Abstract
Purpose.
To investigate the contribution ocular aberrations have on visual performance by quantifying improvements in best-corrected visual acuity (VA) and contrast sensitivity (CS) obtained with higher-order aberration (HOA) correction after penetrating (PK), deep anterior lamellar (DALK), or Descemet's stripping automated endothelial keratoplasty (DSAEK).
Methods.
Sixteen eyes were evaluated from 14 subjects who underwent PK (n = 5), DALK (n = 6), or DSAEK (n = 5) greater than 1 year prior to study enrollment. Ocular aberrations were measured and an adaptive optics system was used to correct ocular lower-order aberration (LOA) and HOA. VA and CS were measured for each subject with LOA or full-aberration correction. CS was measured at each of three spatial frequencies: 4, 8, and 12 cycles/deg.
Results.
All keratoplasty groups had more aberration than that of a normal myopic population and experienced significant VA gains with full-aberration correction (P < 0.0013). PK subjects had better VA than that of DSAEK subjects with LOA correction (logMAR VA 0.03 ± 0.05 vs. 0.25 ± 0.05; P = 0.0870). After HOA correction this trend persisted (P = 0.1734). DSAEK subjects also experienced less VA benefit from full-aberration correction than that of PK and DALK subjects. All keratoplasty groups demonstrated similar CS benefits from full-aberration correction despite differing higher-order root-mean-square magnitudes.
Conclusions.
PK eyes had better logMAR VA than that of DSAEK eyes with LOA correction, whereas DALK eyes performed intermediate between the two. When full correction was applied, the same trend persisted. The findings suggest that factors other than aberration contribute to decrements in VA with DSAEK compared with PK.
Correction of lower- and higher-order aberrations using adaptive optics does not improve visual performance equally across post-keratoplasty groups. Results suggest that other factors, such as scatter or neural adaptation, play a role in visual acuity and contrast sensitivity.
Introduction
With advances in keratoplasty techniques, corneal transplant surgeons may now strategically target replacement of the diseased portion of the cornea. Targeted anterior lamellar substitution via deep anterior lamellar keratoplasty (DALK) can be used for keratectasia, anterior scarring, or infection; likewise, targeted endothelium replacement via Descemet's stripping automated endothelial keratoplasty (DSAEK) is used for endothelial failure. Traditional full-thickness penetrating keratoplasty (PK), wherein the entirety of the cornea is replaced, also remains as a third option. Despite advancements and success with these procedures, all three approaches result in significant perturbations in the optical quality of the cornea. These perturbations include induction of lower- (LOAs) and higher-order aberrations (HOAs), as well as light scatter. The relative contributions of these factors in degrading visual performance in post- keratoplasty eyes are poorly understood.
Penetrating keratoplasty eyes have a large amount of HOA, which cannot be corrected with spectacles. Theoretical calculations predict that these eyes would benefit greatly from correction of these defects.1 Work by Javadi and colleagues2 verified that DALK and PK eyes have similar HOAs and best-corrected visual acuities (BCVAs), that is, 100% ≥ 20/40 and 27% ≥ 20/20.3 However, work by Chen and colleagues4 demonstrated inferior clinical outcomes with DSAEK (90% ≥ 20/40 and 14% ≥ 20/20), despite a previous finding by Bahar et al.5 that DSAEK surgery induced less ocular HOA than PK or DALK. It remains unclear whether the differences in clinical outcomes between DSAEK and PK/DALK are attributed to varying amounts of HOA or some other optical factor (i.e., haze or scatter). Moreover, no prior studies measured contrast sensitivity, another important sensitive measure of visual performance.
Previous work has focused on characterizing the corneal aberration in the above keratoplasty populations6–9; however, visual performance is influenced by total ocular aberration. It is only with characterization and correction of total ocular aberration that an understanding of how it affects visual performance can be obtained. Shack–Hartmann wavefront sensing is an extremely accurate method for quantifying the total ocular aberration. Adaptive optics (AO) is a laboratory-based technology that noninvasively corrects a majority of the aberrations in the eye.10,11 Pairing these powerful tools with psychophysical experiments that test visual acuity (VA) and contrast sensitivity (CS) allows direct quantification of the benefit to be gained from HOA correction. This has been done in subjects with keratoconus, but never in post-keratoplasty populations, where stromal haze and scatter may also play a role.12,13
Aberration, scatter, and neural adaptation all affect visual performance. One of the current limitations in understanding the relative contribution of these factors to visual performance after keratoplasty is the inability to separate/correct them. An AO vision simulator enables the elimination of aberration and allows one to test the hypothesis that scatter and neural adaptation still limit the maximum visual benefit after keratoplasty. The purpose of this study was to quantify the relative contribution of HOAs to visual performance in subjects after these three different corneal transplantation surgeries. Residual decrements in visual performance can then be attributed to other factors (i.e., scatter and neural adaptation).
Methods
Study Participants
The protocol for this study received institutional review board approval from the University of Rochester. Subjects who had previously undergone PK, DALK, or DSAEK surgery greater than 1 year prior to initiation of the study and had clear grafts were contacted and recruited for participation. The study population included 5 PK, 6 DALK, and 5 DSAEK eyes from 14 different subjects. Informed written consent was obtained prior to enrollment. Exclusion criteria included any additional ocular condition known to affect the outcome of visual acuity or contrast sensitivity measurements.
Table 1 illustrates pertinent demographics, indications for surgery, graft size, and length of time since surgery. PK and DALK subjects had undergone selective suture removal for surgically induced astigmatism. At the time of the study, 2 PK subjects had 6 (Eye #13 2) and 16 (Eye #15 4) interrupted sutures in place at the time of enrollment; a third PK subject (Eye #16 5) had a single running suture. One DALK subject (Eye #1 6) had 8 sutures remaining; another subject (Eye #2 7) had 4 remaining. All DSAEK subjects had all sutures removed at the time of enrollment. Due to the differing indications for surgery, the DSAEK subjects were significantly older than DALK subjects (P < 0.01). The time between surgery and study participation was also significantly longer for PK subjects than that for DSAEK subjects (P < 0.05).
Table 1. .
Subject Demographics
|
Eye # |
Surgery |
Age (y) |
Eye |
Diagnosis |
Phakic / Pseudo |
Graft Diameter (mm) |
Time Out from Surgery (mo) |
| 01 | PK | 30 | OD | KCN | Phakic | 8.5 | 74 |
| 02 | PK | 48 | OD | KCN | Phakic | 9.5 | 50 |
| 03 | PK | 29 | OS | KCN | Phakic | 9.0 | 40 |
| 04 | PK | 43 | OD | KCN | Phakic | 9.0 | 20 |
| 05 | PK | 56 | OD | FED / HSV | Phakic | 8.25 | 71 |
| Mean ± SD | PK | 41 ± 12 | 8.85 ± 0.49 | 51 ± 22† | |||
| 06 | DALK | 23 | OD | Corneal scar | Pseudo | 8.25 | 15 |
| 07 | DALK | 30 | OS | KCN | Phakic | 8.5 | 14 |
| 08 | DALK | 29 | OD | KCN | Phakic | 9.0 | 11 |
| 09 | DALK | 48 | OS | KCN | Phakic | 9.0 | 38 |
| 10 | DALK | 29 | OD | KCN | Phakic | 9.0 | 26 |
| 11 | DALK | 40 | OD | Ectasia s/p LASIK | Phakic | 8.5 | 44 |
| Mean ± SD | DALK | 33 ± 9* | 8.71 ± 0.33 | 25 ± 14 | |||
| 12 | DSAEK | 60 | OS | FED | Pseudo | 8.25 | 13 |
| 13 | DSAEK | 66 | OS | FED | Pseudo | 8.25 | 9 |
| 14 | DSAEK | 67 | OS | FED | Pseudo | 8.25 | 19 |
| 15 | DSAEK | 60 | OD | FED | Pseudo | 8.25 | 30 |
| 16 | DSAEK | 69 | OD | FED | Pseudo | 8.25 | 13 |
| Mean ± SD | DSAEK | 64 ± 4 | 8.25 ± 0 | 17 ± 8† |
Kruskal–Wallis test, P = 0.0050; post-test comparison, P < 0.01.
Kruskal–Wallis test, P = 0.0252; post-test comparison, P < 0.05.
Pseudo, pseudophakia; KCN, keratoconus; FED, Fuchs' endothelial dystrophy; HSV, herpes simplex virus (scar).
Surgical Techniques
Surgeries were performed by one of two corneal surgeons (HH or SC) who used identical surgical techniques in a university hospital setting. PK was performed by using a trephine (sizes for host tissue: 8.0–9.5 mm); donor tissue was cut with the same size trephine as the host except for that in one subject, which was oversized by 0.25 mm (trephine sizes for donor tissue: 8.25–9.5 mm). The donor tissue was sutured into the recipient using 12 interrupted and one running 10-0 nylon suture in two subjects, 14 interrupted and one running suture in one subject, and 24 interrupted sutures in the last subject.
DALK was performed using a modified “big bubble” technique, similar to that previously described by Anwar.14 Host trephine sizes ranged between 8.25 and 9.0 mm. The donor cornea was cut with the same size trephine that was used to prepare the host tissue. The donor tissue was placed over the host DM and sutured into position using 16 interrupted 10-0 nylon sutures in five subjects and 20 interrupted sutures in one subject.
In DSAEK, the donor tissue was cut with an 8.25-mm trephine. In the host, DM was removed and the peripheral stroma was roughened using the Terry scraper (Bausch & Lomb, St. Louis, MO) using the technique advocated by Terry.15,16 The scleral tunnel incision was closed with three interrupted 10-0 nylon sutures. All DSAEK subjects had inferior peripheral iridotomies and draining keratotomies at 2, 4, 8, and 10 o'clock.
Large Stroke Adaptive Optics System
An AO system, described previously,13,17 was used to measure and correct the HOAs in all patients with keratoplasty. An annotated photograph of the system, shown in Figure 1, consisted of a large stroke deformable mirror (Mirao 52D; Imagine Eyes, Orsay, France) and a laboratory-based wavefront sensor system (Shack–Hartmann WaveSensor; Trioptics Optical Test Instruments, Wedel, Germany). The system also included a video projector (Model PG-M20X; Sharp Corp., Osaka, Japan) for visual acuity (VA) experiments and a cathode-ray tube (CRT) monitor (MultiSync FP950; NEC, Irving, TX) for displaying horizontal and vertical gratings used to test contrast sensitivity (CS). Both of these display devices produced images that were optically conjugate with the retina.
Figure 1. .
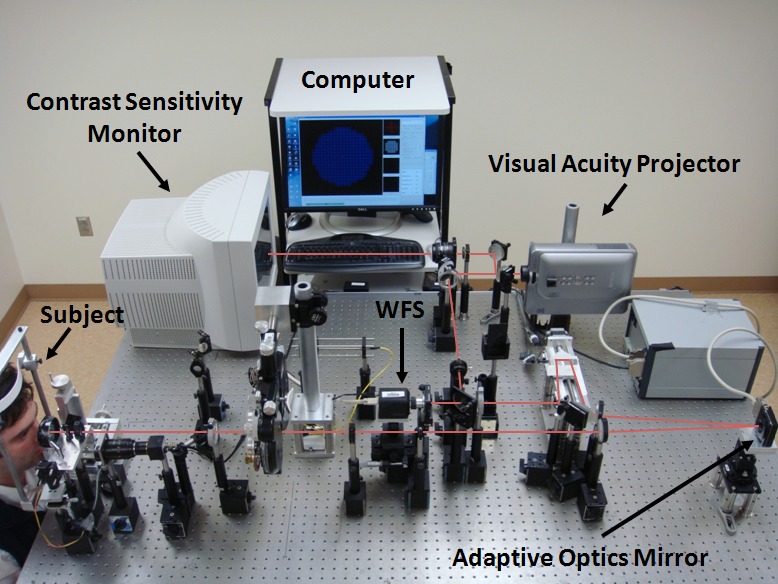
Schematic of the adaptive optics system used to conduct wavefront aberration, visual acuity, and contrast sensitivity measurements. The subject's head is stabilized with a bite-bar (left). The subject looks into the system at either a tumbling “E” target produced by the visual acuity projector (top right) or a grating of varying orientation projected by the contrast sensitivity monitor (top left). Software on the computer analyzes information coming from the Shack–Hartmann wavefront sensor (WFS) and automatically sends corrective adjustments to the AO mirror (bottom right).
Measurement of Wavefront Aberrations
All subjects were dilated and cyclopleged using 1.0% tropicamide. A customized bite bar was prepared for each subject to minimize head movements. Aberrations were measured using the AO system. Zernike polynomials up to 10th order were determined from each spot pattern over the largest pupil size possible. Zernike coefficient magnitudes were renormalized to a 4.5-mm pupil (MATLAB; The MathWorks, Natick, MA). The data from the five best frames were averaged to produce a single set of Zernike coefficients, reported in the Optical Society of America standard format,18 for each eye. Finally, to account for the enantiomorphism present when comparing right and left eyes, the sign of Zernike modes not symmetric about the vertical axis was reversed in all data sets acquired from left eyes (i.e., Z2−2, Z31, Z33, etc.).
Wavefront aberrations in a normal pre-LASIK myopic population (n = 378) were obtained from an existing database (provided by Bausch & Lomb, Rochester, NY). These aberrations were measured over a 6.0-mm pupil using a wavefront sensor (Bausch & Lomb Zywave IIz Shack–Hartmann). Zernike coefficients up to the fifth order were available. For comparison, these data was renormalized to a 4.5-mm pupil.
Assessment of Visual Acuity
VA was measured using a tumbling “E” test at 100% contrast in white light. This is a four-alternative forced choice test in which the subject responds to the orientation of the letter “E.” Letters were black on a white background and the entire field of view subtended 1° on the retina. The test used a psychometric function based on 30 trials (with each letter getting progressively smaller for correct answers and larger for incorrect answers). VA was based on the Snellen letter size for which at least 62.5% of the responses were correct. Details on the optical system and evaluation of AO correction performance were published previously.13
Each subject underwent training on the VA testing task before experimental measurements were collected. Training lasted approximately 1 hour and consisted of 5 to 10 trials of VA testing with AO correction of LOA only. Once the subject was sufficiently comfortable and the results were repeatable, VA was assessed under two conditions: LOA correction only and full-aberration correction (LOA and HOA correction of all Zernike modes up to the 10th order). Randomization of testing conditions (LOA correction versus full correction) ensured that the subjects were blinded to expected performance. In both cases, the maximum available pupil (range: 4.5–6 mm) was used for AO correction, but VA was tested through an artificial 4.5-mm aperture that was optically conjugate with the pupil. Residual aberration root mean square (RMS) was recorded to quantify the fidelity of the AO system's performance. Aberrations were corrected continuously in a closed-loop manner except when subjects blinked, at which time it was suspended. Subjects were asked to blink at will, and did so after every 1- to 2-letter presentations (or every 3–6 seconds). This helped ensure stable optical quality throughout testing of visual performance.
Assessment of Contrast Sensitivity
All subjects completed the CS experiments on a separate day, generally 1–4 weeks after VA measurements were made due to the fatigue that would be associated with acquiring all data in one session. CS was measured using a two-alternative forced choice method where subjects were asked to ascertain whether gratings of a specific spatial frequency and contrast were horizontal or vertical. A psychometric function was used (with progressively lower contrast gratings presented for correct answers and higher contrast gratings for wrong answers), and the contrast threshold was defined as the contrast for which 75% of the responses were correct. Gabor functions (sinusoidal luminance distribution overlaid with a Gaussian envelope routinely used in psychophysical experiments) subtended 2° on the retina, were displayed for 250 ms each with sudden onset and offset, and had spatial frequencies of 4, 8, and 12 cycles/deg. These conditions were chosen because correction of higher-order aberrations has the greatest benefit in high spatial frequency conditions. AO was used to correct aberrations over the largest possible pupil, but testing was done through an artificial 4.5-mm pupil, which was optically conjugate with the subject's pupil.
Training generally lasted 45 minutes. CS threshold was measured at least twice for each spatial frequency (4, 8, or 12 cycles/deg) for both LOA and full-aberration correction. If the first two trials disagreed by more than 0.2 log units, a third trial was completed using the same experimental conditions. Testing conditions (spatial frequency and lower-order versus full-aberration correction) were randomized.
Statistics
The average magnitude of Zernike coefficients for individual modes and higher-order root-mean-square aberration (HORMS) were used to compare wavefront aberrations in the three keratoplasty populations to each other and to the normal data set. Reduction in lower-order root-mean-square aberration (LORMS) and HORMS after full correction was also determined to assess the efficacy of AO correction in each of the three keratoplasty populations. Paired t-tests were used to detect statistically significant differences within a single population after LOA and full correction.
VA measurements were converted to logMAR (logarithm of minimal angle of resolution) scale. Each subject completed six trials for each testing condition; the best and worst trials were discarded, and the remaining four were averaged. VA with only LOA correction was compared with VA after full-aberration correction for each postop group.
As mentioned earlier, two or three contrast sensitivity threshold measurements were taken at each of 4, 8, and 12 cycles/deg, with both LOA and with full-aberration correction. Thresholds for each testing condition were averaged. When studying a single keratoplasty population (i.e., PK), a Friedman matched-pairs test was used to test for differences in CS performance at different spatial frequencies.
When comparing aberrations, VA or CS across the PK, DALK, and DSAEK groups, Kruskal–Wallis tests (nonparametric ANOVA) with Dunn's multiple comparison post-tests and correction for multiple comparisons were performed (i.e., Bonferroni α = 0.05/3 = 0.017). A Wilcoxon matched-pairs signed ranks test was used to assess for differences in VA or CS with LOA and full-aberration correction. Values of P < 0.05 were considered significant.
To quantify the improvement in contrast sensitivity after full HOA correction, we defined observed visual benefit in the following equation:
 |
In addition, we calculated the predicted visual benefit, defined as
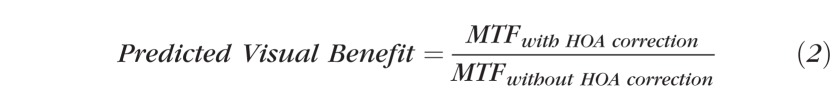 |
where MTF is the modulation transfer function, calculated from the residual aberrations with LOA or with full correction. MTF is an image quality metric calculated solely from the optical aberrations of the eye and, thus, does not account for the effect of other factors on visual performance. Therefore, any discrepancy between the predicted and observed visual benefit implicates factors other than optical aberrations such as scatter or neural adaptation.
Results
Wavefront Aberrations
The magnitudes of lower- and higher-order aberrations over a 4.5-mm pupil for PK, DALK, DSAEK, and normal myopic populations are illustrated in Figure 2. Statistics are shown in Table 2. PK (P < 0.05), DALK (P < 0.01), and normal (P < 0.01) subjects all had significantly more defocus than DSAEK subjects. PK and DALK subjects had more astigmatism and trefoil than did normal subjects. PK, DALK, DSAEK, and normal myopic subjects had HORMS of 0.93 ± 0.19 (mean ± SEM), 0.62 ± 0.27, 0.54 ± 0.15, and 0.16 ± 0.07 μm, respectively (Fig. 3a). All three keratoplasty groups had significantly more HORMS than did normals (P < 0.01), but there was no statistically significant difference between the three groups. The HOAs made up a greater percentage of the total aberration in all three keratoplasty groups when compared with normal myopes (PK = 10 ± 3% [P < 0.01], DALK 5 ± 1% [P > 0.05], DSAEK 29 ± 6% [P < 0.001], normal = 1 ± 1%). DSAEK HOA contribution to total aberration was significantly greater than that of DALK but not PK (Fig. 3b). The PK and DALK subjects' HOAs were dominated by trefoil (69 ± 6% [mean ± SEM] and 39 ± 6% of higher-order variance, respectively), which was a significantly larger percentage than that of normal myopic subjects (2 ± 1%; P < 0.001). Coma made up a significant share of the remaining HOA in DALK subjects (21 ± 10% of higher-order variance). Cumulatively, trefoil (40 ± 19%), coma (18 ± 5%), and spherical aberration (18 ± 9%) accounted for nearly 80% of the HOAs in DSAEK subjects.
Figure 2. .
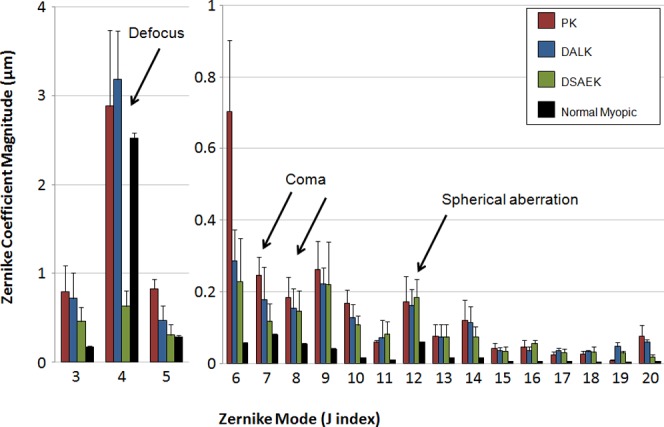
Lower-order and higher-order aberrations of post-keratoplasty and normal populations. Wavefront aberration amplitudes of three keratoplasty populations as compared with a normal myopic group. Error bars: SEM.
Table 2. .
Wavefront Aberration Results
|
Variable |
PK |
DALK |
DSAEK |
Normal |
KW, P Value |
Post-test Comparison |
| Magnitude of defocus (J4) | 2.88 ± 0.86a | 3.20 ± 0.61b | 0.63 ± 0.18abc | 2.53 ± 0.06c | 0.0023* | <0.05a |
| <0.01b | ||||||
| <0.01c | ||||||
| Magnitude of astigmatism (J3 + J5) | 1.62 ± 0.35d | 1.19 ± 0.38e | 0.76 ± 0.22 | 0.46 ± 0.02de | <0.0001* | <0.01d |
| <0.05e | ||||||
| Magnitude of trefoil (J6 + J9) | 0.97 ± 0.21f | 0.51 ± 0.12g | 0.45 ± 0.22 | 0.10 ± 0.00fg | <0.0001* | <0.001f |
| <0.001g | ||||||
| HOA RMS | 0.93 ± 0.19h | 0.62 ± 0.27i | 0.54 ± 0.15j | 0.16 ± 0.07hij | <0.0001* | <0.001h |
| <0.001i | ||||||
| <0.01j | ||||||
| Percentage HOA of total aberration | 10 ± 3l | 5 ± 1k | 29 ± 6km | 1 ± 1lm | ≤0.0101* | <0.01k |
| <0.01l | ||||||
| <0.001m | ||||||
| LOA RMS after AO correction | 0.04 ± 0.01n | 0.07 ± 0.02 | 0.03 ± 0.01n | 0.2861 | >0.05 | |
| HOA RMS after AO correction | 0.11 ± 0.03 | 0.20 ± 0.06 | 0.09 ± 0.01 | 0.2531 | >0.05 |
All data are presented as average ± SEM. KW, Kruskal–Wallis test (nonparametric ANOVA); RMS, root mean square. Superscript letters indicate which keratoplasty groups are being compared with the Dunn's post-test and the corresponding P value for that test.
* indicates statistically significant, P < 0.017 for Kruskal-Wallis tests, P < 0.05 for Dunn's multiple comparison post-tests (P-value accounts for multiple comparisons).
Figure 3. .
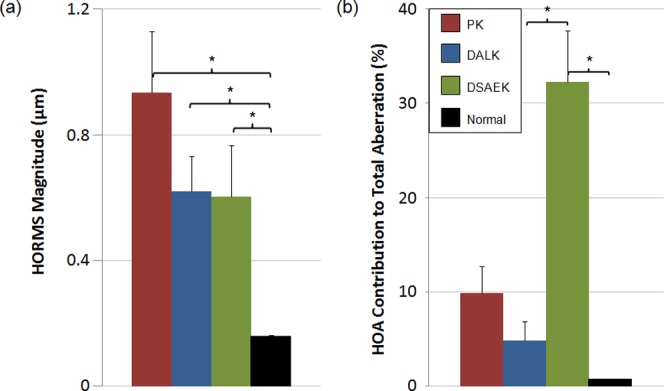
(a) Higher-order aberration RMS and (b) contribution to total aberration of post-keratoplasty and normal populations. All keratoplasty groups had greater HOA RMS than that of normal subjects. DSAEK subjects' HOA contributed a larger percentage to total aberration than other populations; this was likely due to the pseudophakic status of all DSAEK subjects, which resulted in relatively low defocus. Error bars: SEM.
Performance of Adaptive Optics System
When VA or CS was being tested with correction of defocus and astigmatism only (LOA correction), some residual amount of LOAs remained uncorrected in all subjects. The residual uncorrected LORMS in all groups were as follows: PK 0.50 ± 0.11 μm, DALK 0.31 ± 0.06 μm, and DSAEK 0.20 ± 0.04 μm. Residual LORMS in the PK and DALK populations were higher than those in DSAEK, but this difference was not statistically significant (P > 0.05). HOAs were corrected effectively by the AO system. For PK, DALK, and DSAEK, the residual HORMS was similar across groups: 0.11 ± 0.03, 0.20 ± 0.06, and 0.09 ± 0.01 μm, respectively. This eliminated the possibility that differences in the residual LOA or HOA present during VA or CS testing could confound our results.
Visual Acuity
The results of VA testing with LOA and full-aberration correction in the three keratoplasty groups are presented in Table 3 and illustrated in Figure 4a. LogMAR visual acuities with LOA correction were 0.03 ± 0.05 (20/21 Snellen equivalent) in PK, 0.08 ± 0.07 (20/24) in DALK, and 0.25 ± 0.05 (20/36) in DSAEK. Visual acuities improved with full-aberration correction to −0.10 ± 0.06 (20/16) in PK, −0.08 ± 0.07 (20/17) in DALK, and 0.01 ± 0.03 (20/20) in DSAEK. All three keratoplasty groups performed significantly better with full-aberration correction compared with LOA correction (P < 0.0013). With LOA correction, DSAEK subjects had poorer VA compared with that of PK and DALK subjects, although this difference was not significant (Kruskal–Wallis, P = 0.0870). With full-aberration correction, the exact same trend persisted (Kruskal–Wallis, P = 0.1734).
Table 3. .
Visual Acuity Results
|
Keratoplasty Group |
LogMAR VA with LOA Correction (Snellen Equivalent) |
LogMAR VA with Full-Aberration Correction (Snellen Equivalent) |
Paired
t-Test, P Value |
| PK | 0.03 ± 0.05 (20/21) | −0.10 ± 0.06 (20/16) | 0.0013* |
| DALK | 0.08 ± 0.07 (20/24) | −0.08 ± 0.07 (20/17) | 0.0010* |
| DSAEK | 0.25 ± 0.05 (20/36) | 0.01 ± 0.03 (20/20) | 0.0004* |
| KW P value | 0.0870 | 0.1734 |
All data are presented as average ± SEM.
indicate statistically significant, P < 0.05 for paired t-test.
Figure 4. .
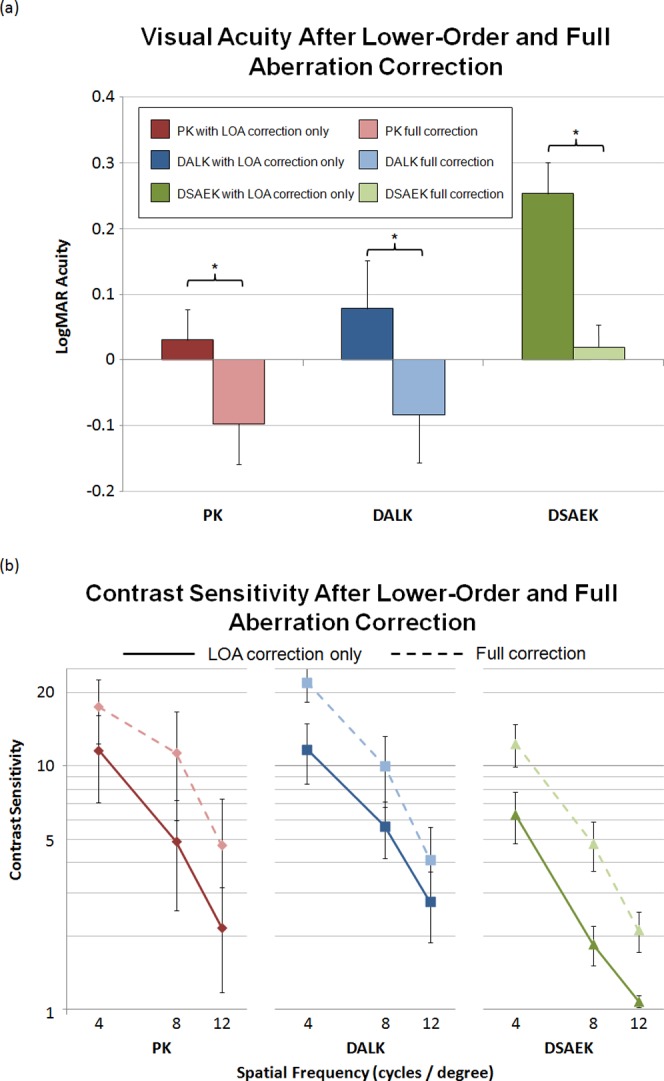
(a) Visual acuity and (b) contrast sensitivity at 4, 8, and 12 cycles/deg in PK, DALK, and DSAEK subjects after lower-order aberration correction and again after full-aberration correction. Full-aberration correction, lower-order and higher-order aberration correction up to the 10th Zernike polynomial order. Error bars: SEM.
Contrast Sensitivity
Results of CS testing with LOA and full-aberration correction in the three keratoplasty groups are presented in Table 4 and illustrated in Figure 4b. When only LOAs were corrected, PK, DALK, and DSAEK CS performance decreased as the spatial frequency of the test stimulus increased. The difference in performance at 4 and 12 cycles/deg was statistically significant for all three groups (Friedman, P < 0.0183; post-test comparison, P < 0.05). With full-aberration correction, all three groups had better CS at 4 than that at 12 cycles/deg (Friedman, P < 0.0008; post-test comparison, P < 0.01). All three keratoplasty groups also performed significantly better after full-aberration correction than with LOA correction at 4 and 8 cycles/deg, but not 12 cycles/deg. There was no statistically significant difference in CS performance between keratoplasty groups for any of the test conditions.
Table 4. .
Contrast Sensitivity Results
|
LOA Correction |
Full-Aberration Correction |
||||||||||
|
Keratoplasty Group |
4 cyc/deg |
8 cyc/deg |
12 cyc/deg |
Friedman,
P
Value |
Post-test Comparison |
4 cyc/deg |
8 cyc/deg |
12 cyc/deg |
Friedman,
P
Value |
Post-test Comparison |
Wilcoxon Signed-Ranks,
P
Value |
| PK | 11.61 ± 4.56a | 4.89 ± 2.34b | 2.17 ± 1.00c | 0.0083* | 4 vs. 12 | 17.48 ± 5.13a | 11.30 ± 5.34b | 4.72 ± 2.60c | 0.0008* | 4 vs. 12 | 0.0313*,a,b |
| *P < 0.01 | *P < 0.01 | 0.1250c | |||||||||
| DALK | 11.68 ± 3.24d | 5.65 ± 1.48e | 2.77 ± 0.89f | 0.0031* | 4 vs. 12 | 22.04 ± 3.69d | 10.00 ± 3.22e | 4.12 ± 1.47f | 0.0001* | 4 vs. 12 | 0.0156*,d,e |
| *P < 0.01 | *P < 0.01 | 0.0625f | |||||||||
| DSAEK | 6.30 ± 1.49g | 1.85 ± 0.34h | 1.08 ± 0.06i | 0.0183* | 4 vs. 12 | 12.37 ± 2.46g | 4.79 ± 1.09h | 2.12 ± 0.40i | 0.0008* | 4 vs. 12 | 0.0313*,g,h |
| *P < 0.05 | *P < 0.01 | 0.0625i | |||||||||
| KW P value | 0.4194 | 0.1287 | 0.0918 | 0.2507 | 0.3294 | 0.5584 | |||||
Superscript letters indicate which spatial frequencies are being compared with the Wilcoxin signed-ranks test and the corresponding P value for that test.
indicates statistically significant, P < 0.017 for Friedman matched-pairs test, P < 0.05 for Dunn's post-test comparison (P-value accounts for multiple comparisons), P < 0.05 for Wilcoxon matched-pairs signed ranks test.
The results of MTF calculations are illustrated in Figure 5. Calculations reflect the predicted visual benefit of correcting the ocular aberrations according to equation 2. Predicted benefit values are shown at 4, 8, and 12 cycles/deg. The MTFs predicted that, based on full correction of LOA and HOA, PK subjects stood to benefit the most and DALK subjects the least, with DSAEK subjects being intermediate between the two. However, none of these differences was statistically significant because of the large amount of heterogeneity within and between the populations.
Figure 5. .
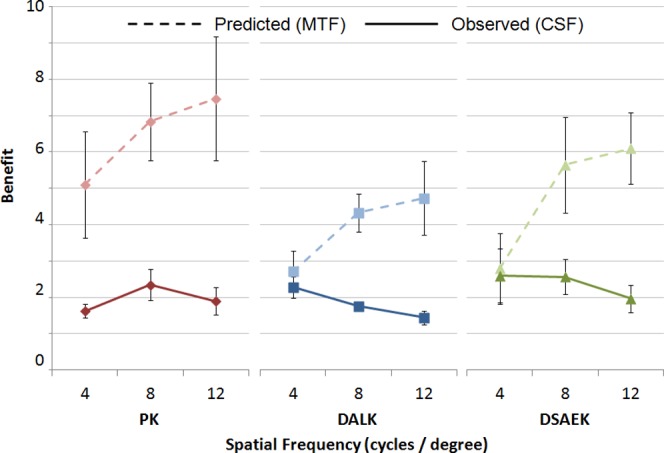
Predicted and observed benefit of higher-order aberration correction on contrast sensitivity. All postop groups experienced benefits significantly greater than 1; however, no one group benefited more than any other group, even though predicted benefits were greatest in the PK group and lowest in the DALK group. Predicted benefit of higher-order aberration correction on contrast sensitivity was calculated based on the modulation transfer function (MTF), an image quality metric based solely on optical parameters. Error bars: SEM.
Actual benefit of full-aberration correction is also illustrated in Figure 5. Two PK, one DALK, and one DSAEK subject could not distinguish the orientation of a Gabor function with 12 cycles/deg, even at 100% contrast, regardless of whether just lower-order or all lower- and higher-order aberrations were corrected. There were no significant differences in the actual benefit different keratoplasty populations experienced from full correction.
Discussion
In this study, we characterized the total ocular wavefront aberrations in three keratoplasty groups (PK, DALK, and DSAEK) and allowed for direct comparison between groups by standardizing pupil size. The results presented here are unique in that almost all of the literature to date that characterizes aberrations in these groups does so by looking at corneal aberrations only.7–9 Although the corneal aberration contributes a greater percentage to the total aberration in eyes with corneal pathology, disregarding the effect of the lens and optical media leads to incomplete assessment of the true aberration magnitudes affecting visual performance. Furthermore, we have used adaptive optics technology to isolate the impact of optical aberrations and to improve our understanding of how other factors limit visual performance post-corneal transplant.
With respect to LOAs, we have shown that PK, DALK, and normal myopic populations all have a greater magnitude of defocus than that of subjects that underwent DSAEK. This can be attributed to the fact that all DSAEK subjects were pseudophakic and the intraocular lens was selected to compensate for their native defocus. PK and DALK eyes also had significantly more astigmatism than did normal myopic eyes, whereas DSAEK eyes did not. This is not the first study to show this1; the large astigmatism in PK and DALK is likely related to the variability associated with suture placement (length, direction, tension, position) and variability in wound healing along the graft–host interface.
The variability in pupil size over which measurements are made across the literature makes direct comparisons with other studies difficult. Nevertheless, our finding that PK subjects had 0.93 ± 0.19 μm (mean ± SE) of total HORMS over a 4.5-mm pupil is consistent with McLaren's observed 1.24 ± 0.4 μm (mean ± SD) of corneal HORMS over a 5.0-mm pupil.7 Similarly, our DSAEK subjects' total HORMS of 0.60 ± 0.16 μm is consistent with Moftuoglu's observed 0.599 ± 0.288 μm of corneal aberration over a 4.0-mm pupil.8 To our knowledge, Ardjomand and colleagues19 constitute the only group to have measured DALK subjects' total ocular HO aberration; they found it to be 0.35 μm on average over a 5.0-mm pupil. This differs from our finding of 0.62 ± 0.11 μm. However, we believe this difference stems from the fact that Ardjomand's group was unable to obtain HOA measurements in approximately half of their subjects and that measurement in the more difficult subjects would have led to a greater amount of HOA. Since characterizing the wavefront aberrations of these three groups was not the primary aim of this report, a more detailed discussion of these results is beyond the scope of this study.
When only LOAs were corrected, PK subjects had better VA than that of DALK, who in turn had better VA than that of DSAEK patients. If the difference in VA were only due to HOAs, VA would have been the worst for PK and better for DALK and DSAEK. The difference between PK and DSAEK VA under these conditions measured just short of statistical significance (Kruskal–Wallis, P = 0.087). We attribute the inability to achieve significance to a single PK outlier (Eye #1) that had between 1.8- and 3.5-fold greater residual LOA (after LOA correction) than any other PK or DSAEK subject. Correspondingly, his VA performance was the poorest of all PK subjects. Had the AO system's correction performance in this PK subject been equal to that of other subjects, the VA difference would have been significant. When AO was used to correct all lower- and higher-order aberrations, the DSAEK group still trended toward poorer VA than that of the other groups.
Multiple factors can limit visual performance. The most obvious is imperfect optics (aberrations), but others include scatter from media opacity, lack of neural adaptation (neurosensory optimization of visual performance under the influence of the subjects' optical aberrations), and retinal disease. In this study, subjects with underlying retinal disease were excluded. Correcting ocular aberrations (both lower- and higher-order) led to dramatic improvements in VA and CS; however, residual contributions from scatter and neural adaptation are difficult to separate and quantify. They may be indirectly assessed by assuming that any residual decrement in visual performance after aberration correction is due to these other factors. Previous studies in normal subjects suggest that the retinal sampling limit to visual acuity is −0.22 logMAR (20/12 Snellen equivalent).20 Unpublished data from our own laboratory on normal subjects suggest that they can actually achieve VA of −0.36 logMAR (20/8 Snellen equivalent) with the same experimental setup and a 6.0-mm pupil. Since none of our keratoplasty groups achieved this visual acuity, we can conclude that all postoperative subjects had contributions from either residual aberration, scatter, or lack of neural adaptation.
That PK subjects performed better than DSAEK subjects suggests that factors other than aberrations play a role in limiting visual performance in DSAEK subjects. PK subjects were tested significantly farther out from surgery than their DSAEK counterparts (51 ± 22.4 vs. 17 ± 8.2 months postop). Therefore, PK subjects may have had more time for neural adaptation to their postoperative aberrations, giving them the ability to see better despite similar residual aberration. This phenomenon has been well described by Sabesan and Yoon,21 wherein keratoconic eyes had better visual performance looking through their native aberrations (present for an extended period of time) than normal eyes that had the exact same aberrations artificially imposed by the adaptive optics system (with no time for neural adaptation).
We hypothesize that the decreased VA in the DSAEK population compared with that in PK and DALK populations, with and without HOA correction, is also partially related to corneal haze. Since all DSAEK subjects were pseudophakic and other keratoplasty subjects except for one were phakic, one can infer that light scatter in the DSAEK group is primarily corneal, whereas lenticular scatter may have played a small role in the other keratoplasty groups. The haze in post-DSAEK subjects may be subepithelial and related to anterior stromal fibrosis in the setting of chronic corneal edema,22 or may be due to donor–host stroma/stroma interactions at the interface. Both may cause light scatter. The present study is not the first to suggest this. A case series published recently by Uchino et al.22 confirmed that there is a significantly greater amount of haze in DSAEK grafts than that in PK at 3 months postoperatively. Confocal microscopy findings in a case series of DSAEK subjects also found that both subepithelial and interface haze decreased from 1 to 6 months postoperatively, and those with the most persistent subepithelial and interface haze at 6 months also had the lowest visual acuity.23 Two studies by Patel et al.24,25 also inversely correlated visual acuity in DSEK (Descemet's stripping endothelial keratoplasty) and DLEK (deep lamellar endothelial keratoplasty) subjects with intraocular forward light scatter. These studies complement the present study in that they all suggest a relationship between stromal haze or scatter in endothelial keratoplasty and poorer visual acuity; however, they do so without addressing the effect of aberrations. The present study suggests that the same trends as those previously observed persist even in the setting of AO correction of the lower- and higher-order aberrations.
This study is the first to show that correcting HOAs improves visual performance in post-keratoplasty populations. All three groups experienced significant gains above and beyond what is possible with LOA correction. Studies by Sabesan and colleagues12,13 showed that subjects with keratoconus experienced significant improvements in visual acuity when fitted with a customized soft contact lens that corrected for their higher-order aberrations. The present study suggests that post-transplant eyes may also benefit from a similar technology.
All three transplant groups had better contrast sensitivity at lower spatial frequencies (4 cycles/deg) than that at higher ones (12 cycles/deg). Contrast sensitivity also significantly improved with correction of the higher-order aberrations at every spatial frequency tested. Our findings suggest that higher-order aberrations also lead to decrements in contrast sensitivity and that correcting aberrations results in improvement. This is important because contrast sensitivity does not necessarily correlate with visual acuity. For instance, subjects with nuclear cataract will initially experience decreased contrast sensitivity, and subsequently develop decreased visual acuity.
This study has several limitations. First, our study groups are small, which limits the power of the study; nonetheless, many significant differences were identified, making it very likely that the differences were real. Psychophysical studies are time intensive. Subjects had to concentrate for several hours to acquire data and had to return for multiple visits. This, along with our need to control for all other potentially vision-limiting conditions, limited recruitment to this study. Second, the study was not controlled for postoperative time point. Since the PK subjects were significantly farther out from surgery than DSAEKs, one cannot exclude the possibility that PK subjects' neural adaptation to their post-keratoplasty aberrations improved with time. This could have contributed to the superior visual acuities in PK subjects over DALK and DSAEK. Although not a limitation to the study, testing contrast sensitivity at higher frequencies might have allowed for more direct comparison to previous studies.
In summary, we have characterized the total ocular wavefront aberrations after PK, DALK, and DSAEK and have shown that correction of these aberrations results in significant improvements in both high-contrast visual acuity and contrast sensitivity. Our data suggest that other factors (i.e., scatter or neural adaptation) limit visual acuity in DSAEK subjects when compared with PK and DALK. Future work measuring forward light scatter in these postoperative groups might further clarify the role of corneal haze in visual performance. Increasing the number of subjects and measuring these image quality metrics at different time points will further solidify our understanding of the contribution of scatter to visual performance in these populations.
Footnotes
Supported in part by National Eye Institute/National Institutes of Health Grants 1K23EY019353, 3K23EY019353-01S1, and 5R01EY014999; a New York State Foundation for Science, Technology and Innovation/Center for Emerging and Innovative Sciences grant; and Research to Prevent Blindness, New York, New York.
Disclosure: S.M. Pantanelli, None; R. Sabesan, None; S.S.T. Ching, None; G. Yoon, None; H.B. Hindman, None
References
- 1.Pantanelli S, MacRae S, Jeong TM, Yoon G. Characterizing the wave aberration in eyes with keratoconus or penetrating keratoplasty using a high-dynamic range wavefront sensor. Ophthalmology. 2007;114:2013–2021 [DOI] [PubMed] [Google Scholar]
- 2.Javadi MA, Feizi S, Yazdani S, Mirbabaee F. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for keratoconus: a clinical trial. Cornea. 2010;29:365–371 [DOI] [PubMed] [Google Scholar]
- 3.Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143:117–124 [DOI] [PubMed] [Google Scholar]
- 4.Chen ES, Terry MA, Shamie N, Hoar KL, Phillips PM, Friend DJ. Endothelial keratoplasty: vision, endothelial survival, and complications in a comparative case series of fellows vs attending surgeons. Am J Ophthalmol. 2009;148:26–31 [DOI] [PubMed] [Google Scholar]
- 5.Bahar I, Kaiserman I, Levinger E, Sansanayudh W, Slomovic AR, Rootman DS. Retrospective contralateral study comparing descemet stripping automated endothelial keratoplasty with penetrating keratoplasty. Cornea. 2009;28:485–488 [DOI] [PubMed] [Google Scholar]
- 6.Koh S, Maeda N, Nakagawa T, et al. Characteristic higher-order aberrations of the anterior and posterior corneal surfaces in 3 corneal transplantation techniques. Am J Ophthalmol. 2012;153:284–290 [DOI] [PubMed] [Google Scholar]
- 7.McLaren JW, Patel SV, Bourne WM, Baratz KH. Corneal wavefront errors 24 months after deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2009;147:959–965 [DOI] [PubMed] [Google Scholar]
- 8.Muftuoglu O, Prasher P, Bowman RW, McCulley JP, Mootha VV. Corneal higher-order aberrations after Descemet's stripping automated endothelial keratoplasty. Ophthalmology. 2010;117:878–884 [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Negishi K, Yamaguchi K, et al. Comparison of anterior and posterior corneal surface irregularity in Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Cornea. 2010;29:1086–1090 [DOI] [PubMed] [Google Scholar]
- 10.Hofer H, Chen L, Yoon GY, Singer B, Yamauchi Y, Williams DR. Improvement in retinal image quality with dynamic correction of the eye's aberrations. Opt Express. 2001;8:631–643 [DOI] [PubMed] [Google Scholar]
- 11.Williams D, Yoon GY, Porter J, Guirao A, Hofer H, Cox I. Visual benefit of correcting higher order aberrations of the eye. J Refract Surg. 2000;16:S554–S559 [DOI] [PubMed] [Google Scholar]
- 12.Sabesan R, Jeong TM, Carvalho L, Cox IG, Williams DR, Yoon G. Vision improvement by correcting higher-order aberrations with customized soft contact lenses in keratoconic eyes. Opt Lett. 2007;32:1000–1002 [DOI] [PubMed] [Google Scholar]
- 13.Sabesan R, Yoon G. Visual performance after correcting higher order aberrations in keratoconic eyes. J Vis. 2009;9:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403 [DOI] [PubMed] [Google Scholar]
- 15.Terry MA, Hoar KL, Wall J, Ousley P. Histology of dislocations in endothelial keratoplasty (DSEK and DLEK): a laboratory-based, surgical solution to dislocation in 100 consecutive DSEK cases. Cornea. 2006;25:926–932 [DOI] [PubMed] [Google Scholar]
- 16.Terry MA, Shamie N, Chen ES, Hoar KL, Friend DF. Endothelial keratoplasty; a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology. 2008;115:1179–1186 [DOI] [PubMed] [Google Scholar]
- 17.Sabesan R, Ahmad K, Yoon G. Correcting highly aberrated eyes using large-stroke adaptive optics. J Refract Surg. 2007;23:947–952 [DOI] [PubMed] [Google Scholar]
- 18.Thibos LN, Applegate RA, Schwiegerling JT, Webb R. Standards for reporting the optical aberrations of eyes. J Refract Surg. 2002;18:S652–S660 [DOI] [PubMed] [Google Scholar]
- 19.Ardjomand N, Hau S, McAlister JC, et al. Quality of vision and graft thickness in deep anterior lamellar and penetrating corneal allografts. Am J Ophthalmol. 2007;143:228–235 [DOI] [PubMed] [Google Scholar]
- 20.Li S, Xiong Y, Li J, et al. Effects of monochromatic aberration on visual acuity using adaptive optics. Optom Vis Sci. 2009;86:868–874 [DOI] [PubMed] [Google Scholar]
- 21.Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol Vis Sci. 2010;51:3835–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchino Y, Shimmura S, Yamaguchi T, et al. Comparison of corneal thickness and haze in DSAEK and penetrating keratoplasty. Cornea. 2011;30:287–290 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi A, Mawatari Y, Yokogawa H, Sugiyama K. In vivo laser confocal microscopy after descemet stripping with automated endothelial keratoplasty. Am J Ophthalmol. 2008;145:977–985 [DOI] [PubMed] [Google Scholar]
- 24.Patel SV, Baratz KH, Hodge DO, Maguire LJ, McLaren JW. The effect of corneal light scatter on vision after descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–160 [DOI] [PubMed] [Google Scholar]
- 25.Patel SV, McLaren JW, Hodge DO, Baratz KH. Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2008;145:97–105 [DOI] [PubMed] [Google Scholar]


