Abstract
The overactive bladder syndrome and detrusor overactivity are conditions that can have major effects on quality of life and social functioning. Antimuscarinic drugs are still first-line treatment. These drugs often have good initial response rates, but adverse effects and decreasing efficacy cause long-term compliance problems, and alternatives are needed. The recognition of the functional contribution of the urothelium/suburothelium, the autonomous detrusor muscle activity during bladder filling and the diversity of nerve transmitters involved has sparked interest in both peripheral and central modulation of overactive bladder syndrome/detrusor overactivity pathophysiology. Three drugs recently approved for treatment of overactive bladder syndrome/detrusor overactivity (mirabegron, tadalafil and onabotulinum toxin A), representing different pharmacological mechanisms; that is, β-adrenoceptor agonism, phosphodiesterase type 5 inhibition, and inhibition of nerve release of efferent and afferent transmitters, all seem to have one effect in common: inhibition of the afferent nervous activity generated by the bladder during filling. In the present review, the different mechanisms forming the pharmacological basis for the use of these drugs are discussed.
Keywords: mirabegron, mucosal signaling, myogenic pathway, onabotulinum toxin A, tadalafil
Introduction
The OAB, defined either symptomatically as the OAB syndrome or urodynamically as DO, is a bladder filling disorder. To exert normal bladder control, adequate sensory input to the CNS is necessary, and it is well established that changes in sensory mechanisms might give rise to disturbances in bladder function. It is therefore logical that pharmacological control of bladder contraction has focused on how afferent nerve activity is generated peripherally and handled by the CNS. Several factors might contribute to the genesis of OAB, and at least two afferent signaling pathways in the bladder can be identified, the “myogenic” and the “urothelial” pathway.1
It is obvious that the mechanisms leading to an increased activity in afferent nerves might be interesting targets for drugs aimed at controlling sensory and motor activity of the bladder. It is now widely accepted that antimuscarinic drugs, which are still first line treatment of OAB/DO, exert effects on afferent signaling important for their clinical efficacy.2 Three novel drugs have recently been approved for treatment of OAB/DO: mirabegron, tadalafil and onabotulinum toxin A. They represent different pharmacological mechanisms; that is, β-AR agonism, PDE5 inhibition, and inhibition of nerve release of efferent and afferent transmitters, and why they all are clinically effective has not been definitely established. In the present review, the mechanisms providing the pharmacological basis for the use of these drugs are discussed.
Bladder afferent signaling
The bladder mucosa is richly innervated with afferent nerves carrying information from the bladder to the CNS, but also the detrusor muscle is supplied with such nerves. The fibers most important in the control of micturition are myelinated Aδ fibers and unmyelinated C-fibers.1 Aδ fibers are mechanosensitive, discharging in response to distension of the bladder wall. C-fibers are normally inactive, but might respond to, for example, chemical irritation or stretch of the urothelium. Under pathological circumstances, C-fibers can be recruited to create new sensory pathways.
As mentioned, afferent fibers in the bladder are activated through nervous pathways in the detrusor muscle (“myogenic”) and the mucosa (“urothelial”).1 During bladder filling, there is no excitatory parasympathetic outflow from the CNS,3 but there is a myogenic contractile activity contributing to bladder tone. This contractile activity is most probably influenced by activity in the sympathetic nerves to the bladder and in local nervous circuits, resulting in what is referred to as spontaneous (autonomous) contractile activity.#b4b5 The constant afferent output from the bladder generated by this autonomous activity has been termed “afferent noise”.6 The urothelium and suburothelial interstitial cells might exert influence over afferent nerves in response to stretch or various chemical mediators,#b7b8 representing another source of constant afferent input.
There are many theories regarding the pathogenesis of OAB (Fig. 1), but it is likely that abnormally increased signaling through afferent pathways might be involved. Therefore, pharmacological manipulation of the mechanisms generating these signals might be effective in the treatment of OAB. It should be emphasized that the afferent signals generated by the bladder have to be processed in the CNS (Fig. 1). This is an important step in OAB pathophysiology, and a promising but challenging therapeutic target.
Fig. 1.
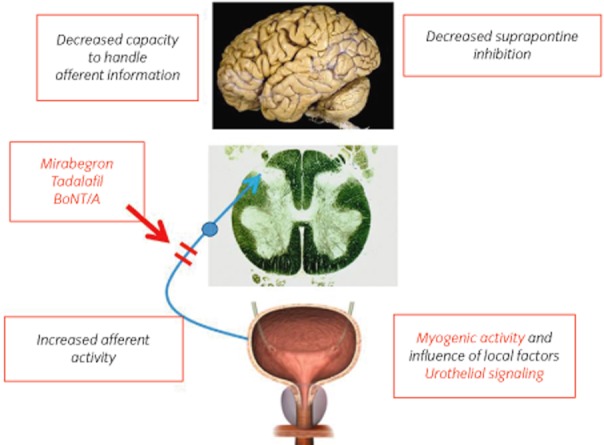
Pathophysiology of the OAB. In the bladder, increased afferent activity generated in the detrusor or mucosa through different mechanisms initiate involuntary detrusor contractions. Such contractions can be initiated independently of changes in afferent activity by changes in the CNS.
Pharmacological control of bladder afferent nerve activity
β-AR agonism
In the human detrusor, the β3-AR has been found to be the predominant subtype.#b9b10b11 The β3-AR, likely along with β2-AR, is directly involved in detrusor smooth muscle relaxation.#b4b10b11 How this effect can be translated into the in vivo effects of β3-AR stimulation seen in numerous preclinical studies and clinical trials – increased bladder capacity without change in micturition pressure or residual volume – has not been clearly established. The normal stimulus for activation of the micturition reflex is distension of the bladder. This initiates activity in “in series”-coupled, low-threshold mechanoreceptive (Aδ) afferents.12 If the compliance of the bladder is increased, the response to distension is decreased; and to recruit afferent activity sufficient to initiate micturition, greater filling volumes are needed – thus bladder capacity increases. One of the factors that generates bladder tone is the autonomous contractile, phasic activity of the detrusor smooth muscle that occurs during filling. These contractions are believed to generate afferent input from both Aδ- and C-fibers, which together with the activity initiated by distension, will start the voiding reflex.
The autonomous activity of the detrusor is effectively reduced by β3-AR agonists,13 and this reduces the afferent output from the bladder. In fact, Aizawa et al. showed in rats that the β3-AR agonist, CL316243, could inhibit filling-induced activity not only in mechanosensitive Aδ-fibers, but also in C-fiber primary bladder afferents provided that these fibers were stimulated by PG E2.14 Even if β3-AR agonists in vitro might be able to relax detrusor muscle contracted by muscarinic receptor stimulation using high concentrations of, for example, ACh or carbachol, they are not very effective. The voiding contraction is caused by a massive release of contractant transmitters (ACh and ATP), and this might be the reason why it is not affected by β3-AR agonists. Providing further evidence that β3-AR agonists might influence the sensory regulation of micturition, Gillespie et al. found that mirabegron (β3-AR agonist) inhibited only non-voiding activity in a rat model of OAB, whereas tolterodine (antimuscarinic) inhibited non-voiding activity, as well as the amplitude of voiding contractions.15
Immunohistochemical staining has identified β3-AR not only on detrusor muscle, but also on the afferent terminals of the dorsal root ganglia of L6-S116, suggesting that β3-AR agonism might directly affect afferent transmission. Several studies have shown β-AR agonist-mediated disruption of afferent nerve signals. As mentioned, Aizawa et al. showed that the β3-AR agonist, CL316243, was able to inhibit filling-induced Aδ-fiber signaling and C-fiber mediated hyperactivity caused by PG E2.14 These results were supported by the study by Kanai et al., who found that the β3-AR agonist, BRL37344, directly inhibited stretch-induced afferent firing in spinal cord transected mouse bladder sheets.16
Investigations of the bladder mucosa have shown that the mechanism of β3-AR agonism might involve interaction with the urothelium/suburothelium. The β3-AR shows intense immunohistochemical staining in the urothelium and suburothelial interstitial cells.#b17b18 It has been shown that norepinephrine can stimulate the release of nitric oxide from the urothelium,19 but β3-AR stimulation might also release other factors. Murakami et al. studied the inhibition of carbachol-induced pig detrusor contractions by isoprenaline and concluded that the relaxation responses did not involve the urothelium, and was not caused by NO.20 A similar study by Otsuka et al. found that the presence of the urothelium caused a right-ward shift of the concentrations–response curve for isoprenaline.21 They suggested that this was a result of β-AR mediated release of an inhibitory factor from the urothelium, which inhibited the β-AR agonist-induced relaxation of the detrusor. However, the influence of stimulation of urothelial β3-AR on detrusor muscle contraction and on afferent signaling requires further study.
Mirabegron is a β3-AR selective agonist that has recently been approved for the treatment of OAB in Japan and the USA, and is under consideration in Europe. Phase IIa, IIb and III drug trials have shown that mirabegron consistently improved the mean number of micturitions in 24 h, and the number of continence episodes in 24 h.#b22b23b24b25 The reported adverse events were similar in placebo and mirabegron groups, and included hypertension, headache, gastrointestinal symptoms, dyspepsia and nausea. There was also a small increase in pulse rate in both trials, but no adverse cardiovascular events were reported. These studies clearly establish β3-AR agonism as an effective treatment of OAB.
PDE5 inhibition
LUTS in men include storage symptoms. It is well documented that PDE5 inhibitors improve male LUTS, whether or not these symptoms are associated with erectile dysfunction.26 The exact mechanisms by which these beneficial effects are exerted have still not been established,27 but this has stimulated research activities with respect to the different signaling pathways controlling the function of the LUT. PDE5 is expressed and has biological activity in all parts of the genitourinary tract, but with respect to its role in LUTS pathophysiology, focus has been on the prostate, bladder and urethra.#b26b28b29 Although PDE5 inhibition in vitro can relax the smooth muscle of the LUT, and specifically the outflow region, symptom improvements have not been associated with significantly improved urinary flow rates,30 implying that there might be some other mechanism(s) involved. PDE5 inhibitors have several effects on the LUT, and as discussed by, for example, Andersson et al.,27 they have shown effects not only on smooth muscle, but also on endothelial cell proliferation, nerve activity and tissue perfusion, all factors that might impact LUTS in men. Interestingly, the mucosal effects of PDE5 inhibitors do not seem to have been studied specifically.
Several in vitro studies on LUT smooth muscle have highlighted the involvement of the NO/cGMP pathway in the mechanism of action of PDE5 inhibitors. Oger et al. investigated the effect of sildenafil on the smooth muscle of the human bladder dome contracted by carbachol and found that sildenafil had a direct relaxant effect.31 However, high concentrations of the drug were needed. The relaxant effects were suggested to involve the cGMP pathway, as well as K+ channels. As the relaxation remained unaltered in the presence of the NO donor, sodium nitroprusside, the authors questioned the role of NO and the CGMP system in human bladder dome smooth muscle. This site of action might not be predictive of the in vivo effects of the PDE5 inhibitors.
In smooth muscle isolated from the periurethral and transition zones of non-diseased human prostates, Ückert et al. carried out in vitro studies showing that PDE5 inhibitors caused a dose-dependent decrease of the tension induced by norepinephrine.32 The relaxant effect, which was associated with increases in cGMP concentrations, varied between 17 and 52%. In isolated human female urethral smooth muscle, the contraction induced by noradrenaline was relaxed in response to sildenafil, vardenafil and tadalafil,33 but high concentrations of the drugs were needed. However, nerve-induced relaxations were enhanced at low drug concentrations. In isolated preparations from the human male proximal penile urethra, the relaxation of norepinephrine contracted preparations produced by sildenafil, vardenafil and tadalafil were modest (20–35%), and associated by elevations in cGMP.34 The predictive value of these experiments for estimating the effects on human urethral pressure do not seem convincing.
In vivo studies have suggested that NO or its downstream signaling could modulate the micturition reflex by reducing the excitability of bladder afferents. Caremel et al. evaluated the role of the NO/cGMP signaling pathway on the micturition reflex in a model of bladder hyperactivity associated with C-fiber activation in the rat.35 They confirmed previous studies#b36b37 that compounds inhibiting the NO/cGMP pathway increased bladder overactivity, whereas compounds activating the NO/cGMP pathway inhibited it. Similar studies have found that nitric oxide generation of cGMP might be the key step in the NO/cGMP pathway responsible for PDE5 inhibition of bladder overactivity.#b38b39
Several lines of evidence further suggest that PDE5 inhibition might affect afferent signaling. PDE5 increases the accumulation of cGMP, which in turn stimulates the activity in PKG. Increased PKG activity might decrease influx through N-type voltage-gated Ca2+ channels in afferent nerve terminals, resulting in decreased neuropeptide release.#b40b41 This would also reduce positive feedback on presynaptic NK1 and NK2 receptors, which in turn might decrease afferent firing.#b41b42b43 Studies have shown direct inhibition of afferent nerve transmission. For example, in a model of bladder hyperactivity involving unanesthetized, decerebrate SCI rats, Behr-Roussel et al. found that administration of vardenafil reduced bladder afferent nerve firing, as well as non-voiding contractions.44 Minagawa et al. found that tadalafil decreased afferent signaling in Aδ and C-fibers in response to both bladder filling and hyperactivity caused by acrolein-induced cystitis with no change in bladder tone.45 This decrease in afferent signaling seemed to be related to PDE5 inhibitor-mediated increased activity in the NO/cGMP pathway, as the administration of L-arginine inhibited Aδ and C-fiber firing, whereas the NOS inhibitor, L-NAME, increased afferent activity.46 It might be questioned if a direct effect on afferent nerves is the only mechanism by which PDE5 inhibitors influences afferent signaling. Assuming that pelvic ischemia contributes to LUTS, improvement of blood flow to the LUT would be expected to also improve LUT function. In a model of chronic bladder ischemia where vascular occlusion was produced by balloon-induced endothelial injury of the iliac arteries combined with a high cholesterol diet, Nomiya et al. found that chronic treatment with tadalafil, even if it did not prevent neo-intimal formation and luminal occlusion, had beneficial effects on bladder function.47 It reduced bladder overactivity, decreased indicators of bladder ischemia, normalized changes in NOS activity (decreases of nNOS and eNOS, increase of iNOS) and prevented collagen deposition.
Thus, there might be several mechanisms by which PDE5 inhibitors affect the bladder, all resulting in reduced afferent signaling, which seem to be the final common pathway of relevance for the generation of LUTS.
To date, only tadalafil has been approved for the treatment of LUTS secondary to BPH. RCT have shown significant improvement in urinary symptoms, and the drug is well tolerated.#b48b49b50b51b52 However, further studies on the long-term effects are required, and whether or not the drug can improve storage symptoms in women remains to be established.
Botulinum toxin
BoNT, the neurotoxin produced by Clostridium botulinum, comprises seven subtypes, of which subtype A (BoNT-A), which has the longest duration of action, is clinically the most relevant. BoNT-A is available in three different commercial forms, which differ in their relative potency: onabotulinum toxin A, abobotulinum toxin A and incobotulinum toxin A. Although there are differences in potency between the forms, there are no reasons to believe that their basic mechanisms of action is different. Most of the information available preclinically and clinically derives from the use of onabotulinum toxin A.
The details of the mechanisms of action of BoNT in the nerve terminal are well-outlined elsewhere.#b53b54 Briefly, it involves cleavage of the attachment proteins involved with the mechanism of fusion of synaptic vesicles to the cytoplasmatic membrane necessary for neurotransmitter release. Attachment proteins (the SNARE complex) include SNAP 25, synaptobrevin (vesicle associated membrane protein) and syntaxin. BoNT-A cleaves SNAP 25, rendering the SNARE complex inactive.#b53b54 In striated muscle, paralysis is produced by inhibition of ACh release from cholinergic motor nerve endings.53
In the human bladder, SNAP-25 expression has been shown in parasympathetic, sympathetic and sensory fibers.55 Blockade of ACh release is believed to play an essential role in the detrusor hypo- or acontractility that follows BoNT-A injection in the bladder. Supporting this view, in normal or SCI animals, BoNT/A treatment decreased the bladder contractions evoked by electrical stimulation of spinal nerves without altering autonomous contractile activity.56 However, BoNT-A might also have effects on sensory fibers, as approximately half of the peptidergic sensory fibers express SNAP 25.55 It has been well documented that BoNT-A can inhibit release from sensory nerves both in the CNS and peripherally.#b57b58b59b60 BoNT-A was found to reduce afferent firing from bladder afferents and antidromic release of neuropeptides.56
Although SNAP 25 immunoreactivity has not been detected in urothelial cells,55 urothelial function also seems compromised after BoNT-A administration. BoNT-A has been shown to inhibit ATP release from the urothelium in animal models of spinal cord injury.#b61b62
In addition to its effect on neurotransmitter release, BoNT-A, injected into the bladder wall seems to influence the receptor profile of important neurotransmitters. Apostolidis et al. found that the mucosal levels of P2X3 and TRVP1 were decreased 4 weeks after BoNT-A injection, and even more so after 16 weeks.#b63 The decrease in the levels of these receptors seemed to correlate with those patients who experienced decreased urgency after the injection. Datta et al. found that patients with OAB had decreased levels of muscarinic receptors in the urothelium/suburothelium and that the levels of muscarinic receptors 1 and 3 were normalized after treatment with BoNT-A.#b64 Furthermore, they found an inverse association with receptor level and patient-reported symptoms. The relationship between mucosal receptor profile and patient symptoms indicates that this might be an important effect mechanism of BoNT-A.
Available evidence thus suggests that BoNT-A, by action on both the motor part of the myogenic (release of contractant transmitters) and on the mucosal (release of sensory transmitters) activation pathways, decreases the afferent nervous activity generated by the bladder during filling.
Several RCT have documented the clinical effects of BoNT-A in both neurogenic and idiopathic DO,#b65 where the drug decreases incontinence episodes, frequency and urgency, and improves quality of life.#b66b67b68 However, successful OAB treatment with BoNT-A does not appear to be related to the existence of DO. No differences in outcomes were found between those with and those without baseline DO.#b69b70 BoNT-A is also effective in patients with OAB syndrome not responding to antimuscarinic drugs.#b69b70
The major adverse effects are urinary retention, sometimes requiring clean intermittent catherization, and urinary tract infections. Beneficial effects were shown not to be dose-dependent, whereas side effects might be lessened at lower doses.#b71
Conclusions
OAB is a filling disorder in which abnormal sensations leads to urinary urgency, frequency and incontinence. The afferent signaling pathways that regulate micturition play a central role in the pathogenesis of OAB, and thus represent important targets for therapy. The three drugs discussed, mirabegron, tadalafil and BoNT-A (although acting through different mechanisms), share a central theme – inhibition of afferent signaling from the bladder.
Glossary
- ACh
acetylcholine
- AR
adrenoceptor
- ATP
adenosine triphosphate
- BoNT
botulinum toxin
- cGMP
cyclic guanosine monophosphate
- CNS
central nervous system
- DO
detrusor overactivity
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NAME
L-NG-nitroarginine methyl ester
- LUT
lower urinary tract symptom
- LUTS
lower urinary tract symptoms
- NOS
nitric oxide synthase
- nNOS
neurogenic nitric oxide synthase
- OAB
overactive bladder
- PDE5
phosphodiesterase type 5
- PKG
cGMP activated protein kinase
- PG
prostaglandin
- RCT
randomized control trials
- SCI
spinal cord injury
- SNAP 25
synaptosome-associated protein 25 kD
Conflict of interest
None declared.
References
- 1.Kanai A, Andersson KE. Bladder afferent signaling: recent findings. J. Urol. 2010;183:1288–1295. doi: 10.1016/j.juro.2009.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson KE. Antimuscarinic mechanisms and the overactive detrusor: an update. Eur. Urol. 2011;59:377–386. doi: 10.1016/j.eururo.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 3.de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int. 2004;93:478–483. doi: 10.1111/j.1464-410x.2003.04667.x. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie JI, van Koeveringe GA, de Wachter SG, de Vente J. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int. 2009;103:1324–1333. doi: 10.1111/j.1464-410X.2009.08377.x. [DOI] [PubMed] [Google Scholar]
- 7.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat. Clin. Pract. Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birder LA. Urothelial signaling. Handb. Exp. Pharmacol. 2011;202:207–231. doi: 10.1007/978-3-642-16499-6_10. [DOI] [PubMed] [Google Scholar]
- 9.Nomiya M, Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and β-adrenoreceptor subtypes and their functional roles in human normal and obstructed bladders. J. Urol. 2003;170(2 Pt 1):649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- 10.Michel MC, Vrydag W. Alpha1-, alpha2- and β-adrenoreceptors in the urinary bladder, urethra and prostate. Br. J. Pharmacol. 2006;147(Suppl 2):S88. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igawa Y, Aizawa N, Homma Y. β3-adrenoreceptor agonists: possible role in the treatment of overactive bladder. Korean J. Urol. 2010;51:811–818. doi: 10.4111/kju.2010.51.12.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iggo A. Tension receptors in the stomach and the urinary bladder. J. Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int. 2006;98:1310–1314. doi: 10.1111/j.1464-410X.2006.06564.x. [DOI] [PubMed] [Google Scholar]
- 14.Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of CL316,243, a β3 adrenoreceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol. Urodyn. 2010;29:771–776. doi: 10.1002/nau.20826. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie JI, Palea S, Guilloteau V, Guerard M, Lluel P, Korstanje C. Modulation of non-voiding activity by the muscarinergic antagonist tolterodine and the β(3) -adrenoceptor agonist mirabegron in conscious rats with partial outflow obstruction. BJU Int. 2012;110(2 Pt B):E132–142. doi: 10.1111/j.1464-410X.2012.11240.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanai A, Wyndaele J, Andersson KE, et al. Researching bladder afferents – determining the effects of β3-adrenergic receptor agonists and botulinum toxin type-A. Neurourol. Urodyn. 2011;30:684–691. doi: 10.1002/nau.21102. [DOI] [PubMed] [Google Scholar]
- 17.Kullmann FA, Downs TR, Artim DE, et al. Urothelial β-3 adrenergic receptors in the rat bladder. Neurourol. Urodyn. 2011;30:144–150. doi: 10.1002/nau.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limberg BJ, Andersson KE, Aura Kullmann F, Burmur G, de Groat WE, Rosenbaum J. S. β-Adrenergic receptor subtype expression in myocyte and non-myocyte cells in human female bladder. Cell Tissue Res. 2010;342:295–306. doi: 10.1007/s00441-010-1053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birder LA, Apodaca G, de Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am. J. Physiol. 1998;275:F226–229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 20.Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R. The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int. 2007;99:669–673. doi: 10.1111/j.1464-410X.2006.06679.x. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of β-adrenoreceptors in the human urinary bladder urothelium. Naunyn. Schmiedebergs Arch. Pharmacol. 2008;377:473–481. doi: 10.1007/s00210-008-0274-y. [DOI] [PubMed] [Google Scholar]
- 22.Chapple CR, Yamaguchi O, Ridder A, et al. Clinical proof of concept study (Blossom) shows novel β3 adrenoreceptor agonist YM178 is effective and well tolerated in the treatment of symptoms of overactive bladder. Eur. Urol. Suppl. 2008;7:239. (abstract 674) [Google Scholar]
- 23.Chapple C, Wyndaele JJ, van Kerrebroeck P, Radziszewski P, Dvorak V. Dose ranging study of once-daily mirabegron (YM178), a novel selective β3 adrenoreceptor agonist, in patients with overactive bladder (OAB) Eur. Urol. Suppl. 2010;9:249. (abstract 774) [Google Scholar]
- 24.Nitti V, Herschorn S, Auerbach S, Ayers M, Lee M, Martin N. The efficacy and safety of mirabegron in patients with overactive bladder syndrome – results from a North American Phase III trial. Eur. Urol. Suppl. 2011;10:278. (abstract 885) [Google Scholar]
- 25.Khullar V, Cabronero J, Strömberg P, et al. The efficacy and tolerability of mirabegron in patients with overactive bladder – results from a European-Australian Phase III trial. Eur. Urol. Suppl. 2011;10:278. doi: 10.1016/j.eururo.2012.10.016. (abstract 886) [DOI] [PubMed] [Google Scholar]
- 26.Uckert S, Stief CG. Treatment of erectile dysfunction and lower urinary tract symptoms by phosphodiesterase inhibitors. Handb. Exp. Pharmacol. 2011;204:307–322. doi: 10.1007/978-3-642-17969-3_13. [DOI] [PubMed] [Google Scholar]
- 27.Andersson KE, de Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol. Urodyn. 2011;30:292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 28.Andersson KE, Uckert S, Stief C, Hedlund P. Phosphodiesterases (PDEs) and PDE inhibitors for treatment of LUTS. Neurourol. Urodyn. 2007;26(6 Suppl):928–933. doi: 10.1002/nau.20485. [DOI] [PubMed] [Google Scholar]
- 29.Fibbi B, Morelli A, Vignozzi L, et al. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J. Sex. Med. 2010;7:59–69. doi: 10.1111/j.1743-6109.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 30.Dmochowski R, Roehrborn C, Klise S, Xu L, Kaminetsky J, Kraus S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J. Urol. 2010;183:1092–1097. doi: 10.1016/j.juro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Oger S, Behr-Roussel D, Gorny D, et al. Signalling pathways involved in sildenafil-induced relaxation of human bladder dome smooth muscle. Br. J. Pharmacol. 2010;160:1135–1143. doi: 10.1111/j.1476-5381.2010.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uckert S, Sormes M, Kedia G, et al. Effects of phosphodiesterase inhibitors on tension induced by norepinephrine and accumulation of cyclic nucleotides in isolated human prostatic tissue. Urology. 2008;71:526–530. doi: 10.1016/j.urology.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 33.Werkström V, Svensson A, Andersson KE, Hedlund P. Phosphodiesterase 5 in the female pig and human urethra: morphological and functional aspects. BJU Int. 2006;98:414–423. doi: 10.1111/j.1464-410X.2006.06217.x. [DOI] [PubMed] [Google Scholar]
- 34.Kedia GT, Sonnenberg JE, Kuczyk MA, Uckert S. In vitro functional responses of isolated human urethral tissue to phosphodiesterase (PDE) inhibitors. Eur. Urol. Suppl. 2011;10:291–292. (abstract 931) [Google Scholar]
- 35.Caremel R, Oger-Roussel S, Behr-Roussel D, Grise P, Giuliano FA. Nitric oxide/cyclic guanosine monophosphate signaling mediates an inhibitory action on sensory pathways of the micturition reflex in the rat. Eur. Urol. 2010;58:616–625. doi: 10.1016/j.eururo.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Persson K, Igawa Y, Mattiasson A, Andersson KE. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br. J. Pharmacol. 1992;107:178–184. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J. Urol. 2000;164:545–550. [PubMed] [Google Scholar]
- 38.Sesatomi K, Hiragata S, Miyazato M, Chancellor M, Morris S, Yoshimura N. Nitric oxide-mediated suppression of detrusor overactivity by arginase inhibitor in rats with chronic spinal cord injury. Urology. 2008;72:696–700. doi: 10.1016/j.urology.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artim DE, Kullman FA, Daughtery SL, Wu H, de Groat WC. Activation of the nitric oxide-cGMP pathway reduces phasic contractions in neonatal rat bladder strips via protein kinase G. Am. J. Physiol. Renal Physiol. 2009;297:F333–340. doi: 10.1152/ajprenal.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura N, Skei S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J. Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- 41.Kanai A, Zabbarova I, Oefelein M, Radziszewski P, Ikeda Y, Andersson KE. Mechanisms of action of botulinum neurotoxins, β3-adrenergic receptor agonists, and PDE5 inhibitors in modulating detrusor function in overactive bladders: ICI-RS 2011. Neurourol. Urodyn. 2012;31:300–308. doi: 10.1002/nau.21246. [DOI] [PubMed] [Google Scholar]
- 42.Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp. Neurol. 2007;205:92–100. doi: 10.1016/j.expneurol.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sculptoreanu A, Artim DE, de Groat WC. Neurokinins inhibit low threshold inactivating K+ currents in capsaicin responsive DRG neurons. Exp. Neurol. 2009;219:562–573. doi: 10.1016/j.expneurol.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behr-Roussel D, Oger S, Caisey S, et al. Vardenafil decrease bladder afferent nerve activity unanesthetized, decerebrate, spinal cord-injured rats. Eur. Urol. 2011;59:272–279. doi: 10.1016/j.eururo.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 45.Minegawa T, Aizawa N, Igawa Y, et al. Inhibitory effects of phosphodiesterase 5 inhibitor, tadalafil, on mechanosensitive bladder afferent nerve activities of the rat and on acrolien-induced hyperactivity of these nerves. BJU Int. 2012;110:E259–266. doi: 10.1111/j.1464-410X.2012.11255.x. [DOI] [PubMed] [Google Scholar]
- 46.Aizawa N, Igawa Y, Nishizawa O, et al. Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesical acrolein treatment. Eur. Urol. 2011;59:264–271. doi: 10.1016/j.eururo.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 47.Nomiya M, Burmeister DM, Sawada N, et al. Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J. Urol. 2012 doi: 10.1016/j.juro.2012.07.141. doi: 10.1016/j.juro.2012.07.141. [DOI] [PubMed] [Google Scholar]
- 48.McVary KT, Roehrborn CG, Kaminetsky JC, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 2007;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 49.Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J. Urol. 2008;180:1228–1234. doi: 10.1016/j.juro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 50.Roehrborn CG, Kaminetsky JC, Auerbach SM, et al. Changes in peak urinary flow and voiding efficiency in men with signs and symptoms of benign prostatic hyperplasia during once daily tadalafil treatment. BJU Int. 2010;105:502–507. doi: 10.1111/j.1464-410X.2009.08822.x. [DOI] [PubMed] [Google Scholar]
- 51.Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur. Urol. 2008;53:1236–1244. doi: 10.1016/j.eururo.2008.01.075. [DOI] [PubMed] [Google Scholar]
- 52.Martínez-Salamanca JI, Carballido J, Eardley I, et al. Phosphodiesterase type 5 inhibitors in the management of non-neurogenic male lower urinary tract symptoms: critical analysis of current evidence. Eur. Urol. 2011;60:527–535. doi: 10.1016/j.eururo.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 53.Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427–446. doi: 10.1016/s0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 54.Chancellor MB, Fowler CJ, Apostolidis A, et al. Drug Insight: biological effects of botulinum toxin A in the lower urinary tract. Nat. Clin. Pract. Urol. 2008;5:319–328. doi: 10.1038/ncpuro1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coelho A, Cruz F, Cruz CD, Avelino A. Spread of onabotulinumtoxinA after bladder injection. Experimental study using the distribution of cleaved SNAP-25 as the marker of the toxin action. Eur. Urol. 2012;61:1178–1184. doi: 10.1016/j.eururo.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 56.Ikeda Y, Zabbarova I, Birder L, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur. Urol. 2012 doi: 10.1016/j.eururo.2012.03.031. doi: 10.1016/j.eururo.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Rapp DE, Turk KW, Bales GT, Cook SP. Botulinum toxin type A inhibits calcitonin gen-related peptide release from isolated rat bladder. J. Urol. 2006;175:1138–1142. doi: 10.1016/S0022-5347(05)00322-8. [DOI] [PubMed] [Google Scholar]
- 59.Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J. Cell Sci. 2007;120(Pt 16):2864–2874. doi: 10.1242/jcs.012211. [DOI] [PubMed] [Google Scholar]
- 60.Lucioni A, Bales GT, Lotan TL, McGehee DS, Cook SP, Rapp DE. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101:366–370. doi: 10.1111/j.1464-410X.2007.07312.x. [DOI] [PubMed] [Google Scholar]
- 61.Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem. Int. 2004;45:987–993. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Smith CP, Gangiano DA, Munoz A, et al. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 2008;52:1068–1075. doi: 10.1016/j.neuint.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apostolidis A, Popta R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J. Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. discussion 982–3. [DOI] [PubMed] [Google Scholar]
- 64.Datta SN, Roosen A, Oullen A, et al. Immunohistochemial exression of muscarinic receptors in the urothelium and suburothelium of neurogenic and idiopathic overactive human bladders, and changes with botulinum neurotoxin administration. J. Urol. 2010;184:2578–2585. doi: 10.1016/j.juro.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Mangera A, Andersson KE, Apostolidis A, et al. Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA) Eur. Urol. 2011;60:784–795. doi: 10.1016/j.eururo.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Sahai A, Khan MS, Dasgupta P. Efficacy of botulinum toxin A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J. Urol. 2007;177:2231–2236. doi: 10.1016/j.juro.2007.01.130. [DOI] [PubMed] [Google Scholar]
- 67.Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J. Urol. 2008;180:217–222. doi: 10.1016/j.juro.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tincello DG, Kenyon S, Abrams KR, et al. Botulinum toxin A versus placebo for refractory detrusor overactivity in women: a randomized blinded placebo-controlled trial of 240 women (the RELAX study) Eur. Urol. 2012;62:507–514. doi: 10.1016/j.eururo.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 69.Rovner E, Kennelly M, Schulte-Baukloh H, Zhou J, Haag-Molkenteller C, Dasgupta P. Urodynamic results and clinical outcomes with intradetrusor injections of onabotulinumtoxinA in a randomized, placebo-controlled dose-finding study in idiopathic overactive bladder. Neurourol. Urodyn. 2011;30:556–562. doi: 10.1002/nau.21021. [DOI] [PubMed] [Google Scholar]
- 70.Kanagarajah P, Ayyathurai R, Caruso DJ, Gomez C, Gousse AE. Role of botulinum toxin-A in refractory idiopathic overactive bladder patients without detrusor overactivity. Int. Urol. Nephrol. 2012;44:91–97. doi: 10.1007/s11255-011-9979-9. [DOI] [PubMed] [Google Scholar]
- 71.Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxin A for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J. Urol. 2010;184:2416–2422. doi: 10.1016/j.juro.2010.08.021. [DOI] [PubMed] [Google Scholar]


