Abstract
Adolescence is a critical developmental period during which most adult smokers initiate their habit. Adolescents are more vulnerable than adults to nicotine’s long-term effects on addictive and cognitive behavior. We investigated whether adolescent nicotine exposure in rats modifies expression of nicotinic acetylcholine receptors (nAChRs) in medial prefrontal cortex (mPFC) in the short and/or long term, and whether this has functional consequences. Using receptor binding studies followed by immunoprecipitation of nAChR subunits, we showed that adolescent nicotine exposure, as compared with saline, caused an increase in mPFC nAChRs containing α4 or β2 subunits (24 and 18%, respectively) 24 h after the last injection. Nicotine exposure in adulthood had no such effect. This increase was transient and was not observed 5 wk following either adolescent or adult nicotine exposure. In line with increased nAChRs expression 1 d after adolescent nicotine exposure, we observed a 34% increase in amplitude of nicotine-induced spontaneous inhibitory postsynaptic currents in layer II/III mPFC pyramidal neurons. These effects were transient and specific, and observed only acutely after adolescent nicotine exposure, but not after 5 wk, and no changes were observed in adult-exposed animals. The acute nicotine-induced increase in α4β2-containing receptors in adolescents interferes with the normal developmental decrease (37%) of these receptors from early adolescence (postnatal day 34) to adulthood (postnatal day 104) in the mPFC. Together, this suggests that these receptors play a role in mediating the acute rewarding effects of nicotine and may underlie the increased sensitivity of adolescents to nicotine.
Keywords: neurophysiology, membrane expression, immunoprecipitation, epibatidine binding, IPSC amplitude, pyramidal neurons
Nicotine is the main addictive and neuroactive compound in tobacco smoke, and smoking is the leading preventable cause of death and disability in the United States (1). Nicotine can disrupt developmental events and cause long-lasting effects when administered during windows of vulnerability, such as the neonatal or adolescent periods (2). Most adult smokers start their habit during adolescence (3), and preclinical studies suggest there are different mechanisms driving nicotine use in adolescents and adults (4). A current hypothesis explaining why adolescents are more vulnerable to nicotine addiction is that nicotine has greater positive effects on adolescents than adults, whereas the negative effects associated with nicotine, such as withdrawal, are smaller in adolescents (5). Adolescence is a critical developmental period during which the brain, in particular the medial prefrontal cortex (mPFC), continues to mature. Processes such as synaptic pruning and myelination are thought to continue until approximately the age of 20 in humans (6). Similar behavioral and developmental changes occur in rats aged ~25–50 d [postnatal day (P) 25–50; ref. 7]. The increased sensitivity to nicotine in adolescents might be due to intrinsic developmental nicotinic receptor expression in the brain and/or the interaction of nicotine with receptor expression over time.
Nicotine acts on nicotinic acetylcholine receptors (nAChRs), belonging to the superfamily of cys-loop ligand-gated ion channels. Eleven nAChR subunits have so far been identified in mammals and are classified into 8 α and 3 β subunits (8). The assembly of 5 subunits forms different subtypes that may be heteromeric (the most prevalent is α4β2) or homomeric (mainly α7). The different receptor subtypes show substantial brain-region-specific expression (9). It has been shown that high-dose chronic and intermittent nicotine exposure increases levels of high-affinity nicotinic receptors in adult humans and rodents (10–14). However, α7-type nAChRs are less prone to regulation, possibly because of their relative low affinity for nicotine (15).
Nicotine administration during, but not following, adolescence has long-lasting effects on cognitive, addictive, and emotional behavior in rats (16–19). Furthermore, adolescent animals are more sensitive to nicotine-conditioned place preference than adults and show this at lower nicotine doses (20–24). Therefore, it is of interest to investigate whether these short- and long-term behavioral effects of adolescent nicotine exposure are caused by a differential regulation of nicotinic receptors following adolescent nicotine exposure.
Previous studies have shown that adolescent nicotine exposure leads to acute and longer-lasting changes in nAChR binding (25, 26) and function (20) in brain regions such as cortex and striatum. In this study, we investigate the short- and long-term aspects of nAChR expression and function in the mPFC following a nicotine exposure regimen that we previously found to induce long-lasting cognitive deficits (17, 18). The use of antibodies specific to the different nAChR subunits enables us to pinpoint whether the subunit composition of nAChRs changes following adolescent nicotine exposure. Because human imaging data suggest that primary cortical areas mature before cortical association areas (27), we studied the mPFC, which is believed to continue developing during adolescence and is relevant for the behavioral effects of nicotine, and the occipital cortex, which contains the primary visual cortex, whose development is thought to be completed by adolescence. We used radioactive ligands to bind the different receptor subtypes and antibodies specific for the different nAChR subunits to determine the nicotine-induced regulation of nAChR subtype expression by means of immunoprecipitation experiments. Finally, we examined the functional consequences of nicotine-induced adolescent nAChR up-regulation on nicotine-mediated augmentation of spontaneous inhibitory transmission in the mPFC.
MATERIALS AND METHODS
Animals
Timed pregnant Wistar female rats arrived at 5 d of gestation (Harlan, Horst, The Netherlands) and were housed individually in Macrolon cages under standard conditions and a reversed day-night cycle (lights on 7 PM–7 AM). On delivery, litters were culled to 8 pups/mother and preferably consisted of males only, but only occasionally were matched with females. At P21, animals were weaned and housed 2 rats/cage. Only males were used in these experiments. The experiments were approved by the Animal Users Care Committee of Vrije Universiteit (Amsterdam, The Netherlands).
Nicotine exposure
Animals were injected subcutaneously with either nicotine (0.4 mg/kg, calculated as the base ([−]nicotine hydrogen tartrate salt; Sigma-Aldrich, St. Louis, MO, USA) or saline 3×/d (10 AM, 1 PM, and 3 PM) for 10 d (n=10 animals/group). A second control group consisted of animals that were not injected, but handled once a week. Nicotine was administered from P34 to P43 (adolescence) or P60 to P69 (adulthood). Saline and no-injection controls were littermates of nicotine-exposed animals in both age groups. Animals were decapitated without anesthesia by an experienced technician on P34 (before nicotine exposure), on the first day of withdrawal (P44/P70) and 5 wk following nicotine exposure (P78/P104). Following decapitation, the brains were removed and quickly frozen in ice-cold isopentane before storage (−80°C). For measurement of plasma nicotine and cotinine levels, 2 different groups of animals (n=8/group, adolescent and adult animals) were injected with nicotine and decapitated at 3 time points: 30 min following the first injection (P34/P60; 10:30 AM), 30 min following the third injection (P34/P60; 3:30 PM), and at the first withdrawal day (P44/P70). Following decapitation, trunk blood was collected and centrifuged at 600 g for 10 min to obtain plasma.
Plasma nicotine and cotinine levels
Extraction procedure
Extraction of nicotine and cotinine was done as described by O’Dell et al. (28) with slight modifications. In short, 100 μl of heparinized plasma was spiked with 2-phenylimidazole to verify extraction efficiency. To this, 20 μl of 20% NaOH was added, before 400 μl of tert-butyl methyl ether was added. After vortexing and centrifugation at 10,000 g (3 min), the organic phase was transferred to a new tube. The extraction was repeated with 200 μl tert-butyl methyl ether. Then MgSO4 was added to pellet any remaining proteins. Following vortexing and centrifugation, the aqueous phase was transferred to a new tube and evaporated to dryness under a gentle stream of nitrogen. The lyophilized samples were reconstituted in 25 μl mobile phase (acetonintrile/methanol/10 mM ammonium acetate, 53:32:15, v/v/v).
Liquid chromatography–electrospray ionization mass spectrometry (LC-ESI/MS)
A Shimadzu LC system of 2 pumps (LC-10ADvp), autosampler (SIL-10ADvp), system controller (SCL-10Avp), and degassing unit (DGU-14A; Shimadzu USA, Canby, OR, USA), was coupled to a Bruker micro-TOF-Q instrument (Bruker Daltonics, Bremen, Germany) equipped with an ESI source. Each LC separation lasted 15 min with a gradient:linear gradient (0% B to 100% B, 7 min), 3 min 100% B, and 5 min equilibration at 0% B, where A is 2 mM ammonium acetate (pH 6.8), and B is MeOH (flow rate 150 μl/min). For all separations, an XTerra MS C18 column was used (2.1×50 mm, 2.5 μm particle size). Positive-mode electrospray was performed at spray voltage 4.5 kV. Scanning was performed over an m/z range from 50 to 3000 Da. The instrument was calibrated by infusing 5 mM sodium formate in 50% MeOH with 0.1% FA at flow rate of 4 μl/min. The data were analyzed with Data Analysis 4.0 software (Bruker Daltonics).
Antibody production and characterization
The subunit-specific polyclonal antibodies were produced in rabbits against peptides derived from the C-terminal and/or intracytoplasmic loop regions of the rat, human, or mouse subunit sequences and affinity purified as described previously (29). Two different peptides were chosen for all of the subunits: one located in the cytoplasmic loop between M3 and M4 (CYT), and the other located at the COOH-terminal (COOH). The antibodies raised against the peptides were purified on an affinity column made by coupling the corresponding peptide to cyanogen bromide-activated Sepharose 4B (Pharmacia, Uppsala, Sweden) according to the manufacturer’s instructions. Antibody specificity was checked by means of quantitative immunoprecipitation or immunopurification experiments using nAChRs from different areas of the CNS of wild-type α4, α5, α6, β2, β3, and β4 (+/+) and null mutant (−/−) mice, which allowed selection of antibodies specific for the subunit of interest, and assessing the immunoprecipitation capacity of each antibody. For full characterization of nAChR subunit antibodies, see Supplemental Table 1 in Grady et al. (29).
Preparation of membranes and 2% Triton X-100 extracts
The mPFC (infralimbic and prelimbic cortex) and caudal (occipital) cortex were removed freehand at −20°C from 1-mm-thick slices. Dissected material was stored at −80°C until further use. In every experiment, tissue from 2 rats (0.04–0.05 g) from each experimental group was pooled and homogenized in 10 ml of 50 mM Na phosphate (pH 7.4), 1 M NaCl, 2 mM EDTA, 2 mM EGTA, and 2 mM PMSF using a potter homogenizer, and homogenates were diluted and centrifuged at 60,000 g (1 h). Total membrane homogenization, dilution, and centrifugation procedures were repeated, after which the cell membrane-enriched pellets were collected; rapidly rinsed with 50 mM TrisHCl (pH 7), 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 2 mM PMSF; and resuspended in the same buffer containing a mixture of 20 μg/ml of each of the following protease inhibitors: leupeptin, bestatin, pepstatin A, and aprotinin. Triton X-100, at a final concentration of 2%, was added to the washed membranes to extract membrane receptors and was incubated at 4°C (2 h). The extracts were centrifuged at 60,000 g (1.5 h) and recovered. An aliquot of the supernatants was collected for protein measurement (BCA protein assay; Pierce, Rockford, IL, USA) with bovine serum albumin as standard.
Binding studies
125I-α-bungarotoxin (125I-αBgt)
125I-αBgt (specific activity 200 Ci/mmol; PerkinElmer, Boston, MA, USA) binding experiments were performed by overnight incubation of mPFC and occipital cortex membranes dissolved in 50 mM Na phosphate (pH 7.4), 1 M NaCl, 2 mM EDTA, 2 mM EGTA, and 2 mM PMSF in a final volume of 100 μl containing a saturating concentration (5 nM) of 125I-αBgt at 20°C and 2 mg/ml bovine serum albumin. Nonspecific binding was determined in parallel by means of incubation in the presence of 1 μM unlabeled α-bungarotoxin. After incubation, the samples were filtered on GFC filters presoaked in polyethylenimine through a harvester apparatus, and the bound radioactivity was directly counted in a γ counter.
3H-epibatidine (3H-Epi)
To ensure that the α7-containing receptor subtypes did not contribute to 3H-Epi (specific activity 50–66 Ci/mmol; GE Healthcare, Little Chalfont, UK) binding to solubilized receptors (30), the binding in the extract and immunoprecipitation experiments was performed in the presence of 2 μM 125I-αBgt, which specifically binds to α7*-nAChRs (and thus prevents 3H-Epi binding to these sites). Binding to the 2% Triton X-100 extracts from mPFC or occipital cortex was carried out overnight by incubating aliquots of extracts with 2 nM 3H-Epi at 4°C. Nonspecific binding (on average 5–10% of total binding) was determined in parallel samples containing 100 nM unlabeled epibatidine. After incubation, the extracts were diluted to 200 μl with H2O and applied to a 500 μl DE52 ion-exchange resin (Whatman, Maidstone, UK). After being washed with 10 ml of wash buffer (10 mM Na phosphate, pH 7.4; 50 mM NaCl; and 0.1% Triton X-100) to remove unbound 3H-Epi, the bound receptors were eluted with 2 ml of 1 N NaOH and, after addition of 10 ml of the liquid scintillation Filter-Count solution (PerkinElmer), counted in a β counter.
Immunoprecipitation of 3H-Epi-labeled receptors by subunit-specific antibodies
Tissue extracts (100 μl) were preincubated with 2 μM 125I-αBgt, labeled with 2 nM 3H-Epi, and incubated overnight with a saturating concentration (20 μg) of anti-subunit-specific affinity-purified IgG produced and characterized by us (see Grady et al., ref. 29).
The immunoprecipitation was recovered by incubating samples with beads containing bound goat anti-rabbit IgG (Technogenetics, Milan, Italy). The beads were washed with 10 ml of 10 mM Na phosphate (pH 7.4), 50 mM NaCl, and 0.1% Triton X-100 to remove unbound 3H-Epi, and the bound receptors were eluted with 2 N NaOH and counted in a β counter.
The level of antibody immunoprecipitation was expressed as percentage of 3H-Epi-labeled receptors immunoprecipitated by antibodies (taking the amount present in the Triton X-100 extracted solution before immunoprecipitation as 100%) or as femtomoles of immunoprecipitated receptors per milligram of protein.
Nicotine-induced changes in synaptic transmission
Adolescent (n=34) and adult (n=24) rats were exposed to nicotine or saline as described above and used for recording of nicotine-induced (10 μM, bath application) spontaneous and miniature inhibitory postsynaptic currents (sIPSCs and mIPSCs), the latter in the presence of tetradotoxin (TTX). After 1 d (acute effect) or 5 to 6 wk (long-term effect) following nicotine exposure, rats were decapitated, and brains were rapidly removed and put in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 3.5 KCl, 2.4 CaCl2, 1.3 MgSO4 · 7H2O, 1.2 KH2PO4, 215.5 sucrose, 26 NaHCO3, and 10 d-glucose, with osmolarity 300 mosmol.
Coronal mPFC slices of 300 μm thickness were prepared in the same sucrose-containing ACSF and then stored in holding chambers containing normal ACSF consisting of (in mM) 125 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 1 CaCl2, 26 NaHCO3, and 10 glucose, bubbled with carbogen gas (95% O2/5% CO2). Pyramidal neurons in layer II/III in the mPFC were visualized using differential interference contrast microscopy, and whole-cell recordings from pyramidal neurons were made using Multiclamp 700B amplifier (Molecular Devices/Axon Instruments, Sunnyvale, CA, USA), digitized by the pClamp software (Molecular Devices/Axon Instruments), and later analyzed offline using Synaptosoft (Delaware, GA, USA) software. To record GABAergic activity at resting membrane potential (−70 mV), pipette medium contained elevated chloride concentration (in mM): 65 K gluconate, 70 KCl; 8 NaCl, 2 MgATP, 10 phosphocreatine, 0.2 EGTA, 10 HEPES, 0.3 Tris GTP, and 1 QX 314-Cl. Recordings were made (32°C) in the presence of 6,7-dinitroquinoxaline-2,3-dione to block glutamatergic currents measuring at baseline (7 min), nicotine wash-in (10 μM; 3 min), and wash-out (7 min). For estimation of the nicotine effect, average sIPSC frequency or amplitude was taken at the peak (last minute of nicotine wash-in and first minute of nicotine washout) and normalized to the last 5 min of baseline recording.
Statistical analyses
Data from plasma nicotine and cotinine measurements were subjected to univariate ANOVA with age of pretreatment (adolescent, adult) and time point as between-subject variables using SPSS 16 (SPSS Inc., Chicago, IL, USA). Developmental data on nAChR expression were subjected to a nonparametric ordered Jonckheere-Terpstra test due to unequal variance per brain region (Levene’s test). Due to their normal distribution, nicotine-dependent regulation of nAChR expression was analyzed by univariate factorial ANOVAs with age of treatment, time point after exposure, brain region, and treatment as between-subject variables. In case of statistically significant main effects and interactions, further breakdown in factors was performed, ultimately followed by post hoc comparisons (Student-Newman-Keuls tests). Due to independent data sets from electrophysiological experiments with different setups, data were analyzed separately for age and time using Student’s t test with nicotine and saline treatment as factors. For cumulative frequencies, a Kolmogorov-Smirnov (K-S) test was performed. The level of probability for statistically significant effects was set at 0.05. All data are displayed as mean values ± se. The effect size in terms of percentage change is calculated as the subtraction of the value from the normalized (vs. saline) nicotine sample minus the value of the saline sample.
RESULTS
Plasma nicotine levels
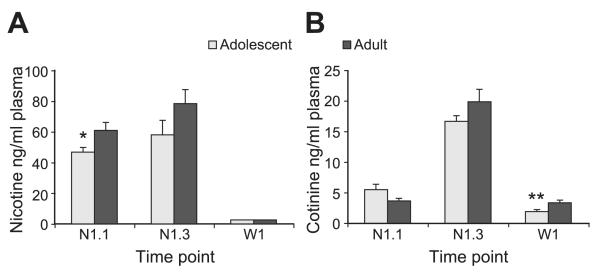
The circulating levels of nicotine and its major metabolite cotinine were measured in plasma of adolescent and adult rats at 3 different time points after nicotine administration (Fig. 1). A single injection of a relatively high dose of nicotine led to plasma nicotine levels of 46.8 ± 3.1 ng/ml in adolescent animals and 60.9 ± 5.2 ng/ml in adults half an hour after the injection (P=0.04; Fig. 1). These levels are comparable with those observed in human smokers (4 to 72 ng/ml) that smoke about a pack of cigarettes a day (31). Repeated nicotine injections led to a slight increase in peak levels in both treatment groups (Fig. 1A; time point F [2,38]=64.15, P<0.001), which has also been observed in human smokers (32, 33). Our administration protocol therefore reflects the circulating dose of nicotine in human smokers.
Figure 1.
Plasma nicotine and cotinine levels during and after nicotine exposure. Plasma nicotine (A) and cotinine levels (B) in animals (n=8) exposed during adolescence (gray) or adulthood (black). Blood was collected 30 min after the first injection (N1.1), 30 min after the third injection on the same day (N1.3), and on the first day of withdrawal in the morning (W1). *P < 0.05, **P < 0.01 vs. adult animals.
Overall, plasma nicotine levels were slightly but significantly higher in adult animals injected with the same concentration of nicotine relative to their bodyweight compared with adolescents (Fig. 1A; age F [1,38]=5.03, P=0.031, age×time point F [2,38]=1.47, ns). However, for circulating levels of cotinine (the major metabolite of nicotine) there was no age effect (Fig. 1B; age F [1,42]=1.43, ns), but only a clear effect of time point (F [2,42]=155.24, P<0.001). Cotinine has a longer half-life than nicotine and therefore accumulates after repeated injections of nicotine, as was observed in both groups. Post hoc analysis of the effect of age × time point (F [2,42]=3.52, P=0.039) does not reveal significant differences between groups at the 2 time points during treatment regimen; only for the first time point a trend (P=0.064 adolescent vs. adult at N1.1) toward higher plasma levels was observed. On the first day of withdrawal, when nicotine was completely cleared from the plasma, the adolescent animals had lower cotinine levels than the adult animals (P=0.007), suggestive of a higher clearance of cotinine in adolescent animals.
3H-epibatidine binding to receptors
Epibatidine is a high-affinity ligand of heteromeric nAChRs. To investigate nAChR expression during late postnatal development, we performed binding studies using 3H-Epi on 2% Triton X-100 extracted membranes from mPFC and occipital cortex, from animals ranging in age from early adolescence (P34) to adulthood (P104). Between P34 and P104, there was an almost linear decrease in nAChR levels (3H-Epi binding) in the mPFC (Supplemental Fig. S1; age, P<0.001), but not in the occipital cortex (age, n.s.). Epibatidine binds to all high-affinity heteromeric nicotinic receptors, of which α4 and β2 are the most commonly expressed subtypes Using immunoprecipitation experiments with antibodies (29) specific to the most abundant nicotinic receptor subunits α4, α5, and β2, we found that both α4 and β2 but not α5 subunits were down-regulated with age specifically in the mPFC (α4, P=0.03; β2, P=0.027), suggesting a specific down-regulation of α4β2-containing receptors.
To determine whether nicotine has a differential effect on the expression of nAChRs when administered during adolescence or adulthood, we examined the mPFC and occipital cortex of animals on the first withdrawal day and 5 wk following 10 d of repeated nicotine injections (3×/d), and we observed altered 3H-Epi-binding as an effect of age of treatment (age, F [1,84]=6.34, P=0.014), time after nicotine exposure (time, F [1,84]=30.54, P<0.001), a 4-way interaction of treatment × age × time × region (F [1,84]=4.63, P=0.034), and a trend for treatment (F [1,84]=3.042, P=0.085) and region (F [1,84]=2.93, P=0.091). When these 2 cortical regions were analyzed separately, only the mPFC showed an effect of age (F [1,47]=23.07, P<0.001) and time (F [1,47]=21.72, P<0.001) and a trend for treatment (F [1,47]=2.77, P=0.10) and interaction age × time × treatment (F [1,47]=3.32, P=0.075). In the occipital cortex, there was only an effect of time (F [1,37]=10.81, P=0.002), and a trend for age (F [1,37]=2.87, P=0.10), but no effect of treatment and no interaction (P=0.43 and 0.21, respectively; Table 1).
TABLE 1.
Binding analysis of nAChRs in the occipital cortex
| Age |
||||||
|---|---|---|---|---|---|---|
| Adolescent exposure, P44 |
Adult exposure, P70 |
|||||
| Abstinence time and binding |
P34 | Nic | Sal | Con | Nic | Sal |
| 1 d | ||||||
| 3H-Epi | 158.57 ± 7.63 | 184.69 ± 13.41 | 181.13 ± 9.98 | 161.97 ± 11.94 | 212.79 ± 12.15 | 173.10 ± 9.72 |
| 125I-αBgt | 18.76 ± 2.84 | 19.31 ± 2.39 | 21.18 ± 4.34 | 20.15 ± 0.82 | 24.54 ± 1.95 | 19.55 ± 2.53 |
| Adolescent exposure, P78 |
Adult exposure, P104 |
|||||
| 5 wk | ||||||
| 3H-Epi | 152.18 ± 8.67 | 161.93 ± 7.52 | 143.56 ± 5.05 | 166.30 ± 7.51 | 176.57 ± 6.19 | |
| 125I-αBgt | 15.48 ± 1.93 | 19.98 ± 2.59 | 17.19 ± 3.38 | 21.44 ± 3.27 | 14.88 ± 2.07 | |
Values represent mean ± se levels of radiolabeled nAChR protein (3H-Epi or 125I-αBgt binding sites, fmol/mg protein) for each treatment (Nic, nicotine; Sal, saline; Con, control) and age group (P44, P70, P78, P104) for animals (n=4–6 pools of 2 animals) exposed to nicotine during adolescence or during adulthood directly 1 d after exposure (P44, P70) or after 5 wk of abstinence (P78, P104). ANOVA did not detect significant effects (P<0.05) of age, treatment, or an interaction for the occipital cortex samples.
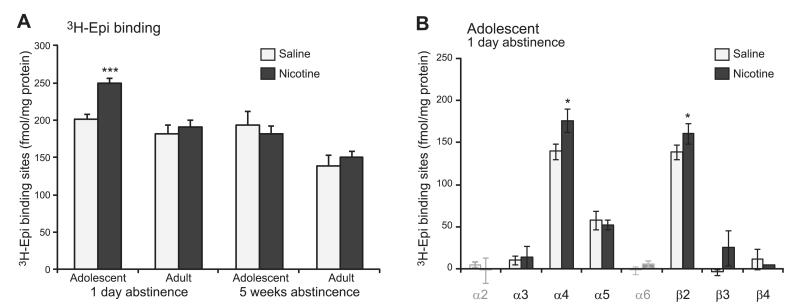
In summary, in the mPFC, the effect of age was apparent with increased 3H-Epi binding due to nicotine treatment, in the absence of an effect due to saline, only 1 d (nicotine adolescent vs. nicotine adult, P<0.001; adolescent nicotine vs. saline, P<0.001), but not 5 wk, following nicotine injections (Fig. 2A).
Figure 2.
Adolescent nicotine exposure increases in 3H-Epi-binding and α4β2-containing receptors in mPFC 1 d following nicotine exposure. A) Levels of radiolabeled nAChR protein (3H-Epi-binding sites, fmol/mg protein) are higher specifically on the first day following adolescent, but not adult, nicotine exposure (n=4–8 pools of 2 animals). B) Difference in levels of 3H-Epi-binding sites (fmol/mg protein) is explained by an increase in receptors containing the α4 and β2 subunits (α4: age F [1,40]=6.27, P=0.016, time F [1,40]=5.79, P=0.021; β2: age F [1,40]=8.53, P=0.006, time F [1,40]=12.36, P=0.001). The α5 subunit that substantially contributes to mPFC nAChRs is not changed. Data for the α2 and α6 subunits are indicated in gray, as they were not detected above background (see Table 2). *P < 0.05, ***P < 0.001 vs. saline control.
3H-Epi-labeled nAChR subunit composition in mPFC
To determine whether the increased 3H-Epi binding in the mPFC acutely after nicotine exposure was due to the up-regulation of α4β2* nAChRs in adolescent animals, we performed immunoprecipitation experiments in the mPFC. Only for the α4 and β2 subunits, but not other subunits, such as α5, did we find a significant increase in expression due to nicotine (α4-containing nAChRs, adolescent nicotine vs. saline, P=0.018; β2-containing nAChRs, adolescent nicotine vs. saline, P=0.030; Fig. 2B). We confirmed that this effect was absent in adult animals, and long after nicotine exposure (Table 2).
TABLE 2.
Immunoprecipitation analysis of the subunit content of nAChRs labeled with 2 nM 3H-Epi in the mPFC
| Age |
||||||
|---|---|---|---|---|---|---|
| Adolescent exposure, P44 |
Adult exposure, P70 |
|||||
| Abstinence time and subunit |
P34 | Nic | Sal | Con | Nic | Sal |
| 1 d | ||||||
| α2 | 15.9 ± 15.4 | ND | 4.6 ± 3.4 | ND | ND | ND |
| α3 | 18.4 ± 8.5 | 13.2 ± 13.4 | 9.8 ± 5.5 | 29.2 ± 24.2 | 14.2 ± 2.4 | 10.7 ± 4.8 |
| α4 | 146.8 ± 10.6 | 176.5 ± 13.6* | 139.8 ± 9.5 | 136.5 ± 11.2 | 126.8 ± 11.9 | 122.6 ± 8.3 |
| α5 | 33.0 ± 16.8 | 52.3 ± 5.4 | 57.6 ± 11.5 | 47.8 ± 15.1 | 22.3 ± 2.8 | 27.5 ± 5.6 |
| α6 | ND | ND | ND | ND | ND | ND |
| β2 | 131.2 ± 10.4 | 160.8 ± 12.6* | 138.6 ± 8.4 | 121.3 ± 4.1 | 119.1 ± 5.2 | 111.1 ± 10.5 |
| β3 | 20.3 ± 1.7 | 24.8 ± 20.7 | ND | 19.3 ± 1.8 | ND | 4.6 ± 5.8 |
| β4 | 2.9 ± 3.9 | 4.7 ± 3.2 | 11.6 ± 12.2 | ND | 4.9 ± 10.4 | 4.9 ± 8.9 |
| Adolescent exposure, P78 |
Adult exposure, P104 |
|||||
| 5 wk | ||||||
| α2 | 1.2 ± 2.1 | 13.4 ± 7.1 | 7.8 ± 5.7 | 5.8 ± 2.4 | 9.9 ± 6.3 | |
| α3 | 9.4 ± 4.6 | 6.8 ± 1.6 | 10.5 ± 1.5 | 16.0 ± 3.4 | 15.6 ± 4.2 | |
| α4 | 131.8 ± 6.8 | 131.0 ± 3.4 | 137.4 ± 8.6 | 116.5 ± 17.3 | 107.2 ± 13.3 | |
| α5 | 34.6 ± 5.3 | 42.6 ± 7.0 | 20.7 ± 5.2 | 26.1 ± 6.9 | 24.4 ± 7.5 | |
| α6 | 21.2 ± 10.5 | ND | ND | ND | ND | |
| β2 | 114.7 ± 21.2 | 116.2 ± 5.2 | 118.2 ± 2.6 | 105.5 ± 10.0 | 97.1 ± 13.6 | |
| β3 | ND | 20.5 ± 30.5 | 8.5 ± 9.4 | 5.7 ± 4.6 | 4.5 ± 1.9 | |
| β4 | ND | ND | ND | 0.4 ± 3.4 | 4.1 ± 8.2 | |
Values represent mean ± se levels of nAChRs (fmol immunoprecipitated receptors/mg protein; see Grady et al., ref. 29) for each treatment (Nic, nicotine; Sal, saline; Con, control) and age group (P44, P70, P78, P104) for animals (n=4–8 pools of 2 animals) exposed to nicotine during adolescence or during adulthood directly 1 d after exposure (P44, P70) or after 5 wk of abstinence (P78, P104). ND, not detected above background.
P < 0.05 vs. saline and control.
125I-αBgt binding
Unlike 3H-Epi binding nAChRs, the 125I-αBgt binding receptors (i.e., α7 homomeric receptors) showed no overall difference in postnatal developmental expression (mPFC, P=0.729; occipital cortex, P=0.292). In addition, for the nicotine treatment paradigm using a 4-factorial ANOVA, we found an effect of age (F [1,50]=7.07, P=0.029), region (F [1,50]=11.12, P=0.002), and time (F [1,50]=4.37, P=0.042), but no treatment effect and no 4-way interaction (P=0.583). Despite this, further analysis per region revealed only an age effect in the mPFC (F [1,24]=22,49, P<0.001) and a trend for time (F [1,26]=4.17, P=0.051) in the occipital cortex, but again no effect of treatment and no interactions. Therefore, we concluded that nicotine during adolescence does not affect the regulation of α7 receptors in cortical areas.
Nicotine modulation of synaptic transmission in mPFC pyramidal neurons
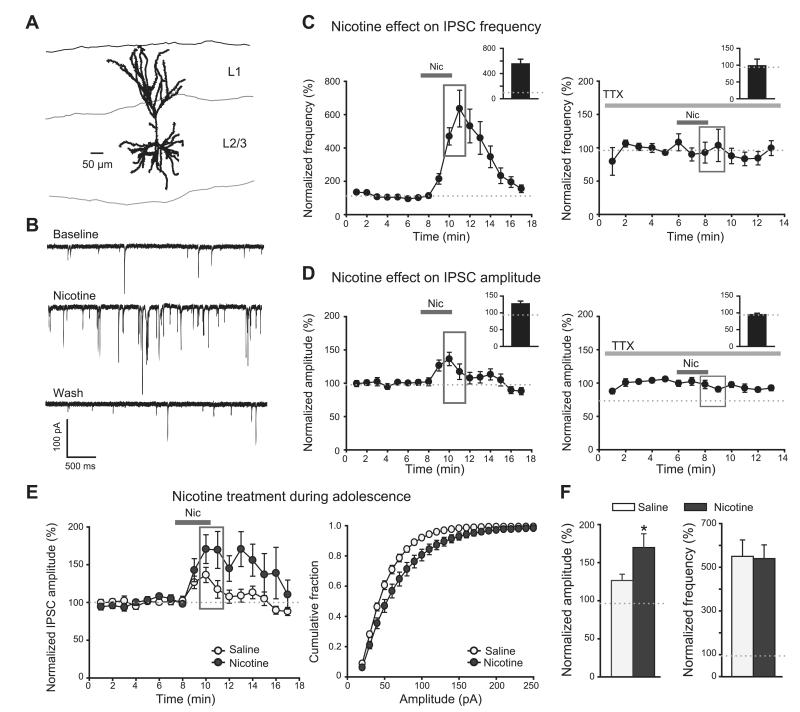
Next, we investigated whether increased expression of α4β2-containing nAChRs in adolescent nicotine-exposed animals had functional consequences for synaptic transmission in the mPFC. Unlike prefrontal cortical layer V, in which nicotine increases inhibitory and excitatory inputs on pyramidal neurons expressed on thalamocortical projections (34, 35), prefrontal cortex layer II/III shows no nicotine-induced increase in excitatory transmission, but nicotine rather augments GABAergic synaptic transmission (ref. 34 and Fig. 3C–D). Nicotinic receptors are expressed by interneurons already in 2–3-wk-old mice (34). To investigate whether nicotine by itself increases spontaneous inhibitory activity in layer II/III of the mPFC of adult animals, we applied nicotine (10 μM) to mPFC slices of saline-treated adolescent animals 1 d after exposure. Nicotine increased frequency (P<0.001; nicotine peak=528.9±76%, baseline=101.9±2.5%) and amplitudes (P=0.003; nicotine peak=126.8±8.1%, baseline=99.9±1.5%) of sIPSCs measured in pyramidal neurons in layer II/III (Fig. 3A–D). Nicotine had no effect on sIPSC frequency or amplitude when TTX was bath applied during the recording (1 μM; Fig. 3C, D), showing that nicotine effects in mPFC rely on firing of inhibitory neurons. The increase in sIPSC frequency reflects a larger number of spontaneous spikes, whereas the rise in sIPSC amplitude probably reflects a larger number of simultaneously occurring events.
Figure 3.
Nicotine affects sIPSC frequency and amplitude in layer II/III pyramidal neurons differently depending on age of nicotine exposure. A) During whole-cell recording, layer II/III pyramidal neurons in mPFC were filled with biocytin for post hoc morphological identification. B) Example of whole-cell recordings during baseline, nicotine application, and washout using animals that were exposed to saline during adolescence. C, D) sIPSC frequency (C) and amplitude (D) are increased by nicotine (10 μM), and these effects are abolished in TTX (1 μM); gray rectangles highlight the time points taken for calculation of the nicotine effect (insets). n = 14 cells from 7 animals, TTX data n = 7 cells from 3 animals. E) Left panel: effect of nicotine (10 μM) application on sIPSC amplitude during the whole-cell recordings in animals 1–2 d after adolescent nicotine or saline exposure (same as in D). Right panel: cumulative distributions of sIPSC amplitudes at the peak of the nicotine effect (at time points highlighted in gray in left panel). F) Left graph: nicotine effect on sIPSC amplitude is larger in animals 1–2 d after adolescent nicotine exposure (average taken during time points highlighted in gray). Right graph: nicotine effect on sIPSC frequency is not altered 1–2 d after nicotine exposure (average taken at same time points as in left panel). Data are from n=6 animals (17–21 cells)/treatment group. *P < 0.05.
We then asked whether the observed increase in α4β2-containing nAChRs acutely after nicotine exposure during adolescence in the mPFC had functional consequences and was paralleled by increased sensitivity to a nicotine challenge. We bath applied nicotine (10 μM) to mPFC slices of animals 1 d following adolescent nicotine or saline exposure, and measured spontaneous inhibitory activity. Nicotine-mediated enhancement of sIPSC frequency and amplitude was more pronounced in animals exposed to nicotine than in saline controls. Nicotine had a greater effect on sIPSC amplitude (P=0.026; nicotine effect in nicotine-treated animals=170.0±18.1%; nicotine effect in saline-treated animals=126.8±8.1%) and shifted the cumulative distribution to larger events (Fig. 3E, F; K-S test, Z=1.5, P=0.02). As a separate control experiment, we exposed animals to nicotine as adults. This group did not show any changes in sIPSC amplitude (Supplemental Fig. S2). Prior nicotine exposure had no effect on nicotine-induced changes in frequency of sIPSCs in any of the pretreatment groups (Fig. 3F, Supplemental Fig. S2). This is probably due to an already saturating effect of nicotine on sIPSC frequency (in some cases >10-fold increase) so that further increase in firing can only lead to more simultaneous events (higher sIPSC amplitude). Furthermore, as an additional control, we repeated these experiments, but then measured the nicotine-mediated enhancement of sIPSC frequency and amplitude 5 wk following nicotine exposure, a time point at which nicotine had no further effect on the number of membrane nAChRs as shown by unchanged levels in 3H-Epi binding (Fig. 2). Indeed, there was no difference in the nicotine-induced changes in sIPSC amplitude 5 wk after nicotine exposure in adolescentor adult-treated animals (Supplemental Fig. S3).
The increase in α4β2-containing nAChRs at 1 d, but not 5 wk, after nicotine exposure during adolescence (but not adulthood) is therefore paralleled by increased interneuron sensitivity to nicotine and leads to increased nicotine-induced firing in the inhibitory circuitry of the mPFC.
DISCUSSION
The current findings demonstrate that adolescents are more sensitive to nicotinic receptor up-regulation in the mPFC than adults. First, we showed that subcutaneous injections of nicotine (0.4 mg/kg) lead to plasma nicotine levels that are comparable with those observed in human smokers (31). We observed that naive rats show an age-related decrease in 3H-Epi-labeled high-affinity nicotinic receptors in the mPFC, but not in occipital cortex. Furthermore, adolescent but not adult nicotine exposure increases 3H-Epi binding of mPFC receptors on the first day of abstinence following 10 d of nicotine injections. This is paralleled by an mPFC-specific increase in expression of nAChRs containing α4 and β2 (but not α5) subunits that is transient in nature, as it is not observed 5 wk later. The increased expression of high-affinity nAChRs in adolescents is accompanied by an increase in nicotine-stimulated GABAergic transmission in the mPFC.
Treatment regimen
We have previously shown that subcutaneous injections of nicotine (0.4 mg/kg), 3×/d for 10 d causes long-term decrements in attention and impulsivity, only when administered during adolescence (17, 18) Here we used the same dose and exposure regimen to study expression of nicotinic receptors. Nicotine injections lead to peak plasma nicotine levels that are more similar to those associated with smoking in (adolescent) humans (31). Moreover, between injections, nicotine is entirely cleared from the plasma, thus leading to repeated activation of nAChRs.
The lower plasma nicotine levels observed in adolescent rats on the first day of nicotine exposure may be due to faster nicotine metabolism, because their plasma levels of cotinine (the active metabolite of nicotine) on the first day were slightly higher. It is known that adolescent rats metabolize nicotine more rapidly than adult rats (28). In addition, adolescent rats had lower plasma cotinine levels than adults on the first day of withdrawal. This could be the cause of known differences in positive and negative effects of nicotine in adolescents compared with adults. To our knowledge, this is the first report comparing plasma nicotine levels in adolescent vs. adult rats following nicotine injections.
nAChRs during development
During postnatal development, there is a decrease in expression of 3H-Epi-labeled nAChRs in rat mPFC that continues after adolescence. Although there is no developmental decline in α4 and β2 mRNA levels in the cortex, binding studies have shown that α4β2 expression is higher in adolescents than in adults (25). Developmental changes in surface expression of nAChRs are not regulated at the transcript level, but by post-translational mechanisms (36, 37). This developmental decline in expression correlates with functional differences, as it has been shown that nicotine-stimulated 86Rb+ efflux in frontal cortex peaks on P35, and then decreases at least until P63 (38).
The developmental decrease in α4β2 nAChR expression is selective to mPFC, as it was not observed in occipital cortex. This suggests developmental differences between primary and higher-order association cortex. The difference in regulation of nAChRs may reflect the different timing of maturation of these areas, as previously shown using proteomics experiments comparing mPFC and motorcortex (39), and for human brain development using imaging studies (27).
The decrease in expression of α4β2 receptors progresses far beyond adolescence (Fig. 2). This has also been shown by means of α4 immunostaining of mouse hippocampus, in which expression of α4 decreases with age from young adults (2–4 mo of age) to very old mice (24–28 mo of age) (40).
nAChRs after nicotine exposure
Repeated or prolonged nicotine exposure increases the number of high-affinity (mainly α4β2*) nicotine-binding sites, in heterologous systems (41, 42), nicotine-treated animals (11, 12), and in post mortem brains of smokers (ref. 10; for review, see ref. 43). Our data showed that the up-regulation of nicotinic receptors depends on age and brain region. Although we used extracts of membrane-enriched fractions, we are confident that the up-regulated 3H-Epi binding in mPFC due to adolescent nicotine exposure represents changes in number of nAChR, because the change is specific for mPFC and not occipital cortex; there is no difference in 125I-αBgt binding, so there is a specificity to the observation; and we have previously reported that there are no gross differences in number of mPFC neurons or number of synapses due to development (39). Furthermore, we confirmed that the increase in 3H-Epi-binding at 1 d after adolescent nicotine exposure was attributable to an increase in α4- and β2-containing receptors by subunit-specific immunoprecipitation. This age effect cannot be caused by higher plasma nicotine in adolescent rats, as our paradigm even caused slightly higher plasma nicotine levels in adults, whereas this did not lead to increased mPFC binding. This perhaps surprising discrepancy with current literature (25, 44) can be explained by the fact that the level of nAChRs in most other reports was determined on the last day of nicotine treatment, whereas we measured it on the first day of withdrawal, to prevent interference with circulating levels of nicotine. Previously Doura et al. (25) found that adult nicotine exposure causes a larger up-regulation of nAChRs in the PFC and most of the brain than adolescent nicotine exposure. However, it should be noted that the use of osmotic minipumps in the study of Doura et al. (25) led to higher plasma nicotine levels (~170–310 ng/ml) than those observed in our animals (~60 ng/ml), which makes it difficult to compare results. In addition, the acute nicotine effect was much larger in adults than in adolescents, thereby not explaining the increased sensitivity of adolescents for nicotine (25, 45). Up-regulated 3H-cystine binding, representing mainly a4b2* receptors, was found in the cerebral cortex of both adult (46) and adolescent (44) rats 2–3 d after the last exposure to nicotine using an osmotic minipump, although plasma nicotine levels were not reported. In comparison, we show that adolescents have a longerlasting and more pronounced up-regulation of mPFC nAChRs in response to an intermittent dosing regimen.
Functional consequences
Nicotinic receptors, to which both the endogenous ligand acetylcholine and nicotine bind, are important for cognitive functioning (47). Despite its acute effects, our data showed that neither adolescent nor adult nicotine exposure have long-term effects on the expression of nAChRs, in accordance with findings of others (46). The long-term effects of adolescent nicotine exposure on cognitive performance during adulthood (18) are therefore not directly caused by different nAChRs expression in the mPFC. However, the enhanced short-term up-regulation of high-affinity nAChRs in adolescent mPFC may have functional consequences and perhaps be the first step in a cascade of events leading to long-term adaptation of neuronal circuitry or other systemic effects.
Here we measured the functional consequences of altered numbers of nAChRs in the mPFC in an indirect way through nicotine-stimulated GABA release from GABAergic neurons and measuring GABA receptor-mediated responses in layer II/III pyramidal neurons. Thus, sIPSCs are used as a proxy to determine numbers of functional nAChRs, and most likely represent only a fraction of the total number of nAChRs in the mPFC. Nonetheless, this fraction represents an important functional contribution to nicotine effects in the PFC, due to the following reasons: nicotine has no effect on glutamatergic spontaneous transmission onto layer II/III pyramidal neurons (unpublished data); the majority of interneurons express nAChRs that are composed of α4β2*, although they also express other subunits (34); all sIPSC measurements were normalized to baseline, which eliminates any influence of possible changes in GABAergic tone with age; and since TTX completely blocks the effect of nicotine on sIPSCs, nAChRs must be located either on somas of interneurons or on the axons of GABAergic projections to PFC. Although there is no documented evidence that the latter exist anywhere in the brain, other than on glutamatergic projections, and we are unaware of any GABAergic projections from other brain regions into the PFC, this possibility cannot be excluded. Regardless of whether nAChRs are on axons of GABAergic projection neurons or PFC interneuron somas, these receptors would still be located within PFC slices in which long-range connections are cut. Thus, layer II/III nAChRs modulate GABAergic inputs onto pyramidal cells in PFC and are up-regulated by adolescent nicotine exposure.
Nicotinic receptors in the mPFC are localized presynaptically on glutamatergic and dopaminergic terminals, where they mediate glutamate (35, 48) and dopamine (49) release, respectively. In addition, different types of PFC interneurons in the mPFC express both α7 and α4β2-containing nAChRs on their cell bodies (34), and nAChR stimulation increases GABAergic transmission (34). We observed increased nicotine-induced GABAergic transmission, and this increased inhibitory neurotransmission has major consequences for the neuronal network. In vitro, the nAChR-stimulated increase in GABAergic transmission prevents induction of LTP by 100 Hz stimulation for 1 s (50). In addition, nicotine in the mPFC changes the rules for spike-timing-dependent plasticity and thereby prevents synaptic potentiation (34). This suggests that repeated nicotine exposure during adolescence, which increases the expression of α4β2-containing nAChRs, might have more pronounced effects on information processing in the mPFC. In particular, the increased activity of interneurons enhances synchronous inhibitory neurotransmission and thus occludes plasticity events to a higher extent. At a system level, this might contribute to differences in the acute rewarding effects of nicotine, and might underlie the increased sensitivity of adolescents (5, 51). In addition, an increase in inhibitory tone relates to higher rates of relapse to drug seeking (52).
CONCLUSIONS
Our data show that adolescent but not adult nicotine exposure increases the expression of nAChRs containing α4 and β2 subunits, specifically and transiently in rat mPFC, and leads to a concomitant increase in nicotine-induced GABAergic transmission. Extending our findings to humans, the difference in basal nAChR expression between adolescents and adults might play a role in the initiation of smoking among adolescents, whereas the short-term increased expression of nAChRs following repeated nicotine may contribute to changing local neuronal network plasticity rules, thus leading to maintenance of smoking behavior and decreasing the likelihood of smoking cessation.
The authors thank Martin Giera for his expertise with the extraction method to measure plasma nicotine levels. The authors also thank Rob Binnekade, Mathijs Stegeman, Dustin Schetters, and Yvar van Mourik for their excellent technical assistance in the behavioral paradigm. Funding was received from the Netherlands Organisation for Scientific Research (NWO-ZonMW) TOP project 91206148 (D.S.C., N.A.G., S.S., H.D.M., and A.B.S.), the European Union Seventh Framework Programme under grant agreement HEALTH-F2-2008-202088 (NeuroCypres project; A.B.S. and C.G.), and from the Italian PRIN 2009R7WCZS (C.G.). The authors report no conflicts of interest.
Supplementary Material
Abbreviations
- ACSF
artificial cerebrospinal fluid
- 125I-αBgt
125I-α-bungarotoxin
- 3H-Epi
3H-epibatidine
- K-S test
Kolmogorov-Smirnov test
- LC-ESI/MS
liquid chromatography–electrospray ionization mass spectrometry
- mIPSC
miniature inhibitory postsynaptic current
- mPFC
medial prefrontal cortex
- nAChR
nicotinic acetylcholine receptor
- P
postnatal day
- sIPSC
spontaneous inhibitory postsynaptic current
- TTX
tetradotoxin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Novick LF. Smoking is the leading preventable cause of death and disability in the United States. J. Public Health Manag. Pract. 2000;6:vi. doi: 10.1097/00124784-200006030-00001. [DOI] [PubMed] [Google Scholar]
- 2.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Chassin L, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychol. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- 4.Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin. Exp. Res. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- 5.O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl. 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev. Cogn. Neurosci. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem. Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J. Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 11.Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J. Pharmacol. Exp. Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- 12.Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- 13.Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol. Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- 14.Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I, Pistillo F, Clementi F, Gotti C. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol. Pharmacol. 2010;78:287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- 15.Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J. Pharmacol. Exp. Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- 16.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J. Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat. Neurosci. 2011;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- 18.Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- 19.Iniguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolanos-Guzman CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kota D, Robinson SE, Imad Damaj M. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem. Pharmacol. 2009;78:873–879. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- 21.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacol. (Berl.) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 22.Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol. Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacol. (Berl.) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 24.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol. Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 25.Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- 27.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacol. (Berl.) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 29.Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulointerpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks MJ, Whiteaker P, Collins AC. Deletion of the alpha7, beta2, or beta4 nicotinic receptor subunit genes identifies highly expressed subtypes with relatively low affinity for [3H]epibatidine. Mol. Pharmacol. 2006;70:947–959. doi: 10.1124/mol.106.025338. [DOI] [PubMed] [Google Scholar]
- 31.Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br. Med. J. 1980;280:972–976. doi: 10.1136/bmj.280.6219.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaac PF, Rand MJ. Cigarette smoking and plasma levels of nicotine. Nature. 1972;236:308–310. doi: 10.1038/236308a0. [DOI] [PubMed] [Google Scholar]
- 33.Hill P, Haley NJ, Wynder EL. Cigarette smoking: carboxyhemoglobin, plasma nicotine, cotinine and thiocyanate vs self-reported smoking data and cardiovascular disease. J. Chronic Dis. 1983;36:439–449. doi: 10.1016/0021-9681(83)90136-4. [DOI] [PubMed] [Google Scholar]
- 34.Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- 36.Ke L, Eisenhour CM, Bencherif M, Lukas RJ. Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes. I. Dose- and time-dependent effects of nicotine treatment. J. Pharmacol. Exp. Ther. 1998;286:825–840. [PubMed] [Google Scholar]
- 37.Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Britton AF, Vann RE, Robinson SE. Perinatal nicotine exposure eliminates peak in nicotinic acetylcholine receptor response in adolescent rats. J. Pharmacol. Exp. Ther. 2007;320:871–876. doi: 10.1124/jpet.106.112730. [DOI] [PubMed] [Google Scholar]
- 39.Counotte DS, Li KW, Wortel J, Gouwenberg Y, Van Der Schors RC, Smit AB, Spijker S. Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. Eur. J. Neurosci. 2010;32:1452–1460. doi: 10.1111/j.1460-9568.2010.07404.x. [DOI] [PubMed] [Google Scholar]
- 40.Rogers SW, Gahring LC, Collins AC, Marks M. Age-related changes in neuronal nicotinic acetylcholine receptor subunit alpha4 expression are modified by long-term nicotine administration. J. Neurosci. 1998;18:4825–4832. doi: 10.1523/JNEUROSCI.18-13-04825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol. Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- 42.Tumkosit P, Kuryatov A, Luo J, Lindstrom J. Beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic alpha6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol. Pharmacol. 2006;70:1358–1368. doi: 10.1124/mol.106.027326. [DOI] [PubMed] [Google Scholar]
- 43.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 45.Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol. Biochem. Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res. Bull. 2008;76:152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 48.Konradsson-Geuken A, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, Bruno JP. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur. J. Neurosci. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- 50.Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 51.Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacol. (Berl.) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN, Mansvelder HD, Smit AB, Spijker S, De Vries TJ. Prefrontal cortex AMPA receptor plasticity is crucial for cueinduced relapse to heroin-seeking. Nat. Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.