Abstract
Aims
To evaluate apixaban single dose safety, tolerability, pharmacokinetics and pharmacodynamics and assess the effect of food on apixaban pharmacokinetics.
Methods
A double-blind, placebo-controlled, single ascending-dose, first-in-human study assessed apixaban safety, pharmacokinetics and pharmacodynamics in healthy subjects randomized to oral apixaban (n = 43; 0.5–2.5 mg as solution or 5–50 mg as tablets) or placebo (n = 14) under fasted conditions. An open label, randomized, two treatment crossover study investigated apixaban pharmacokinetics/pharmacodynamics in healthy subjects (n = 21) administered apixaban 10 mg in fasted and fed states. Both studies measured apixaban plasma concentration, international normalized ratio (INR), activated partial thromboplastin time (aPTT) and prothrombin time (PT) or a modified PT (mPT).
Results
In the single ascending-dose study increases in apixaban exposure appeared dose-proportional. Median tmax occurred 1.5–3.3 h following oral administration. Mean terminal half-life ranged between 3.6 and 6.8 h following administration of solution doses ≤2.5 mg and between 11.1 and 26.8 h for tablet doses ≥5 mg. Concentration-related changes in pharmacodynamic assessments were observed. After a 50 mg dose, peak aPTT, INR and mPT increased by 1.2-, 1.6- and 2.9-fold, respectively, from baseline. In the food effect study: 90% confidence intervals of geometric mean ratios of apixaban Cmax and AUC in a fed vs. fasted state were within the predefined no effect (80–125%) range. Apixaban half-life was approximately 11.5 h. The effect of apixaban on INR, PT and aPTT was comparable following fed and fasted administration.
Conclusions
Single doses of apixaban were well tolerated with a predictable pharmacokinetic/pharmacodynamic profile and a half-life of approximately 12 h. Apixaban can be administered with or without food.
Keywords: anticoagulant, apixaban, factor Xa inhibitor, food effect, pharmacodynamics, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Thrombotic disorders remain the leading cause of death in the Western world, and although current drugs are effective in reducing the risks associated with thrombotic disease, they are associated with various limitations. Therefore, new oral agents targeted to inhibit thrombin or factor Xa have been developed. Apixaban is a highly selective, potent and orally bioavailable inhibitor of both free and prothrombinase-bound factor Xa.
WHAT THIS STUDY ADDS
This report describes the first human assessment of apixaban safety, tolerability, pharmacokinetics and pharmacodynamics in healthy subjects and the effects of food on apixaban pharmacokinetics. The results demonstrate that apixaban, in single oral doses of up to 50 mg, appears to be safe and generally well tolerated in this study population. Apixaban has linear pharmacokinetics, concentration-related pharmacodynamic effects and its exposure is not affected by the administration of a standard high fat, high calorie meal.
Introduction
Thrombotic disorders remain the leading cause of death in the Western world despite the availability of numerous classes of anticoagulants, such as vitamin K antagonists (VKAs), heparin derivatives and direct thrombin inhibitors [1]. Although these drugs are effective in reducing the risks associated with thrombotic disease, they are associated with various limitations [2]. VKAs are currently the most widely used oral anticoagulants. However, management of VKA therapy is complicated due to the narrow therapeutic index of VKAs, slow onset and offset of therapeutic effect and numerous dietary and drug interactions [3–5]. As such, frequent monitoring and dose adjustment are required to manage patients on VKA therapy. Like VKAs, unfractionated heparin also requires frequent monitoring and dose adjustment. The need for parenteral administration of heparin and heparin derivatives is an additional considerable obstacle to the long term use of these agents. Therefore, there is a need for new anticoagulants that can be administered orally and with sufficiently predictable pharmacokinetics/pharmacodynamics such that they would not require regular monitoring or dosage adjustment during routine clinical use.
To address the unmet medical need, oral agents targeted to inhibit thrombin or factor Xa have been developed [1, 6]. Factor Xa, a trypsin-like serine protease, converts prothrombin to thrombin, the final enzyme in the coagulation cascade that is responsible for fibrin clot formation. Evidence from preclinical animal models suggests that direct factor Xa inhibitors exhibit excellent antithrombotic efficacy with minimal bleeding risk when compared with direct thrombin inhibitors. This has been attributed to the attenuation of thrombin generation, but not the activity of thrombin, by factor Xa inhibitors, thereby preserving haemostatic function [7–11]. Results of clinical studies with direct factor Xa inhibitors have supported the preclinical findings that factor Xa inhibitors are effective antithrombotic agents with a minimal bleeding risk [12–16].
Apixaban (BMS-562247) is a highly selective (>30 000-fold selectivity over other coagulation proteases), potent (Ki = 0.08 nm) and orally bioavailable (∼50% bioavailability) [17, 18] inhibitor of both free and prothrombinase-bound factor Xa [19–21]. In a rabbit model of venous thrombosis, apixaban exhibited potent antithrombotic effects at doses that preserved haemostasis [10]. Apixaban was superior to enoxaparin 40 mg daily for the prevention of thromboembolic events in patients who had received total knee or hip replacement surgery [14, 15], and has shown promising results in the treatment of patients with acute symptomatic deep vein thrombosis [22] and a favourable benefit–risk relationship in reducing the risk of stroke and systemic embolism in patients with atrial fibrillation [23, 24].
This report describes the first in-human evaluation of apixaban safety, tolerability, pharmacokinetics and pharmacodynamics in healthy subjects. Results of a second study, conducted to assess the effect of a high fat, high calorie meal on the pharmacokinetics and pharmacodynamics of apixaban, are also presented.
Methods
Subjects
Participants in these studies had to be healthy, non-smoking, non-obese (body mass index: 18–30 kg m−2) male or female subjects (female subjects were required to be non-nursing, non-pregnant and not of childbearing potential) aged 18–45 years. Subjects were excluded for significant, acute or chronic medical conditions (including relevant trauma), any significant head injury within the last 2 years, current or recent (within 3 months) gastrointestinal disease, any recent surgery (within 4 weeks) or planned surgery within 2 weeks of study completion, history of abnormal bleeding or coagulation disorder, history of gastrointestinal conditions that could impact on drug absorption, history of significant drug allergy, recent exposure to over the counter, prescription or investigational medication or a history of drug or alcohol abuse. The use of aspirin and non-steroidal anti-inflammatory agents was prohibited. Eligible subjects provided written informed consent prior to participation in the study.
Study design
The protocol for the single ascending-dose study was approved by the New England Investigational Review Board (Wellesley, Massachusetts, USA) and the protocol for the food effect study was approved by the Western Institutional Review Board (Olympia, Washington, USA). Both protocols complied with local regulations, the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice.
Single ascending-dose study
This was a first-in-human, single centre, double-blind, randomized, placebo-controlled, sequential ascending-dose study conducted from 4 December 2002 to 2 February 2003. Subjects participated in one of seven dose panels. Within each panel, subjects were randomized in a 3 : 1 ratio to receive a single dose of apixaban (n = 6) or matching placebo (n = 2) according to a computer-generated randomization scheme prepared and provided by Bristol-Myers Squibb (BMS). The pharmacist responsible for dispensing the blinded study drug was unblinded with respect to study drug identification, but was not involved in any other aspect of study conduct. All subjects, in addition to the medical staff, were blinded to treatment assignment.
Apixaban was administered as a 0.25 mg ml−1 oral solution for doses of 0.5, 1.0 and 2.5 mg (with solution for placebo doses matched by volume) and as 5 mg oral tablets or matching placebo for doses of 5, 10, 25 and 50 mg. All doses were administered under fasted conditions. Subjects were admitted to the clinical facility (BMS Clinical Research Center, Hamilton, New Jersey, USA) 2 days (day −2) prior to study drug administration for baseline procedures and remained in the facility until discharge (day 5). Escalation to the next dose panel occurred only if safety data through day 3 for six subjects in the ongoing dose panel were considered acceptable by the investigator and sponsor.
Food effect study
This was an open label, randomized, two period, two treatment, crossover study carried out between 20 November 2003 and 20 December 2003. Subjects (n = 24) were admitted to the clinical facility (ProMedica Clinical Research Center, Boston, MA, USA) and randomized to receive a single oral dose of 10 mg apixaban (2 × 5 mg tablets) after a 10 h fast or 5 min after consumption of a standard high fat, high calorie breakfast. The apixaban dose of 10 mg was chosen as it represented the likely clinical dose range. The alternate treatment was administered after a minimum 5 day washout period. Meal composition was based on a standard high fat, high calorie breakfast and consisted of: two fried eggs (∼184 kcal), one tablespoon butter (∼102 kcal), three strips of bacon (∼109 kcal), two slices of white bread toast (∼134 kcal), one tablespoon jelly (54 kcal), 4 oz hash brown potatoes (∼252 kcal) and 8 oz (237 ml) whole milk (∼150 kcal) [25]. Meals were consumed within 30 min. At the time of dosing, 240 ml water was administered to subjects in both treatment periods.
Safety assessments
All available data from subjects who received any study drug (apixaban or placebo) were included in the summaries of safety data. For both studies, safety assessments were performed on admission to the clinical facility, prior to study drug administration and at scheduled intervals thereafter. Safety assessments included monitoring of adverse events (AEs), physical examination, vital signs, clinical laboratory tests and 12-lead electrocardiograms (ECGs). The single ascending-dose study also included assessment of template bleeding time (Simplate®-II R device, Organon Teknika, Durham, North Carolina, USA), measured at screening, before and 3.5 h after dosing, and faecal occult blood. In addition, assessment of arachidonic acid platelet aggregation was performed pre-dose at each treatment period of the food effect study to ensure exclusion of those subjects who had used aspirin within 1 week of enrolment or during the furlough period. Data on AEs were obtained from information volunteered by or solicited from the subjects and by the investigators’ review of their vital signs, ECG and laboratory test results.
Pharmacokinetic assessments
In both studies, blood samples (4.5 ml) for pharmacokinetic assessment were collected from an indwelling catheter or by direct venipuncture. In the single ascending- dose study, serial blood samples were collected prior to administration (t = 0) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 18, 24, 36, 48, 72 and 96 h after apixaban administration. Samples in the food effect study were collected prior to administration and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 9, 12, 24, 48 and 60 h post-dose. Liquid chromatography/tandem mass spectrometry analysis with a lower limit of quantification (LLOQ) of 1 ng ml−1 (range 1 to 1000 ng ml−1) was used to determine apixaban plasma concentration. The methods were validated based upon 2001 FDA method validation guidance. In the single ascending-dose study, the liquid chromatography/tandem mass spectrometry analysis was performed by Bristol-Myers Squibb (New Brunswick, NJ, USA) using a 1/x2 weighted quadratic fit. The inter- and intra-precision runs were ≤9.4% and ≤3.7 %CV, respectively with an accuracy of ±10.2. In the food effect study, the liquid chromatography/tandem mass spectrometry analysis was performed by Alta Analytical (El Dorado Hills, CA, USA) using a 1/x2 weighted linear fit. The inter- and intra-precision runs were ≤0.8% and ≤5.9 %CV, respectively with an accuracy of ±2.3.
Pharmacokinetic parameters (maximum observed plasma concentration (Cmax), the corresponding time to maximum concentration (tmax), area under the plasma concentration–time curve to infinity and to the last observed concentration (AUC(0,∞) and AUC(0,t), respectively) and apparent terminal half-life (t1/2)) were determined using established non-compartmental methods using Kinetica (Version 4.02) software (Thermo Electron Corporation, Philadelphia, PA, USA) [26]. Apixaban Cmax and tmax were determined from experimental observations. The slope (λz) of the terminal phase of the plasma concentration–time profile was determined with a weighting factor of 1 by the method of least squares (log-linear regression of at least three data points). The t1/2 was estimated as ln2/λz. The AUC(0,∞) was determined by summing the areas from zero to the time of last measured concentration, calculated by using conventional trapezoidal and log-trapezoidal methods and the extrapolated area. The extrapolated area was determined by dividing the last measured concentration by the slope of the terminal log-linear phase [27].
Pharmacodynamic assessments
In both studies, blood samples (4.5 ml) at baseline (t = 0) and 0.5, 1.5, 3, 6, 12, 24 and 48 h after administration of study medication were collected in 3.2% citrated tubes for measurement of the pharmacodynamic effects. International normalized ratio (INR) and activated partial thromboplastin time (aPTT) were determined by the BMS Clinical Laboratory (Hamilton, New Jersey, USA). The INR in both studies and prothrombin time (PT) for the food effect study were measured using a Diagnostica Stago STA-Compact® coagulation analyzer (Parsippany, New Jersey, USA) with Stago Neoplastin CI and aPTT was measured using a Diagnostica Stago Compact analyser with PTT Automate 5. Additionally, in the single ascending-dose study, modified prothrombin time (mPT) was measured in apixaban-treated subjects. The assay was performed by Covance Laboratory (Chantilly, Virginia, USA) using a modified thromboplastin reagent on an MLA-1800 coagulation analyzer (Medical Laboratory Automation Inc., Mount Vernon, New York, USA). The thromboplastin reagent (Thromboplastin C + , Dade Behring, Newark, Delaware, USA) was modified by diluting 1 : 2.25 with 100 nm calcium chloride, which slows the clotting reaction and provides a broader dynamic range for measuring the effect of factor Xa inhibitors [28].
Pharmacodynamic parameters determined as part of the single ascending-dose study included area under the response–time curve from the time of dose (0 h) to 24 h post-dose (INR AUC(0,24 h), aPTT AUC(0,24 h) and mPT AUC(0,24 h)), area under the response–time curve to the last sample collected (INR AUC(0,t), aPTT AUC(0,t) and mPT AUC(0,t)), as well as maximum (INRmax, aPTTmax and mPTmax) and minimum (INRmin, aPTTmin and mPTmin) values observed within the 0–24 h post-dose interval. INR, aPTT and PT were summarized by treatment and time point in the food effect study along with their respective changes from baseline.
Statistical methods
Single ascending-dose study
Although the number of subjects was not based on consideration of statistical power, the administration of apixaban to a panel of six subjects would provide an 80% probability of observing at least one occurrence of an AE in any given panel, which occurred with a 24% incidence in the population from which the sample was drawn.
All statistical analyses were carried out using SAS/STAT® Version 8.2 (Cary, NC, USA). Summary statistics were tabulated for apixaban pharmacokinetic parameters by dose. Geometric means and percentage coefficient of variation (CV%) were tabulated for Cmax, AUC(0,∞) and AUC(0,τ). Medians and ranges were presented for tmax, and means and SD on for t1/2. To assess the relationship between pharmacokinetic parameters and dose, scatter plots were created for Cmax and AUC(0,∞) vs. dose.
Summary statistics were tabulated by dose and time point for INR, aPTT and mPT. Additionally, AUC(0,24 h), maximum and minimum INR, aPTT and mPT were summarized by dose. Statistical linear mixed models were estimated to characterize the relationships between apixaban plasma concentration and the clotting measures; INR, aPTT and mPT.
Food effect study
The enrolment of 20 subjects would be expected to provide 95% power to conclude the absence of a food effect with respect to Cmax and 93% power with respect to AUC(0,∞). Calculations were based on the approach described by Diletti et al. [29] and assumed a log-normal distribution of Cmax and AUC(0,∞). Furthermore, it was assumed that variability would be similar to that seen in preliminary data from the apixaban human ascending multiple dose study, i.e. that the intra-subject SD for logCmax and logAUC(0,∞) would not be greater than the inter-subject SD of 0.19 and 0.20 calculated for logCmax and logAUC(0,τ), respectively [30]. All statistical analyses were carried out using SAS/STAT® Version 8.2 (Cary, NC, USA). Absence of a food effect was to be concluded if point estimates and 90% confidence intervals (CIs) for ratios of geometric means for Cmax and AUC(0,∞), with and without food, were contained entirely within the equivalence interval of 80–125%. Point estimates and 90% CIs for the ratios of population geometric means of apixaban fed vs. apixaban fasted were calculated from the results of analysis of variance (anova) on lnCmax and lnAUC(0,∞). The factors in the analyses were treatment (i.e. dietary condition) sequence, period and treatment as fixed effects, and subject within sequence as a random effect.
Results
Subjects
A total of 57 healthy male subjects were randomized to receive apixaban (43 subjects) or placebo (14 subjects) in the single ascending-dose study. Fifty-six subjects (98%) completed the study and one subject (2%) discontinued after withdrawal of consent after dose administration. Baseline demographics were similar across all treatment groups (Table 1).
Table 1.
Baseline subject demographics*
| Single Ascending-Dose Study | Food Effect Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Apixaban dose | |||||||||
| Pooled placebo | 0.5 mg | 1 mg | 2.5 mg | 5 mg | 10 mg | 25 mg | 50 mg | ||
| n | 14 | 6 | 6 | 6 | 6 | 6 | 6 | 7 | 24 |
| Age, mean (SD) (years) | 30 (7) | 35 (5) | 27 (8) | 32 (7) | 30 (7) | 27 (3) | 32 (7) | 30 (9) | 33 (10) |
| Race, n (%) | |||||||||
| White | 6 (43) | 2 (33) | 1 (17) | 3 (50) | 5 (83) | 4 (67) | 5 (83) | 4 (57) | 11 (46) |
| Black | 6 (43) | 4 (67) | 3 (50) | 3 (50) | 0 | 1 (17) | 1 (17) | 1 (14) | 13 (54) |
| Other | 2 (14) | 0 | 2 (33) | 0 | 1 (17) | 1 (17) | 0 | 2 (29) | 0 |
| Weight, mean (SD) (kg) | 77.3 (10.4) | 87.2 (14.3) | 82.2 (13.5) | 87.3 (8.6) | 82.4 (11.4) | 73.8 (11.5) | 73.3 (6.2) | 77.0 (12.2) | 76.6 (10.4) |
| BMI mean (SD) (kg m−2) | 25.5 (3.1) | 25.1 (2.9) | 25.2 (4.0) | 26.4 (2.5) | 25.4 (1.9) | 23.2 (3.2) | 24.5 (0.9) | 25.8 (2.9) | 24.9 (2.8) |
All subjects in both studies were male. BMI, body mass index; SD, standard deviation.
Twenty-four healthy male subjects were enrolled and randomized in the food effect study. Twenty-one (87.5%) subjects received both the fed and fasted treatments and completed the study and three subjects (12.5%) discontinued as detailed below. Baseline demographics for all subjects were similar to those in the single ascending-dose study and are summarized in Table 1.
Safety
Apixaban appeared to be safe and well tolerated, with no serious AEs or major bleeding-related events in either the single ascending-dose or food effect study. Apixaban was not associated with any clinically relevant effects on physical examination, changes in vital signs, ECGs or standard clinical laboratory test results in either study.
Single ascending-dose study
Thirteen AEs were observed in 13 subjects, one of whom received placebo. There was no evidence of a dose-related trend in AEs. The majority of AEs were of mild to moderate intensity and were considered possibly related to study drug. None required treatment. There were two severe AEs (abdominal pain with 10 mg apixaban and musculoskeletal pain with 50 mg apixaban) considered possibly related to study drug. Both subjects completed the study. Three subjects had bleeding-related AEs (one subject with haematochezia who received 25 mg apixaban and two subjects with haematuria who received 50 mg apixaban), all mild in intensity. On follow-up, internal haemorrhoids were subsequently diagnosed in the subject with haematochezia. Apixaban did not have a relevant effect on template bleeding time with little (≤1 min) to no change in bleeding time between pre-dose and 3.5 h post-dose assessments.
Food effect study
Single 10 mg doses of apixaban appeared to be safe and well tolerated, with no bleeding-related AEs. Five AEs were reported by three subjects, who discontinued because of these events. One subject discontinued due to an elevated alanine transaminase level (3× upper limit of normal [ULN]) accompanied by slight elevations in aspartate aminotransferase and gamma-glutamyl transferase levels (<2× ULN), which was considered moderate in intensity and possibly related to study drug. No other relevant laboratory abnormalities or AEs were reported for this subject. While an effect of study drug cannot be ruled out in this study it is not uncommon to observe sporadic elevation of liver function tests in phase I studies even after administration of placebo [31, 32]. Two subjects who reported concurrent upper respiratory tract infections were discontinued due to decreased arachidonic acid-induced platelet aggregation that was considered mild in intensity. These AEs were observed following the return of these subjects from furlough prior to dose administration for the second period. Although not confirmed, it is possible that these subjects consumed aspirin or an aspirin-containing cold medication while on furlough.
Pharmacokinetics
Single ascending-dose study
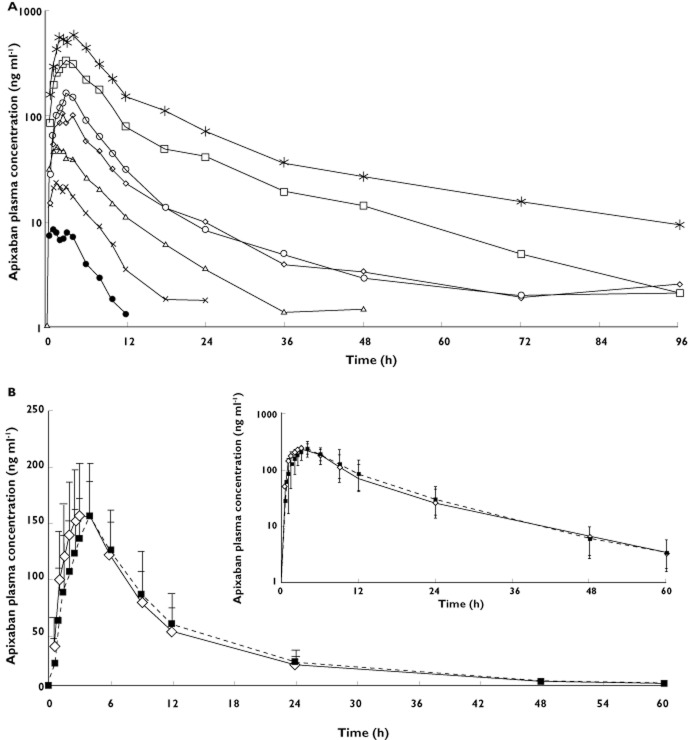
All subjects who received apixaban were included in the pharmacokinetic assessments. The apixaban plasma concentration vs. time profile exhibited a multiphasic elimination profile, with an initial rapid decline followed by a more gradual terminal phase (Figure 1A). Pharmacokinetic parameters are summarized in Table 2. Apixaban exhibited dose-related increases in exposure. Apixaban Cmax, AUC(0,∞) and AUC(0,t) increased approximately proportionally to dose. Cmax, AUC(0,∞) and AUC(0,t) values achieved with the 5 mg tablet were approximately twice those achieved with the 2.5 mg solution, suggesting similar bioavailability between the oral solution and oral tablet (Figure 2, Table 2). Apixaban median tmax occurred between 1.5 and 1.8 h after administration of the oral solution and between 2.5 and 3.3 h after administration of the oral tablet. The mean t1/2 ranged from 3.6 to 6.8 h for oral solution doses ≤2.5 mg and from 11.1 to 26.8 h for the oral tablet doses ≥5 mg. Inter-subject variability in apixaban pharmacokinetics, as indicated by the %CV for Cmax and AUC, was approximately 30% (range 16–42%) across all doses of apixaban, regardless of formulation (Table 2).
Figure 1.
Mean apixaban single dose plasma concentration vs. time profiles for (A) doses from 0.5 to 50 mga (log scale) and (B) a 10 mg dose when administered fasted or after the completion of a high fat, high calorie meal (linear scale [inset is log scale]) (A) Single ascending-dose study.  , 0.5 mg apixaban oral solution (n = 6);
, 0.5 mg apixaban oral solution (n = 6);  , 1.0 mg apixaban oral tablet (n = 6);
, 1.0 mg apixaban oral tablet (n = 6);  , 2.5 mg apixaban oral tablet (n = 6);
, 2.5 mg apixaban oral tablet (n = 6);  , 5 mg apixaban oral tablet (n = 6);
, 5 mg apixaban oral tablet (n = 6);  , 10 mg apixaban oral tablet (n = 6);
, 10 mg apixaban oral tablet (n = 6);  , 25 mg apixaban oral tablet (n = 6);
, 25 mg apixaban oral tablet (n = 6);  , 50 mg apixaban oral tablet (n = 7); (B) Food effect study.
, 50 mg apixaban oral tablet (n = 7); (B) Food effect study.  , Fasted (n = 23);
, Fasted (n = 23);  , Fed (n = 22). Concentration values <LLQ (1 ng ml−1) after dosing were treated as missing. LLQ, lower limit of quantification
, Fed (n = 22). Concentration values <LLQ (1 ng ml−1) after dosing were treated as missing. LLQ, lower limit of quantification
Table 2A.
Summary statistics for apixaban pharmacokinetic parameters
| Dose | n | Cmax (ng ml−1) | AUC(0,∞) (ng ml−1 h) | AUC(0,t) (ng ml−1 h) | tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|---|
| Geometric mean (CV%) | Median (min, max) | Mean (SD) | ||||
| Single ascending-dose study | ||||||
| Oral solution | ||||||
| 0.5 mg | 6 | 9.1 (20) | 61.9 (16) | 52.7 (23) | 1.5 (1.0, 4.0) | 3.6 (1.1) |
| 1.0 mg | 6 | 23.5 (35) | 174.4 (31) | 162.6 (33) | 1.8 (1.0, 3.0) | 4.3 (1.6) |
| 2.5 mg | 6 | 52.5 (35) | 437.5 (41) | 421.1 (42) | 1.5 (1.0, 3.0) | 6.8 (2.0) |
| Oral tablet | ||||||
| 5 mg | 6 | 104.7 (25) | 1016.6 (37) | 976.6 (36) | 3.3 (2.5, 4.0) | 15.2 (8.5) |
| 10 mg | 6 | 176.3 (42) | 1303.6 (40) | 1266.5 (38) | 3.0 (2.0, 4.0) | 11.1 (5.8) |
| 25 mg | 6 | 365.1 (17) | 4010.0 (19) | 3868.9 (22) | 3.0 (2.5, 4.0) | 26.8* (33.7) |
| 50 mg | 7 | 685.2 (22) | 7556.5 (25) | 7096.7 (23) | 2.5 (2.0, 4.0) | 19.7 (15.3) |
| Food effect study, oral tablet | ||||||
| 10 mg fasted | 21 | 150.8 (28) | 1789.0 (31) | 1762.2 (32) | 3.0 (1.5, 6.0) | 11.5 (4.3) |
| 10 mg fed | 21 | 165.0 (18) | 1867.8 (30) | 1811.5 (30) | 4 (1.0, 9.0) | 11.3 (2.9) |
The summary statistics for t½½ in the 25 mg panel were calculated using data from all six subjects, including one subject in whom the calculated t½½ exceeded 95 h.
AUC(0,∞), area under the plasma concentration–time curve from time zero extrapolated to infinity; AUC(0,t), area under the plasma concentration–time curve from time zero to time of the last observed concentration; Cmax, maximum observed plasma concentration; CV, coefficient of variation; SD, standard deviation; t1/2, plasma terminal half-life; tmax, time of maximum observed plasma concentration.
Figure 2.
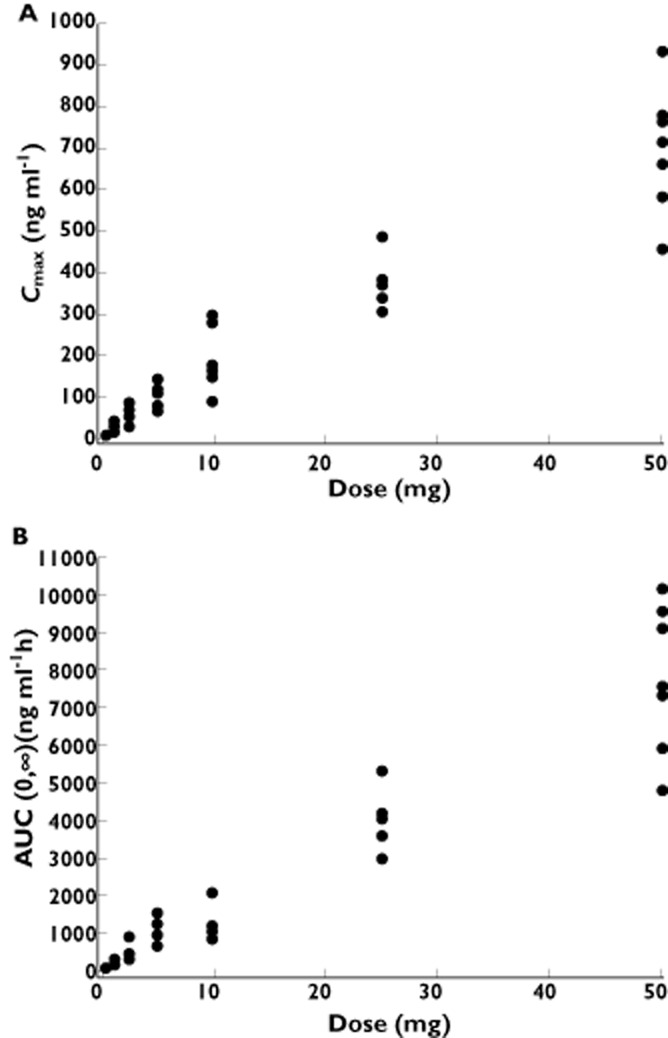
Plot of individual apixaban (A) Cmax and (B) AUC(0.∞) values vs. dose (in single ascending-dose study). AUC(0,∞), area under the plasma concentration–time curve from time zero extrapolated to infinity; Cmax, maximum observed plasma concentration
Food effect study
All available data from subjects who received study drug were included in the pharmacokinetic assessments, but only data from the 21 subjects who completed both treatments were included in the summary statistics and statistical analyses of the pharmacokinetic parameters, as predefined by the study protocol. Apixaban exposure following administration with food was similar to that when administered fasting (Figure 1B). Geometric mean ratios (fed/fasted) for Cmax and AUC(0,∞) (90% CI) were 1.10 (1.004, 1.197) and 1.04 (1.004, 1.086), respectively, and the 90% CI fell completely within the prespecified 80–125% equivalence interval. The t1/2 was similar under both fasted and fed conditions: 11.5 h and 11.3 h, respectively. Median tmax was similar in the fasted state to that seen in the single ascending-dose study, but was increased by 1 h following administration of apixaban with food (4 h vs. 3 h fasted). Apixaban pharmacokinetic parameters are presented in Table 2 and the statistical analysis is summarized in Table 3.
Table 2B.
Statistical analysis of apixaban Cmax, AUC(0,∞) and AUC(0,t)
| Pharmacokinetic parameter | Adjusted geometric means | Ratios of geometric means (fed/fasted) | |
|---|---|---|---|
| 10 mg fasted | 10 mg fed | Fed/fasted point estimate (90% CI) | |
| Cmax(ng ml−1) | 150.8 | 165.3 | 1.10 (1.004, 1.197) |
| AUC(0,∞) (ng ml−1 h) | 1789.3 | 1868.1 | 1.04 (1.004, 1.086) |
| AUC(0,t) (ng ml−1 h) | 1726.5 | 1811.3 | 1.05 (1.002, 1.099) |
AUC(0,∞), area under the plasma concentration–time curve from time zero extrapolated to infinity; AUC(0,t), area under the plasma concentration–time curve from time zero to time of the last observed concentration; CI, confidence interval; Cmax, maximum observed plasma concentration.
Pharmacodynamics
Single ascending-dose study
Subjects with a baseline value and at least one post-dose assessment were included in the summary statistics for each pharmacodynamic parameter. Samples from subjects who received placebo were not analyzed for mPT and, therefore, were not included in the mPT assessments. Additionally, the INR and aPTT values for three subjects on day 1 at 0.5 h post-dose could not be included in the analysis because the samples were clotted.
The mean INR values by dose group at baseline ranged from 1.01 to 1.14. Following administration of apixaban, the INR–time profile closely followed the apixaban plasma concentration–time profile, with no evident temporal lag for doses where changes in INR were observed (Figure 3A). After administration of apixaban, there appeared to be a modest dose-related increase in mean change from baseline (mean % change from baseline) in maximum INR: 1.37 (19.5%), 1.45 (44.1%) and 1.71 (55.8%) for the 10, 25 and 50 mg doses of apixaban, respectively, with minimal, if any, change after lower doses. The relationship between INR change from baseline and plasma concentration appeared to be linear (Figure 4). Similar trends were observed for INR AUC(0,24 h) and AUC(0,t).
Figure 3.
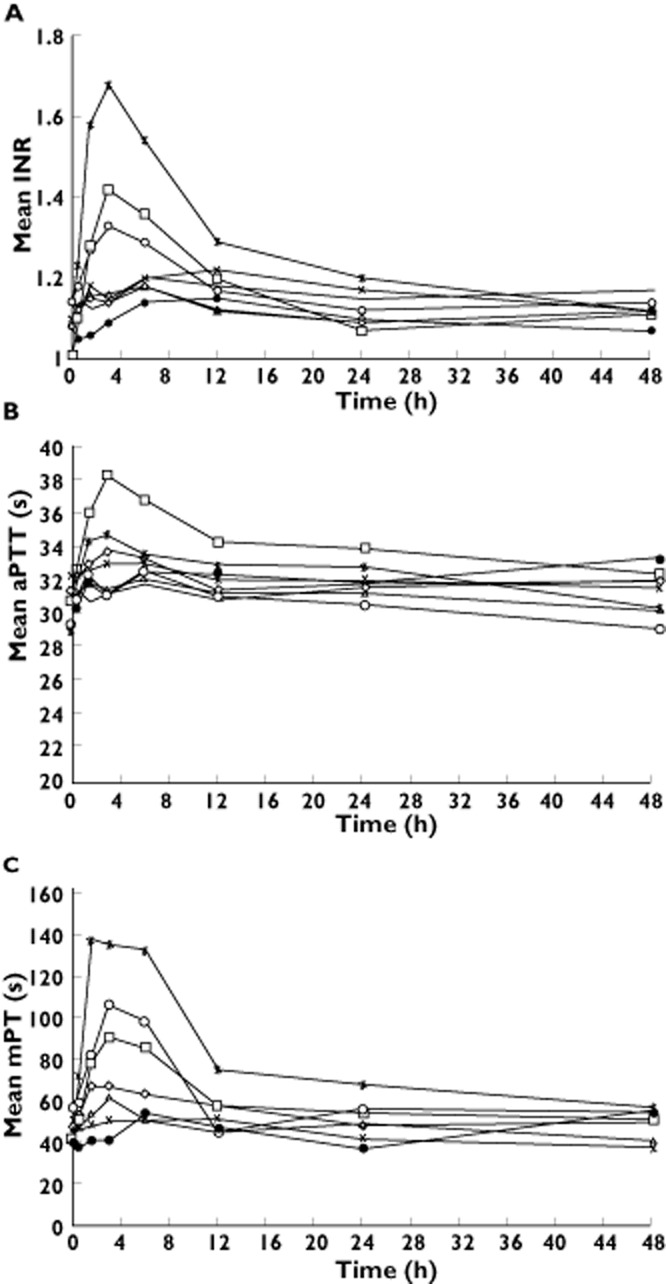
Mean (A) INR, (B) aPTT and (C) mPT vs. time for doses from 0.5 to 50 mg (single ascending-dose study). aPTT, activated partial thromboplastin time; INR, international normalized ratio; mPT, modified prothrombin time.  , 0.5 mg apixaban oral tablet (n = 6);
, 0.5 mg apixaban oral tablet (n = 6);  , 1.0 mg apixaban oral tablet (n = 6);
, 1.0 mg apixaban oral tablet (n = 6);  , 2.5 mg apixaban oral tablet (n = 6);
, 2.5 mg apixaban oral tablet (n = 6);  , 5 mg apixaban oral tablet (n = 6);
, 5 mg apixaban oral tablet (n = 6);  , 10 mg apixaban oral tablet (n = 6);
, 10 mg apixaban oral tablet (n = 6);  , 25 mg apixaban oral tablet (n = 6);
, 25 mg apixaban oral tablet (n = 6);  , 50 mg apixaban oral tablet (n = 7);
, 50 mg apixaban oral tablet (n = 7);  , Placebo. Note: mPT is presented for apixaban-treated subjects only.
, Placebo. Note: mPT is presented for apixaban-treated subjects only.
Figure 4.
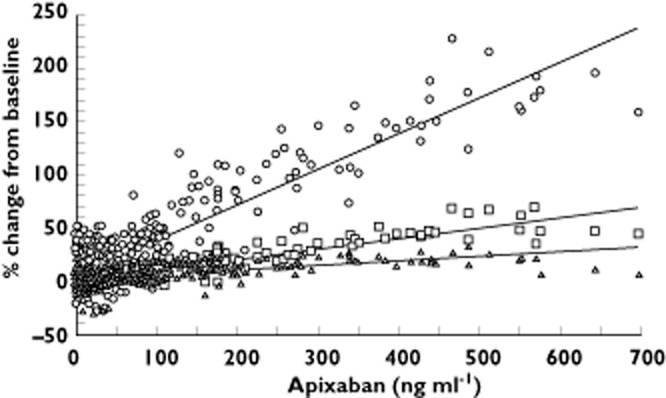
Relationship between different anticoagulation markers and plasma apixaban concentration (single ascending-dose study). Trendlines indicating the relationship between each of the three coagulation markers and apixaban plasma concentration are shown. mPT n = 49 subjects, 391 datapoints, ○, INR n = 49 subjects, 389 data points, □, aPTT n = 49 subjects, 389 data points, ▵. aPTT, activated partial thromboplastin time; INR, international normalized ratio; mPT, modified prothrombin time
The mean baseline aPTT values by dose group ranged from 28.6 to 32.0 s. As with INR, the aPTT–time profile generally followed the apixaban plasma concentration–time profile for doses where changes in aPTT were observed (Figure 3B). For doses ≥25 mg, there appeared to be modest increases in mean maximum aPTT. The mean changes from baseline [mean % change from baseline] in maximum aPTT for the 25 and 50 mg doses were 30.4 to 38.2 s (25.7%) and 28.6 to 35.1 s (23.1%), respectively. The relationship between aPTT change from baseline and plasma concentration appeared to be linear (Figure 4). Similar trends were observed for aPTT AUC(0,24 h) and AUC(0,t).
The mean baseline mPT values for apixaban-treated subjects by dose group were between 40 and 58 s. Again, the mPT–time profile closely followed the apixaban plasma concentration–time profile (Figure 3C). The mean % changes from baseline in maximum mPT for the 2.5, 5, 10, 25 and 50 mg doses were 35.0%, 42.9%, 87.1%, 135.3% and 187.1%, respectively. The relationship between mPT and apixaban plasma concentration appears to be direct and linear (Figure 4) with increases in mPT appearing to be more robust than those for INR or aPTT. For apixaban doses ≥2.5 mg, mPT increased in a dose-proportional manner. Similar trends were observed for mPT AUC(0,24 h) and AUC(0,t).
Food effect study
Following oral administration of apixaban 10 mg, changes in INR, aPTT and PT were modest, similar to those seen for the 10 mg dose in the single ascending-dose study under both fed and fasted conditions, and returned to baseline within 24 h. For example, mean INR at 3 h post-dose was 1.33 and 1.28 under fasted and fed conditions, respectively, and no individual INR exceeded 2.0 (Table 4).
Table 3.
Affect of food on INR, aPTT and PT at 3 h after administration of 10 mg apixaban in the fasted or fed state (food effect study)
| INR | aPTT | PT | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Mean (SD) change from baseline | Mean (SD) | Mean (SD) change from baseline | Mean (SD) | Mean (SD) change from baseline | |
| Food effect study, oral tablet | |||||||
| 10 mg fasted | 23 | 1.33 (0.20) | 0.23 (0.13) | 32.96 (3.59) | 3.05 (2.59) | 14.99 (1.24) | 1.39 (0.89) |
| 10 mg fed | 22 | 1.28 (0.15) | 0.20 (0.12) | 31.55 (3.02) | 2.90 (2.21) | 14.66 (0.97) | 1.30 (0.85) |
aPTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; SD, standard deviation.
Discussion
The purpose of the first-in-human ascending-dose study was to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single oral doses of apixaban (0.5–50 mg) in healthy adult subjects. Apixaban in single oral doses of up to 50 mg appeared to be safe and generally well tolerated in this study population. No dose-limiting AEs were observed and, consequently, a maximum tolerated single dose for apixaban was not determined. Based on the limited number of bleeding-related AEs and no evident effect on template bleeding time, a single dose of apixaban appeared to prolong clotting time in healthy subjects without excessively impairing haemostatic function. Similarly, administration of 10 mg apixaban with or without food was well tolerated, with few AEs observed.
Apixaban is rapidly absorbed following oral administration with maximum concentrations appearing 1.5–3.3 h after administration. Although tmax was reached slightly earlier following administration of the oral solution (1.5–1.8 h) relative to the tablet (2.5–3.3 h), these differences were minimal and indicated that the tablet dissolved rapidly. In the single ascending-dose study, apixaban t1/2 ranged from 7 to 27 h at doses that provided sufficient data to characterize the terminal elimination phase (i.e. 5–50 mg; the shorter t1/2 calculated following oral doses ≤2.5 mg is likely due to the inability to characterize fully the terminal phase with an assay LLOQ of 1 ng ml−1.) Based on the data obtained from the food effect study, the apixaban t1/2 was approximately 12 h. The pharmacokinetics of apixaban were well characterized and exposures (Cmax and AUC) appeared to increase in a dose-proportional manner across the 0.5–50 mg dose range tested.
Apixaban exposure was not affected by administration of a standard high fat, high calorie meal. The only pharmacokinetic parameter that changed with a high fat meal was an increase of 1 h in median tmax. This increase in tmax likely represents the slower gastric emptying observed following consumption of a high fat, high calorie meal [33] and is not clinically relevant as Cmax, AUC and t1/2 remained unchanged and similar to those seen for the same apixaban dose in the single ascending-dose study.
The pharmacodynamic effects of apixaban were consistent with selective inhibition of factor Xa. Single doses of apixaban resulted in rapid and dose-related increases in clotting assay parameters (INR, aPTT and mPT). The consumption of a high fat meal did not alter the effect of apixaban on clotting time assessments. The INR and aPTT assays have traditionally been used to measure the effect of anticoagulants such as VKA and heparin, respectively. While modest dose-related increases in these tests were observed following apixaban administration, the magnitude of the increases in INR or aPTT following apixaban administration would be considered minimal relative to the effect observed following administration of therapeutic doses of a VKA or unfractionated heparin, as the results following apixaban administration were within the normal range for the reporting laboratory (0.7–1.5 and 24–35.9 s, respectively). These results support preclinical evidence that traditional clotting assays, such as INR and aPTT [9, 34, 35] may not be adequately sensitive for reliable evaluation of the pharmacodynamic effects of direct reversible factor Xa inhibitors. Unlike oral VKAs, changes in pharmacodynamic parameters were evident less than 1 h following a single dose of apixaban. The clotting time prolongation closely followed the apixaban concentration–time profile, such that the maximal clotting time occurred near the apixaban tmax, and returned to baseline coincident with the apixaban elimination phase.
The mPT assay provides improved sensitivity over the PT assay, making it suitable for the measurement of anticoagulant activity in individuals receiving factor Xa inhibitors [28]. Among the clotting assays included in the single ascending-dose study, the mPT test demonstrated the greatest sensitivity to apixaban in human plasma. The mPT assay was a more sensitive assessment of apixaban activity than INR or aPTT. These mPT results indicated that traditional clotting time assays may not be suitable for assessing the effect of direct factor Xa inhibitors without modification and further validation.
The pharmacokinetics of certain oral drugs can be affected by the concomitant administration of food. For example, the presence of food has been shown to alter gastric pH, gastric emptying, gastrointestinal motility, bile secretion and may also affect the biotransformation of drugs in the gastrointestinal tract wall and/or liver [27]. A safe, effective oral anticoagulant with predictable pharmacokinetics and pharmacodynamics that are not influenced by food would offer the promise of advantages to patients due to increased convenience of drug administration and reduced potential for complications. The effect of food on the pharmacodynamics of warfarin is well established [3, 4]. Warfarin and other VKAs have several known interactions with food, mostly due to alterations in vitamin K content [3, 36–40]. Variability in warfarin exposure in conjunction with its narrow therapeutic index requires close and constant management of consumed foods, and other factors such as antibiotics that alter gut flora, so as to avoid unwanted AEs. Food has been shown to impact on the pharmacokinetics of another factor Xa inhibitor, rivaroxaban. Administration of a single 10 mg dose of rivaroxaban following consumption of a standard high calorie, high fat meal resulted in a 28% and 41% increase in rivaroxaban AUC(0,∞) and Cmax (P < 0.05 for both), respectively. Administration of a single 20 mg dose of rivaroxaban following a similar meal resulted in a 23% and 74% increase in rivaroxaban AUC(0,∞) and Cmax, respectively. A similar increase in rivaroxaban exposure was observed following administration with a high carbohydrate meal [39]. A high calorie, high fat breakfast did not affect the extent of absorption of the direct thrombin inhibitor, dabigatran [41]. The lack of an effect of food on exposure and pharmacodynamic activity of apixaban represents a desirable feature for an oral anticoagulant.
In summary, single doses of apixaban were well tolerated over a wide dose range and resulted in dose-related prolongation of clotting times across the dose range. The pharmacokinetics of apixaban were predictable, dose-proportional and correlated with pharmacodynamic effects. Food had no effect on the pharmacokinetics and pharmacodynamics of apixaban. Therefore, apixaban can be given without regard to meals.
Acknowledgments
The authors would like to acknowledge Andrew Shenker, MD, PhD and Zhigang Yu, PhD, for their assistance with analysis and reporting the food effect study. Howard Uderman, MD, at Bristol-Myers Squibb Clinical Research Center, was the Principal Investigator of the single ascending-dose study. Miguel A. Zinny, MD, at the Promedica Clinical Research Center, was the Principal Investigator of the food effect study. The authors also thank Dr Robert Wright, PharmD, an employee of Bristol-Myers Squibb at the time of this research, for his critical review of this manuscript. Professional medical writing was provided by Andrew Shepherd, PhD, and Dana Fox, PhD, CMPP, of Caudex Medical, funded by Bristol-Myers Squibb and Pfizer.
Competing Interests
All authors were employees of Bristol-Myers Squibb at the time these studies were conducted. Both studies were funded by Bristol-Myers Squibb and Pfizer.
References
- 1.Weitz JI, Hirsh J, Samama MM. New antithrombotic drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:234S–256. doi: 10.1378/chest.08-0673. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins D. Limitations of traditional anticoagulants. Pharmacotherapy. 2004;24:62S–65. doi: 10.1592/phco.24.10.62s.36120. [DOI] [PubMed] [Google Scholar]
- 3.Harris JE. Interaction of dietary factors with oral anticoagulants: review and applications. J Am Diet Assoc. 1995;95:580–584. doi: 10.1016/S0002-8223(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 4.Rewinkel JB, Adang AE. Strategies and progress towards the ideal orally active thrombin inhibitor. Curr Pharm Des. 1999;5:1043–1075. [PubMed] [Google Scholar]
- 5.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 6.Weitz JI. Emerging anticoagulants for the treatment of venous thromboembolism. Thromb Haemost. 2006;96:274–284. doi: 10.1160/TH06-05-0234. [DOI] [PubMed] [Google Scholar]
- 7.Ieko M, Tarumi T, Takeda M, Naito S, Nakabayashi T, Koike T. Synthetic selective inhibitors of coagulation factor Xa strongly inhibit thrombin generation without affecting initial thrombin forming time necessary for platelet activation in hemostasis. J Thromb Haemost. 2004;2:612–618. doi: 10.1111/j.1538-7933.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 8.Wong PC, Crain EJ, Watson CA, Wexler RR, Lam PY, Quan ML, Knabb RM. Razaxaban, a direct factor Xa inhibitor, in combination with aspirin and/or clopidogrel improves low-dose antithrombotic activity without enhancing bleeding liability in rabbits. J Thromb Thrombolysis. 2007;24:43–51. doi: 10.1007/s11239-007-0017-9. [DOI] [PubMed] [Google Scholar]
- 9.Wong PC, Crain EJ, Xin B, Wexler RR, Lam PY, Pinto DJ, Luettgen JM, Knabb RM. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6:820–829. doi: 10.1111/j.1538-7836.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong PC, Watson CA, Crain EJ. Arterial antithrombotic and bleeding time effects of apixaban, a direct factor Xa inhibitor, in combination with antiplatelet therapy in rabbits. J Thromb Haemost. 2008;6:1736–1741. doi: 10.1111/j.1538-7836.2008.03092.x. [DOI] [PubMed] [Google Scholar]
- 11.Wong PC, Crain EJ, Watson CA, Xin B. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J Thromb Haemost. 2009;7:1313–1320. doi: 10.1111/j.1538-7836.2009.03503.x. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57. doi: 10.1146/annurev-med-062209-095159. [DOI] [PubMed] [Google Scholar]
- 14.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 15.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 16.Ufer M. Comparative efficacy and safety of the novel oral anticoagulants dabigatran, rivaroxaban and apixaban in preclinical and clinical development. Thromb Haemost. 2010;103:572–585. doi: 10.1160/TH09-09-0659. [DOI] [PubMed] [Google Scholar]
- 17.Frost C, Yu Z, Nepal S, Bragat A, Moore K, Shenker A, Barrett YC, LaCreta F. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation [abstract] J Clin Pharmacol. 2008;48:1132. [Google Scholar]
- 18.Vakkalagadda B, Frost C, Wang J, Nepal S, Schuster A, Zhang D, Dias C, Yu Z, Shenker A, LaCreta F. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa [abstract] J Clin Pharmacol. 2009;49:1091–1130. [Google Scholar]
- 19.Jiang X, Crain EJ, Luettgen JM, Schumacher WA, Wong PC. Apixaban, an oral direct factor Xa inhibitor, inhibits human clot-bound factor Xa activity in vitro. Thromb Haemost. 2009;101:780–782. [PubMed] [Google Scholar]
- 20.Pinto DJ, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, Wong PC, Rendina AR, Luettgen JM, Knabb RM, He K, Xin B, Wexler RR, Lam PY. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H -pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50:5339–5356. doi: 10.1021/jm070245n. [DOI] [PubMed] [Google Scholar]
- 21.Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81. doi: 10.1124/dmd.108.023143. [DOI] [PubMed] [Google Scholar]
- 22.Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost. 2008;6:1313–1318. doi: 10.1111/j.1538-7836.2008.03054.x. [DOI] [PubMed] [Google Scholar]
- 23.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 24.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek E, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser S, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon J, Pais P, Parkhomenko A, Verheugt F, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Guidance for Industry: food effect bioavailability and fed bioequivalence studies. Available at http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126833.pdf.2002. FDA. Guidance for Industry. 6-5-2011.
- 26.Perrier D, Gibaldi M. General derivation of the equation for time to reach a certain fraction of steady state. J Pharm Sci. 1982;71:474–475. doi: 10.1002/jps.2600710432. [DOI] [PubMed] [Google Scholar]
- 27.Rowland M, Tozer T. Clinical Pharmacokinetics, Concepts and Applications. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins; 1995. [Google Scholar]
- 28.Barrett YC, Wang Z, Knabb RM. A novel prothrombin time assay for assessing the anticoagulant activity of oral factor Xa inhibitors. Clin Appl Thromb Hemost. 2012 doi: 10.1177/1076029612441859. Apr 2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Diletti E, Hauschke D, Steinijans VW. Sample size determination for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Toxicol. 1991;29:1–8. [PubMed] [Google Scholar]
- 30.Frost C, Yu Z, Moore K, Nepal S, Barrett Y, Mosqueda-Garcia R, Shenker A. Apixaban, an oral direct factor Xa inhibitor: multiple-dose safety, pharmacokinetics, and pharmacodynamics in healthy subjects. J Thromb Haemost. 2007;5:P-M-664. [Google Scholar]
- 31.Groen D, Harris S, Colucci S, Apseloff G. Serum transaminase levels should be measured immediately prior to dosing in early phase I clinical trials. J Clin Pharmacol. 2011;51:252–255. doi: 10.1177/0091270010365548. [DOI] [PubMed] [Google Scholar]
- 32.Pettersson J, Hindorf U, Persson P, Bengtsson T, Malmqvist U, Werkstrom V, Ekelund M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol. 2008;65:253–259. doi: 10.1111/j.1365-2125.2007.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999;36:233–254. doi: 10.2165/00003088-199936030-00004. [DOI] [PubMed] [Google Scholar]
- 34.Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–1271. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 35.Hillarp A, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Stigendal L, Sten-Linder M, Strandberg K, Lindahl TL. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–139. doi: 10.1111/j.1538-7836.2010.04098.x. [DOI] [PubMed] [Google Scholar]
- 36.Ford SK, Misita CP, Shilliday BB, Malone RM, Moore CG, Moll S. Prospective study of supplemental vitamin K therapy in patients on oral anticoagulants with unstable international normalized ratios. J Thromb Thrombolysis. 2007;24:23–27. doi: 10.1007/s11239-007-0014-z. [DOI] [PubMed] [Google Scholar]
- 37.Franco V, Polanczyk CA, Clausell N, Rohde LE. Role of dietary vitamin K intake in chronic oral anticoagulation: prospective evidence from observational and randomized protocols. Am J Med. 2004;116:651–656. doi: 10.1016/j.amjmed.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch IB, Brownlee M. Beyond hemoglobin A1c – need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303:2291–2292. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 39.Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46:549–558. doi: 10.1177/0091270006286904. [DOI] [PubMed] [Google Scholar]
- 40.Kurnik D, Loebstein R, Rabinovitz H, Austerweil N, Halkin H, Almog S. Over-the-counter vitamin K1-containing multivitamin supplements disrupt warfarin anticoagulation in vitamin K1-depleted patients. A prospective, controlled trial. Thromb Haemost. 2004;92:1018–1024. doi: 10.1160/TH04-06-0346. [DOI] [PubMed] [Google Scholar]
- 41.Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H, Rathgen K, Svard R. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–563. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]