Abstract
Objectives. We assessed whether directly observed fluoxetine treatment reduced depression symptom severity and improved HIV outcomes among homeless and marginally housed HIV-positive adults in San Francisco, California, from 2002 to 2008.
Methods. We conducted a nonblinded, randomized controlled trial of once-weekly fluoxetine, directly observed for 24 weeks, then self-administered for 12 weeks (n = 137 persons with major or minor depressive disorder or dysthymia). Hamilton Depression Rating Scale score was the primary outcome. Response was a 50% reduction from baseline and remission a score below 8. Secondary measures were Beck Depression Inventory-II (BDI-II) score, antiretroviral uptake, antiretroviral adherence (measured by unannounced pill count), and HIV-1 RNA viral suppression (< 50 copies/mL).
Results. The intervention reduced depression symptom severity (b = −1.97; 95% confidence interval [CI] = −0.85, −3.08; P < .001) and increased response (adjusted odds ratio [AOR] = 2.40; 95% CI = 1.86, 3.10; P < .001) and remission (AOR = 2.97; 95% CI = 1.29, 3.87; P < .001). BDI-II results were similar. We observed no statistically significant differences in secondary HIV outcomes.
Conclusions. Directly observed fluoxetine may be an effective depression treatment strategy for HIV-positive homeless and marginally housed adults, a vulnerable population with multiple barriers to adherence.
Depressive, pain, and substance use disorders are highly prevalent among persons living with HIV/AIDS1,2 and among the homeless and marginally housed.3–5 The triple diagnosis of depression, HIV, and substance use poses unique treatment challenges for clinicians: successful management of one condition is often dependent on successful management of the others, and the optimal sequencing of depression treatment, substance use treatment, and stabilization of psychosocial comorbidities remains unclear. Adherence to the entire continuum of HIV care is often hampered by depression6–8 and substance use.9,10 For homeless persons, the need to address subsistence concerns such as obtaining food and shelter may not only adversely affect mental well-being11 but may also divert attention away from medication adherence and regular clinic attendance.12 Timely and effective depression treatment is critical for HIV-positive persons, because depression has been associated with CD4+ T-lymphocyte cell count decline,13 progression to AIDS,14 and AIDS-related mortality.15 Yet depression remains pervasively underdiagnosed and undertreated among the homeless16–18 and among HIV-positive persons.19,20
Depression treatment might be expected to improve virological or immunologic outcomes through improved adherence, but this has not been conclusively demonstrated.21–23 We therefore sought to determine whether treatment with once-weekly fluoxetine reduced depression symptom severity among homeless and marginally housed persons with comorbid depression and HIV. Because this population faces many psychosocial barriers to successful medication adherence,12,24 in addition to depression,25 we employed a directly observed treatment strategy similar to that used for treatment and management of patients with tuberculosis and HIV.26 This strategy reduced the potential for incomplete adherence to reduce the effectiveness of antidepressant treatment. A secondary aim was to determine whether depression treatment improved antiretroviral therapy (ART) uptake among persons eligible for treatment and ART adherence and viral suppression among treated persons.
METHODS
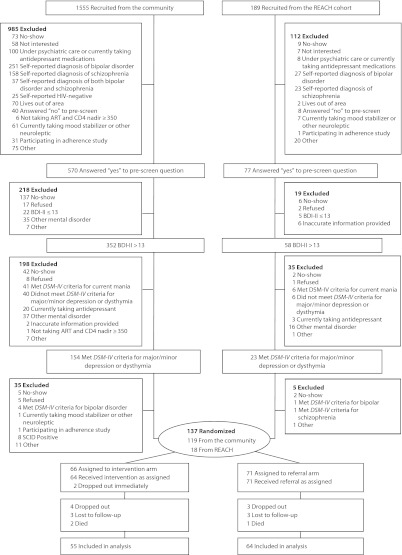
From July 2002 through February 2008, we recruited English-speaking adults with comorbid HIV and depression. We sought participants in homeless shelters, free lunch programs, low-income single-room-occupancy hotels, public HIV clinics, and social service agencies throughout the Tenderloin, South of Market, and Mission districts of San Francisco, California. We recruited a small proportion of participants from Research in Access to Care for the Homeless, an observational, prospective cohort of homeless and marginally housed persons living with HIV (drawn from the same sampling frame).27,28 Potential participants were screened in several sequential steps (Figure 1) before undergoing structured diagnostic assessment29 to determine whether they met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for major depressive disorder, minor depressive disorder, or dysthymia.30 Experienced clinical raters certified to have high interrater reliability and procedural integrity administered all instruments and conducted structured assessments. All potential participants received confirmatory HIV testing to document their serostatus (Quest Diagnostics, Inc, Valencia, CA).
FIGURE 1—
Stages of screening process for homeless and marginally housed participants with HIV and depression for controlled trial of directly observed fluoxetine treatment.
Note. ART = antiretroviral therapy; BDI-II = Beck Depression Inventory-II; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; REACH = Research in Access to Care for the Homeless; SCID = Structured Clinical Interview for DSM-IV. Prescreening with self-report of depression; administration of 21-item BDI-II; structured clinical assessment of candidates with a BDI-II score > 13 to confirm diagnosis of major depressive disorder, minor depressive disorder, or dysthymia; and interview with study psychiatrist to confirm interest and psychiatric diagnosis, assess appropriateness of antidepressant medication treatment, and review coordination of care with potential participant’s primary care provider.
We excluded potential participants if they did not live in San Francisco; were unwilling to take fluoxetine; had a CD4+ T-lymphocyte cell count nadir of 350 cells per milliliter or lower and were not currently taking ART; were unable to commit to the required study visits; reported taking antidepressant medications, mood stabilizers, or other neuroleptics within 3 months prior to study entry; reported being under psychiatric care within 6 months prior to study entry; reported a previous diagnosis of bipolar disorder or schizophrenia; exhibited signs and symptoms consistent with a DSM-IV diagnosis of dementia, any psychotic disorder, or bipolar disorder; were deemed to have a current substance use disorder of a severity requiring immediate residential or inpatient treatment; were at imminent risk of completed suicide; were pregnant; were prescribed a medication or had a history of a medical condition that could harmfully interact with fluoxetine; or were already participating in an ongoing adherence study.
Study Design
Participants who remained eligible at the end of the screening process were randomly assigned to receive fluoxetine treatment or referral to the community for psychiatric care (Figure 1). We employed blocked randomization within categories of DSM-IV diagnosis, substance use, ART use, and CD4 count, with a random choice of 8, 10, or 12 participants in each block. We generated the randomized treatment assignment list prior to study enrollment. Participants were enrolled and assigned to a treatment arm by research staff who obtained the assignment from a password-protected database maintained by the study programmer. Only the study programmer and senior epidemiologist had access to the randomization list (K. R. and E. D. C.).
Participants assigned to the intervention arm received an explanation of their psychiatric diagnosis and were told that they would be treated with fluoxetine. Treatment was directly observed for 24 weeks, introduced in 3 phases of gradually increasing independence from the study provider: (1) 20 milligrams fluoxetine directly observed each weekday and self-administered on weekends, for 2 weeks; (2) 90 milligrams fluoxetine directly observed weekly, for 22 weeks; and (3) 90 milligrams fluoxetine self-administered weekly, for 12 weeks. In 2005, midway through the study, the manufacturer (Eli Lilly & Co, Indianapolis, IN) ceased donating samples of Prozac Weekly. Therefore, we switched participants from that medication to 90 milligrams generic fluoxetine, also taken weekly. All directly observed doses were delivered in the Tenderloin District at the study research site, a system previously shown to be effective for isoniazid distribution in a similar population.31
A study psychiatrist met with intervention arm participants weekly for the first month, every 2 weeks for the second month, and monthly thereafter. At each visit, the psychiatrist conducted a thorough psychiatric interview and mental status exam and inquired about treatment response and possible adverse side effects. Study psychiatrists also used the 17-item Hamilton Rating Scale for Depression (Ham-D)32 and the Clinical Global Impression Severity and Improvement scales33 to guide assessment of treatment response. These instruments were administered without blinding, because the clinical assessments were separate from the blinded study assessments. The dose of fluoxetine was increased to 180 milligrams once weekly for partial responders and nonresponders. If deemed necessary by study psychiatrists, augmenting medications were added to treat symptoms to remission. We reimbursed intervention arm participants $25 per week for completion of all scheduled directly observed doses and $25 per week for the final 12 weeks of self-administered treatment.
Participants randomized to the referral arm received an explanation of their diagnosis and were advised to seek treatment at a public mental health clinic that specialized in the care of HIV-positive persons, located 0.5 mile away along a major public transportation corridor. Referral arm participants received a $25 weekly incentive to come to the research study site to update contact information and undergo data collection procedures.
Outcome Measures and Covariates
Research visits occurred monthly and coincided with psychiatric treatment visits when possible. Structured interviews were used to collect information about participants’ sociodemographic characteristics, health behaviors, and HIV care at baseline, as well as their experience of gastrointestinal, neuropsychiatric, constitutional, and sexual symptoms every 3 months. We determined CD4+ T-lymphocyte cell count through standard techniques (Unilab, San Jose, CA).
The primary outcome of interest was depression symptom severity, assessed with the Ham-D and administered by experienced clinical raters who were blinded to treatment assignment. We defined remission as a virtual absence of depressive symptoms (Ham-D ≤ 7) and response as a clinically meaningful degree of symptom reduction (≥ 50% reduction in symptom severity from baseline).34 Our secondary depression outcome measure was the 21-item Beck Depression Inventory-II (BDI-II)35 with remission (BDI-II ≤ 8) and response defined similarly.
Among study participants who were eligible for ART at baseline (i.e., CD4+ T-lymphocyte cell count nadir < 350 cells/mL), we defined uptake of ART as the patient being on ART as of a given visit. Among study participants on ART at baseline, we measured ART adherence with unannounced pill counts conducted at the participant’s usual place of residence.36 We defined viral suppression as HIV-1 RNA less than 50 copies per milliliter. Plasma was processed and stored at −40°C within 6 hours of collection. We determined HIV-1 viral load with the HIV-1 Amplicor Monitor version 1.5 ultrasensitive assay (Roche Molecular Systems, Alameda, CA), with a lower detection limit of 20 copies per milliliter. We assessed all outcomes monthly.
Statistical Analysis
We used the t test for continuous variables and the χ2 test for categorical variables to compare the 2 study arms on baseline sociodemographic and clinical characteristics. To estimate the average effect of treatment on outcomes over the entire study, we fit generalized linear mixed models to the data with the SAS procedure GLIMMIX (SAS Institute Inc, Cary, NC). For all analyses, we used an unstructured working covariance matrix. For the continuous dependent variables (Ham-D, BDI-II, ART adherence), we assumed a model relating treatment and time effects linearly to the dependent variable, whereas for the binary dependent variables (response, remission, ART uptake, viral suppression), we assumed a model relating treatment and time effects linearly to the logit of the probabilities. We used the RANDOM statement and specified that the linear predictor contained an intercept term that randomly varied at the level of the participant effect. We modeled the effect of time as a series of dummy variables for each month, with the baseline month as the reference category. We also explored adding treatment-by-time interactions to the models for primary outcomes (but did not do so for secondary outcomes, because of the smaller sample size and lack of sufficient degrees of freedom).
We used the F test to assess whether the interaction terms were jointly statistically significant. For example, the models with binary dependent variables were represented mathematically as follows:
where πij denotes the outcome for participant i in month j, Xij denotes the design matrix for the explanatory variables for treatment assignment and time, β denotes the vector of regression coefficients for the explanatory variables, and the random intercepts αi are a linear combination of a grand mean (α) and a normally distributed deviation (ɛij) from the mean. We conducted all analyses in SAS version 9.2.
To estimate the sample size needed for the study, we assumed equal sample sizes for each treatment arm, with 10 proposed monthly time points and 1% relative attrition in both treatment arms at each time point after the first. We also assumed that the pairwise correlations of the quarterly repeated measures on the primary outcome would be 0.6. We then sought to obtain a sample size sufficient to have 80% power for a 2-tailed 0.05 hypothesis test of a medium effect size37 for the primary outcome over the course of treatment. With these assumptions, we used a previously published formula for determining sample size in longitudinal study designs38 to estimate that we would need to enroll 117.7 participants per treatment arm, rounding upward for a total estimated sample size of 236.
RESULTS
We screened 1744 potential participants, 1555 from the community and 189 from the Research in Access to Care for the Homeless cohort (Figure 1). Nearly two thirds were found to be ineligible during the prescreening process; the most common reason was self-report of alternative diagnoses such as bipolar disorder (25.3%) and schizophrenia (16.5%). Of the 647 potential participants who underwent screening with the BDI-II, 471 (73%) had a BDI-II score higher than 13. These potential participants were eligible to undergo structured diagnostic assessment, and nearly two thirds did not meet DSM-IV criteria for inclusion. A study psychiatrist reviewed the remaining 190 potential participants: 137 were confirmed to be eligible and appropriate for the study and consented to participate in the randomized trial. Thus, the trial did not meet its enrollment goal.
Sixty-six participants were randomly assigned to the intervention arm and 71 to the referral arm. Participants recruited from the Research in Access to Care for the Homeless cohort differed from those recruited from the community on history of homelessness (94% vs 64%; P = .012) and recent alcohol use (22% vs 51%; P = .024) but were otherwise comparable. A slightly higher proportion of participants in the intervention arm than in the referral arm reported a history of ever using heroin (36.5% vs 24.3%; P = .125), and a slightly smaller proportion were on ART at baseline (61.8% vs 69.4%; P = .07), but they were otherwise statistically comparable (Table 1). Mean Ham-D scores at baseline (intervention participants = 17.7; referral participants = 17.9; P = .787) indicated moderate levels of depression severity.
TABLE 1—
Baseline Characteristics of Homeless and Marginally Housed Participants with HIV and Depression in Controlled Trial of Directly Observed Fluoxetine Treatment
| Characteristic | Weekly Fluoxetine Arm (n = 64), Mean ±SD or No. (%) | Community Referral Arm (n = 71) Mean ±SD or No. (%) | Test Statistic,a | P |
| Recruited from REACH | 10 (15.2) | 8 (11.3) | 0.45 | .615 |
| Age, y | 44.2 ±9.09 | 42.8 ±8.44 | −0.94 | .348 |
| Female | 6 (9.1) | 8 (11.3) | 0.18 | .781 |
| White | 32 (48.5) | 36 (50.7) | 0.07 | .795 |
| Ever homeless | 45 (72.6) | 45 (64.3) | 1.04 | .307 |
| Illegal drug useb | ||||
| Ever | 52 (82.5) | 60 (85.7) | 0.25 | .642 |
| Past 30 d | 10 (16.1) | 13 (18.6) | 0.14 | .82 |
| Crack cocaine use | ||||
| Ever | 42 (66.7) | 46 (65.7) | 0.01 | .908 |
| Past 30 d | 11 (18.0) | 9 (12.9) | 0.68 | .47 |
| Heroin use | ||||
| Ever | 23 (36.5) | 17 (24.3) | 2.36 | .125 |
| Past 30 d | 2 (3.3) | 3 (4.3) | 0.09 | > .999 |
| Methamphetamine use | ||||
| Ever | 44 (69.8) | 48 (68.6) | 0.03 | .874 |
| Past 30 d | 8 (12.9) | 11 (15.7) | 0.21 | .805 |
| Alcohol use: past 30 d | 29 (46.0) | 34 (48.6) | 0.09 | .77 |
| DSM-IV diagnosis | ||||
| Major depression | 50 (75.8) | 51 (71.8) | ||
| Minor depression | 5 (7.6) | 7 (9.9) | 0.33 | .849 |
| Dysthymia | 11 (16.7) | 13 (18.3) | ||
| CD4+ T-lymphocyte count, cells/mL | 388.8 ±269.86 | 408.9 ±266.73 | 0.44 | .663 |
| CD4+ count nadir < 350 cells/mL | 34 (51.5) | 36 (50.7) | 0.01 | .924 |
| Receiving antiretroviral therapy | 21 (61.8) | 25 (69.4) | 0.07 | > .999 |
Note. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; REACH = Research in Access to Care for the Homeless.
For continuous variables, comparisons used t tests; for categorical variables, χ2 tests.
Crack cocaine, heroin, or methamphetamine use.
Medication Delivery
Participants randomized to the intervention arm were observed receiving 2233 (92.9%) of 2403 scheduled observed doses of daily fluoxetine and 3374 (90.9%) of 3713 scheduled observed doses of weekly fluoxetine, on weekdays. They also reported having taken 1215 (99.5%) of 1221 scheduled self-administered doses of daily fluoxetine on weekends. Among the 55 participants retained at 36 weeks in the intervention arm, 27 (49.1%) reported taking fluoxetine alone, 20 (36.4%) reported taking fluoxetine in combination with another type of psychotropic medication (most commonly mirtazapine [n = 9]), and 5 (9.1%) reported not taking any fluoxetine but reported taking other psychotropic medications (most commonly bupropion [n = 2]).
The mean dose of fluoxetine achieved was 18 milligrams (±4.4 mg) per day among participants still taking daily fluoxetine by the end of the study and 137 milligrams (±73.5 mg) per week among those taking weekly fluoxetine. Among the 64 participants retained at 36 weeks in the referral arm, 7 (10.9%) reported taking fluoxetine and 16 (25.0%) reported taking another type of psychotropic medication (most commonly bupropion [n = 5]).
Treatment Efficacy and Continuation
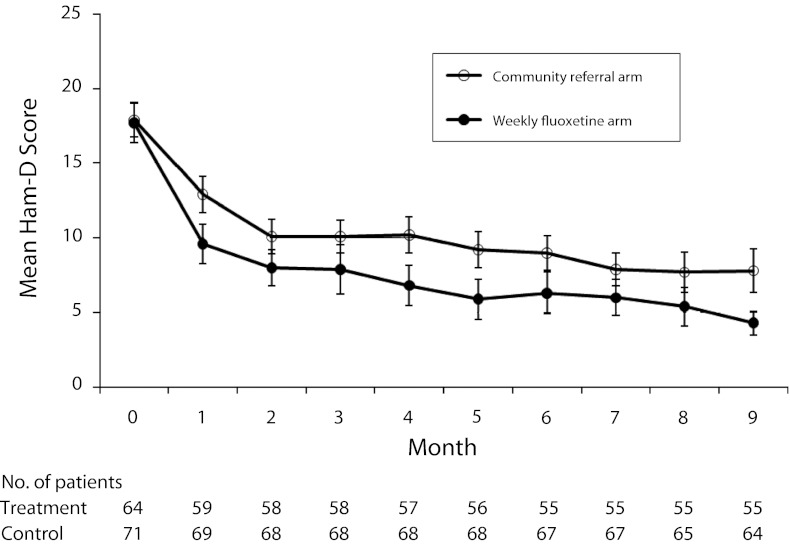
Participants in both study arms experienced improved mood, but mean depression severity was lower in the intervention arm at each assessment (Figure 2). We observed similar trends on the BDI-II and on response and remission (Table 2). The mixed-model analysis confirmed a statistically significant main effect of treatment on the Ham-D (b = −1.97; 95% confidence interval [CI] = −0.85, −3.08; P < .001) and a treatment-by-time interaction (F = 6.25; P = .014). We obtained similar results when we used the Ham-D to categorize participants as responders (adjusted odds ratio [AOR] = 2.40; 95% CI = 1.86, 3.10; P < .001) and remitters (AOR = 2.97; 95% CI = 2.29, 3.87; P < .001). In the mixed-model analysis for BDI-II score, we found a statistically significant main effect of treatment (b = −4.00; 95% CI = −1.65, −6.35; P = .001) but not a treatment-by-time interaction (F = 1.74; P = .19). Use of the BDI-II to categorize participants also yielded similar results (responders, AOR = 2.01; 95% CI = 1.56, 2.59; P < .001; remitters, AOR = 3.11; 95% CI = 2.36, 4.11; P < .001).
FIGURE 2—
Mean depression symptom severity by treatment and time among homeless and marginally housed participants with HIV and depression in controlled trial of directly observed fluoxetine treatment.
Note. HAM-D = Hamilton Rating Scale for Depression. Error bars indicate 95% confidence intervals.
TABLE 2—
Depression Outcomes at Baseline and Follow-Up Among Homeless and Marginally Housed Participants With HIV and Depression in Controlled Trial of Directly Observed Fluoxetine Treatment
| Measure | Weekly Fluoxetine Arm, Mean ±SD or No. (%) | Community Referral Arm, Mean ±SD or No. (%) | Test Statistica | P | Mixed-Effects Estimate,b b or AOR (95% CI) |
| Ham-D | −1.97 (−0.85, −3.08) | ||||
| Baseline | 17.7 ±5.38 | 17.9 ±4.95 | 0.27 | .787 | |
| 12 wk | 7.9 ±6.33 | 10.1 ±4.55 | 2.16 | .033 | |
| 24 wk | 6.3 ±5.10 | 9.0 ±4.58 | 3.00 | .003 | |
| 36 wk | 4.3 ±3.86 | 7.8 ±5.68 | 4.02 | < .001 | |
| Response | 2.40 (1.86, .310) | ||||
| Baseline | NA | NA | NA | NA | |
| 12 wk | 37 (62.7) | 25 (36.8) | 8.51 | .004 | |
| 24 wk | 35 (64.8) | 30 (44.8) | 4.83 | .028 | |
| 36 wk | 46 (82.1) | 37 (56.9) | 8.88 | .003 | |
| Remission (Ham-D ≤ 7) | 2.97 (2.29, 3.87) | ||||
| Baseline | 0 | 0 | NA | NA | |
| 12 wk | 29 (50.9) | 21 (30.9) | 5.17 | .023 | |
| 24 wk | 34 (65.4) | 27 (40.3) | 7.37 | .007 | |
| 36 wk | 48 (88.9) | 35 (53.9) | 17.17 | < .001 | |
| BDI-II | −4.00 (−1.65, −6.35) | ||||
| Baseline | 29.2 ±8.71 | 32.4 ±9.97 | 2.12 | .035 | |
| 12 wk | 14.4 ±10.78 | 20.0 ±10.66 | 2.91 | .004 | |
| 24 wk | 11.3 ±10.28 | 17.3 ±10.98 | 2.98 | .004 | |
| 36 wk | 8.2 ±8.76 | 15.0 ±9.85 | 3.94 | < .001 | |
| Response | 2.01 (1.56, 2.59) | ||||
| Baseline | 30 (52.6) | 27 (39.7) | 2.09 | .148 | |
| 12 wk | 37 (72.6) | 32 (50.0) | 6.01 | .014 | |
| 24 wk | 43 (78.2) | 33 (51.6) | 9.08 | .003 | |
| Remission (BDI-II ≤ 8) | 3.12 (2.36, 4.11) | ||||
| Baseline | 0 | 0 | NA | NA | |
| 12 wk | 20 (35.1) | 12 (17.7) | 4.95 | .03 | |
| 24 wk | 26 (51.0) | 18 (28.1) | 6.28 | .01 | |
| 36 wk | 40 (72.7) | 21 (32.8) | 18.86 | < .001 |
Note. AOR = adjusted odds ratio; BDI-II = Beck Depression Inventory-II; CI = confidence interval; HAM-D = Hamilton Rating Scale for Depression; NA = not applicable.
For continuous variables, comparisons used t tests; for categorical variables, χ2 tests.
Derived from generalized linear mixed models relating treatment and time effects either linearly to the dependent variable (for depression scores) or linearly to the logit of the probabilities (for response and remission).
By the end of the study, similar proportions of ART-eligible participants in each study arm were receiving ART (intervention, 73.1%; referral, 75.0%; P > .999). The mixed-model analysis revealed no statistically significant effects of the intervention on ART uptake (AOR = 1.18; 95% CI = 0.83, 1.68; P = .34). Participants in the intervention and referral arms had a similar average percentage of ART adherence (b = 0.05; 95% CI = −0.02, 0.12; P = .2). We found no statistically significant difference in viral suppression (AOR = 1.04; 95% CI = 0.97, 1.12; P = .23).
Fifty-five (83.3%) of 66 participants assigned to intervention completed the study: 2 died, 6 dropped out (including 2 who dropped out prior to baseline assessment and therefore did not contribute data to Table 1), and 3 were lost to follow-up. Sixty-four (90.1%) of 71 participants assigned to the referral arm completed the study: 1 died, 3 dropped out, and 3 were lost to follow-up. There were no suicides. Eight of 9 dropouts and all deaths occurred in the first 2 months.
DISCUSSION
We showed that directly observed treatment with fluoxetine improved depression symptom severity but not average ART adherence or probability of viral suppression in a group of homeless and marginally housed persons with comorbid HIV and depression. The observed benefit was substantial: at the 36-week follow-up, the average 3.5-point Ham-D treatment difference was equivalent to a Cohen’s d effect size greater than the mean effect size observed in short-term trials of serotonin-specific reuptake inhibitors (d = 0.40).39 Our estimated effect of fluoxetine treatment also compared favorably to the mean estimated effect observed in other classic long-term trials of serotonin-specific reuptake inhibitors (AOR = 1.66; 95% CI 1.12, 2.48).40
Our study added to the literature with 2 notable features. Our intervention specifically targeted homeless and marginally housed HIV-positive persons, a vulnerable population with a tremendous burden of unmet mental health needs16–18 and for whom novel evidence to inform practice and policy is urgently needed.41 Persons with substance use disorders may have difficulty adhering to clinical trial protocols and are frequently excluded from antidepressant medication treatment trials conducted in outpatient settings.42 Homeless and marginally housed persons have high rates of substance use disorders3–5 and are therefore de facto excluded. Yet we obtained adherence rates comparable to those achieved in other studies,43 and our retention rate (85%) over 9 months compared favorably with those observed in both long-term40 and short-term44 studies conducted among outpatients with fewer psychosocial comorbidities. Directly observed ART has been shown to improve ART adherence in marginalized populations with multiple psychosocial adherence barriers.45 Fluoxetine is uniquely suited for directly observed treatment and can easily be incorporated into substance use treatment or other structured counseling programs.
A second notable feature of our study is that it adds to the scant evidence40 on the long-term (≥ 6 months’ duration) treatment of depressed mood. Most of the data supporting this practice come from randomized withdrawal studies, which generalize poorly and can be problematic to interpret.46 The 2-arm parallel (classic) randomized controlled trial has been described as more closely approximating real-world effectiveness,46 but few such trials of antidepressant medications exist. One research team screened 2693 abstracts for a meta-analysis on serotonin-specific reuptake inhibitor treatment of major depression but discovered only 6 long-term, 2-arm parallel randomized controlled studies.40
We observed no statistically significant improvement in secondary HIV outcomes among participants randomized to the intervention arm. Lack of statistical power likely contributed to our lack of a statistically significant estimated effect, because we were unable to recruit the planned number of participants. However, other studies have also had mixed findings. Similar to our analysis, a study of collaborative care for depression implemented in 3 Veterans Affairs HIV clinics showed improvements in depression and HIV symptom severity but not ART adherence.23 A marginal structural model analysis demonstrated an effect of depression treatment on virological outcomes,22 but the authors explicitly noted that the data did not permit them to determine what additional counseling and social support services may have been delivered along with antidepressant medication treatment. An intervention among HIV-positive persons that combined cognitive behavioral therapy and adherence counseling yielded improvements in both depression and ART adherence but not virological outcomes.21 In other fields of medicine, randomized trials of depression interventions have also failed to improve clinical outcomes such as glycemic control,47 suggesting that barriers other than depressed mood are interfering with adherence.48 Taken together, these studies suggest that improving ART adherence may require more than improvements in mood alone and that adherence counseling49 and mobilizing other forms of social support may be necessary to improve adherence.
Limitations
Our study lacked a placebo control group. Even though use of placebos is common in psychopharmacological research,39,50,51 depression is known to adversely affect HIV outcomes and is treatable with medication.13–15 Therefore, we considered offering a 9-month placebo to be unethical. Intervention arm participants had intensive clinical contact with study staff, which itself may be therapeutic.52 However, participants in the referral arm also had significant contact with study staff, which would tend to mitigate this effect. Our positive finding on the primary outcome is all the more notable in light of the documented crossover contamination by referral arm participants who obtained mental health treatment outside the study.
The recruitment phase lasted more than 5 years because of the large screening sample needed to identify eligible participants. Although the refusal rate was low (5.6%), the large proportion of potential participants found to be ineligible may have compromised our ability to generalize the findings to all HIV-positive homeless and marginally housed adults with symptoms of depression. We were unable to formally compare the characteristics of potential participants who were screened for eligibility but declined to participate with those of study participants. However, our study sample was broadly similar to those obtained by systematic sampling.4,16,28,53
Notably, up to 12% of potential participants with symptoms of depression who underwent structured diagnostic assessment may have instead met diagnostic criteria for bipolar disorder, a proportion similar to the findings of a previous study.54 Our results highlight the importance of carefully assessing persons presenting with depressive symptoms, so as to avoid exposing patients with unrecognized bipolarity to antidepressant medications that may be ineffective or destabilizing.55
Conclusions
Our randomized controlled trial demonstrated that directly observed treatment with weekly fluoxetine resulted in improved mood among a cohort of homeless and marginally housed persons living with HIV. The statistically and clinically significant effects on mood that we observed are especially notable because they occurred in a population with ongoing substance abuse problems, homelessness, and other psychosocial comorbidities. Directly observed weekly fluoxetine may be an effective strategy to treat depression and potentially improve HIV treatment outcomes in individuals who might otherwise be considered poor candidates for treatment because of multiple barriers to treatment adherence.
Acknowledgments
This study was funded by the National Institute of Mental Health (NIMH; grants R01 MH 63011-01A1 and K24 MH-087227 to D. R. Bangsberg). The Research in Access to Care for the Homeless cohort, from whom some of the study participants were drawn, was funded by NIMH (grant R01 MH-054907 to D. R. Bangsberg). A. C. Tsai received support from the Robert Wood Johnson Foundation Health and Society Scholars Program. Roche donated HIV RNA kits. Study doses of Prozac Weekly were donated by Eil Lilly from 2002 through 2005.
Early screening data from this research were presented in part at the 15th International AIDS Conference, Bangkok, Thailand, July 11–16, 2004, and at the American Psychiatric Association Annual Meeting, San Diego, CA, May 23, 2007. Outcomes data were presented at the International Association of Physicians in AIDS Care International HIV Treatment Adherence Conference, Miami, FL, April 6, 2009.
We thank Judith Rabkin for critical input on the design of the study and the interpretation of the findings, Steve Safren for helpful comments, the participants who made this study possible by sharing their experiences, and the staff who conducted the interviews.
Note. The sponsors had no role in study design, data collection, or interpretation of the findings. The findings and conclusions are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors.
Human Participant Protection
The University of California, San Francisco committee on human research approved all study procedures. Study participants provided informed consent separately for screening and the subsequent randomized controlled trial. Potential participants identified with a BDI-II higher than 13 who declined to participate were offered referral to an outside agency for further evaluation.
References
- 1.Bing EG, Burnam MA, Longshore Det al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728 [DOI] [PubMed] [Google Scholar]
- 2.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. 2011;12(9):1004–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5(12):e225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushel MB, Evans JL, Perry S, Robertson MJ, Moss AR. No door to lock: victimization among homeless and marginally housed persons. Arch Intern Med. 2003;163(20):2492–2499 [DOI] [PubMed] [Google Scholar]
- 5.Hwang SW, Lebow JM, Bierer MF, O’Connell JJ, Orav EJ, Brennan TA. Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460 [DOI] [PubMed] [Google Scholar]
- 6.Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the Nutrition for Healthy Living Study. J Acquir Immune Defic Syndr. 2010;53(2):266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–171 [DOI] [PubMed] [Google Scholar]
- 8.Carrico AW, Riley ED, Johnson MOet al. Psychiatric risk factors for HIV disease progression: the role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56(2):146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS. 2011;25(8):1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley ED, Moore K, Sorensen JL, Tulsky JP, Bangsberg DR, Neilands TB. Basic subsistence needs and overall health among human immunodeficiency virus-infected homeless and unstably housed women. Am J Epidemiol. 2011;174(5):515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270(21):2568–2573 [PubMed] [Google Scholar]
- 14.Page-Shafer K, Delorenze GN, Satariano WA, Winkelstein W., Jr Comorbidity and survival in HIV-infected men in the San Francisco Men’s Health Survey. Ann Epidemiol. 1996;6(5):420–430 [DOI] [PubMed] [Google Scholar]
- 15.Cook JA, Grey D, Burke Jet al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggett TP, O’Connell JJ, Singer DE, Rigotti NA. The unmet health care needs of homeless adults: a national study. Am J Public Health. 2010;100(7):1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley ED, Gandhi M, Hare C, Cohen J, Hwang S. Poverty, unstable housing, and HIV infection among women living in the United States. Curr HIV/AIDS Rep. 2007;4(4):181–186 [DOI] [PubMed] [Google Scholar]
- 18.Hwang SW, Ueng JJ, Chiu Set al. Universal health insurance and health care access for homeless persons. Am J Public Health. 2010;100(8):1454–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asch SM, Kilbourne AM, Gifford ALet al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003;18(6):450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnam MA, Bing EG, Morton SCet al. Use of mental health and substance abuse treatment services among adults with HIV in the United States. Arch Gen Psychiatry. 2001;58(8):729–736 [DOI] [PubMed] [Google Scholar]
- 21.Safren SA, O’Cleirigh C, Tan JYet al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67(12):1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyne JM, Fortney JC, Curran GMet al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171(1):23–31 [DOI] [PubMed] [Google Scholar]
- 24.Kidder DP, Wolitski RJ, Campsmith ML, Nakamura GV. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am J Public Health. 2007;97(12):2238–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiser SD, Riley ED, Ragland K, Hammer G, Clark R, Bangsberg DR. Factors associated with depression among homeless and marginally housed HIV-infected men in San Francisco. J Gen Intern Med. 2006;21(1):61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmer P, Leandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy). Bull World Health Organ. 2001;79(12):1145–1151 [PMC free article] [PubMed] [Google Scholar]
- 27.Zolopa AR, Hahn JA, Gorter Ret al. HIV and tuberculosis infection in San Francisco’s homeless adults. Prevalence and risk factors in a representative sample. JAMA. 1994;272(6):455–461 [PubMed] [Google Scholar]
- 28.Robertson MJ, Clark RA, Charlebois EDet al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94(7):1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002 [Google Scholar]
- 30.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC; American Psychiatric Association; 1994 [Google Scholar]
- 31.Tulsky JP, Pilote L, Hahn JAet al. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Arch Intern Med. 2000;160(5):697–702 [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guy W. Early Clinical Drug Evaluation Unit Assessment Manual for Psychopharmacology–Revised. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. DHEW publication ADM 76–338 [Google Scholar]
- 34.Frank E, Prien RF, Jarrett RBet al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851–855 [DOI] [PubMed] [Google Scholar]
- 35.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX: Psychology Corp; 1996 [Google Scholar]
- 36.Bangsberg DR, Hecht FM, Charlebois EDet al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366 [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988 [Google Scholar]
- 38.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Stat. 1999;24(1):70–93 [Google Scholar]
- 39.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–1847 [DOI] [PubMed] [Google Scholar]
- 40.Deshauer D, Moher D, Fergusson D, Moher E, Sampson M, Grimshaw J. Selective serotonin reuptake inhibitors for unipolar depression: a systematic review of classic long-term randomized controlled trials. CMAJ. 2008;178(10):1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrman H. Mental disorders among homeless people in western countries. PLoS Med. 2008;5(12):e237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Posternak MA, Zimmerman M, Keitner GI, Miller IW. A reevaluation of the exclusion criteria used in antidepressant efficacy trials. Am J Psychiatry. 2002;159(2):191–200 [DOI] [PubMed] [Google Scholar]
- 43.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310 [DOI] [PubMed] [Google Scholar]
- 44.Gartlehner G, Hansen RA, Carey TS, Lohr KN, Gaynes BN, Randolph LC. Discontinuation rates for selective serotonin reuptake inhibitors and other second-generation antidepressants in outpatients with major depressive disorder: a systematic review and meta-analysis. Int Clin Psychopharmacol. 2005;20(2):59–69 [DOI] [PubMed] [Google Scholar]
- 45.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45(6):770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai AC, Rosenlicht NZ, Jureidini JN, Parry PI, Spielmans GI, Healy D. Aripiprazole in the maintenance treatment of bipolar disorder: a critical review of the evidence and its dissemination into the scientific literature. PLoS Med. 2011;8(5):e1000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katon WJ, Von Korff M, Lin EHet al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–1049 [DOI] [PubMed] [Google Scholar]
- 48.Mills EJ, Nachega JB, Bangsberg DRet al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Bruin M, Viechtbauer W, Schaalma HP, Kok G, Abraham C, Hospers HJ. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: a meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(3):240–250 [DOI] [PubMed] [Google Scholar]
- 50.Brunoni AR, Tadini L, Fregni F. Changes in clinical trials methodology over time: a systematic review of six decades of research in psychopharmacology. PLoS ONE. 2010;5(3):e9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration database. Arch Gen Psychiatry. 2000;57(4):311–317 [DOI] [PubMed] [Google Scholar]
- 52.Kaptchuk TJ, Kelley JM, Conboy LAet al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baggett TP, Singer DE, Rao SR, O’Connell JJ, Bharel M, Rigotti NA. Food insufficiency and health services utilization in a national sample of homeless adults. J Gen Intern Med. 2011;26(6):627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerman M, Mattia JI, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry. 2002;159(3):469–473 [DOI] [PubMed] [Google Scholar]
- 55.Smith DJ, Ghaemi SN, Craddock N. The broad clinical spectrum of bipolar disorder: implications for research and practice. J Psychopharmacol. 2008;22(4):397–400 [DOI] [PubMed] [Google Scholar]