Abstract
Objectives. We examined how risk behaviors differentially connect a population at high risk for sexually transmitted infections.
Methods. Starting from observed networks representing the full risk network and the risk network among respondents only, we constructed a series of edge-deleted counterfactual networks that selectively remove sex ties, drug ties, and ties involving both sex and drugs and a comparison random set. With these edge-deleted networks, we have demonstrated how each tie type differentially contributes to the connectivity of the observed networks on a series of standard network connectivity measures (component and bicomponent size, distance, and transitivity ratio) and the observed network racial segregation.
Results. Sex ties are unique from the other tie types in the network, providing wider reach in the network in relatively nonredundant ways. In this population, sex ties are more likely to bridge races than are other tie types.
Conclusions. Interventions derived from only 1 mode of transmission at a time (e.g., condom promotion or needle exchange) would have different potential for curtailing sexually transmitted infection spread through the population than would attempts that simultaneously address all risk-relevant behaviors.
Although relationships involving sex contacts and those involving needle-sharing contacts are both salient for the transmission of a sexually transmitted infection (STI) from one person to another,1–4 research has demonstrated that these different types of risky contacts provide different probabilities for STI transmission.5–8 New modeling techniques have demonstrated how contact type and partnership networks combine to determine the dynamics of infection transmission through networks.9 Framed in a classical susceptible, exposed, infected, recovered framework, the conclusion that needle-sharing ties tend to provide greater risk than do sexual ties5–8,10 has stemmed from research largely addressing 1 of 2 questions. First, given a population of uninfected individuals, how do differences in risky behaviors lead to differences in subsequent STIs (i.e., focus on the transition between susceptible and infected)? Second, given sexual or needle-sharing contact between serodiscordant individuals, what is the differential likelihood of infection depending on type of contact (i.e., focus on the transition between exposed and infected)? We know comparably less about whether and how sex ties and needle-sharing ties may differentially contribute to the observed connectivity across a full risk network (i.e., focus on the potential transition between susceptible and exposed).
NETWORKS AND SEXUALLY TRANSMITTED INFECTIONS
Although it is well known that both unprotected sexual contact and needle-sharing contact can lead to infection spread, we know little about the role each type of tie plays in connecting a wider population. The potential breadth of an STI epidemic rests on 2 network-related issues, which can either promote or constrain transmission across a population. First, given ties between infected and susceptible individuals, the probability of infection varies by type of risk contact. For example, the probability of an individual being infected with HIV in a single contact varies according to type of sexual contact and is considerably higher for needle-sharing or other “sharps” contact than for any single sex act.5,6,10,11 This network-related aspect directly aligns with research regarding the exposed to infected transition.6,8,11–14 Such work provides important explanations of observed transmission dynamics and is at the core of discussions regarding varying epidemic trajectories in different parts of the world.11–13
Second, the levels of connection between infected individuals and the wider population alters the course of an epidemic, whether via direct ties (those linking partners) or via indirect ties (those linking individuals to their partners’ partners and their partners’ partners’ partners, etc.). Network reach identifies how many people are linked together through direct and indirect paths and thus how widely an infection could potentially spread through a population. Network redundancy identifies the number and pattern of links in that population, which influences the likelihood that infections will actually spread through the population. Previous research demonstrates how reach, redundancy, and other network characteristics shape the potential spread of an infection through a population15 and how common such patterns are in observed networks16 or epidemics.4 In practice, research taking this approach focuses on how readily such measures account for population-level transitions from susceptible to infected.
We focused on a third question that has not been readily addressed previously: the link between being susceptible and being exposed. We examined whether and how sex and drug ties serve to differentially provide STI-exposure potential for uninfected individuals. Although combining each of the approaches in a single study would allow partial estimation of this effect, we have demonstrated that our direct attention to this specific question provides new insights about population STI risk not available through any of the previous approaches. These differences have important implications for future STI research and intervention efforts.
RACE, NETWORKS, AND SEXUALLY TRANSMITTED INFECTIONS
In the United States, research consistently observes that African Americans have substantially higher rates of STIs than do Whites.17–25 Potential explanations of the sources of these differences have ranged over empirical observations of African Americans having more partners,19 higher rates of partnership concurrency (i.e., sex with more than 1 partner in a given time period),26 and different mixing patterns, which include bridging risk groups (e.g., linking network cores to noncore individuals)27 or bridging population groupings that are not directly risk relevant (e.g., race, geography).24 Although each of these differences provides a partial explanation for racial disparities in STI prevalence, the differences remain robust despite controls for these factors.19,21,22 Thus, we examined whether the remaining unaccounted for racial difference in STIs partially stems from the differential network connectivity that different types of risk relationships provide.
We addressed these questions using Colorado Springs Project 90 data28–31 to examine how sex ties, needle-sharing ties, and ties involving both sex and needles differentially connect a high-risk population. By selectively removing types of ties from the contact network, we could evaluate the relative importance of each tie type for various measures of network connectivity. We have elaborated how these would alter the potential spread of an STI through a population.
METHODS
Our data come from the Colorado Springs Project 90 study, which was a Centers for Disease Control and Prevention–funded project focused on HIV transmission in heterosexual and injecting drug user populations. Data come from 595 respondents using face-to-face interviews between 1988 and 1992 using an open cohort design. Data collection focused on eliciting characteristics of risk partnership networks that allowed the research team to identify and interview as many people in the target population as possible (injecting drug users, prostitutes, and their sex and needle-sharing partners) and to assess the size, structure, and epidemic potential of the high-risk partnership network. Detailed overviews of the study and sample design have been published previously,28–30,32 and the data have been used to examine the impact of network structure on disease transmission.3,4,30,33
Analytic Strategy
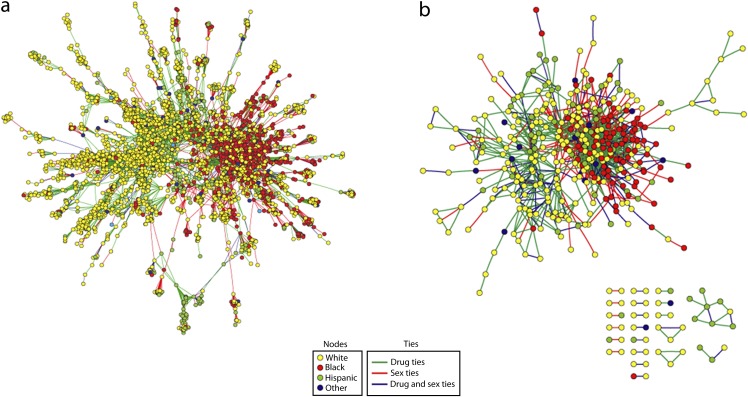
We constructed 2 observed networks, each of which represents ties involving sexual contact, ties involving shared drugs, and ties involving both sexual and drug-sharing contact. The respondent-only network consists of the 595 respondents and the 1296 reported connections among them (Figure 1b). We extracted the respondent-only network from the larger, full network, which additionally includes all ties respondents reported having with other individuals (alters) who were not study participants. In addition to reporting their own ties to these alters, respondents could report on the ties among their alters (“matrix ties”) and ties those alters had with up to 1 other associate who was not among the respondent’s contacts (“associate ties”). Our analyses included all these additional (matrix and associate) tie nominations.34 The full network consists of 6019 individuals (595 respondents and 5424 nonrespondents) and the 13 901 reported ties among them (Figure 1,a). Descriptive statistics for these respondents and their named alters are show in Table A (available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 1—
Ties among (a) the giant connected component for the full network, and (b) the observed respondent-only network: Colorado Springs Project 90, Colorado Springs, CO, 1988–1992.
Note. The full network includes 4319 people (of 6019 total named) and the 13 901 ties between them. The respondent-only network consists of 595 respondents and the 1296 reported connections between them.
We examined how sex ties, drug ties, and ties involving both differentially contribute to the observed connectivity of the network separately for the respondent-only and for the full networks. First, we computed several connectivity measures. We developed a strategy for assessing how each tie type contributes to these network connectivity measures by selectively removing them from the observed networks then quantifying their changes in the edge-removed networks. This edge removal process started with the observed networks, then randomly selected n ties of type k 500 times for each combination of settings. The results show n in increments of 2%, ranging from 2% to 12% of all ties in the full network and up to 14% of all ties in the respondent-only network. We stopped at 12% for the full network because removing that proportion of all ties (those including both sex and drugs) removes all “both” ties. Similarly, for the respondent-only network beyond 14%, removing that proportion of all ties as sex ties removes all sex ties. Extended comparisons of other remaining ties beyond these cutoffs do not appreciably change the described patterns. We, therefore, have presented only the range for which we could compare the contributions of all 3 tie types (the additional comparisons are available from j. a.). To compare the effects of different tie types on connectivity, k included 4 different types of ties. The first 3 are those of analytical interest: sex ties, drug ties, and ties that include both sex and drugs. A baseline for comparison involved the same process of edge removals in which ties were selected completely at random (i.e., indifferent as to whether the tie involved sex, drugs, or both sex and drugs).
Measures
To capture the extent of network connectivity, we computed a series of measures on the observed and edge-removed networks. Table 1 presents the base-level statistics for each of the network-based measures from the observed networks separately for the respondent-only and the full networks. A “component” is a set of persons connected by a path of any length (i.e., a direct or indirect path containing any number of intermediaries). Components can be thought of as capturing the widest potential diffusion of a single STI epidemic. We computed the size of the largest component as the count of persons linked together in the largest component. Most large networks contain a giant component35 comprising more than half of all network members connected through a chain of relations, and this is true of both the observed respondent and the full networks.
TABLE 1—
Observed Dyad and Network Characteristics: Colorado Springs Project 90, Colorado Springs, CO, 1988–1992
| Characteristic | Respondents Plus Named Alters | Respondents Only |
| Dyadic | ||
| Ties, no. | ||
| Sex | 2400 | 200 |
| Drug | 9686 | 853 |
| Sex and drug | 1815 | 243 |
| Total | 13 901 | 1296 |
| Racial/ethnic segregation index | ||
| Sex | 0.02 | −0.07 |
| Drug | 0.38 | 0.03 |
| Sex and drug | 0.18 | 0.03 |
| All | 0.29 | 0.02 |
| Full network | ||
| Nodes, no. | 6019 | 595 |
| Component membership | 0.72 | 0.87 |
| Bicomponent membership | 0.41 | 0.74 |
| Relative reach | 0.0003 | 0.009 |
| Transitivity ratio | 0.26 | 0.33 |
| Racial/ethnic segregation index | 0.41 | 0.23 |
Network components are considered fragile if single nodes or edges are responsible for connecting different portions of the network. Measures of network robustness capture portions of the networks that have greater than minimal connectivity (i.e., constitute a component). A bicomponent identifies subgroups in the largest component in which every person is connected by at least 2 completely independent paths.15,36 In terms of risk contact networks, bicomponents are subsections of the network in which the potential for pathogens to follow multiple distinct routes between pairs of nodes elevates the likelihood of transmission. Because the bicomponent is a subset of the largest component, to avoid documenting the same contributions twice—as would have been the case if we had simply computed the size of the largest bicomponent—we measured the relative size of the largest bicomponent, which identifies the proportion of nodes in the largest component that is also biconnected.
Many observed connected networks display relatively short distances between any 2 randomly selected nodes.37 The geodesic distance between nodes refers to the number of ties on the shortest path between them. In terms of epidemic potential, diseases spread much more efficiently across shorter distances. Thus, we measured the relative distance as a ratio of the average geodesic distance between all observed pairs of nodes in the connected component to the maximum potential length of that path. This measure is necessarily limited to those who are connected (i.e., are part of a single component). The longest a path could be between a pair of nodes in the connected component is the size of the connected component minus 1.
The transitivity ratio identifies the proportion of i-k pairs for which a tie exists given the presence of ties between i-j and j-k.38 A higher transitivity ratio indicates greater clustering of a network, which can be thought of as the amount of recursion in the networks. This would indicate networks that are more locally robust and have a smaller span than do networks with lower transitivity and similar density. In terms of epidemic potential, this can be thought of as indicating the greater likelihood of successful transmission over short distances at the expense of efficient transmission over longer distances.
Racial/Ethnic Segregation
The final characteristic of the networks that we examined was the level of observed racial/ethnic segregation. For this, we used Freeman’s segregation index,39 which captures how much racial segregation in an observed network differs from random mixing. A value of 0 would mean that ties in and between categories are distributed at random; a value of +1 would indicate perfectly segregated networks (all ties in category), whereas negative values indicate greater than random cross-group ties—as would be seen in heterogamous features. The index identifies the extent of cross-race ties compared with what would be expected if ties were formed at random with respect to race, accounting for the racial distribution of the population. Respondents were able to separately report their race and ethnicity. In this population, however, virtually all respondents who identified as Hispanic also identified as White. As such, for this population, race/ethnicity was not an identifiably independent dimension (hence the 4 categories reported in the online supplement). More important for the current examination, although we computed the segregation index using all 4 race/ethnicity categories identified, race (and not ethnicity) was the dominant characteristic on which we observed segregation of ties. As such, although we used both in the construction of the measure, we have focused the discussion on race, which was the salient driver of relational segregation we observed and have attempted to explain.
RESULTS
We present the analyses for the full network edge deletions first, then limit discussion of findings for the respondent-only network to those findings that differ from the full network analyses.
Full Network
Connectivity measures.
Figure 2 presents line graphs for how the edge deletions of the various tie types differentially influenced network connectivity for the full network. The observed full network has a giant component that connects approximately 70% of the population who have any ties involving sex, ties involving drugs, or ties involving sex and drugs. At each level of edge removal, removing sex ties reduces the size of the connected component more severely than does any other tie type, with drug tie removals diminishing the giant component size at a lesser rate than does removing ties at random.
FIGURE 2—
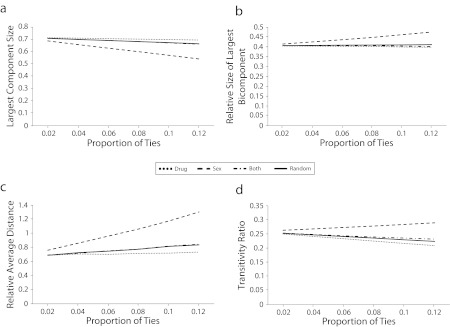
Changes for the simulated counterfactual full network with the indicated percentage of all edges removed as sex ties, drug ties, ties involving sex and drugs, or random ties for (a) largest component size, (b) relative size of largest bicomponent, (c) relative average distance, and (d) transitivity ratio: Colorado Springs Project 90, Colorado Springs, CO, 1988–1992.
Note. All differences are significantly different (P<.01).
Approximately 40% of the individuals in the observed connected component were connected via more than 1 pathway (i.e., are part of the largest observed bicomponent). Removing sex ties increased the proportion of ties in the connected component that are in the biconnected core, whereas drug tie removals decreased the proportion of individuals who are robustly connected in this way. Conceptually, this means that drug ties are more likely to provide additional redundant indirect pathways among those in the connected component, whereas sex ties do not provide this same network robustness. With respect to the average distance, we found that removing sex ties increased the distance between nodes. Drug tie removals also increased the relative average distance observed in the graph but at a lower rate than did random tie removals. With respect to transitivity in the network, among all instances in which 2 people share a common alter, approximately 25% of those pairs are also directly tied to one another. As ties are removed from the network, sex tie removals increased the levels of transitivity in the network. Drug tie and both tie removals each decreased the observed transitivity ratio but at rates greater and less than random tie removals, respectively.
Although each of these individual patterns describes important aspects of sex and drug tie contributions to network connectivity in this population, the full contribution of these analyses comes only through their combination. In general, the combined findings suggest that the most dramatic effects on connectivity are because of sex ties. Specifically, sex ties spread the network to the widest population, but they do so with connectivity that is somewhat fragile: they do not involve the same level of recursion that drug ties do—whether at the local level (via transitivity) or at the level of longer indirect pathways (i.e., bicomponent connectivity). In a network composed exclusively of heterosexual sex ties, transitivity is impossible. From a disease-eye view, however, ties can combine in any pattern; thus, once combined with the population’s drug ties, sex ties can provide for the form of local robustness in the combined network—the focus of our analyses. This combination of findings can be interpreted as thinking of sex ties as producing tendrils that reach out into the wider population but provide (comparatively) fewer reconnections to the strongest core(s) of the network.
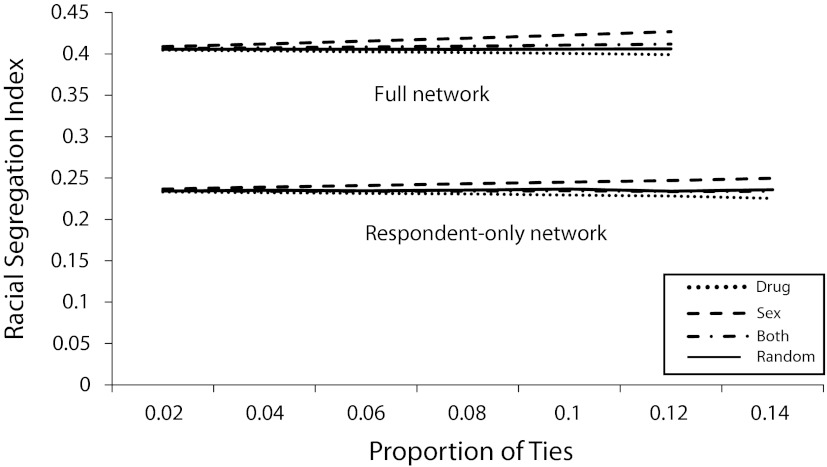
Racial/ethnic segregation.
Figure 4 presents the network racial/ethnic segregation for respective levels of edge deletion. Overall, the level of racial segregation in the observed full network is moderate (∼0.41). Sex ties also uniquely contribute to observed racial/ethnic segregation in the network. Sex tie edge removals increase the level of segregation, whereas drug tie removals decrease the level of racial segregation in the network. Edge removals of ties involving both sex and drugs do not differ substantially from removing random ties, each having virtually no effect on racial segregation in counterfactual networks. This suggests that in the full network, sex ties more frequently serve as a bridge across races in this population, whereas drug ties appear to serve to robustly connect populations of the same race. There are several potential ways that this observed pattern could arise—in particular, potential racial differences in commercial sex work participation among the population. It is also possible that geographic constraints contribute to observed patterns of racial mixing.40
FIGURE 4—
Racial/ethnic segregation for respective levels of edge deletion in the full network, and the network consisting only of P90 respondents: Colorado Springs Project 90, Colorado Springs, CO, 1988–1992.
Respondent-Only Network
Connectivity measures.
More of the respondent-only network is contained in the giant connected component, has more of that component that is also part of the bicomponent, and has nodes that are separated by comparatively shorter distances and are more likely to exhibit transitivity than is the full network (data available as a supplement to the online version of this article at http://www.ajph.org). Figure 3 shows the edge removal effects on each of the network connectivity measures for the respondent-only network.
FIGURE 3—
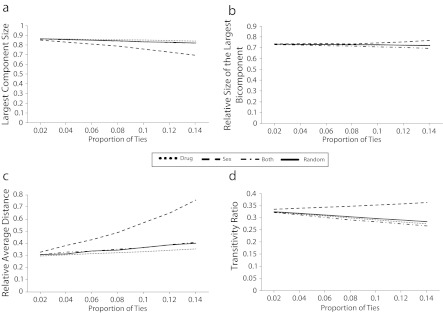
Changes for the simulated counterfactual participant-only network with the indicated percentage of all edges removed as sex ties, drug ties, ties involving sex and drugs, or random ties for (a) largest component size, (b) relative size of largest bicomponent, (c) relative average distance, and (d) transitivity ratio: Colorado Springs Project 90, Colorado Springs, CO, 1988–1992.
Note. All differences are significantly different (P<.01).
In the respondent-only network, edge removals affect connectivity measures in largely the same pattern as observed for the full network. Sex ties occupy unique positions in the network and appear to add tendril-like additional, but sparse connectivity to the network. In the full network (Figure 2), drug ties played the primary counter role to sex ties (e.g., their removal led to declines in measured transitivity, whereas sex tie removals increased transitivity), whereas ties involving both sex and drugs had little appreciably different effect compared with ties removed at random. For the respondent-only network (Figure 3), this counter role is more consistently filled by ties that include both sex and drugs (e.g., they serve the role of providing the redundant ties in the network), whereas it is drug-only ties that do not appreciably differ from removing ties at random.
Racial/ethnic segregation.
With respect to racial segregation, edge removals in the respondent-only network reveal the same pattern for the full network: sex ties are substantially more likely to form bridges across racial groups, and drug ties are more likely to be contained within race. Overall, the level of racial/ethnic segregation in the respondent-only network is substantially lower (∼0.23) than that in the full network.
DISCUSSION
We found that sex ties were key to network expansiveness but that this expansiveness is fragile: sex ties bring in more of the population, but the people reached through sex-only ties tend not to be multiply linked to the core network. Moreover, sex ties are key to bridging races. This pattern is true for both the directly observed respondent-only network and the larger, more racially segregated full network they report on.
These findings have potentially important implications for how we understand STI spread through a population. They suggest that interventions focused on only 1 mode of transmission at a time (e.g., condom promotion or needle exchange programs) would have different potential for curtailing STI spread. For example, interventions related to condom promotion alone might reduce the breadth of a potential epidemic in the full population, whereas needle exchange programs might reduce the likelihood of STI growth in the core of the network.
Because epidemic potential turns on transmissibility, these results suggest that interventions aimed at a highly infectious STI would do best to focus on the broad- but weak-reaching sex ties; whereas interventions focusing on hard to transmit STIs might best be targeted at the redundancies built into drug exchange networks. Ultimately, of course, intervention efforts focused on both would be necessary, because it is clear that neither of these risk behaviors is sufficiently uniquely positioned in the network to alone explain network epidemic potential.
Our findings also have important implications for interpreting STI risk in the specific Project 90 context. For this population, we learned that—compared with other ties involving risky behavior—sex ties provide unique connectivity patterns. In particular, their bridging characteristics seem much more akin to the reach provided by weak social ties,41 an effect that has also been observed among high school romantic relationships.42 This pattern is likely the result of the unique social configurations of drug and sex behavior; that is, the drug ties have more strong tie characteristics with higher density among partners’ partners41—perhaps through trust developed from coparticipating in illegal behavior or through shared relationships that provide robust access to drug supply in the event of a single node’s removal (e.g., through arrest).
What is less clear is how what we learned from this specific sample can be translated to other contexts. First, for the risk population at large in Colorado Springs, we learned that a focus exclusively on the at-risk population would have led to different conclusions than does including their named alters. In particular, the racial composition of ties to the wider sample was more diverse than was that in the high-risk set alone, leading to greater cross-race epidemic potential than might be assumed from the respondents only. Most generally, this work suggests that prediction of effects from targeted intervention attempts rooted in existing network-based approaches (e.g., reducing bridging ties17) may be misestimated if generated from a core sample that might be unrepresentative of the wider at-risk population. As with all case studies, we need to further examine how these differential risk contributions may be different outside the Colorado Springs context.
In general, the network foundations of public health research will likely benefit from taking seriously the multiplex nature of disease-carrying ties. The effect of risk behaviors on corresponding network patterns, and ultimately on epidemic potential, could not have been captured if we had focused on any of these ties to the exclusion of others. Although sex ties play a particularly unique role in connecting members of this population, they do so in a way that is fundamentally intertwined with the unique, and in some ways counterbalancing, patterns contributed by drug ties (in the full network) and ties involving both sex and drugs (in the respondent-only network).
The biggest picture implication of this work, then, is that future public health network research should fully explore the multiple ways people are connected. Rather than simply a connection of pipes that carry disease, network ties likely unfold and evolve in characteristic ways. We have seen the trace of that character in the structural location of types of ties, but we might similarly find differential behavior derived from the life course of a relation. We know that long-term sex partners are less likely to use condoms, for example, but does this life course effect differ if the tie was first embedded in a drug exchange? As we move on to the next generation of public health–relevant network science, integration of these sorts of questions with our networks as pipes models will be crucial.
Acknowledgments
This work was supported in part by the National Institutes of Health (grants DA 12831, HD 41877, and HD 68317-2).
We thank Steven Q. Muth and John Potterat for their help in interpreting these data, especially the racial dynamics of the population. Richard Rothenberg, David Schaefer, the Networks and Health Working Group at Columbia University, and the Structural Dynamics Working Group at Arizona State University provided helpful comments on previous versions of this article.
Note. The views in this article reflect those of the authors and do not necessarily represent those of the National Institutes of Health.
Human Participant Protection
We obtained institutional review board approval for data acquisition and analysis from the University of Washington.
References
- 1.Morris M. Sexual networks and HIV. AIDS. 1997;11(suppl A):S209–S216 [PubMed] [Google Scholar]
- 2.Morris M, Kretzschmar M. Concurrent partnerships and transmission dynamics in networks. Soc Networks. 1995;17(3):299–318 [Google Scholar]
- 3.Potterat JJ, Rothenberg RB, Muth SQ. Network structural dynamics and infectious disease propagation. Int J STD AIDS. 1999;10:182–185 [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg RB, Potterat JJ, Woodhouse DE, Muth SQ, Darrow WW, Klovdahl A. Social network dynamics and HIV transmission. AIDS. 1998;12(12):1529–1536 [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Wawer MJ, Brookmeyer Ret al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1 discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153 [DOI] [PubMed] [Google Scholar]
- 6.Baggaley RF, Boily M-C, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20(6):805–812 [DOI] [PubMed] [Google Scholar]
- 7.Gisselquist DP. Estimating HIV-I transmission efficiency through unsafe medical injections. Int J STD AIDS. 2002;13(3):152–159 [DOI] [PubMed] [Google Scholar]
- 8.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29(1):38–43 [DOI] [PubMed] [Google Scholar]
- 9.Morris M. Local Acts, Global Consequences: Networks and the Spread of HIV. Washington, DC: NIH Director’s Wednesday Afternoon Lecture Series; 2007 [Google Scholar]
- 10.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372(9635):314–320 [DOI] [PubMed] [Google Scholar]
- 11.Boily M-C, Baggaley RF, Wang Let al. Heterosexual risk of HIV-1 infection per sex act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisselquist D, Upham G, Potterat John J. Efficiency of human immunodeficiency virus transmission through injections and other medical procedures: evidence, estimates, and unfinished business. Infect Control Hosp Epidemiol. 2006;27(9):944–952 [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg R, Gisselquist D, Potterat JJ. A simulation to assess the conditions required for high level heterosexual transmission of HIV in Africa. Int J STD AIDS. 2004;15(8):529–532 [DOI] [PubMed] [Google Scholar]
- 14.Wawer MJ, Gray RH, Sewankambo NKet al. Rates if HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409 [DOI] [PubMed] [Google Scholar]
- 15.Moody J, White DR. Structural cohesion and embeddedness: a hierarchical concept of social groups. Am Sociol Rev. 2003;68(1):103–127 [Google Scholar]
- 16.Helleringer S, Kohler H- P. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007;21(17):2323–2332 [DOI] [PubMed] [Google Scholar]
- 17.Aral SO. Understanding racial-ethnic and societal differences in STI. Sex Transm Infect. 2002;78(1):2–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaisson RE, Keruly JC, Moore RD. Race, sex, drug use and progression of human immunodeficiency virus disease. N Engl J Med. 1995;333(12):751–756 [DOI] [PubMed] [Google Scholar]
- 19.Tanfer K, Cubbins LA, Billy JOG. Gender, race, class and self-reported sexually transmitted disease incidence. Fam Plann Perspect. 1995;27(5):196–202 [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention HIV/AIDS Surveillance Report, 2006. Atlanta: US Department of Health and Human Services; 2008 [Google Scholar]
- 21.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(suppl 1):S115–S122 [DOI] [PubMed] [Google Scholar]
- 22.Hallfors DD, Itritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97(1):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kottiri BJ, Friedman SR, Neaigus A, Curtis R, Des Jarlais DC. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. J Acquir Immune Defic Syndr. 2002;30(1):95–104 [DOI] [PubMed] [Google Scholar]
- 24.Laumann EO, Youm Y. Racial/ethnic differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Update to racial/ethnic disparities in diagnoses of HIV/AIDS—33 States, 2001–2005. MMWR Morb Mortal Wkly Rep. 2007;56(9):189–193 [PubMed] [Google Scholar]
- 26.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am J Public Health. 2009;99(6):1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potterat JJ, Rothenberg RB, Zimmerman-Rogers Het al. Sexual network structure as an indicator of epidemic phase. Sex Transm Infect. 2002;78(suppl 1):i152–i158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potterat JJ, Woodhouse DE, Muth SQet al. Network dynamism: history and lessons of the Colorado Springs study. In: Morris M, ed. Network Epidemiology: A Handbook for Survey Design and Data Collection. Oxford: Oxford University Press; 2004:87–114 [Google Scholar]
- 29.Woodhouse DE, Rothenberg RB, Potterat JJet al. Mapping a social network of heterosexuals at high risk of human immunodeficiency virus infection. AIDS. 1994;8(9):1331–1336 [DOI] [PubMed] [Google Scholar]
- 30.Darrow WW, Potterat JJ, Rothenberg RB, Woodhouse DE, Muth SQ, Klovdahl AS. Using knowledge of social networks to prevent human immunodeficiency virus infections: the Colorado Springs study. Sociol Focus. 1999;32(2):143–158 [Google Scholar]
- 31.Klovdahl AS, Potterat JJ, Woodhouse DE, Muth JB, Muth SQ, Darrow SQ. Social networks and infectious disease: the Colorado Springs study. Soc Sci Med. 1994;38(1):79–88 [DOI] [PubMed] [Google Scholar]
- 32.Klovdahl AS, Potterat JJ, Woodhouse DE, Muth J, Muth SQ, Darrow WW. HIV infection in an urban social network: a progress report. Bull Methodol Sociol. 1992;36(1):24–33 [Google Scholar]
- 33.Rothenberg RB, Woodhouse DE, Potterat JJ, Muth SQ, Darrow WW, Klovdahl AS. Social networks in disease transmission: the Colorado Springs study. NIDA Res Monogr. 1995;151:3–19 [PubMed] [Google Scholar]
- 34.adams j, Moody J. Code Book for Colorado Springs Sexual and Drug User Networks. Columbus: Ohio State University; 2002 [Google Scholar]
- 35.Palmer EN. Graphical Evolution: An Introduction to the Theory of Random Graphs. New York: John Wiley and Sons; 1985 [Google Scholar]
- 36.Harary F. Graph Theory. Reading, MA: Addison-Wesley; 1969 [Google Scholar]
- 37.Watts DJ. Small Worlds: The Dynamics of Networks Between Order and Randomness. Princeton, NJ: Princeton University Press; 1999 [Google Scholar]
- 38.Holland PW, Leinhardt S. Some evidence on the transitivity of positive interpersonal sentiment. Am J Sociol. 1972;72(6):1205–1209 [Google Scholar]
- 39.Freeman LC. Segregation in social networks. Sociol Methods Res. 1972;6(4):411–430 [Google Scholar]
- 40.Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse D. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506–512 [DOI] [PubMed] [Google Scholar]
- 41.Granovetter M. The strength of weak ties. Am J Sociol. 1973;81(6):1287–1303 [Google Scholar]
- 42.Bearman PS, Moody J, Stovel K. Chains of affection: the structure of adolescent romantic and sexual networks. Am J Sociol. 2004;110(1):44–91 [Google Scholar]