Abstract
Objective: Although the anterior approach is normally used for elective laparoscopic splenectomy (LS), the posterolateral approach may be superior. We have retrospectively compared the effectiveness and safety of these approaches in patients with non-severe splenomegaly scheduled for elective total LS.
Methods: Patients with surgical spleen disorders scheduled for elective LS between March 2005 and June 2011 underwent laparoscopic splenic mobilization via the posterolateral or anterior approach. Main outcome measures included operation time, intraoperative blood loss, frequency of postoperative pancreatic leakage, and length of hospital stay.
Results: During the study period, 203 patients underwent LS, 58 (28.6%) via the posterolateral and 145 (71.4%) via the anterior approach. Three patients (1.5%) required conversion to laparotomy due to extensive perisplenic adhesions. The posterolateral approach was associated with significantly shorter operation time (65.0 ± 12.3 min vs. 95.0 ± 21.3 min, P < 0.01), reduced intraoperative blood loss (200.0 ± 23.4 mL vs. 350.0 ± 45.2 mL, P < 0.01), and shorter hospital stay (5.0 ± 2.0 d vs. 9.0 ± 3.0 d, P < 0.01) than the anterior approach. The frequency of pancreatic leakage was slightly lower in patients undergoing LS via the posterolateral than the anterior approach (0.0% vs. 3.4%, P > 0.05)
Conclusions: The posterolateral approach is more effective and safer than the anterior approach in patients without severe splenomegaly (< 30 cm).
Keywords: Laparoscopic splenectomy, Posterolateral approach, Anterior approach, Comparative study.
Introduction
Laparoscopic splenectomy (LS) has been increasingly used in general surgery since the first reported adult LS in 19911. LS is primarily used for elective resection in patients with non-traumatic spleen disorders, primarily hematological and oncological diseases, as well as those with extensive splenomegaly, including patients with idiopathic thrombocytopenic purpura (ITP), spleen hamartoma, and hypersplenism. The removal of a much enlarged spleen usually requires the incorporation of hand assistance into laparoscopy2. Refinements in laparoscopic technique and instruments over the last two decades have resulted in multiple technical modifications of LS procedures, such as laparoendoscopic single site (LESS) splenectomy3. Transumbilical LESS splenectomy has been used for the emergency treatment of patients with traumatic spleen rupture4. LS has also been reported effective and safe for patients with massive splenomegaly due to portal hypertension, even without hand assistance5. Many comparative clinical studies have documented the effectiveness and safety of LS versus laparotomy. LS is believed to be superior to open splenectomy due to its minimal invasiveness, which manifests as minimal intraoperative bleeding, less pain, expedited postoperative recovery, shorter hospital stay, and lower complication rate6. Therefore, LS is preferred to open splenectomy in most adults and children. The use of the LigaSure vessel sealing system along with hilum hanging maneuver has been found to further shorten the operating time and intraoperative blood loss7.
Most patients currently scheduled for splenectomy undergo LS via the anterior approach8. This approach provides laparoscopic surgeons with a direct view of the spleen anatomy, similar to that in conventional open splenectomy, as well as extending the indications of LS to patients with massive splenomegaly9. However, this approach also has limitations, with the primary technical concern being the poor visualization of the splenic hilum10, which may increase the risks of bleeding and bleeding-associated complications especially when performed by less experienced surgeons11. Alternatively, LS can be performed using a lateral approach, in which patients are usually placed in the right lateral decubitus position for better exposure of the spleen12. This approach provides surgeons with an excellent view of the splenic vessels, pancreatic tail, and accessory spleens if applicable13. This improved view may reduce bleeding and minimize requirements for blood transfusion. It may be especially beneficial for pediatric patients, as these patients undergo LS primarily for underlying hematological conditions and are therefore susceptible to blood loss and transfusion-associated events14. However, use of the lateral approach requires additional time and labor to adjust the patient's position. Moreover, due to the intrinsic mobility of the spleen, laparoscopic surgeons encounter a distorted splenic anatomy when using this approach. The lateral approach is also less suitable in patients with large spleens, suggesting that the optimal approach for LS be based on spleen size15.
In the anterior approach, the splenic artery is controlled from the ventral side following the transection of the gastrocolic ligament. In contrast, dissection of the splenorenal ligament and the splenodiaphragmatic ligament precedes the mobilization of the splenic pedicle starting from the dorsal side. We have modified the lateral approach to a posterolateral approach by using a position identical to that used in the anterior approach. This modification results in better surgical outcomes and lower complication rate than the anterior approach. We have therefore retrospectively compared the feasibility, effectiveness, and safety of the posterolateral and anterior approaches in patients with non-severe splenomegaly scheduled for elective total LS.
Materials and methods
Patients
Adult patients (n = 203) who required splenectomy due to non-traumatic surgical spleen diseases were consecutively hospitalized in our surgical department between March 2005 and June 2011. Preoperative spleen size was determined using abdominal ultrasonography or computed tomography (CT) scan. Patients with a maximum oblique spleen size >30 cm were excluded. LS was performed using the posterolateral or anterior approach at the discretion of the surgeon in a non-blinded manner. The study protocol was approved by the Institutional Review Board at the First Norman Bethune Hospital of Jilin University, and all patients volunteered to give informed consent in writing prior to surgery.
Preoperative assessment
Patients were assessed for anemia or thrombocytopenia. Patients having a hemoglobin concentration below 70 g/L received red blood cell transfusion, those with a platelet count below 30×109/L received platelet transfusion, and those with a prothrombin time greater than 5×INR received transfusion of fresh plasma or cryoprecipitate. Spleen volume and the severity of splenic vein varicosity were determined by preoperative abdominal CT.
Laparoscopic splenectomy
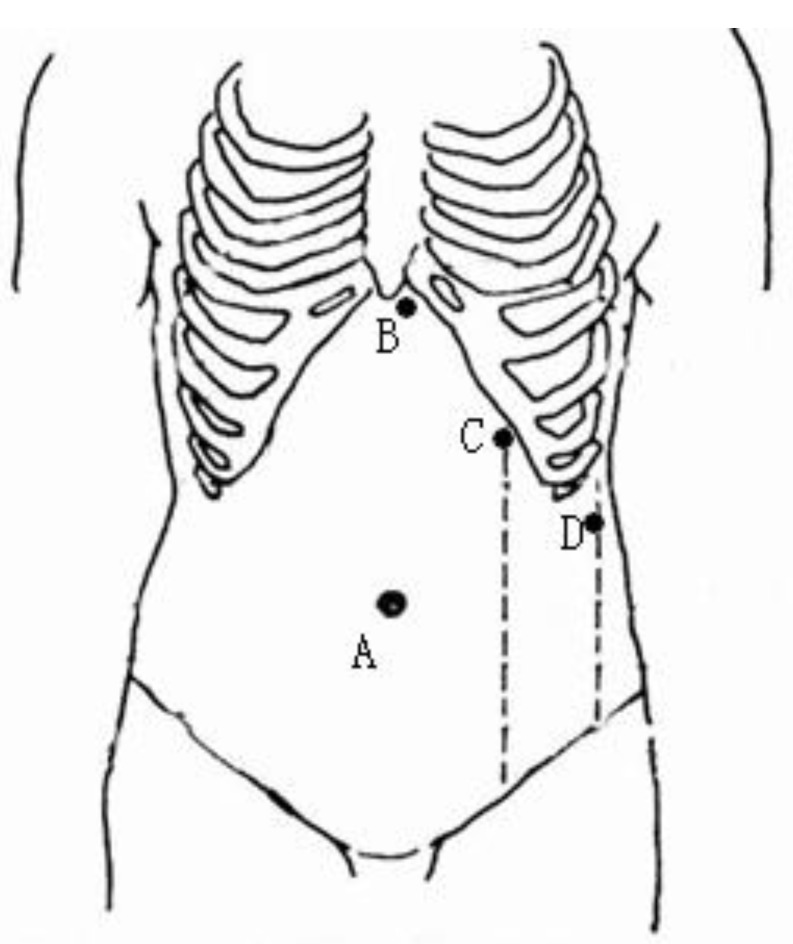
Nasogastric decompression and urethral catheterization were performed routinely, and intravenous prophylactic antibiotics were administered 30 min before the induction of general anesthesia. Following the intubation, the patient was placed in the supine position, with the head and feet tilted down. The chest and pelvis were fixed with straps and cloth cushions to allow a 30° tilt of the operating table towards the right side. A standard four-trocar placement was used to establish access ports for either approach. Following the establishment of pneumoperitoneum, a 10-mm port was placed at the umbilicus, and a 30° laparoscope (KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) was introduced. A 5-mm port was positioned to the left of the falciform ligament below the xiphoid, allowing exposure of the splenic hilum using a grasper. A 10-mm port was placed on the left midclavicular line as the main manipulation port. An additional 5 mm port was sited at the inferior pole of the spleen on the left midaxillary line to retract the spleen (Fig.1). The laparoscopic surgeon and the second assistant stood on the right side of the patient, and the first assistant was positioned on the left side.
Fig 1.
Positions of trocar ports in laparoscopic splenectomy. A: a 10.5-mm port for the laparoscope; B: a 5-mm port for a grasper exposing the splenic hilum; C: a 10.5-mm main manipulation port; and D: an additional 5-mm port for retraction.
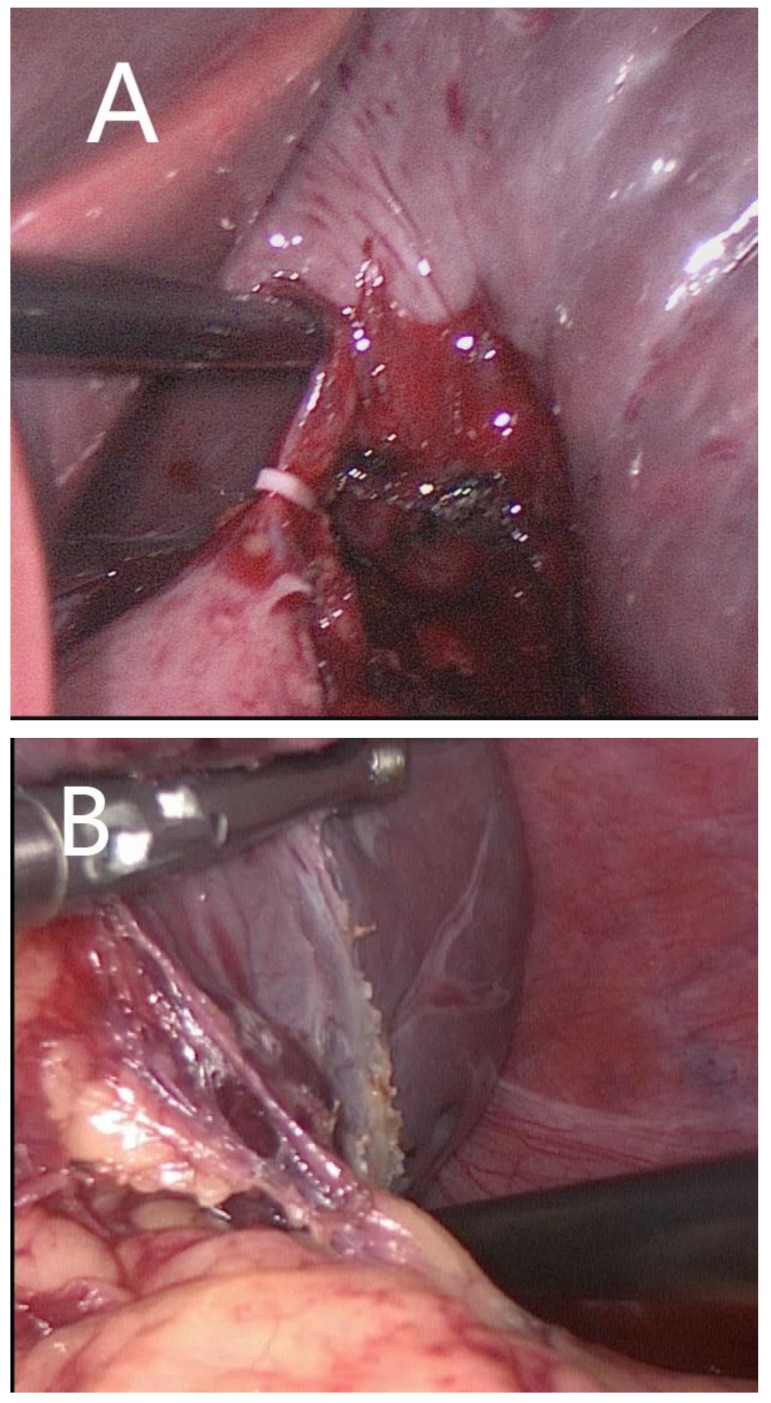
Anterior approach The splenocolic ligament was dissected, followed by upward transection of the splenogastric ligament using the LigaSure vessel sealing system (Covidien Valleylab, Boulder, CO, USA). The splenic artery was identified along the upper margin of the pancreatic body and appropriately dissected using an ultrasonic scalpel (Olympus, Tokyo, Japan). The arterial inflow was interrupted by applying a polymer Hem-o-lock clip (Teleflex) to reduce spleen size and minimize blood loss during splenectomy. The splenic veins were dissected and securely ligated using laparoscopic clips. The splenogastric ligament was transected starting from the upper pole, followed by transection of the splenocolic, splenorenal and splenodiaphragmatic ligaments. The secondary pedicular vessels were transected and ligated using the combination of LigaSure sealing and laparoscopic clips (Fig. 2A).
Fig 2.
Laparoscopic splenectomy via the anterior approach (A) and the posterolateral approach (B).
Posterolateral approach The splenocolic ligament was dissected, followed by transection of the splenorenal and splenodiaphragmatic ligaments. The splenic pedicle was mobilized from the posterior towards the anterior side. Upon dissection of the splenic pedicle, the primary pedicular vessels were mobilized to a sufficient length using the electrosurgical scalpel, and the secondary pedicular vessels were transected and ligated using vascular clips (Teleflex, Athlone, Ireland), starting from the lower pole (Fig. 2B).
Following the resection of the spleen, the trocar on the left midclavicular line was withdrawn and the port site was extended to a 3-cm incision. A previously prepared sterile polymer or 3-liter TPN bag was used to envelop the spleen specimen. The patient's position was adjusted appropriately to facilitate specimen retrieval. The specimen bag was fastened inside the peritoneal cavity, and the specimen was fragmented using a pair of sponge forceps under laparoscopic visualization. The port incision could be further extended to withdraw the intact specimen for pathological examination. The peritoneal cavity was irrigated and examined for any active hemorrhage, and a lavage drain was placed in the spleen fossa.
Postoperative care and follow-up
Platelet counts were assessed routinely. Patients were administered low-molecular-weight heparin, starting on postoperative day 2, to minimize intraportal thrombosis. Peritoneal fluid amylase was assayed daily for the first three postoperative days to monitor for postoperative pancreatic leakage. The lavage drain was maintained and irrigated using negative pressure until the peritoneal fluid was negative for amylase, but was removed when the daily drainage volume was below 50 ml, no perisplenic effusion was detectable on abdominal Doppler ultrasonography, and the peritoneal fluid was negative for amylase. Patients were followed up at outpatient clinics by routine hematological tests to monitor platelet count and abdominal Doppler ultrasonography to monitor intraportal thrombosis.
Outcome measures
The primary outcome measures were operation time; intraoperative blood loss/transfusion; rate of injury to an abdominal organ or major vessel; frequency of conversion to an open procedure; time to restart off-bed activities, bowel movement and oral intake; post-splenectomy drainage volume; time to drain removal; and length of hospital stay.
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL) was used for the statistical analysis. All numeric data were expressed as mean ± SD, and compared by two independent sample Student t-test. All categorical data were expressed as frequency with percentage, and compared by the Fisher's exact probability test. A P-value less than 0.05 was considered statistically significant.
Results
Baseline patient characteristics
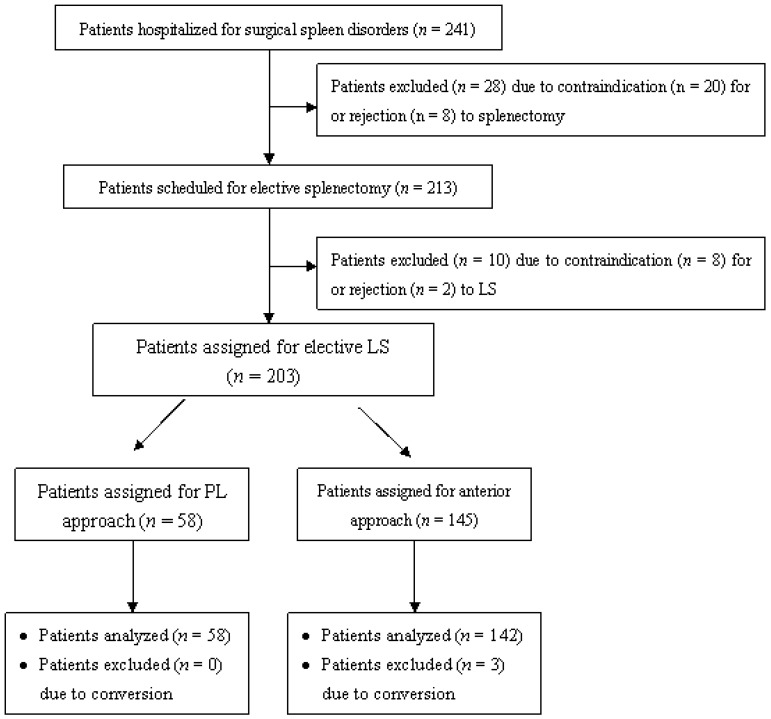
The patients scheduled for elective LS included 74 males and 129 females, at a mean age of 42.5 ± 5.2 years (range, 18-69 years). Out of these patients, 175 were indicated for splenectomy due to histologically documented hematological disorders, including ITP (n = 138), hemolytic anemia (n = 12), and hereditary spherocytosis (n = 25). Of the remaining patients, 19 were indicated for splenectomy due to hypersplenism secondary to portal hypertension, 7 for post-traumatic splenic cysts), and 2 for lymphoma involving the spleen. Among the 203 patients, 58 (28.6%) underwent LS using the posterolateral approach (PLLS group), and 145 (71.4%) underwent LS using the anterior approach (ALS group) (Fig. 3). The baseline characteristics of the two groups are shown in Table 1. The two groups were comparable in terms of age, sex, body mass index, splenic etiology, spleen size, severity of concomitant splenomegaly, Child-Pugh class, previous abdominal surgery, and concomitant medical conditions (all P-values > 0.05).
Fig 3.
Patient assignment flow chart. LS, laparoscopic splenectomy; PL approach, posterolateral approach.
Table 1.
Baseline characteristics of patients (n=203) undergoing elective laparoscopic splenectomy. PLLS, laparoscopic splenectomy via posterolateral approach; ALS, laparoscopic splenectomy via anterior approach; BMI, body mass index.
| PLLS group (n = 58) |
ALS group (n = 145) |
P-value | |
|---|---|---|---|
| Age, year | 40.2 ± 5.6 | 45.3 ± 4.3 | < 0.001 |
| Sex (M:F) | 20/38 | 67/78 | 0.158 |
| BMI, kg/m2 | 23.2 ± 3.6 | 21.2 ± 3.5 | < 0.001 |
| Splenic etiology, n(%) | < 0.001 | ||
| ITP | 24(41.4) | 114(78.6) | |
| Hemolytic anemia | 5(8.6) | 7(4.8) | |
| Hereditary spherocytosis | 11(19.0) | 14(10.0) | |
| Hypersplenism/portal hypertension | 13(22.4) | 6(4.1) | |
| Post-traumatic splenic cyst | 4(6.9) | 3(2.1) | |
| Lymphoma involvement | 1(1.7) | 1(0.1) | |
| Spleen size | |||
| Spleen length, cm | 27.0 ± 3.5 | 28.0 ± 2.8 | 0.0849 |
| Spleen volume, cm3 | 360.0 ± 23.2 | 324.0 ± 31.2 | < 0.001 |
| Concomitant splenomegaly, n(%) | < 0.001 | ||
| Mild | 33(56.9) | 124(85.5) | |
| Moderate | 14(24.1) | 8(5.5) | |
| Severe | 11(19.0) | 13(9.0) | |
| Child-Pugh class | 0.029 | ||
| Class A | 42 | 123 | |
| Class B | 16 | 22 | |
| Previous abdominal surgery (%) | 1 (1.7) | 12 (8.3) | 0.115 |
| Concomitant conditions, n(%) | |||
| Anemia | 29(50.0) | 27(18.6) | < 0.001 |
| Thrombocytopenia | 37(63.8) | 120(82.8) | 0.005 |
| Hypertension | 24(41.4) | 26(17.9) | 0.001 |
| Diabetes mellitus | 13(22.4) | 31(21.4) | 0.853 |
| Cardiovascular diseases | 25(43.1) | 51(35.2) | 0.336 |
Surgical outcomes
Surgical outcomes are shown in Table 2. All patients underwent total LS, except for three patients (1.5%) who required conversion to laparotomy due to extensive perisplenic adhesions. Operating time was significantly shorter (65.0 ± 12.3 min vs. 95.0 ± 21.3 min, P < 0.001) and intraoperative blood loss was significantly lower (200.0 ± 23.4 mL vs. 350.0 ± 45.2 mL, P < 0.001) in the PLLS than in the ALS group. There were 2 cases of transfusion in PLLS group, while 18 cases in ALS group. And the mean volume of transfusion were 200.0 ± 34.5 mL and 350.0 ± 42.5 mL respectively in PLLS group and ALS group( P<0.001). All patients started off-bed activities 24-72 hours following LS and resumed oral intake following removal of the gastric tube. Duration of hospital stay was significantly shorter in the PLLS than in the ALS group (5.0 ± 2.0 d vs. 9.0 ± 3.0 d, P < 0.001) (Table 2).
Table 2.
Surgical outcomes of patients (n = 203) undergoing elective laparoscopic splenectomy via the posterolateral or anterior approach.
| PLLS group (n = 58) |
ALS group (n = 145) |
P-value | |
|---|---|---|---|
| Conversion rate, n (%) | 0(0.0) | 3(2.1) | 0.559 |
| Operative duration, min | 65.0 ± 12.3 | 95.0 ± 21.3 | < 0.001 |
| Volume of blood loss, mL | 200.0 ± 23.4 | 350.0 ± 45.2 | < 0.001 |
| Volume of transfusion, mL) | 200.0 ± 34.5 | 350.0 ± 42.5 | < 0.001 |
| Frequency of transfusion (n[%]) | 2(3.4) | 18(12.4) | 0.067 |
| Time to resume (mean ± SD, day) | |||
| off-bed activities | 2.0 ± 1.5 | 3.0 ± 1.7 | < 0.001 |
| bowel movement | 2.0 ± 0.8 | 2.0 ± 1.2 | 1.000 |
| oral intake | 1.0 ± 0.8 | 2.0 ± 0.5 | < 0.001 |
| Time of drain removal, day | 4.0 ± 1.2 | 5.0 ± 2.1 | < 0.001 |
| Length of hospital stay, day | 5.0 ± 2.0 | 9.0 ± 3.0 | < 0.001 |
Procedural safety and complications
No major intraoperative event occurred in either group. The frequency of pancreatic leakage following PLLS was slightly lower than that following ALS (0.0% [0/58] vs. 3.4% [5/145], P = 0.324).
Pathological and follow-up outcomes
The pathological outcomes were consistent with the preoperative diagnoses. Histological examination identified congestive splenomegaly in 194 patients (95.6%), splenic cysts in two (1.0%), splenic involvement of lymphoma in two (1.0%), and normal spleens in five (2.5%).
Discussion
Elective LS is the preferred therapeutic modality for the treatment of spleen diseases requiring surgery16. The indications for LS are basically the same as those for conventional laparotomy, although LS is less indicated for acute splenic rupture and severe splenomegaly (> 30 cm). Previously, LS was mainly used to treat ITP, since this condition is not accompanied by marked perisplenic varices, which makes the dissection of splenic pedicular vessels relatively easier and less technically complicated17. Although LS is widely used for splenectomy in patients with hematological, oncological and infectious splenic diseases, it is relatively contraindicated for splenomegaly of over 30 cm. Laparoscopic resection is not that technically challenging in patients with splenomegaly secondary to hematological diseases, as these spleens have relatively long secondary pedicles and loose perisplenic ligaments. LS, however, is usually regarded as less safe for patients with splenomegaly complicated by portal hypertension18. Coexisting perisplenic varices are prone to rupture and bleeding, while laparoscopy is less effective in controlling bleeding from secondary pedicular vessels. Most (86.2%) of our patients had hematological spleen diseases, especially ITP (70.4%), with a smaller percentage (9.4%) having hypersplenism and splenomegaly secondary to portal hypertension. LS via either the posterolateral or anterior approach showed good effectiveness and safety profiles in these patients without severe splenomegaly (< 30 cm), with a minimal conversion rate (1.5%) and a low bleeding risk.
The anterior approach is the most frequently used access approach in LS as it is suitable for any laparoscopically eligible patient. This approach permits easy access to the omental pouch and great splenic vessels, similar way to laparotomy9. Using this approach, the splenogastric ligament is transected before the other perisplenic ligaments. The upper pole of the spleen can be elevated using a pair of atraumatic hemostatic forceps to allow ultrasonic dissection. In patients with extensive upper pole adhesions, the splenogastric ligament can be dissected after the other perisplenic ligaments.
Interruption of arterial inflow into the splenic hilum can reduce spleen volume and minimize the risk of bleeding during the dissection of secondary pedicular vessels. In most patients, the main trunk of the splenic vein is located superficially, allowing it to be transected along with the splenic artery. Excessive dissection may result in injury to the pancreatic tail or massive bleeding. Hilar vessel control is usually completed by using Endo-GIA at the pancreatic tail19. This technique requires the use of expansive instruments but is associated with a high risk of pancreatic leakage following splenectomy. We therefore transected the secondary rather than the primary pedicular vessels using the LigaSure sealing device and/or laparoscopic clips, reducing the cost of LS and the risk of postoperative leakage, but prolonging the operating time. The anterior approach is awkward in dissecting splenic retroperitoneal attachments as well as in visualizing and controlling hilar vessels10. These technical disadvantages result in a longer operation time and increase the risk of iatrogenic injuries.
LS using the lateral or posterolateral approach was first described soon after the anterior approach was introduced12. The posterolateral approach is more suitable for obese patients scheduled for elective LS as it is more difficult to visualize and dissect the splenogastric ligament enriched with adipose tissues. Transection of the splenocolic ligament permits the forward dissection of the splenorenal ligament, an approach that entails less dissection of adipose tissue and enables better visualization of the splenic hilum. Following adequate mobilization of the splenic pedicle, the secondary pedicular vessels are mobilized and transected upwards. The direct visualization in this approach renders it easy to manipulate pedicular vessels, minimizing the risk of incident bleeding and iatrogenic injury to the pancreatic tail. However, the lateral or posterolateral approach is less efficient for LS in patients with extensively severe splenomegaly (< 30 cm) 15, since the huge space-occupying spleen cannot be flipped towards the anterior side prior to ligation of the splenic artery.
The primary advantages of LS relative to conventional laparotomy include its minimal invasiveness and better cosmetic outcomes due to the small trocar incisions made in the abdominal wall20. We found that patients can benefit similarly from LS via both approaches, as shown by postoperative recovery, including the resumption of off-bed activities, bowel movement and oral intake. The primary disadvantages of LS compared with open splenectomy include longer operation time and higher bleeding risk21. However, our results demonstrate that the posterolateral approach significantly shortens the LS operation time and reduces the volume of intraoperative blood loss compared with the anterior approach. The lower frequency of laparoscopically uncontrollable bleeding reduces conversion to an open procedure. We observed a very low conversion rate, comparable in patients who underwent LS via the anterior and posterolateral approaches. However, conversion in the three patients was mainly due to extensive perisplenic adhesions rather than bleeding. The reduction in intraoperative blood loss also minimizes the requirement for blood transfusions, although none of our patients who underwent LS via either approach require a transfusion. This benefit is more clinically significant for patients with surgical spleen disorders as they, especially those with complicating coagulopathy, such as ITP, are more prone to surgical bleeding and transfusion-associated adverse effects.
Iatrogenic pancreatic injury is another common procedural complication following splenectomy, resulting in a severe and even fatal outcome in some patients22. The occurrence of pancreatic injury, such as pancreatic leakage, pancreatic bleeding, and pancreatitis, is frequently underestimated, as most patients with complicating pancreatic conditions are asymptomatic. Pancreatic injuries secondary to splenectomy occur mainly during the dissection of the pancreatic tail in proximity to splenic hilum. The rate of pancreatic injury is reported to be up to 16% in patients undergoing laparotomy, but only 1-2% in patients undergoing LS23. The rate (3.4%) of pancreatic leakage in our patients who underwent LS via the anterior approach was similar to that of previous reports, whereas use of the modified posterolateral approach reduced the rate of pancreatic leakage to zero. Inappropriate disposition of the stapler transecting the pancreatic tail and the splenic hilum results in a very high risk of pancreatic leakage. The modified posterolateral approach offers a better visualization of the splenic hilum and facilitates the dissection of the pancreatic tail away from the hilum, thus minimizing the occurrence of pancreatic tail injury and leakage. The monitoring of amylase concentrations in serum and/or peritoneal fluid can be used to detect occult pancreatic leakage22, although abdominal CT scan may identify nonspecific pancreatic tail swelling. Pancreatic leakage following LS is usually transient in duration and mild in severity, with most patients requiring symptomatic treatment alone. Persistent and patent peritoneal drainage is the most effective method of controlling pancreatic leakage, minimizing secondary peritonitis and peritoneal abscess24. The five patients who experienced pancreatic leakage following LS via the anterior approach all responded well to peritoneal drainage, and resolved without significant sequelae. Minimization of pancreatic leakage was the primary contributor to the shorter hospital stay in the PLLS than in the ALS group.
This study had several limitations. The use of either the anterior or posterolateral approach was at the discretion of the surgeon, a non-randomized patient assignment that may have resulted in patient selection bias. In addition, this analysis was retrospective in design, and surgeons were not blinded to the LS approach, thus suggesting that our results may have been subject to observation and confounding biases. To date, however, no prospective randomized control study has compared the effectiveness and safety outcomes in patients undergoing LS via the lateral/posterolateral and anterior approaches.
Conclusion
We found that the posterolateral approach was more effective and safer for LS than the anterior approach in patients without severe splenomegaly (> 30 cm). The posterolateral approach was associated with significantly shorter operation times and hospital stay, significantly reduced intraoperative blood loss, and a lower rate of pancreatic leakage. The posterolateral approach, however, is less suitable in patients with severe splenomegaly (> 30 cm) due to technical limitations. A prospective, randomized, control study may validate the technical advantages of the posterolateral over the anterior approach.
References
- 1.Delaitre B, Maignien B. Laparoscopic splenectomy - technical aspects. Surg Endosc. 1992;6:305–8. doi: 10.1007/BF02498866. [DOI] [PubMed] [Google Scholar]
- 2.Swanson TW, Meneghetti AT, Sampath S. et al. Hand-assisted laparoscopic splenectomy versus open splenectomy for massive splenomegaly: 20-year experience at a Canadian centre. Can J Surg. 2011;54:189–93. doi: 10.1503/cjs.044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colon MJ, Telem D, Chan E. et al. Laparoendoscopic single site (LESS) splenectomy with a conventional laparoscope and instruments. JSLS. 2011;15:384–6. doi: 10.4293/108680811X13125733356918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Y, Wu SD, Siwo EA. Emergency transumbilical single-incision laparoscopic splenectomy for the treatment of traumatic rupture of the spleen: report of the first case and literature review. Surg Innov. 2011;18:185–8. doi: 10.1177/1553350611403767. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Wu Z, Cai Y. et al. The feasibility and safety of laparoscopic splenectomy for massive splenomegaly: a comparative study. J Surg Res. 2011;171:e55–e60. doi: 10.1016/j.jss.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Kucuk C, Sozuer E, Ok E. et al. Laparoscopic versus open splenectomy in the management of benign and malign hematologic diseases: a ten-year single-center experience. J Laparoendosc Adv Surg Tech A. 2005;15:135–9. doi: 10.1089/lap.2005.15.135. [DOI] [PubMed] [Google Scholar]
- 7.Misawa T, Yoshida K, Iida T. et al. Minimizing intraoperative bleeding using a vessel-sealing system and splenic hilum hanging maneuver in laparoscopic splenectomy. J Hepatobiliary Pancreat Surg. 2009;16:786–91. doi: 10.1007/s00534-009-0175-6. [DOI] [PubMed] [Google Scholar]
- 8.de Lagausie P, Bonnard A, Benkerrou M. et al. Pediatric laparoscopic splenectomy: benefits of the anterior approach. Surg Endosc. 2004;18:80–2. doi: 10.1007/s00464-003-9048-2. [DOI] [PubMed] [Google Scholar]
- 9.Choi SH, Kang CM, Hwang HK. et al. Reappraisal of anterior approach to laparoscopic splenectomy: technical feasibility and its clinical application. Surg Laparosc Endosc Percutan Tech. 2011;21:353–7. doi: 10.1097/SLE.0b013e31822d6c8c. [DOI] [PubMed] [Google Scholar]
- 10.Trias M, Targarona EM, Balagué C. Laparoscopic splenectomy: an evolving technique. A comparison between anterior and lateral approaches. Surg Endosc. 1996;10:389–92. doi: 10.1007/BF00191621. [DOI] [PubMed] [Google Scholar]
- 11.Podevin G, Victor A, De Napoli S. et al. Laparoscopic splenectomy: comparison between anterior and lateral approaches. J Laparoendosc Adv Surg Tech A. 2011;21:865–8. doi: 10.1089/lap.2011.0108. [DOI] [PubMed] [Google Scholar]
- 12.Kuriansky J, Ben Chaim M, Rosin D. et al. Posterolateral approach: An alternative strategy in laparoscopic splenectomy. Surg Endosc. 1998;12:898–900. doi: 10.1007/s004649900740. [DOI] [PubMed] [Google Scholar]
- 13.Gossot D, Fritsch S, Célérier M. Laparoscopic splenectomy: optimal vascular control using the lateral approach and ultrasonic dissection. Surg Endosc. 1999;13:21–5. doi: 10.1007/s004649900890. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald PG, Langer JC, Cameron BH. et al. Pediatric laparoscopic splenectomy using the lateral approach. Surg Endosc. 1996;10:859–961. doi: 10.1007/BF00189553. [DOI] [PubMed] [Google Scholar]
- 15.Mahon D, Rhodes M. Laparoscopic splenectomy: size matters. Ann R Coll Surg Engl. 2003;85:248–51. doi: 10.1308/003588403766274953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gigot JF, Etienne J, Lengele B. et al. Elective laparoscopic splenectomy: Person experience and literature review. Semin Laparosc Surg. 1996;3:34–43. doi: 10.1053/SLAS00300034. [DOI] [PubMed] [Google Scholar]
- 17.Friedman RL, Fallas MJ, Carroll BJ. et al. Laparoscopic splenectomy for ITP: The gold standard. Surg Endosc. 1996;10:991–5. doi: 10.1007/s004649900221. [DOI] [PubMed] [Google Scholar]
- 18.Ohta M, Nishizaki T, Matsumoto T. et al. Analysis of risk factors for massive intraoperative bleeding during laparoscopic splenectomy. J Hepatobiliary Pancreat Surg. 2005;12:433–7. doi: 10.1007/s00534-005-1027-7. [DOI] [PubMed] [Google Scholar]
- 19.Vargün R, Göllü G, Fitöz S. et al. En-bloc stapling of the splenic hilum in laparoscopic splenectomy. Minim Invasive Ther Allied Technol. 2007;16:360–2. doi: 10.1080/13645700701699414. [DOI] [PubMed] [Google Scholar]
- 20.Canda AE, Sucullu I, Ozsoy Y. et al. Hospital experience, body image, and cosmesis after laparoscopic or open splenectomy. Surg Laparosc Endosc Percutan Tech. 2009;19:479–83. doi: 10.1097/SLE.0b013e3181c3ff24. [DOI] [PubMed] [Google Scholar]
- 21.Winslow ER, Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: a meta-analysis with an emphasis on complications. Surgery. 2003;134:647–53. doi: 10.1016/s0039-6060(03)00312-x. [DOI] [PubMed] [Google Scholar]
- 22.Chand B, Walsh RM, Ponsky J. et al. Pancreatic complications following laparoscopic splenectomy. Surg Endosc. 2001;15:1273–6. doi: 10.1007/s004640080054. [DOI] [PubMed] [Google Scholar]
- 23.Vecchio R, Gelardi V, Intagliata E. et al. How to prevent intraoperative risks and complications in laparoscopic splenectomy. G Chir. 2010;31:55–61. [PubMed] [Google Scholar]
- 24.Donini A, Baccarani U, Terrosu G. et al. Laparoscopic vs open splenectomy in the management of hematologic diseases. Surg Endosc. 1999;13:1220–5. doi: 10.1007/pl00009625. [DOI] [PubMed] [Google Scholar]