Abstract
The iron storage protein, ferritin, provides an important endogenous MRI contrast that can be used to determine the level of tissue iron. In recent years the impact of modulating ferritin expression on MRI contrast and relaxation rates was evaluated by several groups, using genetically modified cells, viral gene transfer and transgenic animals. This paper report the follow-up of transgenic mice that chronically over-expressed the heavy chain of ferritin (h-ferritin) in liver hepatocytes (liver-hfer mice) over a period of 2 years, with the aim of investigating the long-term effects of elevated level of h-ferritin on MR signal and on the well-being of the mice. Analysis revealed that aging liver-hfer mice, exposed to chronic elevated expression of h-ferritin, have increased R2 values compared to WT. As expected for ferritin, R2 difference was strongly enhanced at high magnetic field. Histological analysis of these mice did not reveal liver changes with prolonged over expression of ferritin, and no differences could be detected in other organs. Furthermore, dietary iron supplementation significantly affected MRI contrast, without affecting animal wellbeing, for both wildtype and ferritin over expressing transgenic mice. These results suggest the safety of ferritin over-expression, and support the use of h-ferritin as a reporter gene for MRI.
Keywords: ferritin, reporter gene, iron, aging
Introduction
Iron is an essential nutrient for the functionality and viability of cells. Due to its ability to mediate one-electron exchange reactions, iron participates in many metabolic pathways and is required for the proper function of numerous essential proteins such as the heme-containing proteins, electron transport chain and microsomal electron transport protein (1-3). However, this vital ability of iron may also be detrimental for living cells, as free radicals, which are potentially harmful to cells, may be generated through the Fenton reaction (that is, Fe-catalyzed hydroxyl radical production). Thus, maintenance of labile free iron homeostasis is highly important to the survival of animals, plants and microorganisms (1). Excess of free cellular iron activates the production of ferritin, which is a ubiquitous, highly conserved protein, that is responsible for controlled iron storage and release (2). Ferritin can store in its central cavity up to 4500 iron atoms as mineral ferrihydrite (Fe5O3(OH)9).
The MR properties of ferritin were the focus of extensive research and showed anomality with high relaxivity at very low iron loading (4,5) and a peculiar linear rather than the expected quadratic dependence on the magnetic field (6). In recent years the possibility for use of ferritin as MR reporter gene was reported by a number of research groups (7-11). Based on the endogenous mechanisms for maintenance of labile iron homeostasis, along with the relatively high R2 relaxivity of ferritin at low iron loading, we previously raised the hypothesis that overexpression of ferritin could augment R2 relaxivity by redistribution of iron among more ferritin complexes as well as by increased total cellular iron level induction of expression of transferrin receptor (TfR) (4,7,8). The ability of the heavy chain of ferritin (h-ferritin), which posses the feroxidase activity, to act as MR reporter was first demonstrated in C6 glioma cells that were transfected with a tetracycline-inducible construct that carried h-ferritin. These cells were tested in vitro and showed a significant increase both in R1 and R2. Inoculation of these cells in nude mice yielded tumors that showed significantly elevated R2 (7). The use of ferritin as MR reporter was demonstrated also by infection of mice brain using adenovirus that encoded for both the heavy and light chains of human ferritin (10). The use of h-ferritin was further demonstrated with co-expression of transferrin receptor in neuronal stem cells that showed signal loss in T2 and T2* weighted MR images in an iron enriched environment (9). Recent studies demonstrated the use of ferritin as MR reporter gene for labeling macrophages (12), monitoring of survival of mouse embryonic stem cells (11) for reporting of the activity of cyclic-AMP dependent protein kinase A by enzyme dependent aggregation (13), and for monitoring of gene transfer and expression in a tumor model (14,15).
The generation of TET-h-ferritin transgenic mice that over-express HA-tagged h-ferritin and enhanced green fluorescent protein (EGFP) in a tissue specific and tetracycline inducible manner opened the possibility for MR application of ferritin as a reporter gene in multiple organs and applications (8). In these mice tissue specific expression of ferritin is achieved by crossing with driver transgenic mice with expression of the tetracycline transactivator (tTA) regulated by the promoter of interest. Addition of tetracycline to the drinking water of double transgenic offspring mice suppresses expression of ferritin, and expression can be induced by tetracycline withdrawal. Endothelial selective expression was achieved using driver mice in which tTA expression is driven by the promoter of vascular endothelial (VE) cadherin (endothelial-hfer mice) (16,17). In these endothelial-hfer mice expression of ferritin resulted in elevation of R2, allowing detection of blood vessel induced expression in the brain of mature mice, as well as fetal vascular development detected non invasively in-utero.
In contrast to the expression of ferritin by endothelial cells which elevated R2 as expected, liver hepatocyte expression of h-ferritin, using mice in which tTA was induced by the liver associated protein (LAP) promoter resulted in a more complicated effect on the MRI contrast. Surprisingly, young liver-hfer mice showed reduced R2 upon acute expression of h-ferritin in liver hepatocytes. This effect was attributed to mild hepatic cytotoxicity leading to water vacuoles with low R2 (8). Indeed previous studies showed varied response to elevated expression of h-ferritin ranging from protection of cells from reactive oxygen species (18,19), to regulation of free iron (20) and increased susceptibility to neurodegeneration in aging mice (21). The critical role of ferritin is further underscored by the in utero mortality of h-ferritin null mice, attributed to cardiac defects (22) and increased oxidative stress in brains of h-ferritin deficient mice (23). The phenotype observed for young liver-hfer mice is reminiscent of that previously reported for mice in which h-ferritin was elevated in response to deficiency in the iron regulatory protein-2 (24). Deposition and accumulation of iron is a well-known phenomenon of aging and was implicated in multiple pathologies such as Hemochromatosis (25), Alzheimer’s disease (AD), and Parkinson’s disease (26).
Since inertness is one of the most important characteristics that a reporter gene should possess, and in view of the fact that endothelial-hfer mice did not exhibit any pathology in utero and in adult, we pursued here the analysis of the impact of chronic elevated h-ferritin expression during aging of liver-hfer mice. The liver has a central role in iron homeostasis and ferritin storage. Given that iron overload is characterized by increased levels of ferritin, haemosiderin, and iron catalyzed lipid peroxidation we examined here the change in R2 upon long-term overexpression of h-ferritin in liver hepatocytes in aging mice as well as the impact of prolonged over expression on increasing the capacity for iron storage during high iron diet.
Materials and Methods
Mice characterization
Animal experiments were approved by the Weizmann Institutional Animal Care and Use Committee. Transgenic LAP:tTA × Tet:EGFP-HAferritin double transgenic mice: Homozygous Tet:EGFP-HAferritin mice (8) were mated with heterozygous LAP:tTA (liver associated protein) (27), double transgenic offspring (liver-hfer) over-express h-ferritin in liver hepatocytes. Wild type mice with matched genetic background (CB6F1; WT) were used as control. Ferritin expression was chronically induced in the double transgenic mice (no tetracycline).
Iron supplementation
MRI was applied for follow up of 4 groups of mice: WT mice and liver-hfer mice (with inducible transgene expression in hepatocytes) with high iron diet (2% carbonyl iron) and with normal diet (10 mice per group). 24 hours prior to each MRI scan the diet of all mice was replaced to regular diet in order to reduce artifacts associated with high iron content in the intestine.
Experimental timeline
The mice were treated with high iron diet from the age of 2 months for a period of 22 months. MRI scans at 4.7T, were performed when the mice were 5.5, 6.5, 8, 9, 20 and 24 months old. MRI scan at 9.4T was performed when the mice were 24 months old. Representative 20 months old mice were sacrificed and organs were taken for histological analysis. The study was completed when mice were 24 months old and liver sections were taken for assessment by electron microscopy. The same time points were used for mice that were treated with normal diet.
MRI studies
Mice anesthesia
i.p. injection of ketamine-xylazine mix (75 mg/kg Ketamine, Fort Dodge Animal Health, Fort Dodge, lowa and 3 mg/kg Xylazine, VMD, Arendonk, Belgium).
MRI was acquired at horizontal 4.7T and 9.4T Bruker spectrometers using a birdcage coil. R2 relaxation was measured using multi echo spin echo sequence with 8 echo times (TR= 2000 ms, TE= 11-88 ms, 2 averages, FOV 6X6 cm, 8 slices, in plane resolution 468 μm, slice thickness 1 mm, matrix 128 × 128, spectral width (SW) 50,000Hz).
R2 values were derived by single exponential fit of the signal intensity decay with echo time (I= I0 e-TE*R2; MATLAB software, MathWorks, Inc. Hill Drive Natick, MA). Analysis included regions of interest (ROI) as well as pixel-by-pixel R2 mapping.
Histopathological analysis
Following MRI analysis, multiple organs (brain, liver, heart, spleen and kidney) were retrieved for histological evaluation (stained at H&E and Prussian blue stains). Liver sections were also analyzed by electron microscopy.
Electron microscopy
Liver sections were examined by scanning electron microscope (SEM) and transmission electron microscope (TEM). For TEM studies the liver was sectioned with a razor blade and mounted in aluminum platelets with a depth of 100 μm, filled with 1-hexadecene (Sigma). The samples were then cryo-immobilized in an HPM 10 high pressure-freezing device (Bal-Tec, Liechtenstein). Freeze substitution was performed using a AFS2 freeze substitution device (Leica Microsystems, Vienna, Austria) in anhydrous acetone containing 2% glutaraldehyde for 3 days at −90°C and then warmed up to −30°C over 24 hours. The samples were washed with anhydrous acetone, infiltrated in a series of increasing concentration of Epon (Agar Scientific, UK) and polymerized at 60°C. Sections (60-80 nm in thickness) were obtained using an Ultracut UCT microtome (Leica Microsystems, Vienna, Austria). Unstained sections were examined in a Tecnai T12 electron microscope (FEI, Eindhoven, the Netherlands) operating at 120 kV. Images were recorded with an eagle 2k × 2k CCD camera (FEI).
For SEM studies livers were placed overnight in 4% PFA, embedded in paraffin and sectioned to 10 μm sections that were placed on a carbon stab after paraffin removal using xylene. Images were taken with FEG ESEM, XL 30 form EFI, the regions that were rich in high atomic number elements were identified with a back scattered electrons detector (BSD) and were selected for element mapping. The X-ray maps were acquired with an energy dispersive X-ray detector (EDS) EDAX with ultra thin window. For Fe mapping the characteristic Kalpha line at 7.056 kV was used.
Results
High iron diet significantly elevates liver R2
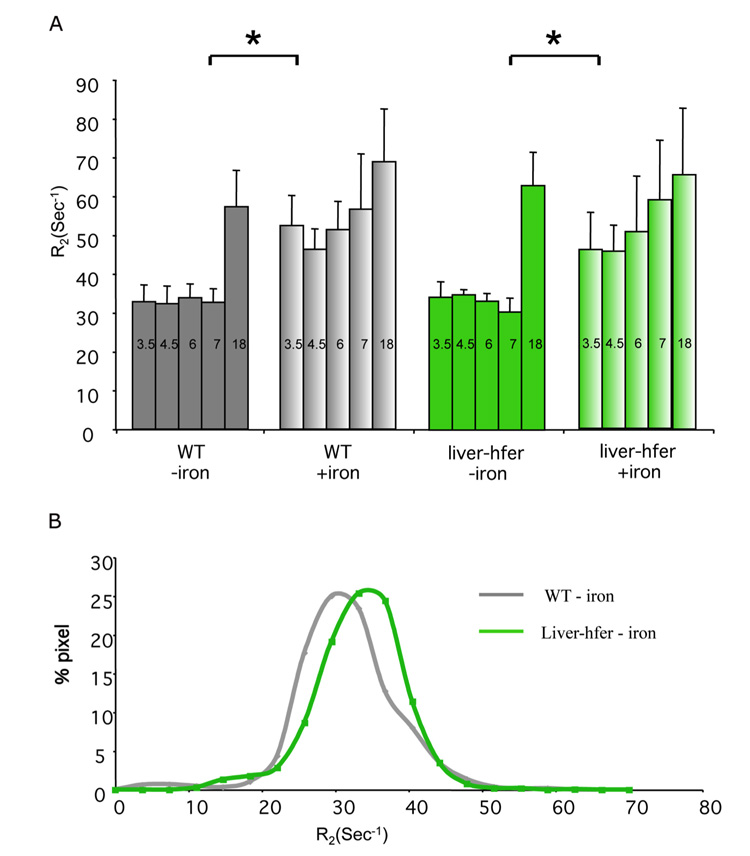
MRI (4.7T) was applied for dynamic follow up of R2 relaxation in 4 groups of mice, including WT mice and liver-hfer mice (n=10 per group) subjected to either high iron diet or normal diet, during a period of 22 months (from 2 to 24 months of age; Figure 1). Under such chronic expression of h-ferritin, ROI analysis did not detect a significant difference in the average R2 (at 4.7T) between liver-hfer and WT mice, neither for regular diet, nor for iron enriched diet (Figure 2A). Iron enriched diet led to a significant elevation in R2 values for both WT and liver-hfer mice with no significant difference between WT and liver-hfer mice (p<0.05 2 tails un-paired Ttest; Figure 2A). Notably, all groups showed a significant rise in R2 with age, which was steeper at early age for the iron-enriched diet and steeper at older age for the normal diet, but similar in both cases for the WT and liver-hfer groups.
Figure 1. MRI gray scale image of mice with regular diet and High iron diet.
4.7T and 9.4T MR images of WT mice (A-D) and liver-hfer mice (E-H) with constitutive transgene expression (2 years old) that were treated with normal diet and high iron diet (TR=2 s, TE=11 ms).
Figure 2. R2 values according to ROI measurements in the liver of the different groups at 4.7T magnet.
Each group was scanned at a 4.7T magnet during 18 months of normal and high iron diet, (A) ROI analysis showed significant difference in R2 between the groups that were treated with high iron diet to groups with regular diet at all time points except for the last time point (*p<0.05 unpaired 2 tail Ttest). Numbers at the top of each bar state the mice age in months (iron supplementation diet was initiated when mice were 2 months old). (B) Analysis of the distribution of R2 values derived from R2 maps showed the spatial distribution of 4.7T R2 values in the liver of 9 months old mice. Overexpression of h-ferritin resulted in elevated R2 values. Wild type mice (gray), liver-hfer mice (green).
Analysis of the spatial distribution of R2 values derived from R2 maps of mice fed with normal diet revealed that overexpression of h-ferritin resulted in a small increase in the fraction of voxels with high R2 values (Figure 2B). These results are consistent with heterogeneous expression of the reporter by a subset of liver hepathocytes. In the presence of high iron diet the R2 values were too high for derivation of reliable R2 maps.
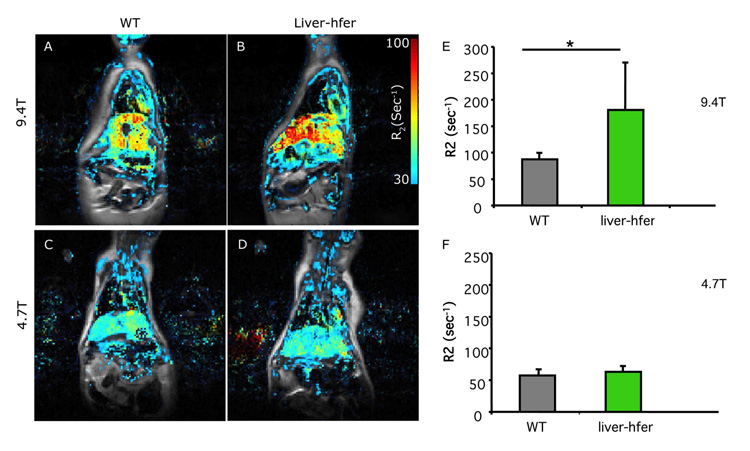
R2 differences between liver-hfer and WT mice are elevated at high magnetic field (4.7T vs 9.4T)
Ferritin has a unique linear R2 dependence on the magnetic field, which can be further enhanced by ferritin aggregation. The mice were scanned at two magnetic fields 4.7T and 9.4T when they were 2 years old. As expected for ferritin, the change in R2 was strongly enhanced at high magnetic field revealing differences between the WT and liver-hfer mice fed normal diet (Figures 3A-D). R2 values at 9.4T but not at 4.7T were significantly higher in normal diet liver-hfer mice compared to WT mice (p<0.05 2 tail unpaired Ttest, Figure 3E-F).
Figure 3. Field dependence of R2 in liver-hfer mice.
Overlay of R2 color maps on a gray scale anatomical images (TE=6.4 ms) of WT mice (A, C) and liver-hfer mice (B, D) (age 24 months). Mice at the age of 24 months were imaged at two magnetic fields, 4.7T and 9.4T. (E, F) ROI analysis reveals significant difference in R2 between WT and liver-hfer mice that were treated with regular diet and scanned at 9.4T (*p<0.05 unpaired 2 tail Ttest).
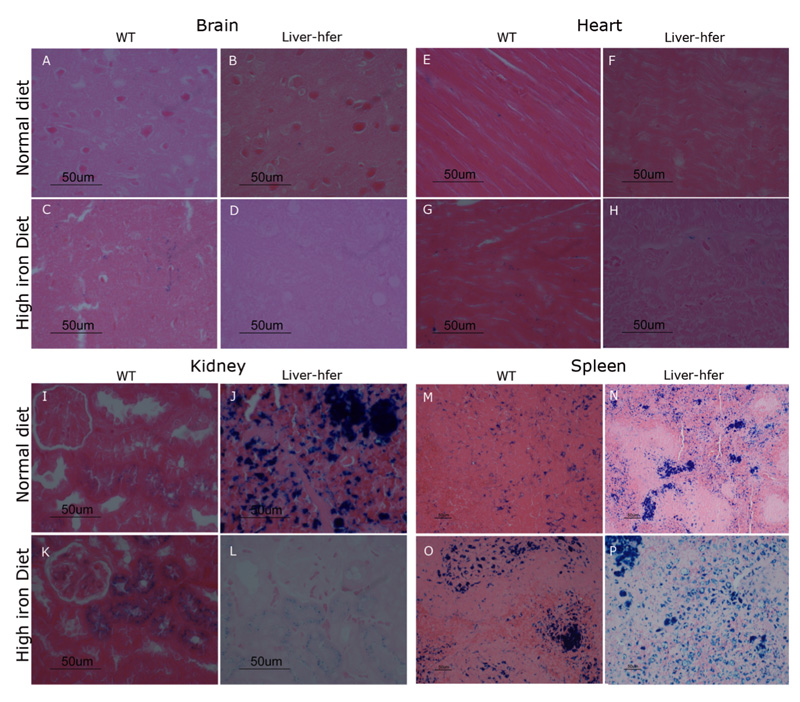
Histological analysis reveals differences in liver iron deposits between WT and liver-hfer mice
Liver sections were taken from representative mice after 18 months of treatment and stained with eosin & hematoxylin (H&E) and Prussian blue. H&E stain revealed similar vacuolar liver changes with aging in all mice groups (Figure 4A-B, E-F). Deposits of iron in the form of hemosiderin were observed in higher concentration only in liver-hfer mice (Figure 4B). High iron diet increased the hemosiderin concentration with clear difference between liver-hfer and WT mice (Figure 4E-F). The differences in iron content were evident in Prussian blue stain, which stains ferric ions (Fe+3) (Figure 4C-D, G-H).
Figure 4. Histological evaluation of the liver.
Liver sections that were taken from representative mice at the age of 20 months were stained with eosin hematoxylin (A-B, E-F) and by Prussian blue, which stains ferric ions (Fe+3) in bright blue color (C-D, G-H). (A, C) WT mice with normal diet, (B, D) liver-hfer mice with normal diet, (E, G) WT mice with high iron diet, (F, H) liver-hfer mice with high iron diet (arrows, hemosiderin deposits).
Over expression of ferritin in hepatocytes does not lead to pathological abnormalities
Brain, heart, kidney and spleen sections were retrieved from representative 20 months old mice and stained with eosin & hematoxylin (H&E) and Prussian blue. No pathological changes were noted in these organs with the chronic ferritin over expression (Figure 5 A-L). However, hemosiderin deposits were observed in the spleen of liver-hfer mice that were treated with normal iron diet but not in WT mice (figure 5 M-N). The difference in iron levels observed by H&E in the spleen of liver-hfer mice was confirmed also by Prussian Blue staining. High iron deposits were found in the kidney and spleen of liver-hfer mice compared to WT mice while treated with normal diet. Prussian blue staining was similar in the brain and heart of all groups (figure 6 A-H).
Figure 5. Histological evaluation of liver-hfer and WT mice.
Different organs sections were taken from representative mice from each group at the age of 20 months and stained with eosin hematoxylin . (A-D) Brain, (E-H) Heart, (I-L) Kidney, (M-P) Spleen (arrows, hemosiderin deposits).
Figure 6. Iron levels evaluation by Prussian blue stain.
Different organs sections were taken from representative mice from each group at the age of 20 months and stained by Prussian blue. (A-D) Brain, (E-H) Heart, (I-L) Kidney, (M-P) Spleen.
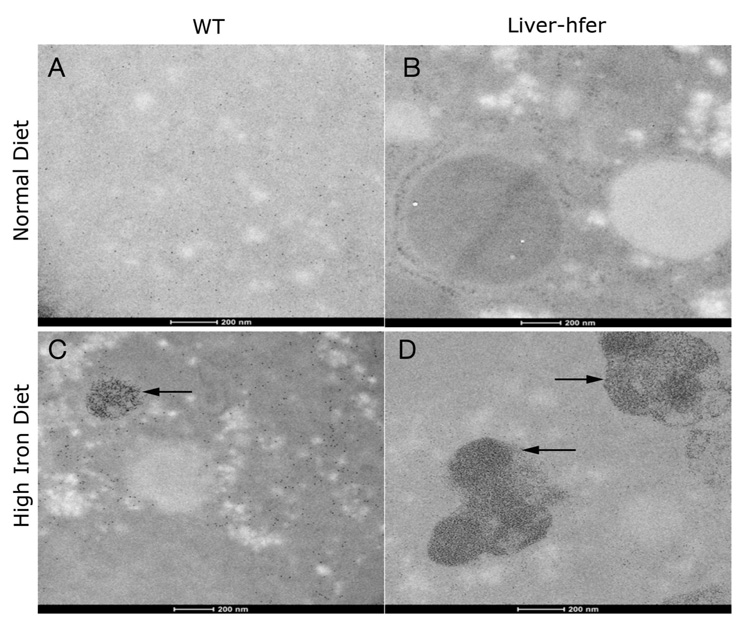
Electron microscopy analysis demonstrated the presence of ferritin and hemosiderin in liver-hfer mice subjected to a high-iron diet
Liver sections were examined by electron microscopy in order to verify the existence of iron and ferritin. SEM analysis was used to map iron in liver section (Figure 7). Regions that were rich in high atomic number elements were identified by back scattered electrons detector (BSD) and were selected for elements mapping. High iron levels were depicted in both WT and liver-hfer mice that were treated with high iron diet (Figure 7J-K), no such regions were observed in the livers of either WT or liver-hfer mice that were raised with normal diet (Figure 7 A, D). The presence of ferritin in liver hepatocytes was confirmed by TEM studies (Figure 8A, B), round structures at the size of 8-10nm are consistent with ferritin. In addition large hemosiderin clusters were observed in mice that were raised on high iron diet (arrows, Figure 8C, D).
Figure 7. Iron mapping of the liver using scanning electron microscope (SEM).
Liver sections that were taken from representative mice at the age of 24 months were mapped for iron levels. (A-D) Livers of mice that were raised with normal diet (A, D back scattered images, B-C iron maps). (E-H) Livers of mice that were raised with high iron diet (E,H back scattered images, F-G iron maps). (I-L) magnification of the white square area.
Scale bar for A-H equals 100μm, Scale bar for I-L equals 50μm.
Figure 8. Transmission electron microscopy images of the liver.
Liver sections that were taken from representative mice at the age of 24 months were examined by Transmission electron microscope. (A) WT mice with normal diet (B) liver-hfer mice with normal diet (C) WT mice with high iron diet (D) liver-hfer mice with high iron diet. Large hemosiderin clusters are observed in mice that were raised on high iron diet (C, D arrows marking). Scale bar equals 200nm.
Discussion
Reporter genes have become an indispensable tool for analysis of gene expression, protein interaction, cell lineage tracing and differentiation. Of particular interest are reporter mice, which can be used for analysis of development, disease and preclinical drug development, providing unique non invasive dynamic information on the genes or cells of interest while significantly reducing the number of animals required for a study (28). The use of reporter genes for the purpose of biological research and biomedical applications is always accompanied by the concern of side effects that may result from the introduction of a foreign gene. Thus, in the search for new reporter genes the critical properties include not only the specificity and sensitivity of the reporter gene but also the safety and inertness during prolonged expression.
Side effects of fluorescent reporters were evaluated for multiple settings including mouse bone marrow cells and cloned transgenic cattle (29)(30). Importantly, indirect side-effects can be associated with the site of insertion, as exemplified by the development of acute myeloid leukemia with EGFP expressing cells in rhesus macaques (31).
In recent years the use of ferritin as MR reporter gene was examined in several systems (7-10,13,14). The advantage of MRI includes its high endogenous anatomical and functional contrast and the ability to acquire high-resolution three dimensional information also in large organs not accessible for high resolution optical imaging. We previously reported the generation of transgenic reporter TET-ferritin mice, which express h-ferritin in a tissue specific and tetracycline inducible manner (8).
Initial assessment of young TET-ferritin mice included analysis of liver and endothelial specific expression. Endothelial-hfer mice revealed no pathologies (16), on the other hand, young mice that expressed h-ferritin in liver hepatocytes showed a transient mild hepatic cytotoxicity leading to vacuolar changes and reduced R2 when h-ferritin expression was acutely induced by withdrawal of tetracycline. The study reported here aimed to evaluate the impact of chronic expression of ferritin by liver hepatocytes in normal husbandry conditions, and when challenged with high iron diet. Mice were monitored continuously over a period of two years.
In contrast to young mice that exhibited a transient reduction of R2 values in response to acute h-ferritin overexpression in the liver (8), in older mice R2 values increased compared to WT. While increased R2 was marginally detectable at a magnetic field of 4.7T, as expected for ferritin, the difference in R2 between WT and liver-hfer mice was significantly enhanced at 9.4T. The high field dependence of R2 could stem from the heterogeneous pattern of transgene expression and iron accumulation. Such non-uniform distribution can lead to quadratic field dependence rather than linear (32,33).
Histo-pathological examination of liver-hfer and WT mice confirmed the increased iron content in the livers of transgenic mice, which was significant but was small relative to the physiological level of liver iron that can be achieved by feeding the mice with iron-enriched diet. In aging mice the vacuolar pattern in the liver was evident and similar for both liver-hfer and WT mice, for both normal and iron-enriched diets, suggesting that prolonged chronic over expression of ferritin does not alter baseline liver function, nor its capacity to respond to iron enriched diet. Histological evaluation of other organs including the brain, heart, spleen and kidney of liver-hfer mice revealed a normal phenotype. These results further substantiate the use of chronic expression of h-ferritin as a reporter gene for MRI analysis of liver hepathocytes. However, due to the central role of iron in multiple pathologies, each new application of ferritin should be carefully evaluated.
Acknowledgments
The Electron Microscopy studies were conducted at the Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging at the Weizmann Institute of Science. This work was supported by the Minerva Foundation, by the European Commission 7th Framework ENCITE Integrated project and by 7th Framework European Research Council Advanced grant 232640-IMAGO (to MN). Michal Neeman is incumbent of the Helen and Morris Mauerberger Chair.
Abbreviations
- h-ferritin
heavy chain ferritin
- wt
wild type
- tTA
tetracycline transactivator
- LAP
liver associated protein
- EGFP
enhanced green fluorescent protein
- SEM
scanning electron microscope
- TEM
transmission electron microscope
- BSD
back scattered electrons detector
- EDS
energy dispersive X-ray detector
- SW
spectral width
References
- 1.Arredondo M, Nunez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26(4-5):313–327. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Chiancone E, Ceci P, Ilari A, Ribacchi F, Stefanini S. Iron and proteins for iron storage and detoxification. Biometals. 2004;17(3):197–202. doi: 10.1023/b:biom.0000027692.24395.76. [DOI] [PubMed] [Google Scholar]
- 3.Siah CW, Trinder D, Olynyk JK. Iron overload. Clin Chim Acta. 2005;358(1-2):24–36. doi: 10.1016/j.cccn.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Gottesfeld Z, Neeman M. Ferritin effect on the transverse relaxation of water: NMR microscopy at 9.4 T. Magn Reson Med. 1996;35(4):514–520. doi: 10.1002/mrm.1910350410. [DOI] [PubMed] [Google Scholar]
- 5.Vymazal J, Brooks RA, Bulte JW, Gordon D, Aisen P. Iron uptake by ferritin: NMR relaxometry studies at low iron loads. J Inorg Biochem. 1998;71(3-4):153–157. doi: 10.1016/s0162-0134(98)10047-8. [DOI] [PubMed] [Google Scholar]
- 6.Gossuin Y, Muller RN, Gillis P. Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification. NMR Biomed. 2004;17(7):427–432. doi: 10.1002/nbm.903. [DOI] [PubMed] [Google Scholar]
- 7.Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 2005;7(2):109–117. doi: 10.1593/neo.04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen B, Ziv K, Plaks V, Israely T, Kalchenko V, Harmelin A, Benjamin L, Neeman M. MRI detection of transcriptional regulation of gene expression in. Nat Med. 2007;13(4):498–503. doi: 10.1038/nm1497. Epub 2007 Mar 2011. [DOI] [PubMed] [Google Scholar]
- 9.Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006;56(1):51–59. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11(4):450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Cheng EC, Long RC, Jr, Yang SH, Wang L, Cheng PH, Yang JJ, Wu D, Mao H, Chan AW. Noninvasive Monitoring of Embryonic Stem Cells in vivo with MRI Transgene Reporter. Tissue Eng Part C Methods. 2009 doi: 10.1089/ten.tec.2008.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida M, Terashima M, Cunningham CH, Suzuki Y, Willits DA, Willis AF, Yang PC, Tsao PS, McConnell MV, Young MJ, Douglas T. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med. 2008;60(5):1073–1081. doi: 10.1002/mrm.21761. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro MG, Szablowski JO, Langer R, Jasanoff A. Protein nanoparticles engineered to sense kinase activity in MRI. J Am Chem Soc. 2009;131(7):2484–2486. doi: 10.1021/ja8086938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aung W, Hasegawa S, Koshikawa-Yano M, Obata T, Ikehira H, Furukawa T, Aoki I, Saga T. Visualization of in vivo electroporation-mediated transgene expression in experimental tumors by optical and magnetic resonance imaging. Gene Ther. 2009 doi: 10.1038/gt.2009.55. [DOI] [PubMed] [Google Scholar]
- 15.Ono K, Fuma K, Tabata K, Sawada M. Ferritin reporter used for gene expression imaging by magnetic resonance. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 16.Cohen B, Ziv K, Plaks V, Israely T, Kalchenko V, Harmelin A, Benjamin LE, Neeman M. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007;13(4):498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 17.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119(4):529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem J. 2001;357(Pt 1):241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, Arosio P. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103(6):2377–2383. doi: 10.1182/blood-2003-06-1842. [DOI] [PubMed] [Google Scholar]
- 21.Kaur D, Rajagopalan S, Chinta S, Kumar J, Di Monte D, Cherny RA, Andersen JK. Chronic ferritin expression within murine dopaminergic midbrain neurons results in a progressive age-related neurodegeneration. Brain Res. 2007;1140:188–194. doi: 10.1016/j.brainres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275(5):3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 23.Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, Hentze MW, Beard J, Connor J. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res. 2003;71(1):46–63. doi: 10.1002/jnr.10463. [DOI] [PubMed] [Google Scholar]
- 24.Grabill C, Silva AC, Smith SS, Koretsky AP, Rouault TA. MRI detection of ferritin iron overload and associated neuronal pathology in iron regulatory protein-2 knockout mice. Brain Res. 2003;971(1):95–106. doi: 10.1016/s0006-8993(03)02366-7. [DOI] [PubMed] [Google Scholar]
- 25.Fowler C. Hereditary hemochromatosis: pathophysiology, diagnosis, and management. Crit Care Nurs Clin North Am. 2008;20(2):191–201. vi. doi: 10.1016/j.ccell.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Zandman-Goddard G, Shoenfeld Y. Hyperferritinemia in autoimmunity. Isr Med Assoc J. 2008;10(1):83–84. [PubMed] [Google Scholar]
- 27.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maggi A, Ottobrini L, Biserni A, Lucignani G, Ciana P. Techniques: reporter mice - a new way to look at drug action. Trends Pharmacol Sci. 2004;25(6):337–342. doi: 10.1016/j.tips.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Tao W, Evans BG, Yao J, Cooper S, Cornetta K, Ballas CB, Hangoc G, Broxmeyer HE. Enhanced green fluorescent protein is a nearly ideal long-term expression tracer for hematopoietic stem cells, whereas DsRed-express fluorescent protein is not. Stem Cells. 2007;25(3):670–678. doi: 10.1634/stemcells.2006-0553. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wu Q, Cui H, Li Q, Zhao Y, Luo J, Liu Q, Sun X, Tang B, Zhang L, Dai Y, Li N. Expression of EGFP and NPTII protein is not associated with organ abnormalities in deceased transgenic cloned cattle. Cloning Stem Cells. 2008;10(4):421–428. doi: 10.1089/clo.2008.0015. [DOI] [PubMed] [Google Scholar]
- 31.Seggewiss R, Pittaluga S, Adler RL, Guenaga FJ, Ferguson C, Pilz IH, Ryu B, Sorrentino BP, Young WS, 3rd, Donahue RE, von Kalle C, Nienhuis AW, Dunbar CE. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood. 2006;107(10):3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen JH, Chandra R. Strong field behavior of the NMR signal from magnetically heterogeneous tissues. Magn Reson Med. 2000;43(2):226–236. doi: 10.1002/(sici)1522-2594(200002)43:2<226::aid-mrm9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.Jensen JH, Chandra R. Theory of nonexponential NMR signal decay in liver with iron overload or superparamagnetic iron oxide particles. Magn Reson Med. 2002;47(6):1131–1138. doi: 10.1002/mrm.10170. [DOI] [PubMed] [Google Scholar]