Abstract
Objectives. We estimated age-standardized ratios of infection and hospitalization among Canadian First Nations (FN) populations and compared their distributions with those estimated for non-FN populations in Manitoba, Canada.
Methods. For the spring and fall 2009 waves of the H1N1 pandemic, we obtained daily numbers of laboratory-confirmed and hospitalized cases of H1N1 infection, stratified by 5-year age groups and FN status. We calculated age-standardized ratios with confidence intervals for each wave and compared ratios between age groups in each ethnic group and between the 2 waves for FN and non-FN populations.
Results. Incidence and hospitalization ratios in all FN age groups during the first wave were significantly higher than those in non-FN age groups (P < .001). The highest ratios were observed in FN young children aged 0 to 4 years. During the second wave, these ratios tended to decrease in FN populations and increase in non-FN populations, especially among groups younger than 30 years.
Conclusions. Incidence and hospitalization ratios in FN populations were higher than or equivalent to ratios in non-FN populations. Our findings support the need to develop targeted prevention and control strategies specifically for vulnerable FN and remote communities.
One striking aspect of the 2009 H1N1 influenza pandemic in Canada was its disproportionate impact on indigenous populations. In particular, on-reserve First Nations (FN) populations experienced severe disease outcomes often necessitating hospitalization and intensive care unit (ICU) admission.1–4 Many of the affected FN communities are located in the northern Manitoba region, which has predominantly Aboriginal populations (76%), and are considered remote or isolated.5 During the first pandemic wave, Winnipeg, an urban center in the province of Manitoba, experienced full occupancy of ICU beds at the peak of the outbreak in June 2009.1 Among laboratory-confirmed cases reported during the first wave in Manitoba, 32% were individuals with registry status as FN, an ethnic group that constitutes approximately 7% of the province’s total population (Table 1). According to the 2006 census data, 45% of this ethnic group in Manitoba resides off reserve. For community cases reported during the 2009 pandemic, FN status was determined by merging demographic data with a copy of the Indian Registry obtained from Indian and Northern Affairs Canada. The FN ethnic group refers to the Canadian Aboriginal peoples (with or without registry status) who are neither Inuit nor Métis.6 As observed in other geographic regions,7 the 2009 pandemic in Manitoba predominantly affected young adults and children in both spring and fall waves; however, the effect of the epidemic on FN populations has not been described.
TABLE 1—
Population Fraction, Laboratory-Confirmed Cases, and Number of Hospitalizations Among FN and Non-FN Populations: Manitoba, Canada; Spring and Fall 2009
| Aged 0–4 Y |
Aged 5–19 Y |
Aged 20–49 Y |
Aged ≥ 50 Y |
|||||
| Variable | FN | Non-FN | FN | Non-FN | FN | Non-FN | FN | Non-FN |
| Population fraction | .01 | .053 | .024 | .18 | .029 | .38 | .0086 | .32 |
| Laboratory-confirmed cases, no. | ||||||||
| First wave | 86 | 52 | 93 | 179 | 82 | 276 | 19 | 96 |
| Second wave | 33 | 154 | 81 | 639 | 90 | 620 | 10 | 155 |
| Hospitalizations, no. | ||||||||
| First wave | 46 | 16 | 19 | 18 | 39 | 38 | 10 | 27 |
| Second wave | 3 | 18 | 5 | 28 | 14 | 55 | 5 | 38 |
Note. FN = First Nations.
We sought to estimate the age distribution of infection and hospitalization among the FN populations and compare those distributions with those estimated for non-FN populations. Our primary objective was to estimate the relative infection and hospitalization ratios by age group using laboratory-confirmed cases of H1N1 infection and to explore possible differences in age-specific patterns of infection and hospitalization. Our secondary objective was to identify possible shifts in patterns in age distribution between the first and second waves of the H1N1 pandemic in Manitoba.
METHODS
We obtained the daily numbers of laboratory-confirmed and hospitalized cases of H1N1 influenza infection in Manitoba from the Manitoba Health influenza H1N1 databases for both waves of the 2009 pandemic: spring (891 cases between May 2 and August 5) and fall (1774 cases between October 1, 2009, and January 3, 2010). We defined a laboratory-confirmed case as that of an individual with influenza-like illness or severe respiratory illness who presented for primary care and tested positive for pandemic H1N1 influenza A virus by real-time reverse-transcriptase polymerase chain reaction or viral culture. All positive laboratory tests and completed case investigation forms were entered into a provincial pandemic influenza surveillance database. Ethnicity in surveillance data for laboratory-confirmed cases was determined through case investigation report forms and, when required for FN status, by merging demographic data with the Indian Registry. We classified the data into 5-year age groups, FN status, and health region of residence (11 health regions in the province of Manitoba); the first case of H1N1 infection was identified (tested positive) on May 2, 2009. Variables available for each patient included hospitalization and ICU admission (which accounted for a subset of laboratory-confirmed cases who were admitted to the hospital or ICU), antiviral use and start date of treatment, and vaccination during the second wave. We included all the variables in the data obtained for laboratory-confirmed cases. The data were reported by the earliest date of symptom onset, initial care, specimen collection, hospital admission, and ICU admission. For the present study, data use was approved by the Human Research Ethics Board of the University of Manitoba (H2009:339) and Health Information Privacy Committee of Manitoba (2009/2010–40). The use of FN data was also approved by the Assembly of Manitoba Chiefs, and the work was reviewed by the Assembly of Manitoba Chiefs before submission.
For demographic data, we used the 2009 population report for the province provided by the Manitoba Health Population Report,8 stratified by 5-year age groups, ethnic status as FN or non-FN, and different health regions.
Relative Ratios
Using laboratory-confirmed cases and demographic data, we calculated relative infection ratios (RIRs) for FN and non-FN populations and used these ratios to compare the age distribution of infection and hospitalization between FN and non-FN populations during the spring and fall waves of the 2009 pandemic. For the RIR,9 we calculated the age-standardized ratio of the proportion of infected cases in a given age group to the proportion of the population in the same age group:
 |
We used a similar expression to calculate the relative hospitalization ratio (RHR) for every age group. For each wave of the 2009 pandemic, we calculated these ratios for FN and non-FN age groups using the associated demographic and epidemiological data. A relative ratio higher than 1 indicates that the corresponding age group experienced a higher incidence of infection (with confirmed cases as the indicator) than the population as a whole.
Statistical Analyses
For each age group, we obtained the binomial confidence intervals for RIR and RHR, considering the total number of confirmed cases in all age groups as the number of observations and the numerator in the RIR expression as the proportion of infections for the specific age group.7 The denominator in the RIR and RHR expressions in this analysis is based on population demographics and thus has a very small uncertainty. The uncertainty in RIR and RHR is thus completely dominated by the numerator, which is binomially distributed. In the cases in which the normal approximation for the binomial confidence interval does not apply, we calculated the Wilson score interval.10 For comparative analysis of age distribution of FN and non-FN populations between the 2 waves, we performed nonparametric analysis of variance using the Mann–Whitney test11 for the samples of RIR and RHR drawn from their associated binomial distributions. We also performed the nonparametric Kruskal–Wallis test for comparative analyses of different age groups in each ethnic group within each wave.12 We used a 2-sided significance level of .05.
RESULTS
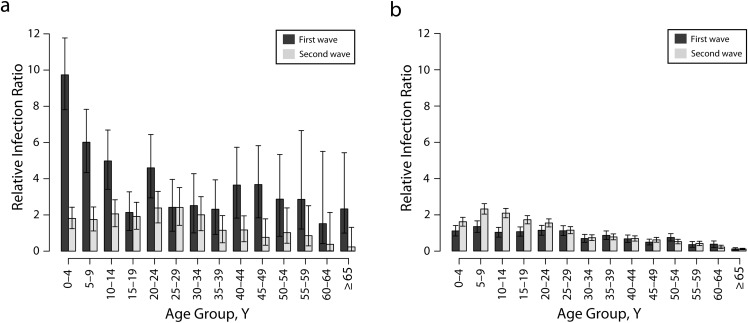
Overall, we observed decreasing RIR values from younger to older age groups for both FN and non-FN populations during spring and fall waves of the H1N1 pandemic (Figure 1a and 1b). During the first wave, the average RIR for all FN age groups was more than 1, with a significant difference between the mean RIR values for the groups younger than 15 years compared with those aged 15 years or older (with P < .001 when comparing different FN age groups using the Kruskal–Wallis test). The difference in the mean RIR corresponds to a more than 4-fold decline from 9.76 (95% CI = 7.83, 11.81) for the 0 to 4 years age group to 2.14 (95% CI = 1.14, 3.28) for the 15 to 19 years age group. Compared with the first wave, the RIR in FN groups younger than 15 years were lower in the second wave (maximum P < .007), with the largest drop for children younger than 5 years.
FIGURE 1—
Relative infection ratios for (a) First Nations (FN) age groups and (b) non-FN age groups: Manitoba, Canada; Spring and Fall 2009.
By contrast, the mean values of RIR for non-FN age groups were significantly higher for the groups younger than 25 years in the second wave compared with the first wave (maximum P < .001), in particular for school-aged children. Furthermore, groups older than 30 years had RIR values less than 1 during both the first and second waves. Comparative analyses of RIR for the first wave indicated considerably higher infection ratios in all FN age groups compared with non-FN age groups (maximum P < .001), with the maximum difference in the 0 to 4 years age group (Figure 1a and 1b; dark gray bars). Despite a significant drop in RIR during the second wave for all FN age groups, the RIR of FN groups older than 20 years was higher than the corresponding ratio for non-FN groups (maximum P < .001), in particular for the age groups between 20 and 30 years (Figure 1a and 1b; light gray bars).
Relative Hospitalization Ratio
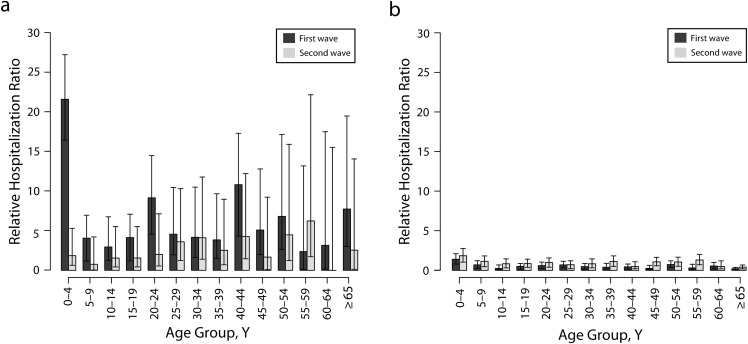
The mean RHR was greater than 1 during the first wave for all FN age groups (Figure 2a; dark gray bars). The mean RHR for the 0 to 4 years age group was 21.62 (95% CI = 16.45, 27.27) for the first wave but decreased significantly to 1.85 (95% CI = 0.63, 5.31) for the second wave (P < .001). In contrast to the FN groups, RHR for non-FN groups younger than 25 years increased during the second wave compared with the first wave (maximum P < .001; Figure 2a and 2b). Comparative analyses during the first wave indicated that the mean value of RHR for all FN age groups was higher than that for non-FN age groups, with a maximum difference of approximately 15-fold in the 0 to 4 years age groups (Figure 2a and 2b; dark gray bars). We also observed higher RHR in FN young adults than in non-FN young adults aged between 20 and 45 years during the second wave (maximum P < .001). Overall, the age distribution of hospitalization largely resembled that of the infection during both pandemic waves.
FIGURE 2—
Relative infection ratios for (a) First Nations FN age groups and (b) non-FN age groups: Manitoba, Canada; Spring and Fall 2009.
Aggregated Analyses of Ratios
Because of the low number of events in some of the age groups, especially for RHR, we also performed a comparative analysis of RIR and RHR by 4 broad age groups: 0 to 4 (preschool), 5 to 19 (school-aged), 20 to 49 (young adults), and 50 years and older (older adults; Table 2). RIR was greater than 1 for all FN age groups during the first wave but significantly reduced during the second wave (maximum P < .001). By contrast, RIR increased for non-FN groups younger than 50 years, with the largest increase in school-aged children (P < .001). Higher RIR was associated with all FN age groups compared with non-FN age groups during the first wave and also in groups younger than 5 and older than 20 years during the second wave. We made a similar observation for comparative analyses of RHR for FN groups younger than 50 years; however, RHR for all non-FN age groups was higher in the second wave than in the first wave (maximum P < .002) and stayed greater than 1 for the 0 to 4 years age group during both waves.
TABLE 2—
Aggregated Relative Infection and Hospitalization Ratios With 95% Confidence Intervals for FN and Non-FN Populations: Manitoba, Canada; Spring and Fall 2009
| Aged 0–4 Y |
Aged 5–19 Y |
Aged 20–49 Y |
Aged ≥ 50 Y |
|||||
| Variable | FN, RR (CI) | Non-FN, RR (CI) | FN, RR (CI) | Non-FN, RR (CI) | FN, RR (CI) | Non-FN, RR (CI) | FN, RR (CI) | Non-FN, RR (CI) |
| Infection | ||||||||
| First wave | 9.76 (7.83, 11.81) | 1.11 (0.83, 1.41) | 4.40 (3.60, 5.25) | 1.14 (1.00, 1.30) | 3.24 (2.61, 3.91) | 0.82 (0.74, 0.90) | 2.49 (1.44, 3.67) | 0.34 (0.28, 0.41) |
| Second wave | 1.81 (1.25, 2.43) | 1.62 (1.37, 1.86) | 1.91 (1.51, 2.33) | 2.03 (1.91, 2.16) | 1.73 (1.40, 2.09) | 0.91 (0.85, 0.97) | 0.65 (0.26, 1.11) | 0.28 (0.24, 0.32) |
| Hospitalization | ||||||||
| First wave | 21.62 (16.45, 27.27) | 1.42 (0.80, 2.12) | 3.72 (2.16, 5.49) | 0.48 (0.26, 0.69) | 6.38 (4.58, 8.19) | 0.47 (0.33, 0.60) | 5.43 (2.17, 8.69) | 0.40 (0.27, 0.55) |
| Second wave | 1.85 (0.00, 4.33) | 1.86 (1.05, 2.79) | 1.29 (0.26, 2.58) | 0.94 (0.63, 1.29) | 3.01 (1.51, 4.52) | 0.88 (0.68, 1.07) | 3.57 (0.71, 7.14) | 0.74 (0.54, 0.95) |
Note. CI = confidence interval; FN = First Nation; RR = relative ratio.
DISCUSSION
Using comprehensive laboratory testing and hospitalization data for the province of Manitoba, in which approximately 7% of the population has FN status, we observed that the infection and hospitalization ratios for FN populations were higher than expected in all age groups during both waves of the 2009 H1N1 influenza pandemic. Moreover, these ratios in FN populations were higher or equivalent to ratios in non-FN populations throughout the pandemic. The highest infection and hospitalization ratios among FN populations were observed in young children, aged 0 to 4 years, during the first wave. Of 213 hospitalized cases in the first wave, 54% were FN populations, of whom 21% were admitted to the ICU. The largest fraction of hospitalization among FN populations was associated with children younger than 5 years (40%). Several factors may have contributed to severe outcomes necessitating hospitalization or ICU admission, including longer delay in start of antiviral treatment since the onset of clinical symptoms, in particular for individuals with a preexisting comorbidity.4 The ratios of infection and hospitalization tended to be lower for FN populations during the second wave than during the first wave, whereas they appeared to be higher for non-FN populations in the second wave than in the first wave, especially among younger age groups.
The tendency for FN populations to experience a higher incidence and greater severity of acute respiratory illness than non-FN populations is well documented.4,13–18 The reasons for this increased risk are not well understood, but important factors may include the prevalence of predisposing health conditions and barriers to and disparities in health care access. For example, among hospitalized cases during the 2009 pandemic in Manitoba, 50.6% (first wave) and 53.7% (second wave) had 1 or more chronic conditions, with asthma, diabetes, and chronic lung diseases as the most prevalent comorbidities. Corresponding rates for chronic conditions among ICU-admitted cases were higher (first wave = 62.3%; second wave = 75%). FN ethnicity has also been identified as an independent determinant of severe infection leading to ICU admission.4 Demographic characteristics and geographic patterns of disease spread also influence the risk of infection. The laboratory and epidemiological data collected during the 2009 pandemic suggest a geographic shift in outbreaks from northern Manitoba (e.g., Burntwood, North Eastman, and Norman) in the first wave to more southern parts of the province (e.g., Assiniboine, Central, and Brandon) in the second wave.19
Most remote and isolated communities and FN reserves are located in northern Manitoba, where access is mainly by air travel. For example, the Burntwood health region in northern Manitoba has a large FN population, of whom 82% live on reserve. In these isolated northern communities, limited access to health care resources, crowded living conditions that allowed the virus to spread rapidly and readily between individuals, and limitations in critical infrastructure, including access to clean water for nonpharmaceutical interventions such as hand washing, as well as other social and demographic factors may all have contributed to the increased burden of disease.20,21 Some of these factors may have worked in combination to increase risk of infection. For example, housing conditions of some FN populations with multigenerational households including many children may tend to increase exposure of young children, who transmit the virus more effectively than adults because of social interaction patterns.22,23 In addition to these potential mechanisms for increasing risk, the substantial difference in the age profiles between FN and non-FN populations may explain the large number of pediatric FN cases. For example, the average age in Burntwood, in which 62% of the population has FN status, is 24 years, which is approximately 15 years younger than the average age in Winnipeg (38.7 years), the largest urban center in southern part of the province.24
A strength of our study was the use of data for an entire province covered by a single health care system and with a large FN population. Most studies on this topic have used ecological designs or limited their scope to small FN populations.14,25 We also performed a comparative analysis between the 2 waves of pandemic using data for the entire province. Not only does this analysis quantify the reduction in incidence and hospitalization ratios in FN populations during the second wave compared with the first wave, but it also shows that these ratios remained higher than those in non-FN populations throughout the pandemic. However, our study had limitations. In the absence of data on the true burden of infection, we used laboratory-confirmed diagnoses, and these data likely introduce some bias resulting from differential rates of testing across age groups, over time, and possibly between FN and non-FN populations. Assessing the magnitude and direction of such biases in laboratory data is difficult, but we do not expect hospitalization data to contain such biases, and we observed similar age-specific patterns for laboratory-confirmed and hospitalized cases. Although we observed higher relative ratios for FN populations than for non-FN populations, the nature of the data did not allow us to evaluate the influence on these ratios of on-reserve versus off-reserve status. Because only FN status was included in the data for the ethnicity variable, our analysis does not include other aboriginal people (e.g., FN or Indian people without status, Inuit, and Métis), which constitute approximately 6% of the Manitoba population.
The experience of the 2009 pandemic adds further support for the development of intervention strategies specifically for vulnerable FN and remote communities.4 Public health interventions in northern latitudes must place a strong emphasis on reducing disease transmission in the community (preventing individuals from becoming sick in the first place), thereby minimizing morbidity and mortality. Such a reduction in infection within specific communities would also serve to slow the spread of infection between remote and isolated communities and FN reserves because of the limited between-populations connectedness. However, the social structure in FN reserves must be considered when developing interventions.23 For example, in northern communities in which many family units are multigenerational, having infected children stay home from school may increase the rate of secondary household transmission, particularly among older individuals who share the same dwelling. Avoiding these unintended outcomes requires the development and evaluation of population-specific strategies for the implementation of transmission-reduction measures, such as early treatment of ill individuals and prophylaxis of close contacts. Moreover, prioritization of groups in these community settings for preventive measures such as vaccination may require specific consideration of familial relationships and social network patterns,26 which can be used to evaluate the impact of population heterogeneity on disease impact and transmission. As shown in our recent study,23 demographic parameters (e.g., age and household composition) of the population play a critical role in epidemic spread. In a noncrowded setting with a relatively low average of people per household, the protection of young individuals remains a determining factor, regardless of the age distribution of the population. However, in crowded settings, age distribution of the population significantly influences the impact of protection levels of different age groups on epidemic control.23
Previous work has demonstrated that the strength of social ties, rather than shared geography, may be extremely important in determining who acquires infection from whom.27–29 The effect of such a social network appears particularly important in underserved communities, including FN populations. These fundamental population differences have not been taken into account in previous work and warrant further investigation to identify tailored, community-specific disease intervention strategies.
Acknowledgments
We acknowledge the support of the Canadian Institutes of Health Research (operating grant, 114932) and the Mathematics of Information Technology and Complex Systems.
Note. The funders had no role in study design, data collection and analysis, preparation of the article, or decision to publish.
Human Participant Protection
Data use was approved by the Human Research Ethics Board of the University of Manitoba, Winnipeg, Manitoba, Canada, and Health Information Privacy Committee of Manitoba.
References
- 1.Kumar A, Zarychanski R, Pinto Ret al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879 [DOI] [PubMed] [Google Scholar]
- 2.Campbell A, Rodin R, Kropp Ret al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ. 2010;182(4):349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondro W. Dispensing antivirals in underserved communities. CMAJ. 2009;181(9):E199–E200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarychanski R, Stuart TL, Kumar Aet al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182(3):257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health Agency of Canada Considerations for definitions of “remote” and “isolated” in the context of pandemic (H1N1) 2009. Available at: http://www.phac-aspc.gc.ca/alert-alerte/h1n1/guidance_lignesdirectrices/cdricp-cdeicp-eng.php#fn2. Accessed November 15, 2011
- 6.Canada’s System of Justice: Rights and Freedoms in Canada. Department of Justice Canada Website. Available at: http://www.justice.gc.ca/eng/dept-min/pub/just/06.html. Updated August 3, 2012. Accessed March 16, 2012
- 7.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010;2(RRN1153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manitoba Health and Healthy Living Population Report. Available at: http://www.gov.mb.ca/health/population/2009/pr2009.pdf. Published June 1, 2009. Accessed March 16, 2012.
- 9.Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4th ed. Malden, MA: Blackwell Science; 2002 [Google Scholar]
- 10.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–212 [Google Scholar]
- 11.Birnbaum ZW. On a use of the Mann-Whitney statistic. : Neyman J, Third Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, CA: University of California Press; 1955 [Google Scholar]
- 12.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583–621 [Google Scholar]
- 13.Crighton EJ, Elliott SJ, Moineddin R, Kanaroglou P, Upshur R. A spatial analysis of the determinants of pneumonia and influenza hospitalizations in Ontario (1992–2001). Soc Sci Med. 2007;64(8):1636–1650 [DOI] [PubMed] [Google Scholar]
- 14.Fraser-Lee NJ, Hessel PA. Acute respiratory infections in the Canadian Native Indian population: a review. Can J Public Health. 1994;85(3):197–200 [PubMed] [Google Scholar]
- 15.Houston CS, Weiler RL, MacKay RW. Native children’s lung. J Can Assoc Radiol. 1979;30(4):218–222 [PubMed] [Google Scholar]
- 16.Marrie TJ, Carriere KC, Jin Y, Johnson DH. Hospitalization for community acquired pneumonia in Alberta First Nations Aboriginals compared with non-First Nations Albertans. Can Respir J. 2004;11(5):336–342 [DOI] [PubMed] [Google Scholar]
- 17.Mahoney MC, Ellrott MA, Michalek AM. A mortality analysis of Native American in New York State, 1980–86. Int J Epidemiol. 1989;18(2):403–412 [DOI] [PubMed] [Google Scholar]
- 18.Tomashek KM, Qin C, Hsia J, Iyasu S, Barfield WD, Flowers LM. Infant mortality trends and differences between American Indian/Alaska Native infants and white infants in the United States, 1989–1991 and 1998–2000. Am J Public Health. 2006;96(12):2222–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandemic H1N1 Influenza Historical Data. Manitoba Health Website. Available at: http://www.gov.mb.ca/health/publichealth/surveillance/h1n1/index.html. Accessed March 16, 2012.
- 20.Clark M, Riben P, Nowgesic E. The association of housing density, isolation and tuberculosis in Canadian First Nations communities. Int J Epidemiol. 2002;31(5):940–945 [DOI] [PubMed] [Google Scholar]
- 21.FitzGerald JM, Wang L, Elwood RK. Tuberculosis: 13. Control of the disease among aboriginal people in Canada. CMAJ. 2000;162(3):351–355 [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw M. Housing and public health. Annu Rev Public Health. 2004;25:397–418 [DOI] [PubMed] [Google Scholar]
- 23.Laskowski M, Mostaço-Guidolin LC, Greer A, Wu J, Moghadas SM. The impact of demographic variables on disease spread: influenza in remote communities. Sci Rep. 2011;1:105 [Google Scholar]
- 24. Statistics Canada. Winnipeg, Manitoba (Code 4611040). 2006 Community Profiles. Catalogue no. 92-591-XWE. Statistics Canada Website. Available at: http://www12.statcan.ca/census-recensement/2006/dp-pd/prof/92-591/index.cfm?Lang=E. Released March 13, 2007. Accessed March 16, 2012.
- 25.Young TK. Review of research on aboriginal populations in Canada: relevance to their health needs. BMJ. 2003;327(7412):419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dushoff J, Plotkin JB, Viboud Cet al. Vaccinating to protect a vulnerable subpopulation. PLoS Med. 2007;4(5):e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potterat JJ, Muth SQ, Rothenberg RBet al. Sexual network structure as an indicator of epidemic phase. Sex Transm Infect. 2002;78(suppl 1):i152–i158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weis SE, Pogoda JM, Yang Z. Transmission dynamics of tuberculosis in Tarrant County, Texas. Am J Respir Crit Care Med. 2002;166(1):36–42 [DOI] [PubMed] [Google Scholar]
- 29.Klovdahl AS, Graviss EA, Yaganehdoost Aet al. Networks and tuberculosis: an undetected community outbreak involving public places. Soc Sci Med. 2001;52(5):681–694 [DOI] [PubMed] [Google Scholar]