Abstract
While cautious criteria for selection of living kidney donors are credited for favorable outcomes, recent practice changes may include acceptance of less than ideal donors. To characterize trends in donor acceptance, the Renal and Lung Living Donors Evaluation (RELIVE) Study evaluated 8,951 kidney donors who donated between 1963 and 2007 at three major U.S. transplant centers. Over the study interval, there was an increase in the percentage of donors >40 years old from 38% to 51%; donors >60 years varied between 1% and 4%. The proportion of donors with obesity increased from 8% to 26% and with glucose intolerance from 9% to 25%. The percentage of hypertensive donors was consistent (5%–8%). Accepted donors ≥60 years old were more likely to have obesity, glucose intolerance, and/or hypertension compared to younger donors (p<0.0001). Our results demonstrate important trends in acceptance of older and more obese donors. The fraction of older donors accepted with glucose intolerance or hypertension remains small and for the majority includes mild elevations in glucose or blood pressure that were previously classified as within normal limits.
Keywords: living kidney donor, metabolic characteristics, obesity, hypertension, glucose intolerance, trends
Introduction
Failure of the deceased donor organ supply to meet the needs of the growing number of end-stage renal disease (ESRD) patients awaiting transplantation motivates donation from living donors. Data from national registries and single center series suggest that the lifetime risks of chronic kidney disease (CKD) and its associated comorbidities resulting from live kidney donation are minimal (1–7). Historically, these excellent outcomes are believed to result, at least in part, from careful pre-donation evaluation and conservative living donor eligibility standards (8).
More recently, acceptance criteria at some transplant centers have relaxed to allow living kidney donation by older individuals, and by donors with some known risk factors for the eventual development of CKD including obesity, glucose intolerance, and treated hypertension (9–12). These newer donor acceptance practices may be partially explained as the continuation of previous acceptance criteria that were based upon older and less stringent definitions of glucose intolerance, diabetes mellitus (13), and hypertension (14). Acceptance of donors who might be considered less than ideal, due to relaxed eligibility criteria or expanded diagnostic definitions of CKD risk factors, has brought into question the applicability of prior living donor outcome studies to current practices.
The Renal and Lung Living Donors Evaluation (RELIVE) Study is a research consortium funded by the National Institute of Allergy, Immunology and Infectious Diseases (NIAID), Health Resources and Services Administration (HRSA), and National Heart Lung and Blood Institute (NHLBI). RELIVE was established in 2006 to examine the epidemiology of living kidney donation at three large U.S. transplant centers (Mayo Clinic, Rochester, MN; University of Alabama at Birmingham, AL; and University of Minnesota, Minneapolis, MN) with a Data Coordinating Center (DCC) at the University of Michigan and Arbor Research Collaborative for Health, Ann Arbor, MI. In this study, our aim was to characterize temporal trends in living kidney donor demographics, and potential risk factors for CKD at the time of donation at the three RELIVE kidney transplant centers over five decades.
Materials and Methods
The study was approved by the Institutional Review Boards (IRBs) at NIAID, the study sites, and the DCC.
There were 8,951 live kidney donations at the three study sites from 1963 to 2007. The medical record of each donor was manually abstracted for pre-donation demographic information (date of birth, race, ethnicity), anthropometric measurements (height, weight, blood pressure), nicotine use, prior or current diagnosis or treatment for hypertension or hyperlipidemia, and laboratory data (fasting blood glucose, serum cholesterol, and triglycerides and serum creatinine). Blood pressure readings were collected from up to three separate time points during the donor evaluation. Family history of hypertension, diabetes mellitus, kidney disease, transient ischemic attack/stroke, or heart disease in the donor’s first degree relatives was recorded if documented. Obesity was defined as a body mass index (BMI) ≥30 kg/m2. Glucose intolerance was defined as a fasting blood glucose >100 mg/dL. Hypertension was defined as use of anti-hypertensive medications, a systolic blood pressure (SBP) ≥140 mmHg, or a diastolic blood pressure (DBP) ≥90 mmHg, using the lowest of up to three recorded blood pressure readings, as described above.
This study also used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN),. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Statistical methods
We examined trends in the domains of interest over time, with the years of donation grouped into quartiles: 1963–1974, 1975–1985, 1986–1996, and 1997–2007. We report means or percentages of donors with pre-donation characteristics of interest in each interval. Quantile regression was used to model the relation between year of donation and each of the five quantiles (5th, 25th, 50th, 75th, and 95th) of pre-donation characteristics (e.g., blood pressure). Quantile regressions were limited to years in which there were at least 20 donors with non-missing data. Donors were also grouped based on the presence or absence of pre-donation obesity, glucose intolerance, and hypertension. Percentages of younger (≤60 years old) and older (>60 years old) donors with one or more of these three conditions were compared by chi-square test. A p-value <0.05 was considered significant.
To address the degree to which our donor population represents the national donor population, we compared RELIVE donors to their complement in the larger cohort of SRTR donors for donations between 1991 and 2007, where the comparison SRTR data are at least 90% complete. A second analysis compared RELIVE and SRTR data for donations between 1995 and 2007, where the comparison SRTR data are 99% complete.
Statistical analyses were performed with SAS software, version 9.2 (SAS Institute; Cary, North Carolina, USA). The authors have followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines.
Results
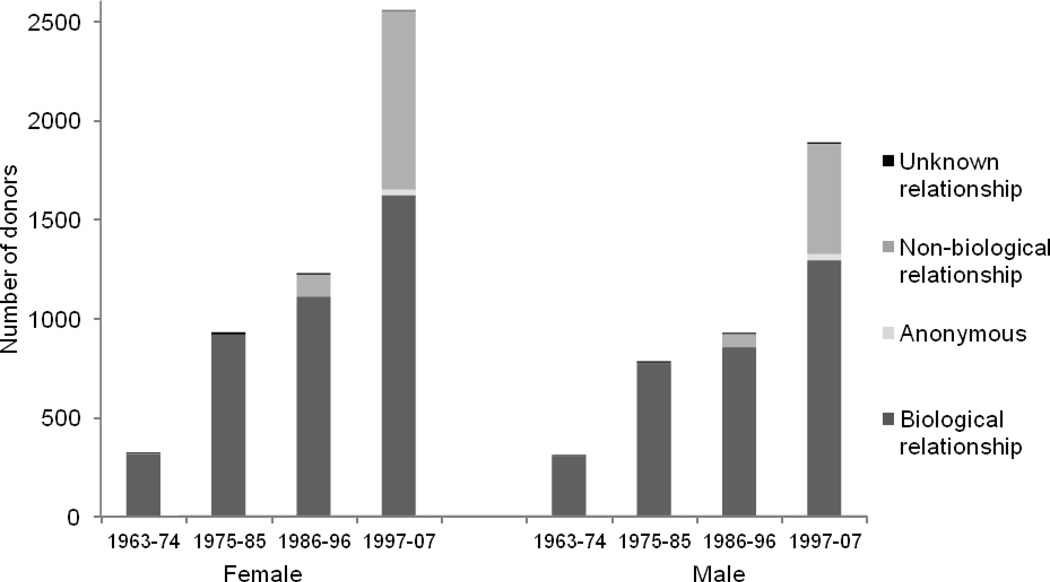
Demographic data are shown in Table 1. The number of living donors increased substantially at each center over time, overall nearly tripling from quartile 1 to quartile 2 and more than doubling from quartile 3 to quartile 4. Females made up 56% of living kidney donors and there was a trend on both an absolute and percentile basis for more females to be donors in recent years. Donors were predominantly white (86%). Nine percent of living donors were African American and the majority of these were at the University of Alabama at Birmingham site, where there was an increase in the proportion of African American donors over time. Overall, 80% of living kidney donors were biologically related to their recipient. However, in the most recent quartile (1997–2007), there was an increase in the percentage of non-biologically related donors and recipients (Figure 1). Non-biologically related donors increased from 1% to 35% among females and 1% to 29% among males from quartile 1 to quartile 4. Very few donors were non-directed (1%), and nearly all of those were at the University of Minnesota. Of note, the cohort pre-dated the use of paired donor exchanges at the three participating centers.
Table 1.
General characteristics of kidney donors at time of donation
| Era of Donation | |||||
|---|---|---|---|---|---|
| Quartile 1 (1963–1974) |
Quartile 2 (1975–1985) |
Quartile 3 (1986–1996) |
Quartile 4 (1997–2007) |
Total | |
| n (%)** | |||||
| Total | 635 (100) | 1,715 (100) | 2,155 (100) | 4,446 (100) | 8,951 (100) |
| Transplant center | |||||
| Mayo Clinic | 167 (26) | 300 (17) | 351 (16) | 1,523 (34) | 2,341 (26) |
| UAB | 102 (16) | 487 (28) | 797 (37) | 1,526 (34) | 2,912 (33) |
| UMN | 366 (58) | 928 (54) | 1,007 (47) | 1,397 (31) | 3,698 (41) |
| Female | 323 (51) | 931 (54) | 1,228 (57) | 2,557 (58) | 5,039 (56) |
| Race | |||||
| White | 580 (91) | 1,481 (86) | 1,846 (86) | 3,820 (86) | 7,727 (86) |
| African American | 22 (3) | 139 (8) | 229 (11) | 455 (10) | 845 (9) |
| Other or unknown | 33 (5) | 95 (6) | 80 (4) | 171 (4) | 379 (4) |
| Age (Y)* | 37.1 (11.9) | 35.9 (12.0) | 39.5 (11.2) | 41.5 (10.8) | 39.6 (11.5) |
| ≤ 30 | 228 (36) | 702 (41) | 534 (25) | 811 (18) | 2,275 (25) |
| 31 – 40 | 155 (24) | 454 (26) | 747 (35) | 1,333 (30) | 2,689 (30) |
| 41 – 50 | 153 (24) | 304 (18) | 516 (24) | 1,430 (32) | 2,403 (27) |
| 51 – 60 | 85 (13) | 182 (11) | 266 (12) | 718 (16) | 1,251 (14) |
| > 60 | 9 (1) | 55 (3) | 86 (4) | 154 (3) | 304 (3) |
| Unknown | 5 (1) | 18 (1) | 6 (0) | 0 (0) | 29 (0) |
| Tobacco Use | |||||
| Current | 273 (43) | 668 (39) | 699 (32) | 1,235 (28) | 2,875 (32) |
| Former | 47 (7) | 213 (12) | 308 (14) | 733 (16) | 1,301 (15) |
| Never | 234 (37) | 680 (40) | 1,094 (51) | 2,436 (55) | 4,444 (50) |
| Unknown | 81 (13) | 154 (9) | 54 (3) | 42 (1) | 330 (4) |
| Relationship to recipient | |||||
| Biological | 621 (98) | 1,692 (99) | 1,961 (91) | 2,914 (66) | 7,188 (80) |
| Non-biological | 6 (1) | 3 (0) | 182 (8) | 1,459 (33) | 1,650 (18) |
| Anonymous | 0 (0) | 0 (0) | 0 (0) | 62 (1) | 62 (1) |
| Unknown | 8 (1) | 20 (1) | 12 (1) | 11 (0) | 51 (1) |
Mean (SD)
Missing values occurred in age (0.3%), history of tobacco use (0.01%), and relationship to recipient (0.6%).
Figure 1. Total number of donors and relationship of donor to recipient by sex.
The number of living donors increased substantially, from quartile 1 to quartile 4. There was a trend on both an absolute and percent basis for more female donors in recent years. There was a notable increase in the percentage of non-biologically related donors in the 1997–2007 quartile.
The mean age was lowest in quartile 2, and rose in quartiles 3 and 4, reaching 41.5±10.8 years in the most recent quartile (Table 1 and Figure 2). This trend primarily reflects an increase in the percentage of donors aged 41 – 60 and a decline in those ≤ 30 years old. Notably, from quartile 2 on, the percent of donors >60 remained constant at 3% to 4%. Only 6 of 304 older donors were black. There was a progressive increase in the percentage of donors who were former smokers or who never smoked, and a corresponding decrease in the percentage of current smokers. Among those donating to a first degree biological relative, 77% to 94% indicated a family history of kidney disease and 34% to 45% indicated a family history of diabetes. However, these rates may include the donor’s recipient. Rates of kidney disease or diabetes in first degree relatives were lower for donors who were either more distant relatives or were unrelated to their recipient (3%–12%, p<0.0001, and 20%–25%, p<0.0001, respectively) than rates among first degree biological relatives.
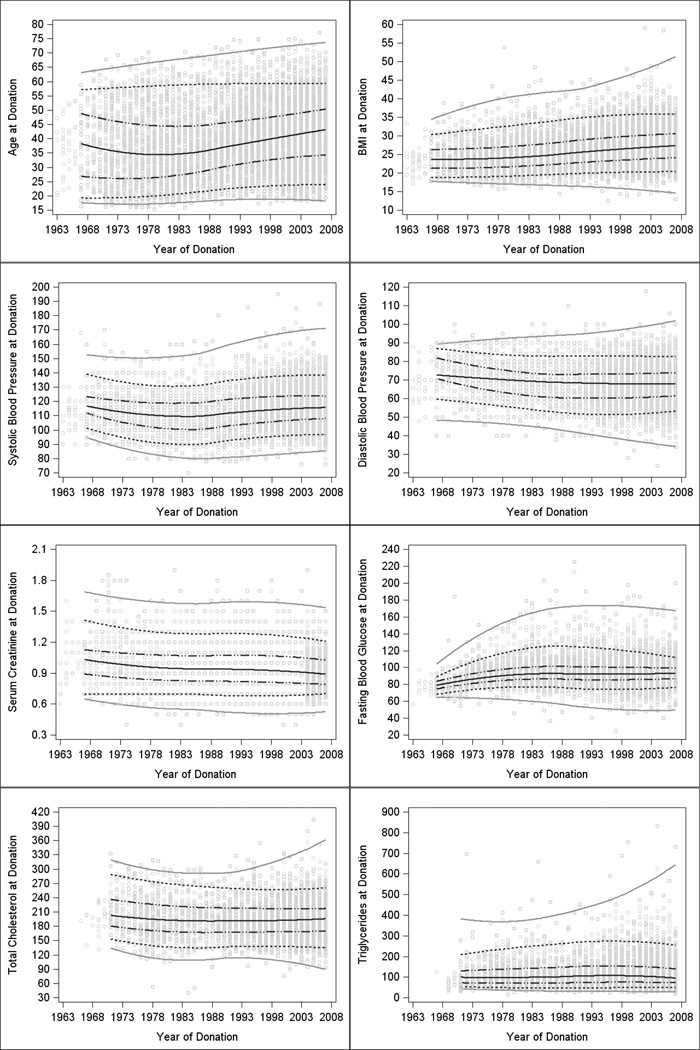
Figure 2. Characteristics of living donors by year of donation.
Plots of pre-donation characteristics by year of donation with smoothed trends (loess curves) over time for each quantile (5th, 25th, 50th, 75th, and 95th). Gray circles represent data points. The middle solid black line represents the median. The black dotted-dashed lines are the 25th and 75th percentiles. The black dotted lines are the 5th and 95th percentiles and the solid grey lines are smoothed through the minima and maxima. The slopes and significance of tests for linear trends are shown in Table 4.
Additional analyses of trends in pre-donation characteristics over time are presented in Table 2 and Figure 2. The median values for age at donation, BMI, fasting blood glucose, and SBP increased over time, while DBP decreased over the study interval (p<0.0001 for each, Table 4). Mean BMI at donation increased progressively from quartile 1 to 4, as did the percentage of living kidney donors meeting the World Health Organization definition for overweight (BMI from 25–29.9 kg/m2) and obese (BMI ≥30 kg/m2). Overall, 20% of all donors had a BMI ≥30 kg/m2. In the most recent quartile, 26% of donors were obese compared to 8% in quartile 1 (Table 2). Pre-donation glucose intolerance increased from 9% in quartile 1 to 21% in quartile 2 and was present in almost one-quarter of living donors thereafter. There was no statistically significant change in median cholesterol, triglycerides, or creatinine. There were changes in the maximum values over time, which trended upward for each characteristic except creatinine (Table 4 and Figure 2).
Table 2.
Metabolic parameters
| Era of Donation | ||||||
|---|---|---|---|---|---|---|
| N** | Quartile 1 (1963–1974) |
Quartile 2 (1975–1985) |
Quartile 3 (1986–1996) |
Quartile 4 (1997–2007) |
Total | |
| BMI* | 8,598 | 24.3 (3.9) | 24.7 (4.4) | 26.2 (4.6) | 27.3 (4.8) | 26.4 (4.7) |
| n(%) | ||||||
| < 25 | 333 (52) | 965 (56) | 891 (41) | 1,524 (34) | 3,713 (41) | |
| 25 – 29.9 | 143 (23) | 467 (27) | 768 (36) | 1,734 (39) | 3,112 (35) | |
| 30 – 34.9 | 43 (7) | 137 (8) | 298 (14) | 868 (20) | 1,346 (15) | |
| 35 – 39.9 | 8 (1) | 30 (2) | 83 (4) | 226 (5) | 347 (4) | |
| ≥ 40 | 0 (0) | 10 (1) | 14 (1) | 56 (1) | 80 (1) | |
| Unknown | 108 (17) | 106 (6) | 101 (5) | 38 (1) | 353 (4) | |
| Glucose* | 8,298 | 86.0 (13.0) | 94.2 (14.2) | 94.9 (16.2) | 93.5 (13.5) | 93.5 (14.4) |
| < 100 | 403 (63) | 1,160 (68) | 1,382 (64) | 3,273 (74) | 6,218 (69) | |
| 100 – 125 | 48 (8) | 300 (17) | 466 (22) | 1,016 (23) | 1,830 (20) | |
| 126 – 139 | 5 (1) | 31 (2) | 50 (2) | 56 (1) | 142 (2) | |
| ≥ 140 | 2 (0) | 27 (2) | 39 (2) | 40 (1) | 108 (1) | |
| Unknown | 177 (28) | 197 (11) | 218 (10) | 61 (1) | 653 (7) | |
| Cholesterol* | 4,251 | 209.1 (41.3) | 194.0 (41.9) | 195.5 (38.1) | 194.4 (37.2) | 195.1 (38.3) |
| Triglycerides* | 4,358 | 108.2 (71.9) | 116.7 (64.1) | 123.9 (71.9) | 124.3 (75.0) | 122.4 (72.6) |
Mean (SD)
Missing values occurred in cholesterol (490 (77%) in 1963–1974, 1,063 (62%) in 1975–1985, 1,294 (60%) in 1986–1996 and 1,853 (42%) in 1997–2007), and triglyercides (497 (78%) in 1963–1974, 963 (56%) in 1975–1985, 1,274 (59%) in 1986–1996 and 1,859 (42%) in 1997–2007).
Table 4.
Relation between living donor characteristics and year of donation, per 10 year increase.
| 5th percentile | 25th percentile | 50th percentile | 75th percentile | 95th percentile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Age at donation | 1.55 | <0.0001 | 2.88 | <0.0001 | 2.87 | <0.0001 | 1.43 | <0.0001 | 0.51 | 0.0133 |
| BMI | 0.56 | <0.0001 | 0.90 | <0.0001 | 1.14 | <0.0001 | 1.26 | <0.0001 | 1.33 | <0.0001 |
| Systolic BP | 2.00 | <0.0001 | 2.00 | <0.0001 | 1.74 | <0.0001 | 1.62 | <0.0001 | 2.42 | <0.0001 |
| Diastolic BP | −1.25 | 0.0008 | −0.67 | 0.0461 | −0.77 | <0.0001 | −1.35 | <0.0001 | −0.56 | 0.0558 |
| Fasting blood glucose | 0.38 | 0.1695 | 1.07 | <0.0001 | 1.18 | <0.0001 | 0.71 | 0.0002 | −1.36 | 0.1276 |
| Cholesterol | 0.71 | 0.4591 | 0.00 | 1.0000 | 0.00 | 1.0000 | −2.31 | 0.0066 | −7.20 | <0.0001 |
| Triglycerides | 0.67 | 0.3041 | 0.34 | 0.5633 | 0.29 | 0.7973 | 1.61 | 0.2550 | 11.82 | 0.0217 |
| Serum creatinine | −0.00 | 0.0011 | −0.00 | <0.0001 | −0.01 | 0.1212 | −0.00 | 1.0000 | −0.00 | 1.0000 |
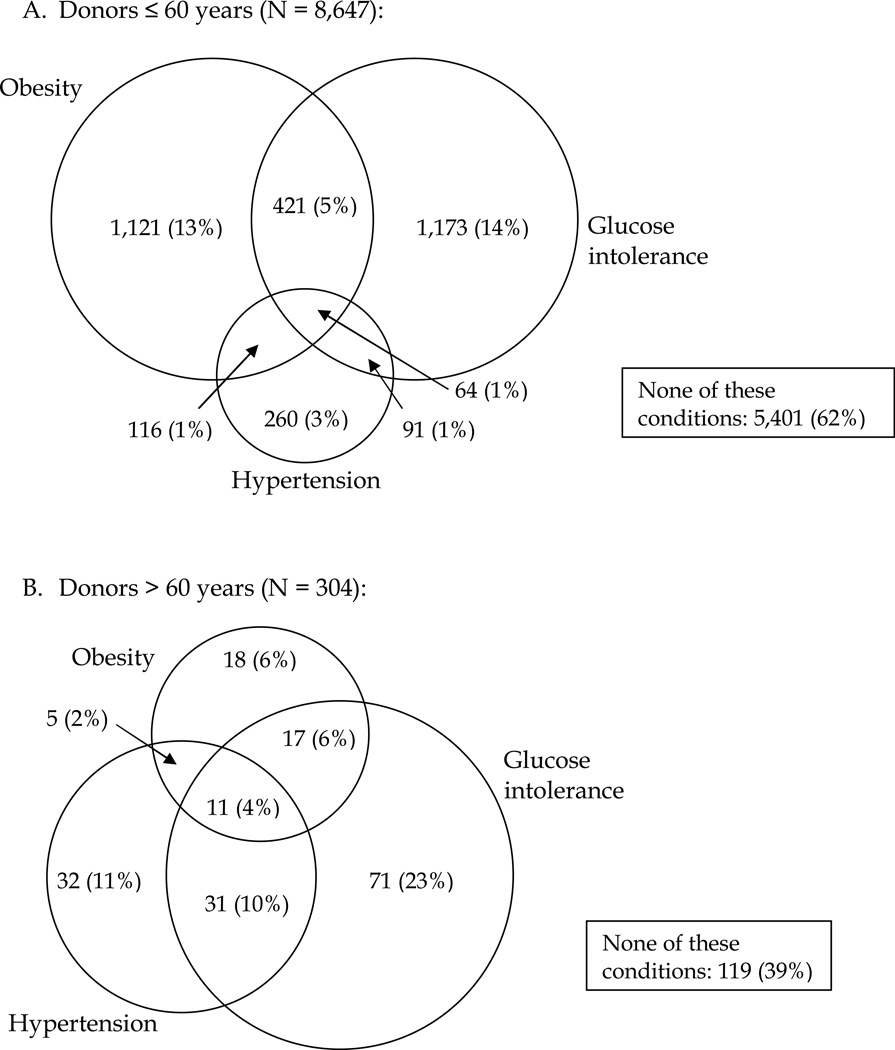
We also assessed the presence of one or more of the following conditions: obesity, glucose intolerance, and hypertension in two donor age groups (≤ 60 and > 60 years) (Figure 3). The prevalence of obesity was similar in donors ≤60 years and those >60 years (20% vs. 17%, respectively; p=0.1772). Glucose intolerance and hypertension were both significantly less prevalent in donors ≤ 60 years than >60 years (20% vs. 43%, p < 0.0001 and 6% vs. 26%, p < 0.0001, respectively). The proportion of donors with more than one of these three conditions was significantly higher among the older donors (21% vs. 8%, p < 0.0001). Sixty-two percent of the younger donors and 39% of the older donors had none of these three conditions, 7% and 17% had two, and 1% and 4% had all three conditions, respectively.
Figure 3. Clustering of obesity, glucose intolerance, and hypertension*.
Donors are categorized into groups based on pre-donation BMI ≥ 30 kg/m2, fasting blood glucose > 100 mg/dL, and diagnosis of hypertension using Venn diagrams and compared by age (younger ≤60 years old and older >60 years old) using the chi-square test.
* The overall Chi-square statistic is significant (χ2 = 318.2, p < 0.0001), indicating the clustering of obesity, glucose intolerance, and hypertension differs by age category.
Among donors 60 years of age and younger, the percentage with obesity, glucose intolerance, hypertension, or a combination of these conditions differed by sex. Younger male donors were more likely to have hyperglycemia only, while younger female donors were more likely to have none of the three conditions (p < 0.0001). Obesity, glucose intolerance, and hypertension prevalence did not differ by sex among donors older than 60 (p = 0.064). Black donors aged 60 years and under were more likely than younger non-black donors to be obese only or to have both obesity and hyperglycemia; younger non-black donors were more likely to have none of the three conditions (p < 0.0001). There were only 6 black donors older than age 60, of whom 4 had one or more of obesity, glucose intolerance, and hypertension (1 with one, 2 with two, 1 with three characteristics). The mean pre-donation systolic blood pressure (SBP) increased over time, primarily for male and black donors; mean diastolic blood pressure (DBP) decreased from quartile 1 to quartile 3 primarily for female donors (Table 3). A minority of accepted donors (n = 230) were taking antihypertensive medications at the time of donation, and 78% of these donated after 1997. The overwhelming majority (92%) of treated hypertensive donors had a SBP <140 mmHg, and 5 had a DBP ≥90 mm Hg.
Table 3.
Blood pressure
| Era of Donation | ||||||
|---|---|---|---|---|---|---|
| N** | Quartile 1 (1963–1974) |
Quartile 2 (1975–1985) |
Quartile 3 (1986–1996) |
Quartile 4 (1997–2007) |
Total | |
| n(%) | ||||||
| Hypertension | ||||||
| No | 591 (93) | 1,633 (95) | 2,036 (94) | 4,081 (92) | 8,341 (93) | |
| Yes | 44 (7) | 82 (5) | 119 (6) | 365 (8) | 610 (7) | |
| SBP* | 8,741 | 114.9 (12.5) | 110.2 (12.0) | 112.3 (13.3) | 116.4 (13.0) | 114.1 (13.1) |
| < 120 | 339 (53) | 1,226 (71) | 1,467 (68) | 2,617 (59) | 5,649 (63) | |
| 120 – 139 | 198 (31) | 370 (22) | 581 (27) | 1,615 (36) | 2,764 (31) | |
| ≥ 140 | 28 (4) | 38 (2) | 77 (4) | 185 (4) | 328 (4) | |
| Unknown | 70 (11) | 81 (5) | 30 (1) | 29 (1) | 210 (2) | |
| DBP* | 8,741 | 72.2 (9.0) | 69.1 (8.9) | 67.2 (9.8) | 67.7 (9.5) | 68.1 (9.5) |
| < 70 | 151 (24) | 705 (41) | 1,139 (53) | 2,464 (55) | 4,459 (50) | |
| 70 – 89 | 392 (62) | 891 (52) | 945 (44) | 1,910 (43) | 4,138 (46) | |
| ≥ 90 | 22 (3) | 38 (2) | 41 (2) | 43 (1) | 144 (2) | |
| Unknown | 70 (11) | 81 (5) | 30 (1) | 29 (1) | 210 (2) | |
| SBP in treated donors* | 230 | 119.8 (5.2) | 115.2 (12.6) | 117.2 (14.1) | 123.5 (13.1) | 122.0 (13.3) |
| < 120 | 4 (67) | 10 (48) | 13 (57) | 59 (33) | 86 (37) | |
| 120 – 139 | 2 (33) | 11 (52) | 7 (30) | 105 (58) | 125 (54) | |
| ≥ 140 | 0 (0) | 0 (0) | 3 (13) | 16 (9) | 19 (8) | |
| DBP in treated donors* | 230 | 78.0 (4.2) | 74.0 (9.4) | 71.0 (9.7) | 72.8 (8.8) | 72.9 (8.9) |
| < 70 | 0 (0) | 6 (29) | 9 (39) | 51 (28) | 66 (29) | |
| 70 – 89 | 6 (100) | 15 (71) | 13 (57) | 125 (69) | 159 (69) | |
| ≥ 90 | 0 (0) | 0 (0) | 1 (4) | 4 (2) | 5 (2) | |
Mean (SD)
Missing values occurred in systolic and diastolic blood pressure (2.3% overall) but not among donors known to be treated with medication for hypertension.
Comparison of the RELIVE donor population to non-RELIVE donors in SRTR data during the time period from 1991–2007, or restricted to 1995–2007 where the comparison data were 99% complete, indicated similar characteristics (data not shown). Linear trends in age, sex, and race were not different between donors at RELIVE centers and donors in non-RELIVE centers. We were not able to compare BMI since it was missing for 42% of the SRTR sample.
Discussion
In this large cohort of living kidney donors, we observed notable trends in donor characteristics over the last five decades: the number of living kidney donors at the three study centers increased steadily over time, there was a marked rise in the proportion of donors unrelated to their recipient, and a higher proportion of female donors. While the mean age at donation increased, donors > 60 years constituted a stable minority of up to 4% over the period studied.
We observed trends over time toward higher values in the upper end of the distributions of all characteristics reported except serum creatinine. In contrast, mean values for fasting blood glucose and blood pressure changed little over time. In terms of applying a label of glucose intolerance to measured blood glucose values or a label of hypertension to blood pressure measurements, the use of both older and more contemporary label definitions demonstrated fairly stable donor acceptance practice across the most recent three time quartiles. There were very few donors (1%–2%) with fasting blood glucose above 125 mg/dL in any quartile. While an increasing number of programs reported willingness to accept hypertensive donors, particularly older ones, practices among the three RELIVE study sites extended only to those with milder forms of hypertension. The majority of recorded blood pressure measurements were below 140/90 mm Hg, and these donors may have been accepted after factoring in measurement circumstances (e.g., white coat hypertension) that were not available for analysis. Ambulatory blood pressure monitoring was not performed in earlier years or at all centers. While it would be misleading to label an individual as hypertensive by a single reading, even using the highest of three possible recorded blood pressure values, there was not a trend toward higher pre-donation blood pressure over time.
The national trend of an increasing prevalence of obesity in the general population as reported in NHANES (15) is clearly mirrored among donors. It is notable that 26% of all donors had a BMI ≥ 30 kg/m2 in the most recent era compared to 8% in 1963–1974. We believe some of those donors were accepted after careful evaluation, based on the absence of other adverse metabolic conditions or were muscular in build. Similarly, 22% of donors in the most recent era had glucose intolerance compared to 7% in 1963–1974. The acceptance of donors with recorded glucose measurements clearly outside of acceptable range for any era does raise concerns. There may have been additional normal values documented by a local provider, but not captured by our abstraction approach. While cholesterol and triglyceride levels were relatively stable over time, there were fewer donors with high cholesterol values and more donors with high triglyceride levels, tracking with increased BMI.
The lifetime risk of hypertension is very high in the general population. Hypertension prevalence increases with age in Western societies and the treatment is generally effective and well-accepted. In fact, one’s risk of developing this condition, even if normotensive at age 55, is around 90% by age 80 (16). This is of great relevance, particularly to the younger donor, as optimal health at a young age does not guarantee continued health decades later, particularly if the young donor does not adopt healthy lifestyle practices. Reports of higher hypertension prevalence rates in donors (17) or of incrementally higher blood pressure after living kidney donation (18) may reflect earlier occurrence, or earlier detection and treatment. It is reassuring to see that only 6% of donors 60 years and younger were hypertensive in contrast to 26% of those over 60.
We did observe greater tolerance for multiple potential risk conditions in donors over age 60. A minority of even these older donors had all three conditions of obesity, glucose intolerance, and hypertension, but 61% had at least one, compared to 38% of donors age 60 or lower. We conclude that these transplant centers were potentially willing to tolerate one or two of these conditions primarily in the older donor but less commonly would accept all three. Stricter criteria appear to be applied to younger donors, with lower percentages with even a single risk factor for CKD, in particular hypertension or glucose intolerance.
Data on acceptance of donors with isolated medical abnormalities (IMAs) are limited, often to small case series. A systematic review identified 37 reports, each including at least three donors with IMAs; seven of these were reported only as abstracts (9). Twenty-two studies included older donors, ten studies obese donors, and six studies hypertensive donors. Whether there is a trend to greater acceptance of donors with IMAs was not clear from prior analyses, as results were not stratified by donation era and definitions were not standardized. Our results illustrate that the trend of increasing obesity seen in the general population is reflected in accepted donors but also indicate stable rates of glucose intolerance or hypertension in donors, likely related to the continuation of previous acceptance criteria despite adoption of lower numerical thresholds for diagnosis of these conditions in other settings. Still, the inclusion of donors with elevated blood pressure or hyperglycemia merits review and reinforces the need for follow-up.
This study was limited by the use of cross-sectional data not collected specifically for research purposes and not verified at the time of collection. Comparison of RELIVE donors to the national donor population recorded by the SRTR suggests that the donor trends seen in RELIVE were not different from the larger SRTR cohort. We may have overestimated the prevalence of pre-donation hypertension. We recorded up to three readings, but measurement techniques have changed over time and we could not document use of inappropriate cuff size given the retrospective study design. We did not have sufficient information on ambulatory blood pressure measurements to exclude white coat hypertension that may have been otherwise ascertained by the evaluating teams. Similarly, we recorded the glucose value closest to but prior to donation. We did not record glucose measurements done locally or at the center prior to the pre-operative visit that may have been taken under strict fasting conditions. There could have been additional values that were normal that were taken into consideration in the process of donor approval. Data on hemoglobin A1c or glycosylated hemoglobin were available in only 767 (8.6%) cases. They were mostly normal. Oral glucose tolerance testing was not used routinely across centers, and we did not record this information.
With three centers located in two geographic regions of the United States, we chose to analyze our data as an aggregated cohort. We examined only the characteristics of actual donors and cannot assess acceptance rates among those who presented as potential donors. With only three centers, we could not comprehensively analyze center-based practice variation.
Living donors have historically been held to a higher health standard than that of the general population, both to ensure long-term health and stability for the donor and to protect the recipient from transplantation with a less than optimal organ. As the transplant candidate population ages, they may have fewer healthy prospective living donors available, resulting in consideration of those with isolated imperfections. If an organ with fewer potential future years of function is to be transplanted into an older recipient with a shorter remaining lifespan, organ function may be adequate to serve both donor and recipient over their remaining years of life. Furthermore, as most renal diseases present in mid-life or later, thorough evaluation of a young individual does not necessarily provide clues or guarantee protection from the later onset of disease. Based on our data and those reported by others, tolerance for associated medical risk factors among young donors has been reported to be declining (19), perhaps contributing to a greater willingness to consider imperfections among older donor candidates with fewer future years at risk for late complications of donation. Short and mid- term reports regarding outcomes for hypertensive (20) and obese (21) donors suggest that these donors have similar outcomes to donors without these conditions.
Living kidney donation experienced explosive growth over the study period, with greater use of donors between the ages of 41 and 60 years and of obese donors. At the same time, utilization of donors with hypertension or glucose intolerance by current definitions is not new and in fact has remained steady. While the percentage of hypertensive donors was 5% to 8%, glucose intolerance was more common, affecting 19% to 24% of donors from 1975 on. The percent of donors age 30 years and younger declined from 41% to 18%, while the percentage of donors older than 60 remained low at 3% to 4%. A possible explanation for these trends is greater focus by transplant programs on long-term outcomes for living kidney donors with perhaps lower inclination to accept young donors due to uncertainties regarding their lifetime risk for future diseases. Stable and limited acceptance of older donors, donors with obesity or glucose intolerance, and hypertensive donors supports the adoption of thoughtful individual case review in the donor approval process.
Taken together, this combined data set from three large transplant centers indicates that the overwhelming majority of accepted donors met consistent glucose tolerance and blood pressure criteria. In fact, with the exception of age and BMI, there were only minimal changes observed for most medical characteristics of living kidney donors over the course of the last five decades. We did see an increase in the maximal values for all reported parameters, highlighting the need for prospective data collection and long term follow-up.
Acknowledgments
This research was performed as a project of the Renal and Lung Living Donors Evaluation Study (RELIVE), a collaborative clinical research consortium sponsored by the National Institute of Allergy and Infectious Diseases, Health Resources and Services Administration and National Heart, Lung, and Blood Institute.
Some data reported here have been supplied by Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government
Abbreviations
- ESRD
end-stage renal disease
- CKD
chronic kidney disease
- RELIVE
Renal and Lung Living Donors Evaluation Study
- NIAID
National Institute of Allergy, Immunology and Infectious Diseases
- HRSA
Health Resources and Services Administration
- NHLBI
National Heart Lung and Blood Institute
- DCC
Data Coordinating Center
- IRBs
Institutional Review Boards
- BMI
body mass index
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- IMAs
isolated medical abnormalities
Footnotes
Disclosure
The authors of the manuscript have no conflicts of interest to disclose as described by the Americal Journal of Transplantation.
References
- 1.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tyden G, Groth CG. Kidney donors live longer. Transplantation. 1997;64(7):976–978. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 2.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-Term Consequences of Kidney Donation. N Engl J Med. 2009;360(5):459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramcharan T, Matas AJ. Long-Term (20–37 years) follow-up of living kidney donors. Am J Transplant. 2002;2:959–964. doi: 10.1034/j.1600-6143.2002.21013.x. [DOI] [PubMed] [Google Scholar]
- 5.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340(8823):807–810. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb DA, Matin SF, Braun WE, Schreiber MJ, Mastroianni B, Papjcik D, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047. [PubMed] [Google Scholar]
- 7.Anderson CF, Velosa JA, Frohnert PP, Torres VE, Offord KP, Vogel JP, et al. The risks of unilateral nephrectomy: Status of kidney donors 10 to 20 years postoperatively. Mayo Clin Proc. 1985;60:367–374. doi: 10.1016/s0025-6196(12)60845-3. [DOI] [PubMed] [Google Scholar]
- 8.Davis CL, Delmonico FL. Living-Donor Kidney Transplantation: A Review of the Current Practices for the Live Donor. J Am Soc Nephrol. 2005;16(7):2098–2110. doi: 10.1681/ASN.2004100824. [DOI] [PubMed] [Google Scholar]
- 9.Young A, Storsley L, Garg AX, Treleaven D, Nguan CY, Cuerden MS, et al. Health Outcomes for Living Kidney Donors with Isolated Medical Abnormalities: A Systematic Review. Am J Transplant. 2008;8(9):1878–1890. doi: 10.1111/j.1600-6143.2008.02339.x. [DOI] [PubMed] [Google Scholar]
- 10.Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, et al. The Medical Evaluation of Living Kidney Donors: A Survey of US Transplant Centers. Am J Transplant. 2007;7(10):2333–2343. doi: 10.1111/j.1600-6143.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis CL, Cooper M. The State of U.S. Living Kidney Donors. Clin J Am Soc Nephrol. 2010;5(10):1873–1880. doi: 10.2215/CJN.01510210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reese P, Feldman H, McBride M, Anderson K, Asch D, Bloom R. Substantial Variation in the Acceptance of Medically Complex Live Kidney Donors Across US Renal Transplant Centers. Am J Transplant. 2008;8(10):2062–2070. doi: 10.1111/j.1600-6143.2008.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chobanian AV, Committee NC. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US Adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 16.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: Thre Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 17.Garg AX, Prasad GVR, Thiessen-Philbrook HR, Ping L, Melo M, Gibney EM, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86(3):399–406. doi: 10.1097/TP.0b013e31817ba9e3. [DOI] [PubMed] [Google Scholar]
- 18.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, et al. Meta-Analysis: Risk for Hypertension in Living Kidney Donors. Ann Intern Med. 2006;145(3):185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 19.Steiner RW. ‘Normal for Now’ or ‘At Future Risk’: A Double Standard for Selecting Young and Older Living Kidney Donors. Am J Transplant. 2010;10(4):737–741. doi: 10.1111/j.1600-6143.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 20.Textor SC, Taler SJ, Driscoll N, Larson TS, Gloor J, Griffin M, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78:276–282. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 21.Heimbach JK, Taler SJ, Prieto M, Cosio FG, Textor SC, Kudva YC, et al. Obesity in living kidney donors: Clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant. 2005;5:1057–1064. doi: 10.1111/j.1600-6143.2005.00791.x. [DOI] [PubMed] [Google Scholar]