Abstract
Background
Urinary N‐acetyl‐β‐D‐glucosaminidase (NAG) excretion is increased in patients with impaired glucose tolerance (IGT). This study investigated when during the oral glucose tolerance test (OGTT) the plasma glucose, urine glucose, and insulin levels correlate most strongly with urinary N‐acetyl‐β‐d‐glucosaminidase (NAG) levels in prediabetic subjects.
Methods
The OGTT was administered to 80 subjects who had not yet received a diagnosis of diabetes mellitus (DM) and in whom HbA1c levels were ≤6.8% and fasting plasma glucose levels were <7.0 mmol/l. Forty‐two subjects had normal glucose tolerance (NGT), 31 had impaired glucose tolerance (IGT), and 7 had DM according to World Health Organization criteria. Serum levels of cystatin C, the estimated glomerular filtration rate, the urinary albumin‐to‐creatinine (Cr) ratio, urinary and serum β2‐microglobulin, and urinary NAG were measured as markers of renal function.
Results
NAG levels were significantly higher in subjects with DM and in subjects with IGT than in subjects with NGT. No significant associations were observed between glycemic status and other markers of renal function. Multiple linear regression analysis showed that the NAG level was positively correlated with plasma glucose levels at 120 min of the OGTT and was associated with the glycemic status of prediabetic patients.
Conclusion
These results suggest that postprandial hyperglycemia is an independent factor that causes renal tubular damage in prediabetes patients.
Keywords: cystatin C, N‐acetyl‐β‐d‐glucosaminidase, tubular dysfunction, impaired glucose tolerance, diabetic nephropathy
INTRODUCTION
Diabetic nephropathy is a major cause of death worldwide in patients with diabetes mellitus (DM). Microalbuminuria has been recognized as an early predictor of nephropathy and has, therefore, become an important target for screening 1. The urinary albumin excretion rate has been selected as a urinary marker because of its significance in the pathophysiology of diabetic nephropathy and its potential associations with the other markers being studied 2, 3. Furthermore, urinary N‐acetyl‐β‐d‐glucosaminidase (NAG) has also been identified as a possible marker for diabetic nephropathy 4, 5. The Diabetes Control and Complications Trial has recently shown that early urinary elevations of albumin and NAG in type 1 DM independently predict future microalbuminuria and macroalbuminuria 3.
NAG is a widely distributed lysosomal enzyme that has a molecular weight of 140 kDa and is present at highest concentrations in the renal proximal tubules 6. Because urinary levels of NAG are increased in glomerulonephritis, tubulointerstitial diseases, renal allograft rejection, and toxic renal injury 7, 8, 9, 10, they have been used to evaluate and predict subtle degrees of renal injury. Increased urinary levels of NAG have been reported in both type 1 and 2 DM 4, 11, 12. Moreover, a new marker for glycemia developed in Japan, 1,5‐anhydroglucitol (1,5‐AG), is a specific index of postprandial hyperglycemia. A relationship between serum 1,5‐AG and urinary NAG in type 2 DM has been reported 13. However, to date, only a few studies have examined urinary NAG levels in subjects with impaired glucose tolerance (IGT) 14, 15. Furthermore, the relationships among urinary NAG, plasma glucose levels, and other markers have not been examined at preloading or during the oral glucose tolerance test (OGTT).
Urinary levels of NAG vary with changes in plasma glucose levels in patients with type 1 or type 2 DM 11, 16. In addition, our recent longitudinal study has shown that urinary levels of NAG are significantly related to long‐term blood glucose control in elderly patients with type 2 DM 17.
The first major aim of the present study was to examine how plasma glucose, urine glucose, and insulin levels during the OGTT are related to urinary NAG levels in subjects in the prediabetic state. The second aim was to examine when during the OGTT the plasma glucose most closely relates to urinary NAG levels in subjects with prediabetes. In addition, the final aim of this study was to examine the relationships between glycemic status and other markers of renal function.
MATERIALS AND METHODS
Diagnostic 75‐g OGTTs were administered at the Nippon Medical School Hospital from December 2005 through August 2010 to 80 patients (33 men and 47 women, aged 18–79 years) who had not yet received a diagnosis of DM and in whom HbA1c levels were ≤6.8% and fasting plasma glucose levels were <7.0 mmol/l. On the basis of the OGTT and World Health Organization (WHO) criteria 18, patients were placed into a normal glucose tolerance (NGT) group, an IGT group, or a DM group.
Subjects were excluded on the basis of the following criteria: pregnancy, previous gastrectomy, anemia, severe illness, serum creatinine (Cr) level ≥ 97.24 μmol/l (1.10 mg/dl), urine protein test > 1+ (equivalent to > 0.3 g/l), renal glucosuria, liver cirrhosis, chronic hepatitis, history of coronary artery disease (defined as symptoms of ischemia with simultaneous ischemic changes on electrocardiography or a history of myocardial infarction), urolithiasis, and chronic urinary tract infection.
The OGTTs were performed after an overnight fast of 12–14 hr. Subjects ingested a simple carbohydrate solution equivalent to 75 g of glucose (Torelan‐G, Shimizu Pharmaceuticals, Shimizu, Japan), and blood samples were obtained after 0, 30, 60, and 120 min. Plasma glucose levels were determined from specimens of venous blood by means of the glucose oxidase method. Plasma immunoreactive insulin (IRI) levels were determined with solid‐phase radioimmunoassay. Levels of HbA1c (normal range, 4.1–5.9%) were assayed with high‐performance liquid chromatography (Auto A1C analyzer; Arkray, Inc., Kyoto, Japan) according to the method of the Japanese Diabetes Society (JDS), which is equivalent to the internationally used HbA1c (%) (HbA1c [NGSP]) defined by the National Glycohemoglobin Standardization Program (NGSP), expressed by adding 0.4% to the HbA1c (JDS) (%) 19.
Urinary glucose levels and serum Cr levels were measured with enzymatic assays. The serum levels of cystatin C were measured with the N Latex Cystatin C kit (Siemens Healthcare Diagnostics, Inc., Marburg, Germany) and the fully automated particle‐enhanced nephelometric immunoassay. Urinary NAG levels were measured spectrophotometrically with sodio‐meta‐cresolsulfonphthaleinyl‐N‐acetyl‐β‐D‐glucosaminide as a substrate (NAG assay kit, Shionogi & Co., Ltd., Osaka, Japan) 20. This assay is specific for NAG, has no detectable reaction with other glycosidases, and has excellent sensitivity, with a linear range up to 200 U/l and excellent precision (coefficient of variation <5%). The ratio of the urinary activity of NAG to Cr (NAG index) was determined in spot urine samples.
The estimated glomerular filtration rate (eGFR) was determined from serum Cr values and patient age using the new Japanese equation as follows 21:
| (1) |
Serum and urinary concentrations of β2‐microglobulin (β2MG) were measured with a latex immunoassay kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The urinary albumin‐to‐Cr ratio (ACR) was measured with radioimmunoassay. Specimens of blood and urine were obtained while the subjects were fasting. The urinary NAG index, levels of β2MG, and the ACR were determined in spot samples of second morning urine specimens. Blood pressure was measured with the subject in a sitting position. The body‐mass index (BMI) was calculated. Insulin resistance was measured with the homeostasis model assessment of insulin resistance (HOMA‐R) using fasting IRI and the fasting plasma glucose level during the OGTT 22 as follows:
| (2) |
Measurements were made using the area under the curve of glucose (AUC [glu]) andinsulin during the OGTT.
The Mann–Whitney U‐test, the Kruskal–Wallis H‐test, Pearson's correlation, and multiple linear regression analysis were used for statistical analysis of the data. A value of P < 0.05 was considered to indicate statistical significance. All statistical tests were performed with a statistical software program (IBM SPSS statistics, version 12, IBM Corp., Armonk, NY). All data are presented as means ± SD.
Subjects were divided into three groups according to their glycemic response to the OGTT. No subjects showed impaired glucose tolerance (IGT) with fasting alone (fasting glucose ≥6.1 and < 7.0 mmol/l and 120‐min glucose <7.8 mmol/l). The three groups were defined according to WHO criteria 18 as follows: the NGT group: fasting glucose <6.1 mmol/l and 120‐min glucose <7.8 mmol/l; the IGT group: fasting glucose <7 mmol/l and 120‐min glucose ≥7.8 and <11.1 mmol/l; and the DM group: fasting glucose ≥7 mmol/l or 120‐min glucose ≥11.1 mmol/l or both.
All examinations were performed in the outpatient clinic of our hospital. Before the start of the study, informed consent was obtained from all subjects.
RESULTS
On the basis of the OGTT and WHO criteria, NGT was diagnosed in 42 subjects, IGT in 31, and DM in 7. The clinical characteristics of the subjects are shown in Table 1, and glucose levels at each time point during the OGTT are shown in Figure 1. The BMI was higher in the DM group than in the NGT group or the IGT group, but the difference was not statistically significant.
Table 1.
Clinical Characteristics of Study Subjects
| Clinical characteristics OGTT status | NGT | IGT | DM | All | P‐value |
|---|---|---|---|---|---|
| N | 42 | 31 | 7 | 80 | – |
| Age (years) | 61.1 ± 17.2 | 66.3 ± 9.4 | 57.7 ± 15.1 | 62.8 ± 14.6 | 0.441 |
| Men/women | 15/27 | 13/18 | 5/2 | 33/47 | 0.209 |
| BMI (kg/m2) | 23.4 ± 3.9 | 24.6 ± 3.2 | 27.1 ± 4.4 | 24.2 ± 3.7 | 0.050 |
| Systolic blood pressure (mmHg) | 123.8 ± 14.1 | 129.7 ± 16.1 | 133.1 ± 10.7 | 126.9 ± 14.9 | 0.129 |
| Diastolic blood pressure (mmHg) | 73.8 ± 9.6 | 75.9 ± 11.5 | 82.6 ± 4.9 | 75.4 ± 10.3 | 0.092 |
| Hypertension (%) | 47.6 | 77.4 | 71.4 | 61.3 | 0.031* |
| Antihypertensive medication (%) | 38.1 | 51.6 | 71.4 | 46.3 | 0.199 |
| ACE inhibitors/ARBs (%) | 23.8 | 41.9 | 42.9 | 32.5 | 0.327 |
| Total cholesterol (mmol/l) | 5.05 ± 0.72 | 5.30 ± 1.21 | 4.99 ± 0.72 | 5.14 ± 0.94 | 0.784 |
| LDL cholesterol (mmol/l) | 2.86 ± 0.58 | 3.05 ± 0.91 | 2.99 ± 0.56 | 2.94 ± 0.72 | 0.733 |
| Triglycerides (mmol/l) | 1.20 ± 0.62 | 1.92 ± 3.06 | 1.54 ± 0.72 | 1.51 ± 1.99 | 0.294 |
| Serum albumin (g/l) | 44.0 ± 3.0 | 44.0 ± 2.0 | 45.0 ± 3.0 | 44.0 ± 3.0 | 0.930 |
| Uric acid (μmol/l) | 309.3 ± 89.2 | 303.3 ± 65.4 | 333.1 ± 35.7 | 309.3 ± 77.3 | 0.476 |
| FPG (mmol/l) | 5.03 ± 0.42 | 5.63 ± 0.60 | 6.35 ± 0.85 | 5.38 ± 0.67 | <0.001** |
| HbA1c (%) | 5.78 ± 0.33 | 6.16 ± 0.36 | 6.61 ± 0.47 | 6.00 ± 0.47 | <0.001** |
| Serum Cr (μmol/l) | 60.2 ± 10.6 | 59.3 ± 14.2 | 59.3 ± 10.6 | 59.3 ± 11.5 | 0.730 |
| Urinary pH | 6.19 ± 0.89 | 5.97 ± 0.71 | 6.00 ± 0.58 | 6.09 ± 0.80 | 0.491 |
Data are expressed as mean ± SD.
BMI, body mass index; LDL cholesterol, low‐density lipoprotein cholesterol; ACE inhibitors, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; FPG, fasting plasma glucose.
*P < 0.05; **P < 0.005.
Figure 1.
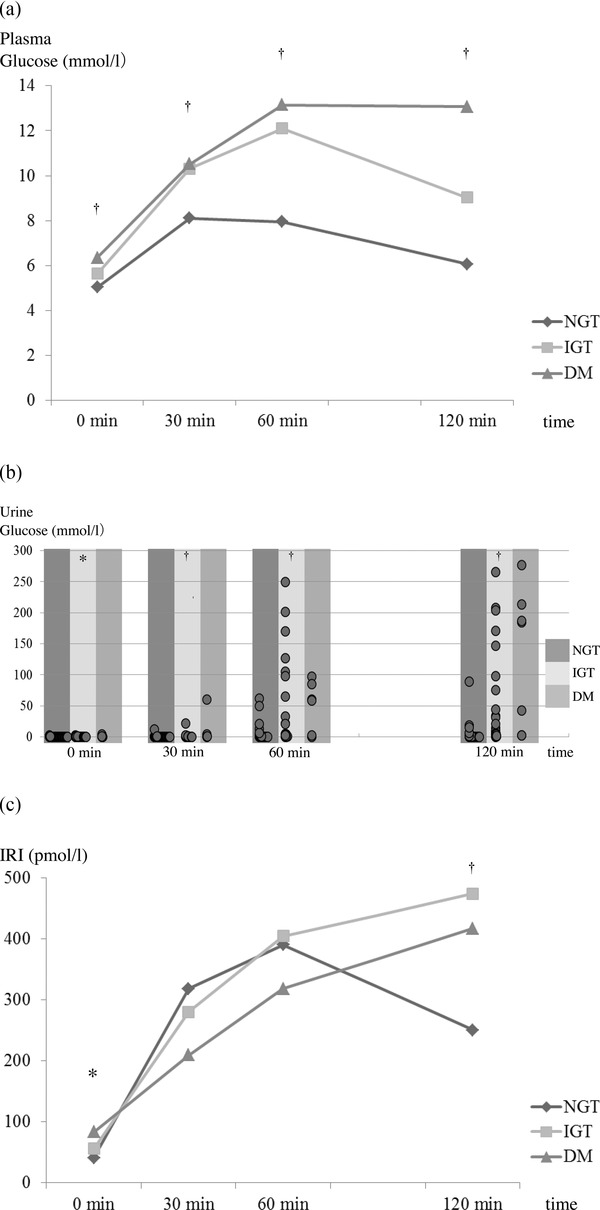
Changes in plasma glucose (a), urine glucose (b), and IRI (c) concentrations during a 75‐g oral glucose tolerance test (OGTT). IRI, immunoreactive insulin; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; DM, diabetes mellitus.
† P < 0.005, *P < 0.05.
The HbA1c level was positively associated with glycemic status (the Kruskal–Wallis H‐test), and the HbA1c level was significantly higher in the DM group (P < 0.05, Mann–Whitney U‐test). The three groups did not differ significantly in regard to age, sex, systolic or diastolic blood pressure, total cholesterol, low‐density lipoprotein cholesterol, triglycerides, serum albumin, uric acid, serum Cr, or urinary pH. The prevalence of hypertension was positively associated with glycemic status and the development of glucose intolerance.
The plasma and urine levels of glucose and IRI during the OGTT are shown in Figure 1. Mean plasma and urine glucose levels differed significantly between the NGT, IGT, and DM groups at each time point of the OGTT (Fig. 1a, b). Mean plasma IRI levels differed significantly between the NGT, IGT, and DM groups at 0 and 120 min of the OGTT (Fig. 1c).
Figure 2 shows the associations between markers of renal function and glycemic status (assessed with OGTT: NGT, IGT, and DM). The three groups did not differ significantly with regard to levels of cystatin C, eGFR, serum β2MG, urinary β2MG, or urinary ACR. Only urinary NAG showed a significant difference (P = 0.016): the urinary NAG level in the DM group (8.53 ± 4.96 U/ g UCr) was significantly higher than that in the NGT group (5.40 ± 3.82 U/g UCr; P = 0.020), and that in the IGT group (6.60 ± 2.88 U/g UCr) was significantly higher than that in the NGT group (5.40 ± 3.82 U/g UCr; P = 0.027). The urinary NAG level in the DM group was slightly but not significantly higher than that in the IGT group (P = 0.460). The urinary NAG level was positively correlated with patient age (r = 0.404, P < 0.001) and with the plasma glucose level at 120 min of the OGTT (r = 0.320, P = 0.004). However, age was not positively correlated with the plasma glucose levels at 120 min (r = 0.162, P = 0.151).
Figure 2.
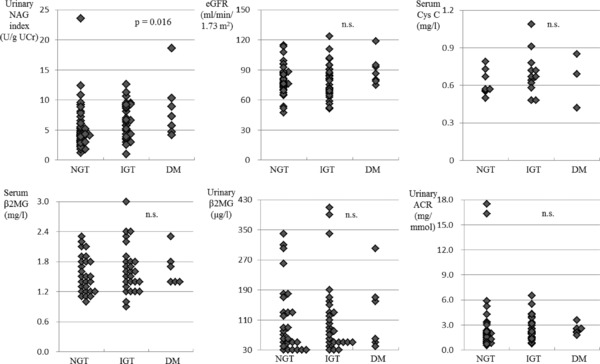
Renal markers in 80 subjects in the prediabetic state. Urinary NAG index (U/g UCr), eGFR (ml/min/1.73 m2), serum cystatin C (mg/l), serum β2MG (mg/l), urinary β2MG (μg/l), urinary ACR (mg/mmol) by glycemic status (assessed with a 75‐g oral glucose tolerance test [OGTT]). Urinary NAG index, urinary N‐acetyl‐β‐d‐glucosaminidase index; eGFR, estimated glomerular filtration rate; β2MG, β2‐microglobulin; ACR, albumin‐to‐Cr ratio; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; DM, diabetes mellitus; ns, not significant. Data are expressed as mean ± SD.
Multiple linear regression analyses showed that the urinary NAG level was significantly and positively associated with the plasma glucose level at 120 min of the OGTT when corrected for age, sex, systolic blood pressure, serum levels of Cr and total cholesterol, and BMI (Table 2). Furthermore, multiple linear regression analysis showed a similar relation between the urinary NAG level and glycemic status.
Table 2.
Multiple Linear Regression Analyses of Urinary NAG Index in Relation to Other Variables
| Dependent variable: | Dependent variable: | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| urinary NAG index | urinary NAG index | ||||||||||
| Independent | Full‐ | Independent | Full‐ | ||||||||
| variables | Correction | β | t | P‐value | model R 2 | variables | Correction | β | t | P‐value | model R 2 |
| Plasma glucose | Model 1 | 0.095 | 0.846 | 0.400 | 0.009 | IRI | Model 1 | 0.001 | 0.006 | 0.995 | <0.001 |
| 0 min | Model 2 | 0.135 | 1.141 | 0.258 | 0.208 | 0 min | Model 2 | 0.163 | 1.386 | 0.170 | 0.214 |
| Plasma glucose | Model 1 | 0.063 | 0.561 | 0.576 | 0.004 | IRI | Model 1 | −0.082 | −0.730 | 0.468 | 0.007 |
| 30 min | Model 2 | −0.037 | −0.309 | 0.758 | 0.195 | 30 min | Model 2 | −0.067 | −0.565 | 0.574 | 0.197 |
| Plasma glucose | Model 1 | 0.081 | 0.718 | 0.475 | 0.006 | IRI | Model 1 | −0.158 | −1.421 | 0.159 | 0.025 |
| 60 min | Model 2 | 0.034 | 0.284 | 0.777 | 0.194 | 60 min | Model 2 | −0.154 | −1.268 | 0.209 | 0.210 |
| Plasma glucose | Model 1 | 0.320 | 3.003 | 0.004** | 0.102 | IRI | Model 1 | 0.080 | 0.717 | 0.476 | 0.006 |
| 120 min | Model 2 | 0.319 | 2.975 | 0.004** | 0.282 | 120 min | Model 2 | 0.113 | 0.979 | 0.331 | 0.204 |
| Urine glucose | Model 1 | 0.013 | 0.112 | 0.911 | <0.001 | AUC (glu) | Model 1 | 0.154 | 1.383 | 0.170 | 0.024 |
| 0 min | Model 2 | 0.138 | 1.230 | 0.223 | 0.209 | Model 2 | 0.116 | 0.982 | 0.329 | 0.204 | |
| Urine glucose | Model 1 | 0.079 | 0.705 | 0.483 | 0.006 | HbA1c | Model 1 | 0.161 | 1.451 | 0.151 | 0.026 |
| 30 min | Model 2 | 0.128 | 1.182 | 0.241 | 0.208 | Model 2 | 0.184 | 1.661 | 0.101 | 0.223 | |
| Urine glucose | Model 1 | −0.033 | −0.295 | 0.768 | 0.001 | OGTT status | Model 1 | 0.253 | 2.320 | 0.023* | 0.064 |
| 60 min | Model 2 | −0.009 | −0.072 | 0.943 | 0.193 | NGT/IGT/DM | Model 2 | 0.297 | 2.723 | 0.008* | 0.269 |
| Urine glucose | Model 1 | 0.046 | 0.412 | 0.682 | 0.002 | HOMA‐R | Model 1 | 0.050 | 0.443 | 0.659 | 0.002 |
| 120 min | Model 2 | 0.104 | 0.890 | 0.376 | 0.202 | Model 2 | 0.208 | 1.806 | 0.075 | 0.228 | |
Urinary NAG index, urinary N‐acetyl‐β‐d‐glucosaminidase index; IRI, immunoreactive insulin; AUC (glu), the area under the curve of glucose; HOMA‐R, homeostasis model assessment of insulin resistance; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; DM, diabetes mellitus; BMI, body mass index; ACR, urinary albumin‐to‐Cr ratio.
Number of dummy variables: 1, OGTT‐NGT; 2, IGT; 3, DM.
Model 1: uncorrected; Model 2: corrected as for age, sex, systolic blood pressure, serum Cr, total cholesterol, BMI, and ACR.
*P < 0.05; **P < 0.005.
No statistically significant associations were observed between the urinary NAG and plasma glucose levels at preloading, 30 min or 60 min of the OGTT, urine glucose levels at preloading or during the OGTT, AUC (glucose), HbA1c, or HOMA‐R. Furthermore, there were no statistically significant differences between the insulin levels at preloading or during the OGTT, the homeostasis model assessment of β‐cell function (HOMA‐β), or the insulinogenic index.
DISCUSSION
The main finding of the present study is that urinary NAG levels are significantly and positively correlated with plasma glucose levels at 120 min of the OGTT in the prediabetic state. Increased urinary levels of NAG indicate proximal tubular damage, because the highest concentrations of NAG are located in the renal proximal tubules 6. To date, few studies have examined the association of tubular markers with the severity of renal function in diabetic nephropathy. Previous studies have shown that the urinary level of NAG and the levels of other markers of tubular damage are correlated with urinary albumin excretion 23, 24. Several studies have found that even in patients with normoalbuminuric DM, urinary NAG levels are already higher than in subjects without diabetes 24, 25. On the basis of these data, Nauta et al. 25 have proposed that the tubulointerstitium plays a role in the pathogenesis and progression of nephropathy in patients with DM.
Urinary NAG levels generally rise and fall according to the degree of glycemic control in patients with diabetes 26. Increased urinary NAG levels have been reported in both type 1 and 2 DM 11, 12. Furthermore, Fujita et al. 14 have found that urinary NAG excretion is slightly but significantly higher in subjects with sustained IGT than in control subjects with NGT. Another study has shown that urinary NAG excretion is significantly increased in patients with IGT 15. The present study also found that the urinary excretion of NAG was correlated with glycemic status (assessed with an OGTT). These findings of some previous studies are consistent with our present findings, because the most difference between NGT and IGT is the postload glucose levels of the OGTT. In addition, Hiratsuka et al. has shown no significant associations between urinary albumin, urinary β2‐microglobulin, and glycemic status (i.e., NGT, IGT, or DM) 15. The present study obtained similar findings.
Urinary NAG levels increase with age 27, 28, 29. To examine the relationship between urinary NAG levels and age, we have determined the NAG index in 137 healthy subjects, aged 19–88 years 28. This cross‐sectional study found that urinary NAG levels increase with age at a rate of 0.65 U/g UCr per decade. In the present study, nearly two‐thirds of the subjects (65%) were older than 60 years. Multiple linear regression analysis of all models in the present study identified only age as an independent predictor of increased urinary NAG. Therefore, this result confirms the relationship between urinary NAG levels and age. Hyperglycemia, IGT, and type 2 DM become progressively more common with advancing age 30, 31, 32, 33. The increase in plasma glucose levels at 120 min of the OGTT is significantly greater in elderly subjects than in younger subjects 33. Furthermore, another study has suggested that the age‐related decline in glucose metabolism has only a small effect on the fasting plasma glucose concentration, which increases by 1 mg/dl per decade. In contrast, following oral glucose ingestion, the 60‐min plasma glucose response has been shown to increase by 0.22–0.78 mmol/l (4–14 mg/dl) (mean = 0.5 mmol/l [mean = 9 mg/dl]) per decade and the 120‐min plasma glucose value by 0.06–0.61 mmol/l (1–11 mg/dl) (mean = 0.28 mmol/l [mean = 5 mg/dl]) per decade 34.
In the present study, the urinary excretion of NAG was correlated with the plasma glucose level at 120 min of the OGTT when the data were corrected for age, sex, systolic blood pressure, serum levels of Cr and total cholesterol, BMI, and urinary ACR. Neither the plasma glucose level at preloading of the OGTT nor the HbA1c level was correlated with the urinary excretion of NAG. To investigate the correlation between the glucose levels after load and urinary NAG in younger patients, we excluded elderly subjects and performed multivariate analysis in 29 subjects (60 years or younger). The glucose value at 120 min (P = 0.003) was the most significantly related with urinary NAG index and was followed by the 60 min glucose value (P = 0.030), but the 30 min glucose value was not significantly related (data not shown). In our analysis, the most significant correlation was seen in the 120 min glucose value in younger patients as well. However, the 60 min glucose value also had significant correlation. Unfortunately, the plasma glucose level at 90 min of the OGTT was not measured in the present study, but the 90 min glucose value may have had the strongest correlation in younger patients. We would like to study a larger number of younger patients and measure 90 min glucose values in the future.
Recently, many studies have found that the serum 1,5‐AG level generally reflects postprandial hyperglycemia 35 but not fasting hyperglycemia 36. A strong negative correlation has also been found between the serum 1,5‐AG level and the plasma glucose level at 120 min of the OGTT in subjects with IGT 35. A study by Yamanouchi et al. has found that the serum 1,5‐AG level and the urinary NAG level are related 13; this study also found that urinary excretion of NAG is associated with a change in the serum 1,5‐AG level and is quickly reversible when the serum 1,5‐AG level improves in type 2 DM. In the 3 years after the onset of diabetes, Yamanouchi et al. 13 obtained at least 18 measurements of one variable for each patient (n = 112) and calculated the means. The urinary NAG level was found to be significantly correlated with the fasting plasma level of glucose (r = 0.512, P < 0.0001), the level of HbA1 (r = 0.351, P = 0.001) and, especially, with the level of 1,5‐AG (r = −0.790, P < 0.0001). These findings are consistent with our present findings that the urinary NAG level was correlated with the plasma glucose level at 120 min of the OGTT, and the possibility tubular damage is induced by postprandial hyperglycemia. Between 99% and 100% of 1,5‐AG is reabsorbed in normoglycemia, but the reabsorption rate decreases significantly in hyperglycemia in approximate proportion to the degree of the hyperglycemia above the renal threshold for glucosuria 37.
Moreover, although there are individual differences in the renal threshold for glucosuria, when the plasma glucose level is higher, the urinary excretion of glucose increases. In general, the renal threshold for glucosuria is a plasma glucose level of 9–10 mmol/l (160–180 mg/dl) or more. Therefore, in patients in a prediabetic state, glucosuria appears during the OGTT but not at preloading of the OGTT. When the previous study of the relationship between urinary NAG levels and the serum 1,5‐AG levels is taken into account, it is necessary to examine both the relationship between urinary NAG levels and urine glucose levels and the relationship between urinary NAG levels and plasma glucose levels during the OGTT. To our knowledge, no previous reports have examined the relationship between urinary NAG levels and plasma or urine glucose levels during the OGTT. In the present study, we investigated the relationship between urinary NAG levels and plasma or urine glucose levels during the OGTT and found no statistically significant association between urinary NAG levels and urine glucose levels at preloading or during the OGTT.
Several studies have found that urinary NAG levels are not consistently correlated with the severity of hypertension 4, 27, 38. Schnoell et al. 39 have reported that urinary NAG did not differ significantly between hypertensive and normotensive patients with diabetes. In addition, our previous study showed no evidence that urinary NAG levels are related to blood pressure 28. Furthermore, Sano et al. 40 found that in patients with type 2 DM and persistent microalbuminuria, although the angiotensin‐converting enzyme (ACE) inhibitor enalapril reduced urinary albumin excretion in both normotensive patients and patients with well‐controlled hypertension, urinary NAG levels were not decreased in either group. However, combined administration of the ACE inhibitor perindopril and the diuretic indapamide reduces urinary NAG excretion in type 2 DM 26. In the present study, we found that neither systolic nor diastolic blood pressure differed among subjects with NGT, IGT, or DM. There was no difference in the percentages of patients being treated with ACE inhibitors or angiotensin receptor blockers (ARBs) among the NGT, IGT, and DM groups. There was no simple correlation between urinary NAG and systolic blood pressure or between urinary NAG and diastolic blood pressure in any subject. Multiple linear regression analysis found no correlation between systolic blood pressure and urinary NAG levels when the data were corrected for demographic factors. This finding is in concordance with those of most previous studies. The results of linear regression analysis remained valid when the data were corrected for a history of ACE inhibitor or ARB use but not for systolic blood pressure. A similar result was obtained when the data were corrected for both the systolic blood pressure and history of ACE inhibitor or ARB use.
In the present study, the ratio of the urinary activity of NAG to Cr (NAG index) was determined in spot urine specimens. The NAG index in random urine specimens provides a useful and convenient means of assessing daily NAG excretion and avoids many of the problems of 24‐hr collection. A study by Ellis et al. 11 has found that the correlation coefficient for the NAG index in random early morning urine specimens versus 24‐hr NAG excretion in children with diabetes was 0.80.
Urinary levels of NAG are increased in various renal diseases, including glomerulonephritis, tubulointerstitial diseases, renal allograft rejection, and toxic renal injury 7, 8, 9, 10. These observations suggest that the glucose levels at 120 min of the OGTT must be taken into account in subjects without diabetes when the urinary NAG index is examined. Although a positive correlation between glucose tolerance and NAG activity has been reported 14, before the present study little was known about the relationship between urinary NAG levels and glucose or insulin levels at each time point of the OGTT.
The present study had several limitations. First, this study was a single‐center study and might have involved self‐selection bias. Second, because our study used a cross‐sectional design, cause–effect relationships cannot be inferred. The urinary NAG level was measured at only one time point, and the OGTT was performed only once. Third, the mechanism by which hyperglycemia increases urinary NAG excretion remains unclear. When we reviewed earlier studies and considered the limitations mentioned above, we concluded that glucose intolerance was probably not caused by the increased urinary levels of NAG. When a possible correlation between postprandial glucose levels and the OGTT is considered, postprandial hyperglycemia may cause tubular damage. This study suggests that the urinary excretion of NAG with other urinary enzymes in the prediabetic state is an appropriate biomarker for screening for prediabetic renal dysfunction. The present data suggest that the increasing urinary levels of NAG are related to both the structural damage and the functional damage caused by hyperglycemia. Longitudinal studies are needed to fully clarify the underlying causes. When the postprandial glucose levels decrease, urinary NAG levels may decrease as well. Therefore, markers other than postprandial glucose related to the decrease in urinary NAG levels should be investigated.
To our knowledge, this cross‐sectional study is the first to show, by means of multivariate analysis, a relationship between urinary NAG levels and plasma glucose, urine glucose, and IRI levels at each time point of the OGTT. In conclusion, these results suggest that postprandial hyperglycemia is an independent factor that causes renal tubular damage in prediabetic patients.
ACKNOWLEDGMENTS
The authors are grateful to Masao Okazaki, MD, of the Academic Information Center, The Jikei University School of Medicine, for his careful revision of the English of the manuscript.
REFERENCES
- 1. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–S266. [PubMed] [Google Scholar]
- 2. Kowluru A, Kowluru R, Bitensky MW, Corwin EJ, Solomon SS, Johnson JD. Suggested mechanism for the selective excretion of glucosylated albumin. The effects of diabetes mellitus and aging on this process and the origins of diabetic microalbuminuria. J Exp Med 1987;166:1259–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kern EF, Erhard P, Sun W, Genuth S, Weiss MF. Early urinary markers of diabetic kidney disease: A nested case‐control study from the Diabetes Control and Complications Trial (DCCT). Am J Kidney Dis 2010;55:824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study Group . UK Prospective Diabetes Study (UKPDS). IX: Relationships of urinary albumin and N‐acetylglucosaminidase to glycaemia and hypertension at diagnosis of type 2 (non‐insulin‐dependent) diabetes mellitus and after 3 months diet therapy. Diabetologia 1993;36:835–842. [PubMed] [Google Scholar]
- 5. Okazaki K, Oba K, Nakano H. Urinary N‐acetyl‐β‐D‐glucosaminidase activity predicts development of diabetic nephropathy. Geriatr Gerontol Int 2005;5:22–28. [Google Scholar]
- 6. Le Hir M, Dubach UC, Schmidt U. Quantitative distribution of lysosomal hydrolases in the rat nephron. Histochemistry 1979;63:245–251. [DOI] [PubMed] [Google Scholar]
- 7. Diener U, Knoll E, Langer B, Rautenstrauch H, Ratge D, Wisser H. Urinary excretion of N‐acetyl‐β‐D‐glucosaminidase and alanine aminopeptidase in patients receiving amikacin or cis‐platinum. Clin Chim Acta 1981;112:149–157. [DOI] [PubMed] [Google Scholar]
- 8. Dance N, Price RG, Cattell WR, Lansdell J, Richards B. The excretion of N‐acetyl‐β‐glucosaminidase and β‐galactosidase by patients with renal disease. Clin Chim Acta 1970;27:87–92. [DOI] [PubMed] [Google Scholar]
- 9. Gibey R, Dupond JL, Alber D, Leconte des Floris R, Henry JC. Predictive value of urinary N‐acetyl‐beta‐D‐glucosaminidase (NAG), alanine‐aminopeptidase (AAP) and beta‐2‐microglobin (β2 M) in evaluating nephrotoxicity of gentamicin. Clin Chim Acta 1981;116:25–34. [DOI] [PubMed] [Google Scholar]
- 10. Sandman R, Margules RM, Kountz SL. Urinary lysosomal glycosidases after renal allotransplantation: Correlation of enzyme excretion with allograft rejection and ischemia. Clin Chim Acta 1973;45:349–359. [DOI] [PubMed] [Google Scholar]
- 11. Ellis EN, Brouhard BH, Lagrone L, Travis LB. Urinary excretion of N‐acetyl‐beta‐D‐glucosaminidase in children with type 1 diabetes mellitus. Diabetes Care 1983;6:251–255. [DOI] [PubMed] [Google Scholar]
- 12. Martin P, Hampton KK, Walton C, Tindall H, Davies JA. Microproteinuria in type 2 diabetes mellitus from diagnosis. Diabet Med 1990;7:315–318 [DOI] [PubMed] [Google Scholar]
- 13. Yamanouchi T, Kawasaki T, Yoshimura T, et al. Relationship between serum 1,5‐anhydroglucitol and urinary excretion of N‐acetylglucosaminidase and albumin determined at onset of NIDDM with 3‐year follow‐up. Diabetes Care 1998;21:619–624. [DOI] [PubMed] [Google Scholar]
- 14. Fujita H, Narita T, Morii T, et al. Increased urinary excretion of N‐acetylglucosaminidase in subjects with impaired glucose tolerance. Ren Fail 2002;24:69–75. [DOI] [PubMed] [Google Scholar]
- 15. Hiratsuka N, Shiba K, Nishida K, Iizima S, Kimura M, Kobayashi S. Analysis of urinary albumin, transferrin, N‐acetyl‐β‐D‐glucosaminidase and β2‐microglobulin in patients with impaired glucose tolerance. J Clin Lab Anal 1998;12:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brouhard BH, LaGrone L, Travis LB, Pollard TG. Response of urinary N‐acetyl‐β‐D‐glucosaminidase to rapid decreases in blood glucose. Clin Chim Acta 1984;140:197–202. [DOI] [PubMed] [Google Scholar]
- 17. Oba K, Igari Y, Matsumura N, et al. Effect of control of blood glucose on urinary excretion of N‐acetyl‐β‐D‐glucosaminidase in elderly type 2 diabetes mellitus. J Nippon Med Sch 2000;67:143–145. [DOI] [PubMed] [Google Scholar]
- 18. Alberti KG, Zimmet PZ . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 19. Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus; The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Diabetol Int 2010;1:2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noto A, Ogawa Y, Mori S, et al. Simple, rapid spectrophotometry of urinary N‐acetyl‐β‐D‐glucosaminidase, with use of a new chromogenic substrate. Clin Chem 1983;29:1713–1716. [PubMed] [Google Scholar]
- 21. Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum Cr in Japan. Am J Kidney Dis 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 22. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 23. Piwowar A, Knapik‐Kordecka M, Fus I, Warwas M. Urinary activities of cathepsin B, N‐acetyl‐β‐D‐glucosaminidase, and albuminuria in patients with type 2 diabetes mellitus. Med Sci Monit 2006;12:CR210–CR214. [PubMed] [Google Scholar]
- 24. Mohammadi‐Karakani A, Asgharzadeh‐Haghighi S, Ghazi‐Khansari M, Hosseini R. Determination of urinary enzymes as a marker of early renal damage in diabetic patients. J Clin Lab Anal 2007;21:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nauta FL, Boertien WE, Bakker SJ, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 2011;34:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basturk T, Altuntaş Y, Kurklu A, Aydin L, Eren N, Unsal A. Urinary N‐acetyl β glucosaminidase as an earlier marker of diabetic nephropathy and influence of low‐dose perindopril/indapamide combination. Ren Fail 2006;28:125–128. [DOI] [PubMed] [Google Scholar]
- 27. Agirbasli M, Radhakrishnamurthy B, Jiang X, Bao W, Berenson GS. Urinary N‐acetyl‐β‐D‐glucosaminidase changes in relation to age, sex, race, and diastolic and systolic blood pressure in a young adult biracial population. The Bogalusa Heart Study. Am J Hypertens 1996;9:157–161. [DOI] [PubMed] [Google Scholar]
- 28. Oba K, Hirai M, Ajiro Y, et al. Effect of age on urinary excretion of N‐acetyl‐β‐D‐glucosaminidase. Nihon Ika Daigaku Zasshi 1999;66:33–36. [DOI] [PubMed] [Google Scholar]
- 29. Dehne MG, Sablotzki A, Mühling J, Dehne KL, Röhrig R, Hempelmann G. Renal effects of cardiopulmonary bypass in the elderly. Perfusion 2002;17:205–209. [DOI] [PubMed] [Google Scholar]
- 30. Sekikawa A, Tominaga M, Takahashi K, et al. Prevalence of diabetes and impaired glucose tolerance in Funagata area, Japan. Diabetes Care 1993;16:570–574. [DOI] [PubMed] [Google Scholar]
- 31. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787. [DOI] [PubMed] [Google Scholar]
- 32. The DECODE Study Group . Age‐ and sex‐specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–69. [DOI] [PubMed] [Google Scholar]
- 33. Stevic R, Zivkovic TB, Erceg P, Milosevic D, Despotovic N, Davidovic M. Oral glucose tolerance test in the assessment of glucose‐tolerance in the elderly people. Age Ageing 2007;36:459–462. [DOI] [PubMed] [Google Scholar]
- 34. DeFronzo RA. Glucose intolerance and aging. Diabetes Care 1981;4:493–501. [DOI] [PubMed] [Google Scholar]
- 35. Yamanouchi T, Inoue T, Ogata E, et al. Post‐load glucose measurements in oral glucose tolerance tests correlate well with 1,5‐anhydroglucitol, an indicator of overall glycaemic state, in subjects with impaired glucose tolerance. Clin Sci (Lond) 2001;101:227–233. [PubMed] [Google Scholar]
- 36. Matsumoto K, Yano M, Miyake S, et al. Effects of voglibose on glycemic excursions, insulin secretion, and insulin sensitivity in non‐insulin‐treated NIDDM patients. Diabetes Care 1998;21:256–260. [DOI] [PubMed] [Google Scholar]
- 37. Stickle D, Turk J. A kinetic mass balance model for 1,5‐anhydroglucitol: Applications to monitoring of glycemic control. Am J Physiol 1997;273:E821–E830. [DOI] [PubMed] [Google Scholar]
- 38. Alderman MH, Melcher L, Drayer DE, Reidenberg MM. Increased excretion of urinary N‐acetyl‐β‐glucosaminidase in essential hypertension and its decline with antihypertensive therapy. N Engl J Med 1983;309:1213–1217. [DOI] [PubMed] [Google Scholar]
- 39. Schnoell F, Weitgasser R, Straberger A, Pretsch I. Urinary activity of N‐acetyl‐β‐D‐glucosaminidase (NAG) in non‐insulin‐dependent diabetics (abstract). Diabetologia 1990;33:A153. [Google Scholar]
- 40. Sano T, Kawamura T, Matsumae H, et al. Effects of long‐term enalapril treatment on persistent micro‐albuminuria in well‐controlled hypertensive and normotensive NIDDM patients. Diabetes Care 1994;17:420–424. [DOI] [PubMed] [Google Scholar]


