively reduced LV volumes and myocardial shortening associated with ageing and/or hypertension.
Figure 4.
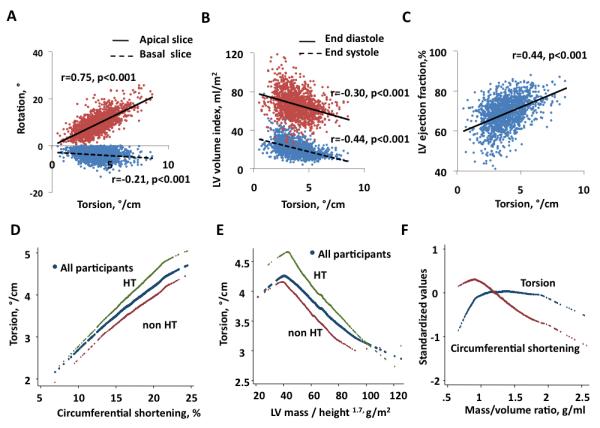
The correlations between the torsion and rotation, ejection fraction and LV volume by CMR. There was a strong correlation between apical rotation and torsion (r=0.75, p<0.001). However the correlation between basal rotation and torsion was statistically significant but relatively weak (r=−0.21, p<0.001). 222 cases were excluded due to positive rotation at basal slice (A). Torsion correlated negatively with LVESV index (r=−0.44, p<0.001), LVEDV index (r=−0.30, p<0.001) (B), positively with LVEF (r=0.44, p<0.001) (C). Torsion correlated positively with circumferential shortening in all participant subgroups, and at any level of circumferential shortening, the torsion was higher in hypertensive participants (n=705) compared to participants without hypertension (D). Torsion correlated negatively with LV mass/height1.7 in all groups, and torsion was higher in hypertensive participants at any level of LV mass/height1.7 (E). M/V ratio correlated negatively with circumferential shortening and the relations with torsion was nonlinear (F).
Figure 5.
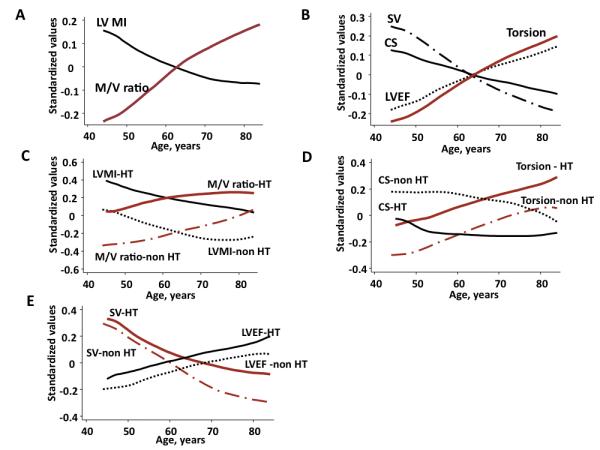
The influence of hypertension on torsion and age-related remodeling. Age was inversely associated with LVMI and positively with M/V ratio (A), and inversely with SV and circumferential shortening. However, torsion was positively correlated with age, along with LVEF (B). In hypertensive participants, remodeling was characterized primarily by an upward parallel shift compared to non hypertensive participants. At any age, hypertensive participants had higher LVMI and M/V ratio (C). Torsion was higher despite lower circumferential shortening compared to participants without hypertension (D). SV was lower with age, but was maintained to a larger degree in hypertensive individuals, indicating that increased torsion in part compensates for the reduced circumferential shortening in individuals with hypertension (E). CS; circumferential shortening, HT; hypertension, LVEF; left ventricular ejection fraction, LVMI; left ventricular mass index, M/V; mass/volume, SV; stroke volume
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures: O.G. received support from the Fulbright Foundation, the Norwegian Medical Association and the Norwegian Research Council.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 2.Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, Oliveira A. Prevalence of chronic heart failure in southwestern europe: The epica study. Eur J Heart Fail. 2002;4:531–539. doi: 10.1016/s1388-9842(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 3.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 4.Streeter DD, Jr., Spotnitz HM, Patel DP, Ross J, Jr., Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 5.Ashikaga H, Criscione JC, Omens JH, Covell JW, Ingels NB., Jr Transmural left ventricular mechanics underlying torsional recoil during relaxation. Am J Physiol Heart Circ Physiol. 2004;286:H640–647. doi: 10.1152/ajpheart.00575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with mr-tagging in a multi-center study: Interobserver and intraobserver agreement of fast strain analysis with harmonic phase (harp) mri. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta PP, Khandheria BK, Narula J. Twist and untwist mechanics of the left ventricle. Heart Fail Clin. 2008;4:315–324. doi: 10.1016/j.hfc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Arts T, Veenstra PC, Reneman RS. Epicardial deformation and left ventricular wall mechanisms during ejection in the dog. Am J Physiol. 1982;243:H379–390. doi: 10.1152/ajpheart.1982.243.3.H379. [DOI] [PubMed] [Google Scholar]
- 9.Beyar R, Sideman S. Effect of the twisting motion on the nonuniformities of transmyocardial fiber mechanics and energy demand--a theoretical study. IEEE Trans Biomed Eng. 1985;32:764–769. doi: 10.1109/TBME.1985.325491. [DOI] [PubMed] [Google Scholar]
- 10.Beyar R, Sideman S. Left ventricular mechanics related to the local distribution of oxygen demand throughout the wall. Circ Res. 1986;58:664–677. doi: 10.1161/01.res.58.5.664. [DOI] [PubMed] [Google Scholar]
- 11.Burns AT, La Gerche A, MacIsaac AI, Prior DL. Augmentation of left ventricular torsion with exercise is attenuated with age. J Am Soc Echocardiogr. 2008;21:315–320. doi: 10.1016/j.echo.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006;19:1077–1084. doi: 10.1016/j.echo.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.van Dalen BM, Soliman OI, Vletter WB, ten Cate FJ, Geleijnse ML. Age-related changes in the biomechanics of left ventricular twist measured by speckle tracking echocardiography. Am J Physiol Heart Circ Physiol. 2008;295:H1705–1711. doi: 10.1152/ajpheart.00513.2008. [DOI] [PubMed] [Google Scholar]
- 14.Notomi Y, Srinath G, Shiota T, Martin-Miklovic MG, Beachler L, Howell K, Oryszak SJ, Deserranno DG, Freed AD, Greenberg NL, Younoszai A, Thomas JD. Maturational and adaptive modulation of left ventricular torsional biomechanics: Doppler tissue imaging observation from infancy to adulthood. Circulation. 2006;113:2534–2541. doi: 10.1161/CIRCULATIONAHA.105.537639. [DOI] [PubMed] [Google Scholar]
- 15.Goffinet C, Chenot F, Robert A, Pouleur AC, le Polain de Waroux JB, Vancrayenest D, Gerard O, Pasquet A, Gerber BL, Vanoverschelde JL. Assessment of subendocardial vs. Subepicardial left ventricular rotation and twist using two-dimensional speckle tracking echocardiography: Comparison with tagged cardiac magnetic resonance. Eur Heart J. 2009;30:608–617. doi: 10.1093/eurheartj/ehn511. [DOI] [PubMed] [Google Scholar]
- 16.Notomi Y, Setser RM, Shiota T, Martin-Miklovic MG, Weaver JA, Popovic ZB, Yamada H, Greenberg NL, White RD, Thomas JD. Assessment of left ventricular torsional deformation by doppler tissue imaging: Validation study with tagged magnetic resonance imaging. Circulation. 2005;111:1141–1147. doi: 10.1161/01.CIR.0000157151.10971.98. [DOI] [PubMed] [Google Scholar]
- 17.Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H, Smiseth OA. New noninvasive method for assessment of left ventricular rotation: Speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 18.Buchalter MB, Weiss JL, Rogers WJ, Zerhouni EA, Weisfeldt ML, Beyar R, Shapiro EP. Noninvasive quantification of left ventricular rotational deformation in normal humans using magnetic resonance imaging myocardial tagging. Circulation. 1990;81:1236–1244. doi: 10.1161/01.cir.81.4.1236. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 21.Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events. The mesa (multi-ethnic study of atherosclerosis) study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John-Sutton M, Rietzschel ER. Left ventricular mass: Allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamas GA, Vaughan DE, Parisi AF, Pfeffer MA. Effects of left ventricular shape and captopril therapy on exercise capacity after anterior wall acute myocardial infarction. Am J Cardiol. 1989;63:1167–1173. doi: 10.1016/0002-9149(89)90173-2. [DOI] [PubMed] [Google Scholar]
- 24.Rosen BD, Gerber BL, Edvardsen T, Castillo E, Amado LC, Nasir K, Kraitchman DL, Osman NF, Bluemke DA, Lima JA. Late systolic onset of regional lv relaxation demonstrated in three-dimensional space by mri tissue tagging. Am J Physiol Heart Circ Physiol. 2004;287:H1740–1746. doi: 10.1152/ajpheart.00080.2004. [DOI] [PubMed] [Google Scholar]
- 25.Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD. Ventricular untwisting: A temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol. 2008;294:H505–513. doi: 10.1152/ajpheart.00975.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lumens J, Delhaas T, Arts T, Cowan BR, Young AA. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using mri. Am J Physiol Heart Circ Physiol. 2006;291:H1573–1579. doi: 10.1152/ajpheart.00074.2006. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Toorn A, Barenbrug P, Snoep G, Van Der Veen FH, Delhaas T, Prinzen FW, Maessen J, Arts T. Transmural gradients of cardiac myofiber shortening in aortic valve stenosis patients using mri tagging. Am J Physiol Heart Circ Physiol. 2002;283:H1609–1615. doi: 10.1152/ajpheart.00239.2002. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Bui AL, Katz R, Kestenbaum B, de Boer IH, Fried LF, Polak JF, Wasserman BA, Sarnak MJ, Siscovick D, Shlipak MG. Cystatin c and carotid intima-media thickness in asymptomatic adults: The multi-ethnic study of atherosclerosis (mesa) American Journal of Kidney Diseases. 2009;53:389–398. doi: 10.1053/j.ajkd.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russel IK, Gotte MJ, Kuijer JP, Marcus JT. Regional assessment of left ventricular torsion by cmr tagging. J Cardiovasc Magn Reson. 2008;10:26. doi: 10.1186/1532-429X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oxenham HC, Young AA, Cowan BR, Gentles TL, Occleshaw CJ, Fonseca CG, Doughty RN, Sharpe N. Age-related changes in myocardial relaxation using three-dimensional tagged magnetic resonance imaging. J Cardiovasc Magn Reson. 2003;5:421–430. doi: 10.1081/jcmr-120022258. [DOI] [PubMed] [Google Scholar]
- 32.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: The multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: The multi-ethnic study of atherosclerosis. Circulation. 2005;112:984–991. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 35.Nikitin NP, Witte KK, Thackray SD, de Silva R, Clark AL, Cleland JG. Longitudinal ventricular function: Normal values of atrioventricular annular and myocardial velocities measured with quantitative two-dimensional color doppler tissue imaging. J Am Soc Echocardiogr. 2003;16:906–921. doi: 10.1016/S0894-7317(03)00279-7. [DOI] [PubMed] [Google Scholar]
- 36.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: Earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchalter MB, Rademakers FE, Weiss JL, Rogers WJ, Weisfeldt ML, Shapiro EP. Rotational deformation of the canine left ventricle measured by magnetic resonance tagging: Effects of catecholamines, ischaemia, and pacing. Cardiovasc Res. 1994;28:629–635. doi: 10.1093/cvr/28.5.629. [DOI] [PubMed] [Google Scholar]
- 38.Kroeker CA, Tyberg JV, Beyar R. Effects of ischemia on left ventricular apex rotation. An experimental study in anesthetized dogs. Circulation. 1995;92:3539–3548. doi: 10.1161/01.cir.92.12.3539. [DOI] [PubMed] [Google Scholar]
- 39.Ingels NB, Jr., Hansen DE, Daughters GT, 2nd, Stinson EB, Alderman EL, Miller DC. Relation between longitudinal, circumferential, and oblique shortening and torsional deformation in the left ventricle of the transplanted human heart. Circ Res. 1989;64:915–927. doi: 10.1161/01.res.64.5.915. [DOI] [PubMed] [Google Scholar]
- 40.Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD, Drazner MH. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: The dallas heart study. Circulation. 2006;113:1597–1604. doi: 10.1161/CIRCULATIONAHA.105.574400. [DOI] [PubMed] [Google Scholar]
- 41.Notomi Y, Martin-Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB, Garcia MJ, Greenberg NL, Thomas JD. Enhanced ventricular untwisting during exercise: A mechanistic manifestation of elastic recoil described by doppler tissue imaging. Circulation. 2006;113:2524–2533. doi: 10.1161/CIRCULATIONAHA.105.596502. [DOI] [PubMed] [Google Scholar]
- 42.Adhyapak SM, Parachuri VR. Architecture of the left ventricle: Insights for optimal surgical ventricular restoration. Heart Fail Rev. 2010;15:73–83. doi: 10.1007/s10741-009-9151-0. [DOI] [PubMed] [Google Scholar]
- 43.Stuber M, Scheidegger MB, Fischer SE, Nagel E, Steinemann F, Hess OM, Boesiger P. Alterations in the local myocardial motion pattern in patients suffering from pressure overload due to aortic stenosis. Circulation. 1999;100:361–368. doi: 10.1161/01.cir.100.4.361. [DOI] [PubMed] [Google Scholar]
- 44.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res. 1992;71:490–502. doi: 10.1161/01.res.71.3.490. [DOI] [PubMed] [Google Scholar]
- 45.Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused st-elevation myocardial infarction: A comprehensive tissue doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging. 2008;1:189–196. doi: 10.1161/CIRCIMAGING.108.784900. 182 p following 196. [DOI] [PubMed] [Google Scholar]
- 46.Knudtson ML, Galbraith PD, Hildebrand KL, Tyberg JV, Beyar R. Dynamics of left ventricular apex rotation during angioplasty: A sensitive index of ischemic dysfunction. Circulation. 1997;96:801–808. doi: 10.1161/01.cir.96.3.801. [DOI] [PubMed] [Google Scholar]


