Abstract
This study assessed how conformational information encoded by ligand binding to δ-opioid receptors (DORs) is transmitted to Kir3.1/Kir3.2 channels. Human embryonic kidney 293 cells were transfected with bioluminescence resonance energy transfer (BRET) donor/acceptor pairs that allowed us to evaluate independently reciprocal interactions among signaling partners. These and coimmunoprecipitation studies indicated that DORs, Gβγ, and Kir3 subunits constitutively interacted with one another. GαoA associated with DORs and Gβγ, but despite being part of the complex, no evidence of its direct association with the channel was obtained. DOR activation by different ligands left DOR-Kir3 interactions unmodified but modulated BRET between DOR-GαoA, DOR-Gβγ, GαoA-Gβγ, and Gβγ-Kir3 interfaces. Ligand-induced BRET changes assessing Gβγ-Kir3.1 subunit interaction 1) followed similar kinetics to those monitoring the GαoA-Gβγ interface, 2) displayed the same order of efficacy as those observed at the DOR-Gβγ interface, 3) were sensitive to pertussis toxin, and 4) were predictive of whether a ligand could evoke channel currents. Conformational changes at the Gβγ/Kir3 interface were lost when Kir3.1 subunits were replaced by a mutant lacking essential sites for Gβγ-mediated activation. Thus, conformational information encoded by agonist binding to the receptor is relayed to the channel via structural rearrangements that involve repositioning of Gβγ with respect to DORs, GαoA, and channel subunits. Further, the fact that BRET changes at the Gβγ-Kir3 interface are predictive of a ligand’s ability to induce channel currents points to these conformational biosensors as screening tools for identifying GPCR ligands that induce Kir3 channel activation.
Introduction
G protein–gated inwardly rectifying K+ (GIRK or Kir3) channels mediate slow postsynaptic inhibitory potentials and help regulate cell excitability in the heart and brain (Hibino et al., 2010). Their activity is controlled by neurotransmitters such as monoamines, amino acids, and peptides, which, on binding to their respective receptors, trigger Kir3 channel opening via stimulation of pertussis toxin (PTX)-sensitive Gi/o proteins (Hibino et al., 2010). The specificity and temporal precision of this signaling process have been attributed to the constitutive association of G protein–coupled receptors (GPCRs), G proteins, and channel subunits (Rebois et al., 2006; Riven et al., 2006; Pineyro, 2009). In particular, in vitro and in vivo studies indicate that Gβγ subunits may associate directly with cytosolic domains of the channel (Huang et al., 1995; Rebois et al., 2006), an interaction that is present both before and during channel activation (Riven et al., 2006; Berlin et al., 2011). Gαi/o is also thought to be part of the Kir3-G protein complex, but the way in which this subunit interacts with other complex components, particularly the channel, remains to be fully elucidated. Indeed, biochemical and biophysical studies show that Gα-GTP may interact directly with channel subunits (Berlin et al., 2010; Mase et al., 2012), whereas Gα-GDP would associate with the complex via the Gβγ dimer (Clancy et al., 2005; Berlin et al., 2011). Functional studies indicate that Gα actively participates in channel modulation in both its GDP- and GTP-bound states (Berlin et al., 2010; Berlin et al., 2011) and have identified Gβγ interactions with Kir3 subunits as the mediator of channel activation (Logothetis et al., 1987; Riven et al., 2006).
Together, the data available indicate that Kir3 activity is regulated by well coordinated interactions at the interface of the channel and heterotrimeric Gαβγ subunits. What is less well defined are the dynamics of channel activation via GPCRs. In particular, little is known regarding how conformational information encoded by ligand binding to the receptor is transmitted to the channel. Here we used BRET to investigate how ligand binding to δ-opioid receptors (DORs) modulated constitutive interactions among receptors, Gαoβ1γ2 subunits, and neuronal Kir3.1/Kir3.2 channels. The results obtained showed that ligand binding to the receptor induced correlated conformational changes at BRET pairs assessing Gβγ interaction with DORs and Kir3 subunits. DOR activation also induced conformational rearrangements between DORs and Gαo and among the latter and Gβγ subunits. However, no evidence was found for conformational changes being transferred directly to the channel via Gαo. Similarly, despite close proximity between DORs and Kir3 subunits, BRET assays did not reveal relevant structural rearrangements at this interface. We conclude that conformational information encoded by ligand binding to the receptor is essentially transferred to the channel via the Gβγ dimer and propose that a BRET biosensor that captures these changes may be a useful tool to screen for GPCR ligands that modulate Kir3 channel function.
Materials and Methods
Reagents.
Buffer chemicals, protease inhibitors, [D-Pen(2), D-Pen(5)]-enkephalin (DPDPE), morphine, naloxone, naltrindole, pertussis toxin, anti-FLAG M2 affinity resin, and FLAG peptide were purchased from Sigma (St. Louis, MO). [35S]GTPγS and coelanterazine were from PerkinElmer (Waltham, MA). SNC-80 was from Tocris Cookson (Ellisville, MO), TIPP and TICPΨ were kindly provided by Dr. Schiller (Université de Montréal, QC, Canada), and UFP512 was a generous gift from Dr. Balboni (University of Cagliari, Italy). Anti-rabbit Alexa-488 and anti-mouse Alexa 647-conjugated antibodies were from Molecular Probes (Eugene, OR).
DNA Constructs.
Constructs encoding the yellow fluorescent protein (YFP) or Renilla luciferase (RLuc) fused in frame at the C-terminus of human DORs have been previously described (Breit et al., 2006), as have plasmids encoding YFP fused at the N-terminus of human Gγ2 or at the C-terminus of CD8-YFP (Gales et al., 2005). Kir3.1/3.2 human subunits bearing Rluc at their C-terminus have also been described (Robitaille et al., 2009). The recombinant plasmid encoding for GαoA tagged with luciferase at position 99 (GαoA99-Luc) was created from overlapping polymerase chain reaction (PCR) from three fragments, then digested with the restriction enzymes SalI + NheI, and cloned in the XhoI + NheI sites of the expression vector pCDNA3.1 zeo(+) (Invitrogen, Carlsbad, CA). Two of the PCR fragments were created from pcDNA3.1(+) G protein alphaoA EE-tagged (cat number: GNA0OAEI00 from Missouri S&T cDNA Resource Center, Rolla, MO) with the following primers: GNAO NheI forward: 5′-CAATGCTAGCGATATCGGTAC CACCATGGGATGTACTCTGAGCGCA-3′, GNAoA Nterm-NAAIRS L reverse: 5′-CACCTTGCTGGTCATGGACCGAATGGCGGCATTCTCCTTATCACCATATTCGATGCCCAA-3′, GNAoA NAAIRS-Cterm forward: 5′-AATGCCGCTATTCGGTCCACTTTGGG CATCGAATATGGT-3′, GNAOa SalI reverse: 5′-CCCTCTAGAGTCGACTCAGTAC AAGCCGCAGCCCCG-3′. The third fragment encoding RlucII flanked by NAAIRS linkers was created by PCR from pCDNA3.1 Hygro (+) EPAC db bret2 sensor (http://www.ncbi.nlm.nih.gov/pubmed/19584306) with the following primers: NAAIRS-RlucII forward:5′-AATGCCGCCATTCGGTCCATGACCAGCAAGGTGTACGACC-3′ and RlucII-NAAIRS reverse: 5′-GGACCGAATAGCGGCATTCTGCTCGTTCTTCAGCACT CT-3′. Once assembled in one fragment by an overlapping PCR, the resulting product encoded a human EE-tagged GαoA G protein subunit with an internal RlucII flanked by NAAIRS linkers and the loop connecting helices αA and αB of the helical domain. The mutant human Kir3.1-Luc subunit lacking residues 183-342 (Kir3.1-RLuc183-342) was produced using overlapping PCR on a Kir3.1-Rluc in pRC-CMV. Primers used were T7 forward primer and 5′-GGAACTGGGAGAT-GAACATGCAGCCGATG-3′ (PCR1). Forward 5′-CATGTTCATCTCCCAGTTCCACG-CAAC-3′ and reverse 5′-GGATCA TAAACTTTCGAAGTCATGG’3′ (PCR2) were followed by forward T7 primer and reverse 5′-GGATCATAAACTTTCGAAGTCATGG-3′ (PCR on the products of PCR1 and PCR2). The resulting product was subcloned into Kir3.1-Rluc in pRC-CMV using the NheI site. Kir3.1 subunits bearing a Flag tag at position 114 (extracellularly tagged FLAG-Kir3.1) were kindly provided by Dr. Deborah J. Nelson (University of Chicago, Chicago, IL).
Cell Culture and Transfections.
Human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 unit/ml penicillin-streptomycin, at 37°C in a humidified atmosphere at 95% air and 5% CO2. For transient expression of recombinant proteins, HEK293 cells were seeded at a density of 3 × 106 cells in 100-mm Petri dishes, cultured for 24 hours, and transfected with vectors encoding BRET constructs for DORs, G protein, and Kir3.1/3.2 subunits, along with untagged complementary complex components as specified in Table 1. Unless otherwise specified, transfections were performed with polyethylenimine (Polysciences, Warrington, PA) as previously described (Boussif et al., 1995). Forty-eight hours after transfection, cells were used for BRET, immunoprecipitation, or electrophysiological experiments. For [35S]GTPγS binding assays, HEK293 cells were stably transfected with Flag-DORs. Clonal cell lines were produced using Lipofectamine (Invitrogen) to transfect 6 µg of DNA/100-mm Petri dish followed by selection with G418 (500 μg/ml).
TABLE 1.
BRET constructs used to assess protein interactions within the signaling complex formed by DOR-GαoAβ1γ2-Kir3.1/Kir3.2
| Interaction | BRET Donor/Acceptor Pairs | Untagged Complex Components |
|---|---|---|
| DOR and Kir3.1 subunits | Kir3.1-Luc/DOR-YFP | GαoAβ1γ2 + Kir3.2 |
| DOR and Kir3.1 subunits | DOR-Luc/Kir3.1-GFP | GαoAβ1γ2 + Kir3.2 |
| DOR and Kir3.2 subunits | Kir3.2-Luc/DOR-YFP | GαoAβ1γ2 + Kir3.2 |
| DOR and Gβγ dimer | DOR-Luc/YFP-Gγ2 | GαoAβ1 + Kir3.1 + Kir3.2 |
| DOR and GαoA subunits | GαoA99-Luc/DOR-YFP | Gβ1g2 + Kir3.1 + Kir3.2 |
| Gβγ dimer and GαoA subunits | GαoA99-Luc/Gγ2-YFP | DOR + Gβ1 + Kir3.1+ Kir3.2 |
| Gβγ dimer and Kir3.2 subunits | Kir3.2-Luc/Gγ2-YFP | DOR + GαoAβ1 + Kir3.2 |
| Gβγ dimer and Kir3.1 subunits | Kir3.1-Luc/Gγ2-YFP | DOR + GαoAβ1 + Kir3.2 |
| GαoA and Kir3.1 subunits | GαoA99-Luc/Kir3.1-YFP | DOR + Gβ1 + Kir3.1+ Kir3.2 |
BRET, bioluminescence resonance energy transfer; DOR, δ-opioid receptor; GFP, green fluorescent protein; YFP, yellow fluorescent protein.
BRET Experiments.
Titration BRET assays were performed as previously described (Audet and Pineyro, 2011) to determine the relative amount of DNA constructs necessary to achieve a maximal BRET signal. Briefly, a fixed amount of donor-tagged proteins was coexpressed with increasing amounts of the vector coding for the acceptor. Untagged complementary signaling partners were also included at levels that would support membrane expression of the complete signaling complex at all points of the titration curve. Donor-acceptor DNA ratios corresponding to the beginning of the saturation plateau were subsequently used for single-point assays. Two days after transfection, HEK293 cells expressing different BRET pairs were washed with phosphate-buffered solution (PBS) and distributed into 96-well microplates (white Optiplate; PerkinElmer Life Sciences, Boston, MA) at a concentration of 1–2 mg/ml. Cells were then incubated for 3 minutes with coelanterazine h (1 µg/ml) before the addition of the indicated ligands (10 µM), which in turn were introduced 2 minutes before taking BRET1 readings. The latter were obtained using a Victor3 plate reader (PerkinElmer Life Sciences), which allows the sequential integration of signals detected in the 440- to 480-nm (RLuc emission) and 520- to 550-nm (YFP emission) windows using filters with the appropriate band pass (Chroma). The BRET signal generated by each sample was determined by calculating the ratio of light emitted by YFP over the light emitted by Rluc. These values were then corrected by subtracting the background signal (detected when the Rluc-tagged construct was expressed without acceptor) from the BRET ratio detected in cells coexpressing both Rluc and YFP (netBRET). For kinetic assays, cells were suspended and transferred to 96-well plates, after which BRET was measured using a Mithras LB940 plate reader (Berthold Technologies, Bad Wildbad, Germany) equipped with a microinjector that allowed automatic delivery of coelanterazine h and SNC-80 (10 µM). The agonist was injected 5 minutes after the RLuc substrate, and BRET1 measures were obtained at room temperature, every 0.8 seconds for 6 minutes. Corrected netBRET1 values were obtained as described. Finally, for the calculation of a Z’ factor describing the reliability of the Kir3.1-RLuc/YFP-Gγ2 as a screening biosensor, the construct and complementary complex components were transfected onto cells plated in 100-mm dishes (4 million) using Lipofectamine (Life Technologies, Carlsbad, CA). The day after the transfection, the cells were transferred onto 96-well plates (150,000 cells/well), and on the following day, readings were taken on attached cells (to emulate the conditions used in screening campaigns). The experiment cells were exposed to coelanterazine h 5 minutes before the addition of SNC-80 (10 µM) or vehicle (dimethylsulfoxide). Immediately after these injections, 48 alternate readings were obtained for each condition using the Mithras LB940 plate reader. Because filters in the different plate readers were not the same, net BRET values obtained for Z’ calculations were different from those obtained with the Victor3 plate reader. The Z’ factors for these assay conditions were calculated from the following formula: 1 – (3σligand + 3σCTL) /│µligand - µCTL│, as described by Zhang et al. (1999).
Immunoprecipitation Assays.
Immunoprecipitation assays were carried out as previously described (Audet et al., 2008). Two days after transfection, HEK293 cells were exposed to DPDPE (10 µM; 2 minutes) or vehicle and, at the end of incubation, immediately used for membrane preparation. Membranes were prepared by suspending cells in lysis buffer (5 mM Tris pH 7,4, 3 mM MgCl2, 2 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 5 μg/ml leupeptin, 5 μg/ml soybean trypsin inhibitor, and 10 μg/ml benzamidine) and homogenizing them in an Ultra-turrax homogenizer (IKA, Wilmington, NC). After centrifugation at 500 × g to pellet mitochondria and nuclei, the supernatant containing membranes and cytosol was recovered and centrifuged at 30,000 × g for 20 minutes. The resulting membrane pellet was resuspended in lysis buffer for a second round of centrifugation (30,000 × g; 20 minutes). Finally, membranes were suspended in solubilization buffer (0.5% n-dodecyl-maltoside, 25 mM Tris pH 7.4, 140 mM NaCl, 2 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 5 μg/ml leupeptin, 5 μg/ml soybean trypsin inhibitor, and 10 μg/ml benzamidine) and agitated at 4°C for 2 hours, after which the solubilized material was centrifuged at 20,000 × g for 30 minutes. Flag-DORs or extracellularly tagged FLAG-Kir3 subunits were immunoprecipitated from the supernatant using anti-FLAG M2 antibody resin. Briefly, 40 µl of antibody-coupled resin equilibrated in solubilization buffer and supplemented with 0.1% bovine serum albumin (BSA; w/v) was added to 500 µl of solubilized membranes and incubated overnight at 4°C under gentle agitation. The next morning, the resin was centrifuged, washed twice with 500 µl of solubilization buffer, and washed four times with 500 µl of modified solubilization buffer [containing 0.1% instead of 0.5% n-dodecyl-maltoside (w/v)]. Flag-tagged proteins were then eluted by incubating the resin for 10 min at 4°C with 100 µl of modified solubilization buffer containing the FLAG peptide (150 µg/ml). This elution was repeated three times, and the eluates were combined and concentrated by membrane filtration over Microcon-30 concentrators (Millipore, Billerica, MA). SDS sample buffer was then added, and samples were used for SDS-PAGE. Resulting gels were transferred onto nitrocellulose (GE Healthcare, Pittsburgh, PA) and Kir3.1-Luc or YFP-Gγ2, respectively, recovered with Flag-DORs, or extracellularly tagged FLAG-Kir3. Subunits were revealed using mouse anti-Luc (1:1,000 Millipore) or rabbit anti-green fluorescent protein (1:10,000; Abcam) antibodies, followed by corresponding secondary horseradish peroxidase-conjugated antibodies (1:40,000; Amersham Biosciences, Piscataway, NJ). Chemiluminescence detection reagents (GE Healthcare) were used to reveal blotted proteins.
Immunofluorescence and Confocal Microscopy.
Forty-eight hours after transfection, HEK293 cells were seeded on L-polylysine-coated coverslips and grown as described already. Labeling of surface Flag-DORs was done by a “feeding technique” in which rabbit anti-Flag M2 antibody (1:100; Sigma) was introduced into the incubation medium for 30 minutes. At the end of this incubation period, cells were washed in PBS and fixed with 3% paraformaldehyde (15 minutes, at room temperature) followed by permeabilization with 0.1% Triton X-100 (15 minutes). Cells were then washed three times in PBS-1% BSA and incubated with mouse anti-Luc (1:100; Millipore) to label different Kir3.1-RLuc constructs. Corresponding fluorescence-conjugated anti-rabbit antibody Alexa 467 or anti-mouse antibody Alexa 488 (1:1000) was then introduced for 30 minutes. At the end of incubation, cells were extensively washed and coverslips were mounted onto slides using mounting medium (Immu-Mount; Fisher). Immunofluorescence microscopy was performed using a Leica DM550 Q microscope. Images were analyzed using ImageJ software.
[35S]GTPgS Binding Assays.
[35S]GTPγS binding assays were carried out on membrane preparations as previously described (Pineyro et al., 2001). Membranes were prepared as already described and immediately resuspended in [35S]GTPγS assay buffer (50 mM Hepes, 200 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT, 0.5% BSA, and 3 μM GDP; pH 7.4) to yield 10 μg protein per tube. [35S]GTPγS was used at 50 nM, and nonspecific binding was determined in the presence of 100 μM cold GTP. Ligands were introduced at a final concentration of 100 nM, and incubation was allowed to proceed for 1 hour at room temperature. The reaction was terminated by rapid filtration onto Whatman GF/C glass filters presoaked in water. Filters were washed twice with ice-cold wash buffer (pH 7) containing 50 mM Tris, 5 mM MgCl2, and 50 mM NaCl; the amount of radioactivity retained was determined by liquid scintillation.
Electrophysiological Experiments.
Twenty-four hours after transfection, the cells were trypsinized and resuspended in DMEM before plating on poly-d-lysine–coated 35-mm culture dishes. The following day, the cells were used for electrophysiological recordings. On the day when the experiments were conducted, culture media were replaced with high-potassium solution (HK+ external, 5 mM NaCl,140 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, 10 mM glucose pH 7.4, 300 mOsmol/l). The plate was placed on the microscope stage, and the cells were perfused with HK external by a large-volume, gravity-fed perfusion system until seal formation began. Patch pipettes were pulled from borosilicate glass, and when filled with a HK ATP/GTP-containing solution (140 mM KCl, 20 mM NaCl, 5 mM EGTA, 5.4 mM MgCl2, 10 mM HEPES, 2.5 mM K2ATP, 0.3 mM Li2GTP), they had an internal resistance between 2 and 5 MΩ. Cells were patched in the whole-cell configuration and voltage clamped with 85% compensation for series resistance using an Axopatch 200B and ensuring that cell capacitance was <30 pF. Cells were then subjected to a ramp protocol, which changes the membrane potential from −100 mV to +50 mV at a rate of 1 mV/ms using Clampex 10.2 software. The ramping protocol was then repeated every 250 milliseconds for 75 seconds. Agonists were suspended in HK external (1 µM final) and applied to the cell by a small-volume gravity-fed perfusion system directly next to the patched cell. Once peak current was observed, agonists were washed out of the bath by perfusing HK external from a large-volume gravity-fed perfusion system. Trace data were presented by plotting the current at −100 mV during the ramp protocol against time using Clampfit 10.2. Data were collected with a low-pass Bessel filter at 5 kHz and digitized with a Digidata 1440A.
Results
Constitutive Association of DORs, Gβ1γ2, and Kir3.1/3.2 Subunits.
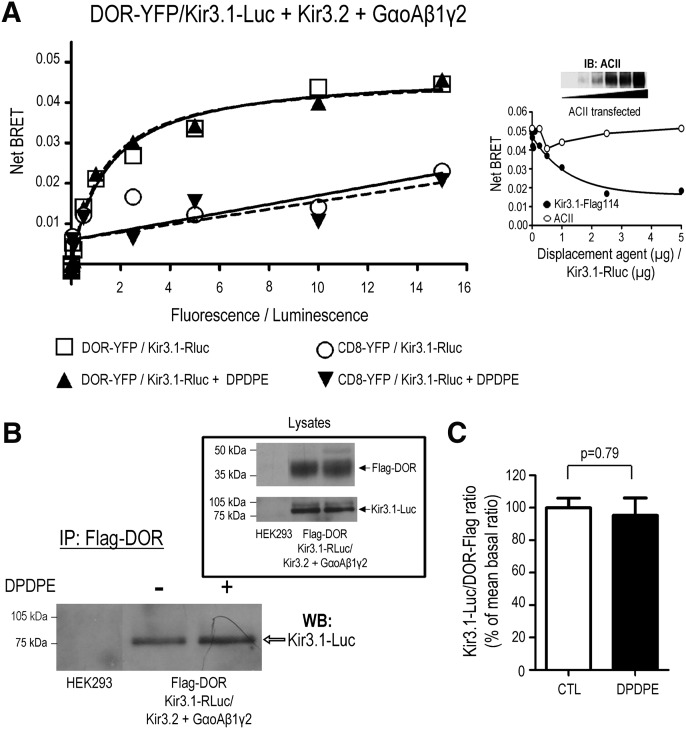
Kir3.1/3.2 subunits form the most abundant type of neuronal Kir3 channel and mediate the actions of numerous inhibitory neurotransmitters, including GABA, dopamine, and opioids (Luscher and Slesinger, 2010). In the particular case of opioids, Kir3.1/3.2 channels are essential for the analgesic actions of these drugs (Marker et al., 2002). Within this context, we were interested in determining how agonist binding to DOR results in Kir3.1/3.2 channel activation. We had previously shown that Kir3.1/3.2 channels interact with GABA-B (David et al., 2006) and dopamine D2 receptors in HEK293 cells and native tissue (Lavine et al., 2002) and had demonstrated that Kir3 subunits (David et al., 2006; Rebois et al., 2006) and GPCRs (Gales et al., 2005), including DORs (Audet et al., 2008), both constitutively and specifically associate to the Gβγ dimer. To corroborate whether DORs were able to constitutively associate with Kir3.1/3.2 channels, we used BRET and coimmunoprecipitation assays, two approaches that we have previously applied to monitor protein interactions within multimeric signaling complexes ( David et al., 2006; Rebois et al., 2006; Audet et al., 2008). The association between DORs and Kir3.1/3.2 channels was first assessed by generating BRET titration curves in which a fixed amount of the donor Kir3.1-Rluc was cotransfected with increasing concentrations of the acceptor DOR-YFP, as well as with all relevant complementary complex components indicated in Fig. 1A. As shown therein, cumulative amounts of DOR-YFP progressively increased energy transfer until reaching a plateau, indicating the existence of a spontaneous and specific interaction between Kir3.1-Luc and DOR-YFP (Mercier et al., 2002). Indeed, cotransfection of Kir3.1-Luc subunits with a CD8-YFP construct that has similar distribution as the receptor, but does not interact with the channel or G proteins (Gales et al., 2005), produced a marginal energy transfer that did not follow saturation kinetics. The specificity of DOR interaction with the channel effector was also established in displacement assays where increasing amounts of untagged Kir3.1 subunits, but not adenylyl cyclase II (ACII), competed for the BRET signal generated by the Kir3.1-Luc/DOR-YFP pair. Moreover, the failure of ACII to compete for the Kir3.1-Luc/DOR-YFP BRET signal was not due to inadequate expression, as indicated by the presence of increasing amounts of ACII immunoreactivity in progressive transfections (Fig. 1A, inset).
Fig. 1.
Kir3.1 subunits constitutively and specifically associate with DORs. (A) Titration assays were performed in HEK293 cells transfected with increasing concentrations of DOR-YFP or CD8-YFP and a fixed amount of Kir3.1-Luc, together with untagged Kir3.2 channel subunits and the Gαoβ1γ2 heterotrimer. Results correspond to a representative example of three independent experiments carried out in duplicate (means of duplicates are shown). BRET measures were taken in the absence or presence of DPDPE (10 µM; 2 min). Inset: Competition assay in which a fixed amount of the Kir3.1-Luc/DOR-YFP pair was coexpressed with the same complementary signaling partners as indicated previously, plus increasing amounts of Kir3.1-Flag or ACII. Data correspond to the means obtained in one experiment carried out in triplicate (means of triplicates are shown). Western blot shows expression of ACII at increasing levels of transfection. (B) HEK293 cells were transiently transfected to express the indicated proteins and exposed or not to DPDPE (10 µM, 2 min). At the end of treatment, cells were washed and lysed, and Flag-DORs were immunopurified. Resulting products were separated by SDS-PAGE, and Kir3.1-Luc subunits recovered with the receptor were revealed by immunoblot. Inset shows proteins present in cellular lysates before immunopurification. (C) Histograms show results obtained by compiling densitometric measures of four coimmunoprecipitation experiments. Data were normalized to the mean of the Kir3.1-Luc/Flag-DOR ratio observed in nonstimulated controls. Statistical comparison of immunoreactivity ratios was done by paired Student’s t test, and results are shown on the figure.
DOR stimulation with the agonist DPDPE (10 µM, 2 minutes) did not modify the titration curve generated for the Kir3.1-Luc/DOR-YFP pair. Comparison of titration curves by simultaneous fitting (Berchiche et al., 2011) indicated that the addition of agonist did not alter BRET50 (CTL (cytotoxic T lymphocyes): 1.5 ± 0.4, DPDPE: 1.3 ± 0.3, P = 0.78, n = 3) or BMAX (CTL: 0.048 ± 0.004, DPDPE: 0.047 ± 0.003, P = 0.85, n = 3) values. This observation is consistent with the notion that neither the total amount of receptor associating with the channel nor the way in which both proteins interact with one another was modified by receptor activation. The spontaneous interaction between DORs and Kir3 subunits was then corroborated in coimmunoprecipitation assays carried out in HEK293 cells coexpressing Flag-DOR, heterotrimeric Gαoβ1γ2 subunits, and Kir3.1-Luc/3.2 channel subunits. In keeping with the constitutive interaction observed in living cells using BRET, immunoprecipitation of the receptor resulted in recovery of Kir3 channel subunits. Also consistent with BRET assays, the amount of channel subunits copurified with the receptor was not modified by DOR stimulation with the agonist DPDPE (Fig. 1B).
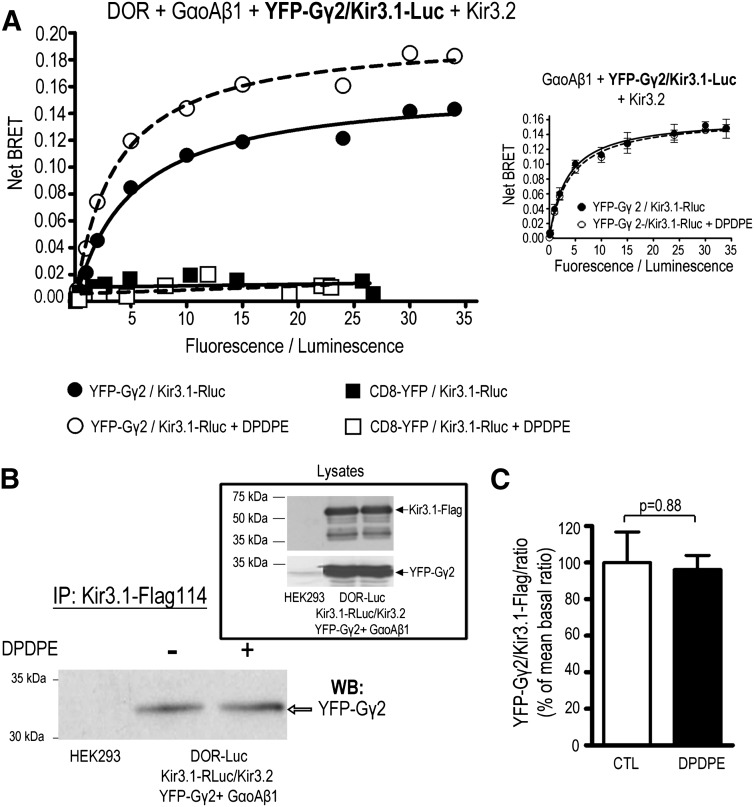
A second series of BRET assays also revealed a constitutive and specific transfer of resonance energy in the Kir3.1-Luc/YFP-Gγ2 pair (Fig. 2A), corroborating the established ability of Kir3.1/3.2 channels to associate with Gβγ subunits (Rebois et al., 2006; Riven et al., 2006; Rubinstein et al., 2009). This interaction could be observed both in cells that expressed DOR (Fig. 2A) and in those that did not (inset), but only DOR-expressing cells displayed a BRET signal that was sensitive to receptor stimulation by DPDPE (10 µM, 2 min). Simultaneous curve fitting revealed that in cells expressing the receptor, DPDPE significantly increased BMAX (CTL: 0.16 ± 0.01, DPDPE: 0.20 ± 0.01, P < 0.01, n = 3) without modification of BRET50 values (CTL: 5.1 ± 1.2, DPDPE: 3.6 ± 0.6, P = 0.23, n = ). In contrast, the nonspecific interaction between Kir3.1-Luc and CD8-YFP did not respond to agonist. Immunoprecipitation experiments showing that Gγ2 subunits could be coimmunopurified with Kir3.1 subunits corroborated the constitutive association of the channel and the Gβγ dimer (Fig. 2B). On the other hand, pre-exposure to DPDPE failed to modify the amount of Kir3.1-Gγ2 complex recovered. Thus, taken together, the data characterizing Kir3/Gβγ subunits interaction are consistent with a scenario in which receptor activation does not necessarily change the total amount of complexes that are present in the cell but imposes conformational changes at the Gβγ-Kir3.1 interface.
Fig. 2.
Kir3.1 subunits constitutively and specifically associate with the obligatory Gβγ dimer. (A) Titration assays to monitor the in cellulo interaction between Kir3.1 and the Gβ1γ2 dimer were performed by transfecting cells with the Kir3.1-Luc donor and increasing amounts of the acceptor YFP-Gγ2 together with DOR, Kir3.2, and GαoAβ1 subunits. Results correspond to a representative example of three independent experiments carried out in duplicate (means of duplicates are shown), in absence or presence of DPDPE (10 µM; 2 min). Inset shows the lack of any effect of DPDPE in cells that were transfected in the same way as described expect for the absence of DOR. Data correspond to the means obtained in one of two independent experiments carried out in triplicate. (B) HEK293 cells were transiently transfected to express the indicated proteins and treated or not with DPDPE (10 µM, 2 min) as indicated. Kir3.1-Flag subunits were immunopurified, and the resulting products were separated by SDS-PAGE. YFP-Gγ2 purified with channel subunits was revealed by Western blot. Inset shows proteins present in cellular lysates before immunopurification. (C) Histograms show the results obtained by compiling densitometric measures of three independent experiments. Data were normalized to the mean of the YFP-Gγ2/Flag-DOR ratio observed in nonstimulated cells. Statistical comparison of immunoreactivity ratios was done by paired Student’s t test, and results are shown on the figure.
Activation of DORs Does Not Modify Their Interaction with the Channel but Evokes Conformational Rearrangements at the Receptor Gbg Interface.
Results from the previous section indicate that DOR, Gβγ, and Kir3 channel subunits associate in a constitutive complex whose components remain together despite conformational changes that occur during the early stages of signal transduction. To characterize more fully how receptor activation–modified interactions among these different complex components, we used a range of DOR ligands and assessed BRET changes from a variety of conformational vantage points within the signaling complex.
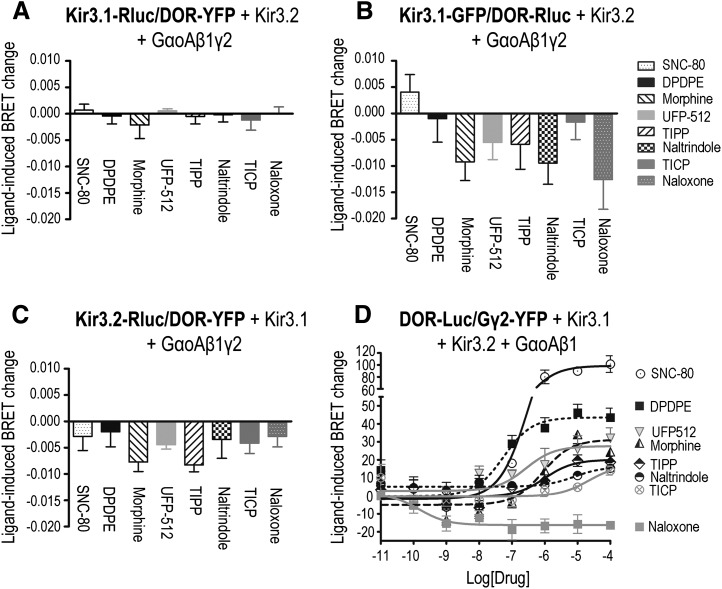
In a first series of experiments, we assessed whether ligands other than DPDPE could modify BRET at the Kir3.1-Luc/DOR-YFP BRET pair. These included drugs classified as full agonists (SNC-80), partial agonists (morphine, UFP512, and TIPP), inverse agonists (TICPs), and neutral antagonists (naloxone and naltrindole) for DOR (Schiller et al., 1999; Pineyro et al., 2005; Vergura et al., 2008). As shown in Fig. 3A, none of the ligands tested significantly modified the basal BRET signal generated by this BRET pair. To rule out that the absence of response might have been caused by the location of BRET tags, experiments were repeated using a pair in which donor and acceptor positions were switched (DOR-Luc/Kir3.1-green fluorescent protein; Fig. 3B) and another pair in which the donor was placed at the C-terminal end of the Kir3.2 subunit (Kir3.2-Luc/DOR-YFP; Fig. 3C). Except for TIPP (Fig. 3C), ligand-induced BRET changes did not attain significance and were not efficacy related, implying that ligand-encoded conformational information is not transferred to the channel via this interaction.
Fig. 3.
DOR stimulation did not modify receptor interaction with Kir3.1/3.2 channel subunits but induced conformational rearrangements between receptor and the Gβ1γ2 dimer. HEK293 cells were transfected with the indicated BRET pairs together with untagged signaling partners to assess DOR interaction with Kir3 (A–C) or Gγ2 (D) subunits. (A–C) BRET measures were obtained in the presence or absence of indicated DOR ligands (10 µM; 2 min), and results were expressed as the difference between these readings, corresponding to mean ± S.E.M. of three to seven independent experiments. Statistical comparisons for each BRET pair were carried out on net BRET values using repeated measures one-way analysis of variance, followed by Bonferroni posthoc test for multiple comparisons. * P < 0.05 indicates significance compared with untreated controls. Spontaneous net BRET for the Kir3.1-Rluc/DOR-YFP pair: 0.042 ± 0.002 (n = 3). Spontaneous net BRET for the DOR-Rluc/Kir3.1-GFP (green fluorescent protein) pair: 0.032 ± 0.002 (n = 5). Spontaneous net BRET for the Kir3.2-Rluc/DOR-YFP pair: 0.087 ± 0.003 (n = 7). (D) Drug ability to modify DOR-Luc/YFP-Rluc interaction was assessed in dose-response curves. Results were normalized to the maximal effect observed for SNC-80 and correspond to mean ± S.E.M. of four experiments. Curves were compared by two-way analysis of variance (ANOVA), which revealed an effect of drug (P < 0.0001) and concentration (P < 0.0001) as well as an interaction (P < 0.0001). Subsequent comparison by simultaneous curve fitting yielded the rank order of Emax values detailed in the text. Spontaneous net BRET for the DOR-Luc/YFP-Rluc pair: 0.106 ± 0.013 (n = 4).
Since three different BRET pairs failed to reveal direct transfer of conformational information between the receptor and the channel, we sought alternative sources for information transfer. We previously established that DOR activation modified the interaction of the receptor C-terminus with Gα and βγ subunits in the absence of channel effectors (Audet et al., 2008). To determine whether this was also the case when Kir3 channels were present, we assessed whether the same DOR ligands that were without effect at the DOR-channel interface could modify the transfer of energy between DOR and Gβγ subunits. To do so, cells were transfected with a BRET pair constituted by DOR-Luc/YFP-Gγ2, together with the Kir3.1/Kir3.2 and complementary GαoA and Gβ1 subunits. As shown in Fig. 3D, DOR activation (2 min) by different ligands induced a concentration-dependent increase in energy transfer. Simultaneous fitting established that the dose-response curve generated by SNC-80 resulted in larger maximal effect (Emax) than that generated by DPDPE (P < 0.0001; n = 4). Further, Emax values for both these agonists were larger than those observed for UFP512 and morphine (P < 0.05; n = 4). In turn, the Emax for morphine was not different from Emax values for TIPP, naltrindole, and TICP, whereas the Emax for TICP was higher than that determined for naloxone (P < 0.05; n = 4). Thus, the resulting rank order of magnitude for BRET changes observed at the DOR-Luc/YFP-Gγ2 pair was as follows: SNC-80 > DPDPE > UFP512 = morphine ≥ TIPP ≥ naltrindole = TICP > naloxone.
Ligand-Induced Conformational Rearrangements at the DOR-Gbg Interface are Correlated with Those Observed at a BRET Pair Assessing Gbg Interaction with Channel Subunits.
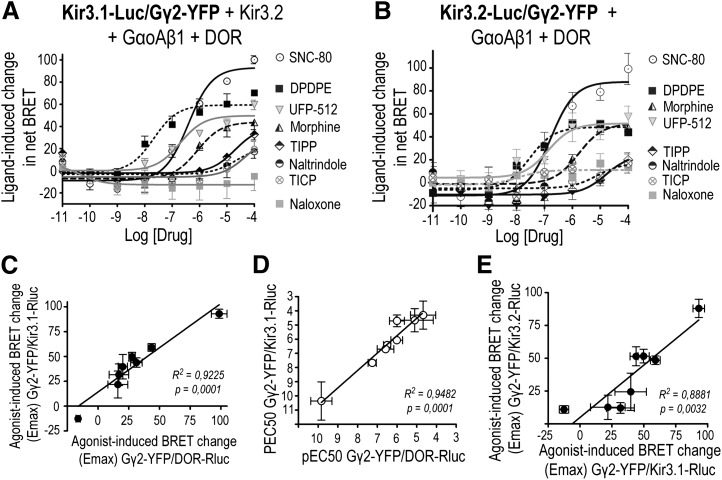
DOR activation modified the relative positioning of the receptor C-terminus with respect to Gγ2 but not with respect to Kir3 subunits, suggesting that the Gβγ dimer could be the mobile species among all three interacting partners. Therefore, we next examined whether and how DOR activation modified Gβγ interaction with other complex components. We first monitored Gβγ movement from two different vantage points within the cytosolic portion of the channel: the C-termini of Kir3.1 and Kir3.2 subunits. Figure 4A shows that DOR ligands produced a concentration-related increase in the BRET signal generated by the Kir3.1-Luc/YFP-Gγ2 pair, indicating that the free end of the Gβγ dimer was drawn closer to the C-terminus of these Kir3 subunits. Comparison of Emax values from the different dose-response curves revealed the following rank order: SNC-80 > DPDPE ≥ UFP512 ≥ morphine ≥ TIPP ≥ naltrindole = TICP > naloxone (SNC-80 versus DPDPE P < 0.0001; DPDPE versus morphine P < 0.05; TICP versus naloxone P = 0.05; n = 4). DOR activation by different agonists also induced concentration-dependent BRET changes at the Kir3.2-Luc/YFP-Gγ2 interface (Fig. 4B), with the magnitude of Emax values displaying the following rank order: SNC-80 > DPDPE = morphine = UFP512 ≥ TIPP = naltrindole ≥ TICP = naloxone (SNC-80 versus DPDPE P < 0.0001; DPDPE versus naloxone P < 0.05; n = 3). Ligand-dependent BRET changes at both interfaces were correlated (Fig. 4C), which is consistent with the notion that Gβγ repositioning with respect to either channel subunit corresponds to similar phenomena. The idea that the Gβγ dimer was the mobile species contributing to BRET changes at its interaction with the receptor and channel subunits is further supported by two observations: ligand potency to produce conformational changes at the Gβγ/DOR and Gβγ/Kir3 interfaces were correlated (Fig. 4D), as were Emax values for BRET changes observed at both interfaces (Fig. 4E).
Fig. 4.
DOR stimulation induces BRET changes between Gβγ and channel subunits. HEK293 cells were transfected with the indicated BRET pairs and untagged signaling partners to evaluate Gβγ interactions with Kir3.1 (A) or Kir3.2 (B) channel subunits. BRET measures were taken in the absence or presence of increasing concentrations of indicated DOR ligands, and ligand-induced BRET changes were normalized to the maximal effect observed for SNC-80. Results correspond to mean ± S.E.M. of three or four experiments. Curves generated by different ligands were compared by two-way analysis of variance, revealing an effect of drug (P < 0.0001), concentration (P < 0.0001) and an interaction (P < 0.0001) for each of the interactions. Subsequent comparison by simultaneous curve fitting yielded rank order of Emax values as detailed in text. (C) Correlation of Emax values for ligand-induced BRET changes at Kir3.1-Rluc/YFP-Gγ2 versus Kir3.2-Rluc/YFP-Gγ2. Correlation of EC50 (D) and Emax (E) values for ligand-induced BRET changes at pairs assessing Gβγ interaction with DOR (DOR-Luc/Gγ2-YFP) and Kir3.1 subunits (Kir3.1-Luc/Gγ2-YFP).
Agonist-Evoked Conformational Rearrangements at the Gbg/Channel Interface Require the Proximal Portion of the C-Terminal Domain of Kir3.1 Subunits.
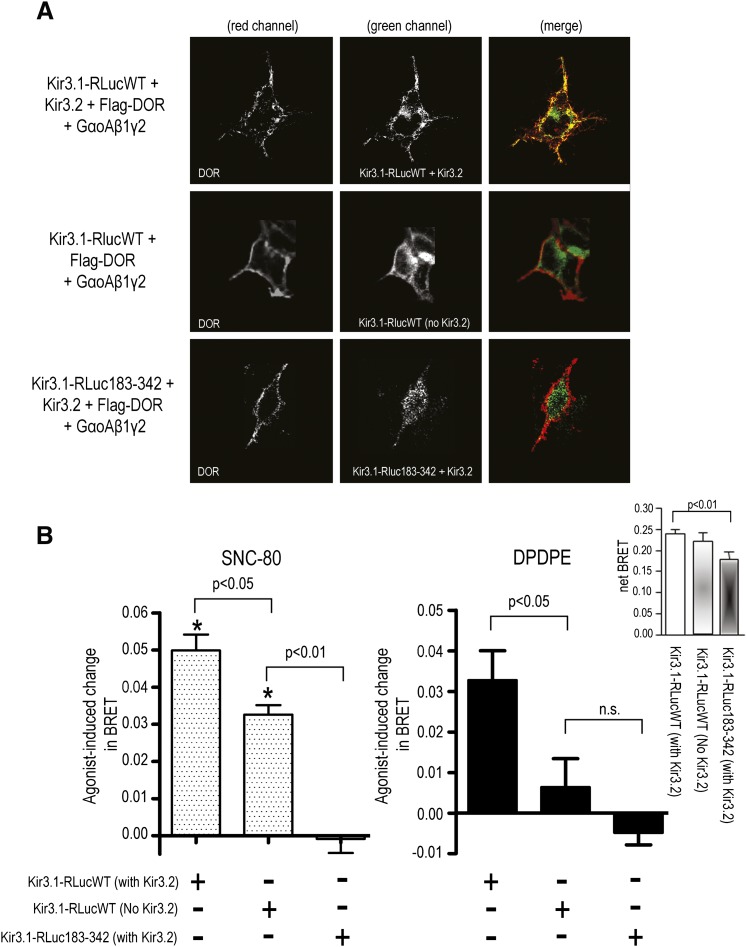
Gβγ binding sites have been mapped to proximal and distal portions of the C-terminal end of Kir3 subunits (Huang et al., 1997; Ivanina et al., 2003). Whereas the most distal sites are unique to Kir3.1 subunits (Huang et al., 1997), more proximal residues within βD-βE and βL-βM strands have been identified as critical for Gβγ-mediated activation of different Kir3 subunits, including Kir3.1 (He et al., 1999, 2002). To determine the relative contribution of proximal C-terminal regions to the dynamics of DOR-mediated channel activation, we generated Kir3.1 donor BRET constructs lacking βD-βE and βL-βM strands together with connecting residues (Kir3.1-RLuc183-342). Membrane expression of wild-type and mutant constructs was first assessed as a prelude to their use in BRET experiments. To do so, wild-type and mutated Kir3.1 BRET donors were cotransfected with DORs bearing the Flag epitope at the N-terminus (Flag-DOR), as well as with GαoAβ1γ2 and Kir3.2 subunits. Receptors present at the cell surface were labeled by antibody feeding of live cells, and donor Kir3.1-Luc constructs were subsequently labeled in vitro by permeabilizing the cells and exposing them to anti-Luc antibody. Figure 5A shows that when coexpressed with Kir3.2 subunits, Kir3.1-Lucwt constructs colocalized with membrane DOR. However, expression of Kir3.1-Lucwt constructs in the absence of Kir3.2 subunits resulted in intracellular retention of Kir3.1-Lucwt. Kir3.1-RLuc183-342 constructs were similarly retained within the intracellular compartment, despite coexpression with Kir3.2 subunits.
Fig. 5.
Agonist-evoked conformational rearrangements at the Gβγ-channel interface require the proximal portion of the C-terminal domain of Kir3.1 subunits. (A) HEK293 cells were transfected with wild-type or mutant Kir3.1-Luc constructs together with Flag-DORs and GαoAβ1γ2 subunits. Kir3.2 subunits were included or not in the transfection as indicated. On the day of the experiment, cells were incubated in vivo (30 min) with primary antibody to label surface Flag-DOR, after which they were washed, fixed, and permeabilized before being exposed to anti-Luc antibody to label Kir3.1-Luc constructs. Immunoreactivity was then revealed using secondary antibodies conjugated either to Alexa467 (red channel; Flag-DOR) or Alexa488 (green channel; Kir3.1-Luc). (B) HEK293 cells were transfected with the indicated Kir3 subunits together with YFP-Gγ2, GαoAβ1, and DORs. On the day of the experiment, cells were exposed to vehicle, SNC-80 (left panel), or DPDPE (right panel) (2 min; 10 µM). Results are expressed as the difference between BRET measures obtained in the presence and absence of agonists and correspond to mean ± S.E.M. of five to eight experiments. Statistical analyses for SNC-80 (left panel) effects on Kir3.1-LucWT/YFP-Gγ2 interaction were done on net BRET values by means of two-way ANOVA. Comparisons among cells expressing or not expressing Kir3.2 subunits showed an effect of drug (P < 0.05) but no effect for the presence or absence of Kir3.2 (P = 0.6178; n = 5). Analysis of net BRET values for the effect of SNC-80 on Kir3.1-LucWT versus Kir3.1-RLuc183-342 constructs showed an effect of mutation (P < 0.0001; n = 7). Comparison of net BRET values for experiments carried out in the presence or absence of DPDPE (right panel) revealed an effect of Kir3.2 (P < 0.01; n = 7), and analysis of net BRET values for Kir3.1-LucWT versus Kir3.1-RLuc183-342 constructs also showed an effect of mutation for this ligand (P < 0.0001; n = 7). BRET changes induced by the two agonists across different Kir3 constructs were compared by one-way ANOVA with Bonferroni correction, and results are shown on the figure. Inset shows basal net BRET values in cells transfected with different Kir3.1/3.2 combinations. Results correspond to mean ± S.E.M. of nonstimulated values from experiments described previously (n = 7-13). Statistical comparisons were done using one-way ANOVA followed by Dunnet posthoc test.
Different Kir3.1-Luc constructs were then coexpressed together with YFP-Gγ2 and the corresponding signaling partners to assess whether the mutation disrupted channel/Gβγ interaction. The inset in Fig. 5B shows constitutive BRET generated by Kir3.1-Lucwt in the presence and absence of Kir3.2 subunits. Despite the absence of Kir3.2 subunits, basal BRET generated by Gβγ and intracellular Kir3.1-Lucwt subunits was not different from that measured in Kir3.1-Lucwt/Kir3.2 channels that were adequately distributed. Consistent with its intracellular location, Kir3.1-Lucwt interaction with Gβγ was insensitive to DOR activation by DPDPE (Fig. 5B, right panel), a hydrophilic, peptidic ligand that does not readily cross the cell membrane. In contrast, the hydrophobic, cell-permeable agonist SNC-80 evoked a significant increase in the basal BRET signal (Fig. 5B, left panel). Unlike intracellularly trapped wild-type constructs, the basal net BRET signal generated by the Kir3.1-RLuc183-342/YFP-Gγ2 pair was lower than that observed for the WT channel (Fig. 5B, inset), and, more importantly, it was not modified by exposure to SNC-80 (Fig. 5B, left panel). As already described, DPDPE was also without effect in cells expressing the cytosolic Kir3.1-RLuc183-342 mutant (Fig. 5B, right panel).
DOR-Evoked Changes at the Gbg/Kir3 Subunit Interface are Concomitant with Conformational Rearrangements Among GaoA/Gbg Subunits and Predictive of Ligand Ability to Induce Channel Currents.
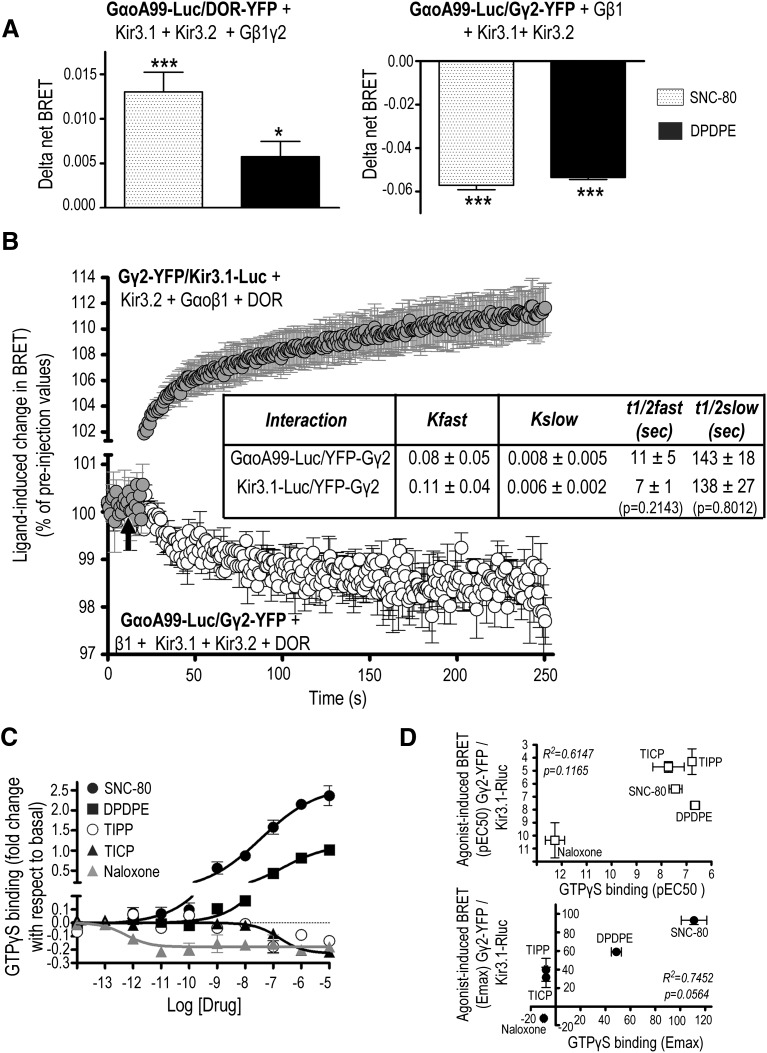
Gα subunits are thought to modulate channel activity both through direct interaction with Kir3 subunits and by making Gβγ readily available in the proximity of channel subunits (Peleg et al., 2002; Clancy et al., 2005; Rubinstein et al., 2009; Mase et al., 2012). We were therefore interested in characterizing whether DOR activation modified Gα interactions with different complex components. To address this question, we expressed the GαoA99-Luc donor together with any of the following acceptors: DOR-YFP, YFP-Gγ2, or Kir3.1-YFP, as well as the corresponding complementary signaling partners. We noted a specific (unpublished data), constitutive BRET signal for GαoA99-Luc/DOR-YFP (BRETMax: 0.086 ± 0.006, n = 5) and for GαoA99-Luc/YFP-Gγ2 (BRETMax: 0.135 ± 0.002, n = 5) but not for the GαoA99-Luc /Kir3.1-YFP pair.
In a second series of experiments, we assessed how two of the most efficacious agonists, SNC-80 and DPDPE, influenced GαoA interactions with other complex components. Neither of these agonists induced BRET at the GαoA/Kir3.1 interface, where there was none detected in the absence of ligand. However, BRET changes observed at GαoA/DOR and GαoA/Gβγ interfaces indicated that DOR activation caused loop αA-αB in the helical portion of GαoA subunits to approach the receptor C-terminus and separate from the free end of Gβγ (Fig. 6A). Moreover, BRET changes at the GαoA/Gβγ and Gβγ/Kir3.1 interfaces followed similar kinetics (Fig. 6B), indicating that the conformational changes that separated Gβγ from Gα were concomitant with those that brought it into closer proximity of the channel. Indeed, Gβγ separated from GαoA in a two-phase movement whose kinetics were not different from those observed for Gβγ approaching Kir3.1 channel subunits (see inset in Fig. 6B).
Fig. 6.
DOR-induced conformational changes between Gβγ and Kir3.1 channel subunits are associated with conformational rearrangements among GαoA/Gβγ subunits. (A) HEK293 cells were transfected with the indicated BRET pairs and untagged signaling partners to evaluate GαοA interaction with DOR and the Gβγ dimer. BRET measures were taken in the presence or absence of DPDPE or SNC-80 (10 µM; 2 min). Results were expressed as the difference between measures obtained in presence and the absence of ligand and correspond to mean ± S.E.M. of five experiments. Statistical comparisons for each BRET pair were carried out on net BRET values using repeated measures one-way ANOVA, followed by Dunnett posthoc test. * P < 0.05; *** P < 0.001 compared with untreated controls. (B) HEK293 cells were transfected as indicated, and BRET kinetics were obtained at room temperature using a Mitras plate reader with automatic injector. SNC-80 injection is indicated by a black arrow. Data are expressed as percent of preinjection values and correspond to mean ± S.E.M. of three experiments. Slow and fast t1/2 values for each interaction were compared by non-paired t test and appear in the inset. (C) HEK293 cells stably expressing Flag-DOR were exposed to the indicated ligands to determine [35S]GTPγS binding. Results are expressed as fold of change with respect to basal binding and correspond to mean ± S.E.M. of two or three experiments carried out in triplicate (naloxone n = 2; rest n = 3). Curves were compared by two-way ANOVA, revealing an effect of drug (P < 0.0001) and concentration (P < 0.0001) as well as an interaction (P < 0.0001). (D) Correlation of EC50 (above) and Emax (below) values for ligand-induced GTPγS binding and BRET changes at the pairs assessing Gβγ interaction with Kir3.1 subunits (Kir3.1-Luc/Gγ2-YFP).
Mutagenesis studies (Ford et al., 1998) and structural analyses (Yokogawa et al., 2011) have shown that Kir3 association with Gβγ subunits takes place at a surface that normally interacts with Gα subunits, known as the “hot spot.” For the hot spot to become available for effector interaction, Gα subunits must exchange GDP for GTP (Smrcka et al., 2008). In keeping with this mechanism, ligand-induced BRET changes at the pair monitoring Gβγ/Kir3.1 interaction were sensitive to pertussis toxin (DPDPE-induced BRET changes in CTL cells: 0,022 ± 0.005 and in cells pre-exposed to PTX: −0,002 ± 0,005; P < 0.01; n = 6, unpaired t test). Interestingly, and despite coordinated conformational rearrangements at the Gβγ interfaces with Gα and Kir3 subunits, we found no significant correlation between EC50 (Fig. 6C, upper right panel) and Emax (Fig. 6C, lower right panel) values obtained in GTPγS binding and in BRET assays assessing ligand-promoted conformational changes at the Gβγ/Kir3.1 interface. This dissociation between readouts of G-protein activation and Gβγ interaction with the channel implies the possibility of effector responses being determined by ligand-specific structural determinants as much as GDP/GTP exchange by the Gα subunit.
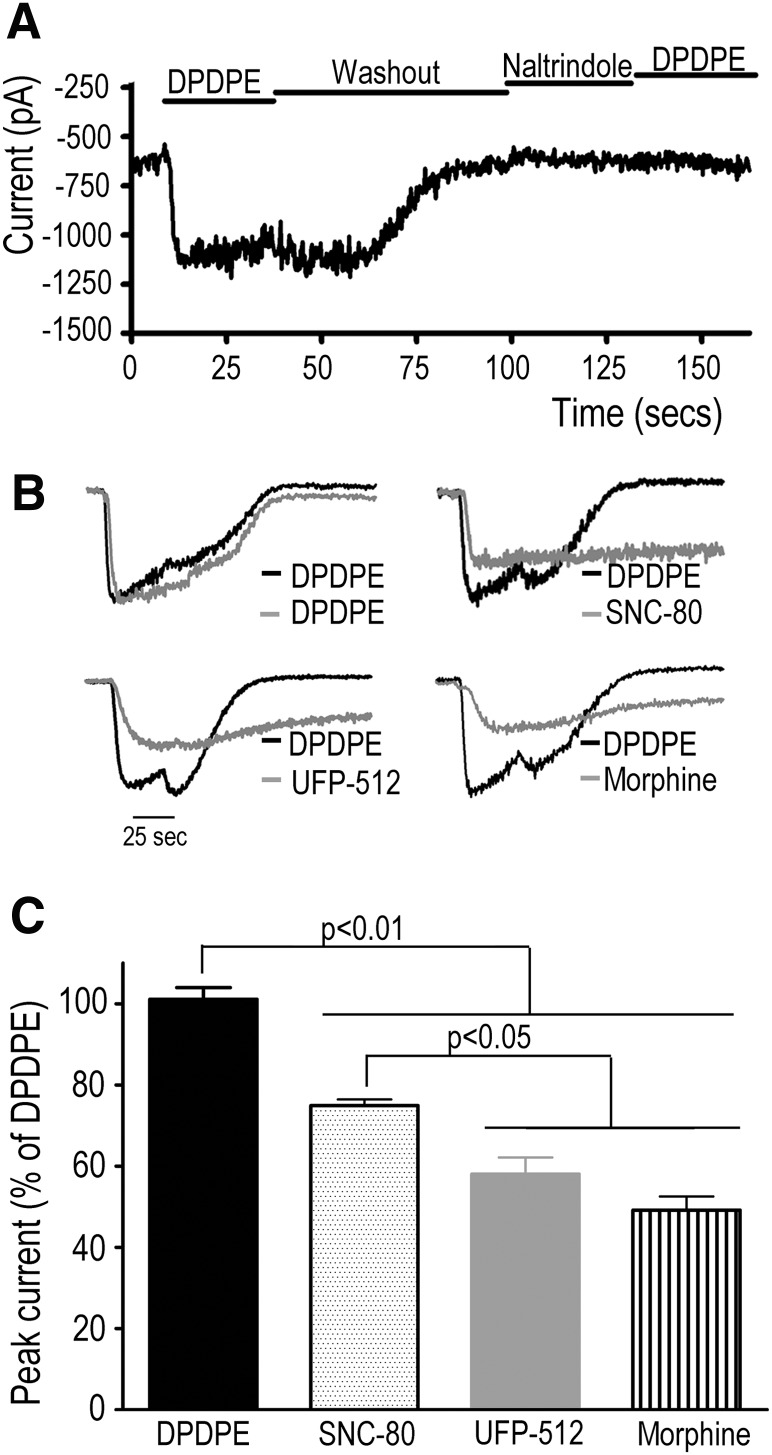
Finally, we assessed agonist ability to evoke peak channel currents. To do so, HEK293 cells expressing the Kir3.1-Luc/YFP-Gγ2 pair, DORs, and Kir3.2 subunits were studied using whole-cell patch clamp. Transfected cells displayed characteristic basal currents (1122 ± 213 pA; n = 9) in the presence of a high extracellular K+ concentration (140 mM). The stimulation of DOR by DPDPE induced an evoked current (−3593 ± 615pA; n = 16) that could be blocked by preincubation with the antagonist naltrindole (1 μM) and disappeared on agonist removal (Fig. 7A). Other agonists, like SNC-80, UFP512, and morphine (1 µM), also produced channel currents (Fig. 7, B and 7C). At this concentration, peak currents produced by DPDPE were larger than those induced by SNC-80, which in turn produced greater hyperpolarization than UFP512 and morphine. Although the magnitude of these currents was not correlated with Emax values of ligand-induced conformational changes, they are not incompatible with ligand-induced conformational changes observed at 1 µM concentration, where SNC-80 did not attain its maximal effect (Fig. 4, A and B). Independent of the latter, agonists that displayed the highest potency and efficacy to induce conformational changes at the Gβγ/Kir3.1 and Gβγ/Kir3.2 interfaces were also found to activate the channel, pointing to the predictive validity of the conformational changes captured by the Kir3/ Gγ2 biosensors as a rapid means for identifying ligands that activate Kir3 channels via GPCR activation.
Fig. 7.
Ligands that induce conformational rearrangements at the biosensors monitoring Gβγ interaction with channel subunits also induce whole-cell currents. HEK293 cells transfected with the Kir3.1-Luc/Gγ2-YFP pair DOR and Kir3.2 were studied using whole-cell patch-clamp voltage clamped at 100 mV. (A) The presence of a basal current was determined by incubating cells in a high K+ solution (140 mM). Addition of the agonist DPDPE (1 µM) evoked a current that returned to basal values on removal of the agonist. Preincubation with the antagonist naltrindole (1 µM) blocked currents induced by DPDPE. (B) Individual traces of whole-cell currents induced by different agonists. DPDPE was used to determine functional channel current in each cell, and traces for all agonists were normalized to the maximum current produced by DPDPE. (C) Agonist-evoked whole-cell currents (1 µM) were normalized to DPDPE, and results are expressed as percent of maximal response induced by this agonist. Values represent mean ± S.E.M. of three experiments. Statistical comparisons were done by one-way analysis of variance, followed by Bonferroni posthoc test for multiple comparisons.
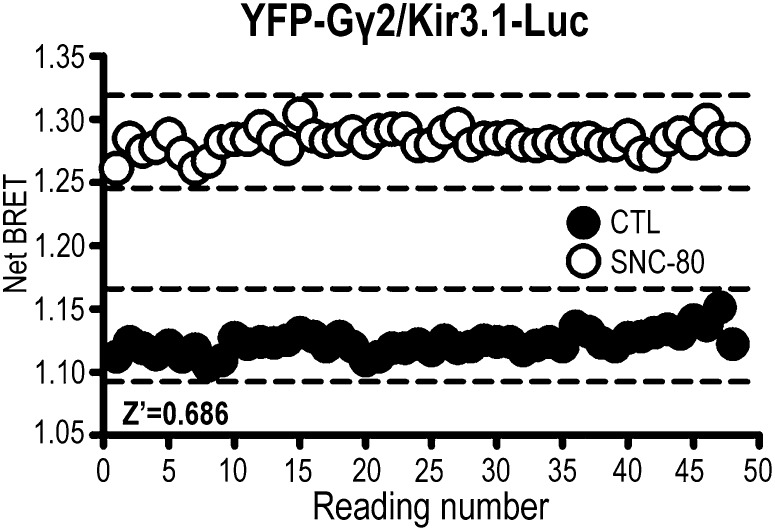
One of the prerequisites for establishing whether a biosensor can be reliably used in an assay with screening validity is its ability to discriminate confidently signal from background. The evaluation of the Kir3.1-Luc/YFP-Gγ2 BRET biosensor with respect to these parameters was done by means of a Z’ factor (Zhang et al., 1999). To obtain the latter, we monitored basal and agonist-induced netBRET readings over 3 min (Fig. 8). The Z’ factor was calculated as described in experimental procedures, yielding a value of 0.686.
Fig. 8.
The Kir3.1-Rluc/YFP-Gγ2 biosensor reliably discriminates signal from noise with a Z’ value of 0.686. HEK293 cells were transfected Kir3.1-Luc subunits together with YFP-Gγ2, Gαoβ1, and DORs. On the day of the experiment, cells were exposed to SNC-80 (10 µM) or vehicle [dimethylsulfoxide (DMSO)], and 48 alternate readings were obtained for each condition. Values for the Z’ factor were calculated as described in experimental procedures. Full lines represent mean reading, and broken lines represent the variability for basal and stimulated conditions.
Discussion
Although it is now well established that Kir3 channels, Gαi/o proteins, and GPCRs may constitutively interact (Lavine et al., 2002; Fowler et al., 2007; Luscher and Slesinger, 2010), the way in which these interactions are modified during signal transduction remains to be fully elucidated. Biochemical and biophysical results obtained in this study confirmed and extended our previous observations by showing that DOR spontaneously associates not only with heterotrimeric G proteins (Audet et al., 2008) but also with Kir3.1 channel subunits. Additionally, Gβ1γ2 dimers associated with GαoA, Kir3.1, and Kir3.2 subunits, indicating that with the exception of GαoA, all signaling partners in the complex constitutively interacted with one another. Indeed, in keeping with previous reports, we observed that GαoA interacted with the receptor and Gβγ subunits (Gales et al., 2005; Audet et al., 2008) but obtained no evidence for its direct association to the channel. Previous studies had similarly reported that inactive Gα did not directly interact with Kir3.1/3.2 subunits (Fowler et al., 2007; Berlin et al., 2011) and had proposed that this subunit associates with Kir3 channels via the Gβγ dimer (Berlin et al., 2011). On the other hand, NMR studies have revealed a direct interaction between the switch 2 region of GTP-bound Gαi3 and the αA helix of Kir3.1 subunits (Mase et al., 2012), suggesting that Gα and the channel may come into direct contact on G protein activation. We did not find evidence for this type of reorganization since DOR stimulation by highly efficacious agonists failed to show BRET at the GαoA/Kir3.1 interface. However, since tagging of Gα subunits interferes with the activation of Kir3 effectors (Berlin et al., 2011), we cannot exclude that defects in Kir3 signal transduction might have prevented conformational changes between GαoA and channel subunits. In contrast, the BRET pair assessing GαoA99-Luc interaction with YFP-Gγ2 revealed that DOR activation caused donor/acceptor moieties at the GαoA/Gβγ interface to separate from one another. Furthermore, conformational changes that took place at the GαoA/Gβγ interface followed similar two-phase kinetics as rearrangements that repositioned Gβγ with respect to the channel. In addition, it was also established that similar to conformational changes undergone by heterotrimeric subunits (Audet et al., 2008), rearrangements between Gβγ and Kir3.1 subunits were sensitive to PTX. This was evidenced by sensitivity of conformational rearrangements between Gβγ and Kir3.1 subunits to PTX. Thus, taken together, these observations support a scenario where DOR activation promotes an initial conformational change that leads to nucleotide exchange by Gα, followed by a conformational alteration in Gβγ for its interaction with the channel.
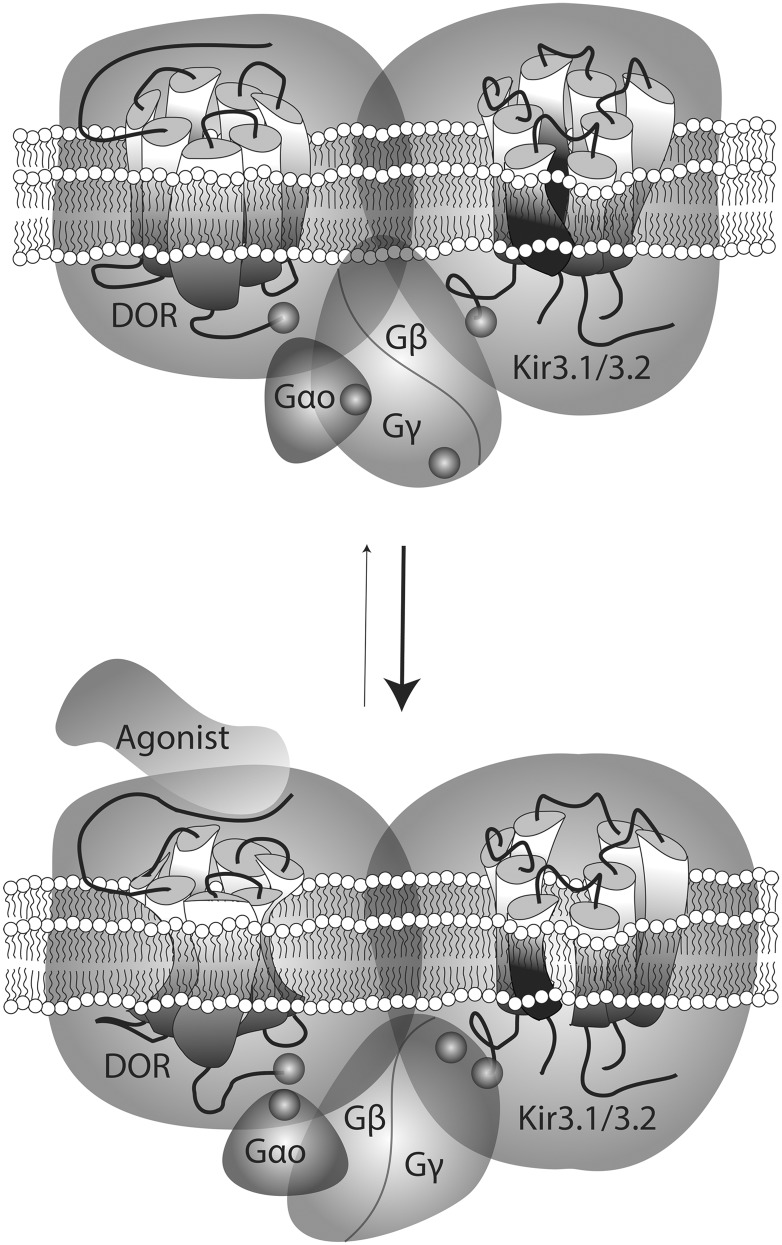
The DOR agonists enhanced BRET at pairs assessing interactions between the receptor C-terminus and Gβγ, as well as GαoA subunits. The fact that these increases in energy transfer took place while the receptor C-terminus remained stationary with respect to channel subunits suggests that ligand-evoked BRET changes at the DOR interface with G protein components were most likely due to the displacement of heterotrimeric subunits, not the C-terminal domain of the receptor. This reasoning would imply that instead of actively participating in the transfer of conformational information from activated DORs, the C-terminus would be a bystander for rearrangements taking place within its proximity. Such an interpretation is consistent with NMR and crystallization studies of an activated β2AR-Gαsβγ complex, where the agonist-stabilized receptor was associated with Gα, not via its C-terminus, but through its second intracellular loop and transmembrane domains 5 and 6 (Rasmussen et al., 2011; Westfield et al., 2011). These studies also reported the absence of direct interaction between the receptor and Gβγ (Rasmussen et al., 2011; Westfield et al., 2011), pointing to Gα as the initial relay for conveying conformational information from the receptor to the effector. Agonist-induced conformational changes that were captured by the different biosensors used in this study are schematically represented in Fig. 9.
Fig. 9.
Schematic representation of conformational rearrangements undergone by different complex components on DOR activation by an agonist. Biosensors assessing interactions between DORs, GαoAβ1γ2, and Kir3.1/3.2 subunits revealed the spontaneous association of these proteins within a multimeric array. In the nonstimulated complex, the receptor C-terminus was within a distance of 100 Å of the C-terminal end of Kir3 subunits, the N-terminus of Gγ2, and position 99 in the αA-αB loop of GαoA subunits. On stimulation by an agonist, DORs and Kir3 C-termini underwent minimal displacement with respect to one another, but the tag at the N-terminal domain of Gγ2 approached these two regions. This rearrangement was paralelled by Gγ2 separation from the helical domain of GαoA which, in turn, moved closer to the C-terminal tag on the receptor. No basal or ligand induced transfer of energy was observed at the BRET pair assessing GαoA and Kir3.1 interaction.
The DORs were previously shown to adopt agonist-specific conformations that distinctively interact with heterotrimeric G proteins (Audet et al., 2018, 2012). In particular, this was evidenced by the fact that ligand-induced BRET changes among DOR-Gαβγ complex components did not correlate with functional responses evoked at cyclase or ERK effectors (Audet et al., 2008). Here we compared ligand-induced structural rearrangements at the interface of the receptor with Gβγ subunits and that of the Gβγ dimer with Kir3.1 and 3.2 channel subunits. The data obtained revealed that different ligands induced correlated conformational changes at these interfaces. On the other hand, we found that Emax and EC50 values that described ligand tendency to induce nucleotide exchange by Gα subunits did not correlate with parameters describing agonist tendency to induce different conformational rearrangements nor the magnitude of channel currents produced by the different agonists. One possible interpretation of these observations is that effector responses (in this case, magnitude of channel currents) are determined not only by ligand ability to promote GDP/GTP exchange but also by the unique set of conformational changes that each ligand imposes among complex components. However, although it is tempting to speculate that organization within a signaling complex allows channel subunits to be influenced by the conformational diversity of the ligand-receptor pair, we cannot exclude that mismatches between BRET and GTPγS binding data could have been related to the fact that the former were obtained in presence of overexpressed GαoAβ1γ2, whereas the latter describe nucleotide exchange by endogenous G proteins.
On the other hand, conformational changes at either Gβγ/Kir3.1 or Gβγ/Kir3.2 interfaces were predictive of whether a ligand could evoke channel currents since drugs that displayed the highest potency and relative efficacy to modify BRET at these pairs also increased channel permeability. Within this context, we propose that a conformational biosensor that monitors Gβγ/Kir3 subunit interactions could prove a valuable screening tool for identifying GPCR ligands that are capable of modulating Kir3 channel function. To assess the reliability of this type of tool, we calculated a Z’ factor as an indicator of biosensor ability to discriminate signal from noise (Zhang et al., 1999). The value obtained was well above the 0.5 limit that ensures robust screening capacity (Zhang et al., 1999), further underscoring the potential of these conformational biosensors as a viable means for fast and precise screening of ligands that modulate Kir3 channel function via GPCRs. Importantly, although these biosensors may function as a screening tool, they do not necessarily represent conformational changes undergone by these signaling proteins in their natural neuronal environment.
Mutagenesis and functional studies have identified leucine residues within βD-βE (He et al., 2002) and βL-βM strands (He et al., 1999; Finley et al., 2004) as being essential for evoking Kir3 channel activation via GPCRs. Both these strands have also been confirmed as direct binding sites for Gβγ subunits (Yokogawa et al., 2011). Based on these observations, it was predicted that if the Kir3.1-Luc/YFP-Gγ2 BRET pair did indeed capture conformational changes associated with GPCR-mediated activation of Kir3 channels, then removal of βD-βE and βL-βM strands (Kir3.1-RLuc183-342) should interfere with structural rearrangements as monitored by the Gβγ/Kir3 biosensor. This assumption was confirmed by showing that a highly efficacious agonist like SNC-80 failed to modify the basal signal produced by the Kir3.1-RLuc183-342/YFP-Gγ2 pair. Since the mutant construct did not reach the cell surface, it was first necessary to establish whether the lack of response was due to the mutation or to intracellular retention of the complex. To discriminate between the two possibilities, SNC-80 was also tested on cells expressing the Kir3.1-Lucwt/YFP-Gγ2 BRET pair, together with all complex components except for Kir3.2 subunits. Since Kir3.1 subunits lack the endoplasmic reticulum export signal that is present in Kir3.2 (Ma et al., 2002), Kir3.1-Lucwt constructs remained trapped in the intracellular compartment when Kir3.2 subunits were absent. We previously showed that different complex components associate early during synthesis (Rebois et al., 2006) and that such complexes respond to cell-permeable agonists without reaching the cell surface (David et al., 2006). In keeping with these observations, the membrane-permeable agonist SNC-80 enhanced energy transfer in cells in which the Lucwt/YFP-Gγ2 pair was retained intracellularly, whereas the membrane-impermeable agonist DPDPE was without effect. Thus, despite being able to evoke a conformational change at intracellular Kir3.1Lucwt constructs, SNC-80 failed to modify BRET between YFP-Gγ2 and Kir3.1-RLuc183-342 mutants that were also retained intracellularly. This deficient response cannot be attributed to lack of Gβγ interaction with the mutant channel subunit since, despite being reduced with respect to the spontaneous BRET signal generated by normally distributed channels, the Kir3.1-RLuc183-342/YFP-Gγ2 pair also displayed basal energy transfer. We therefore conclude that a biosensor in which Kir3.1 subunits lack βD-βE and βL-βM strands loses its ability to respond adequately to GPCR stimulation. Further, the observed reduction in spontaneous BRET is not only consistent with previous reports showing that leucine residues within βD-βE and βL-βM strands help determine basal currents (Ivanina et al., 2003), but it also supports the idea that these channel regions contribute to an optimal constitutive interaction between Gβγ and Kir3.1 subunits.
In conclusion, data obtained in this study provide evidence that DOR, G proteins, and Kir3 channel subunits form a signaling complex whose integrity is maintained in the early stages of signal transduction. Despite multiple interactions among different components, conformational information encoded by agonist binding to the receptor was relayed to the channel via a specific route of structural rearrangements that involved repositioning of heterotrimeric subunits among themselves with respect to the receptor and the channel. In particular, the occurrence of measurable conformational changes at BRET pairs assessing Gβγ interaction with Kir3 subunits was predictive of ligand ability to evoke channel currents and could function as a conformational biosensor to screen for GPCR ligands that induce Kir3 channel opening.
Abbreviations
- ACII
adenylyl cyclase II
- BRET
bioluminescence resonance energy transfer
- BSA
bovine serum albumin
- CTL
cytotoxic T lymphocytes
- DOR
δ-opioid receptor
- DPDPE
[D-Pen(2), D-Pen(5)]-enkephalin
- Emax
maximal effect
- GPCR
G protein–coupled receptor
- HEK
human embryonic kidney
- HK
high potassium
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PTX
pertussis toxin
- SNC-80
(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3- methoxybenzyl]-N,N-diethylbenzamide
- TICP
H-Tyr-Ticpsi[CH2-NH] Cha-Phe-OH
- TIPP
H-Tyr-Tic-Phe-Phe-OH
- UFP512
H-Dmt-Tic-NH-CH(CH2-COOH)-Bid
- YFP
yellow fluorescent protein
Authorship Contributions:
Participated in research design: Hébert, Pineyro.
Conducted experiments: Richard-Lalonde, Nagi, Audet, Sleno, Amraei.
Contributed new reagents or analytic tools: Hogue, Balboni, Schiller, Bouvier.
Performed data analysis: Richard-Lalonde, Nagi, Audet, Sleno.
Wrote or contributed to the writing of the manuscript: Richard-Lalonde, Nagi, Sleno, Hébert, Pineyro.
Footnotes
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada [311997] (G.P.); the Canadian Institutes of Health Research (CIHR) [MOP 79432] (G.P.), [MOP 79354] (T.E.H.), and P.W.S. [MOP 89716] (P.W.S.); and the Consortium québécois sur la découverte du médicament (G.P., T.E.H., M.B.); and by National Institutes of Health [Grant DA004443] to (P.W.S.). T.E.H. holds a Chercheur National award from the Fonds de la Recherche en Santé du Québec. M.B. holds a Canada Research Chair in Cell Signalling and Molecular Pharmacology. R.S. holds a studentship from the McGill-CIHR Drug Development Training Program. K.N. holds a studentship from Ste-Justine Hospital Research Center.
References
- Audet N, Charfi I, Mnie-Filali O, Amraei M, Chabot-Doré AJ, Millecamps M, Stone LS, Pineyro G. (2012) Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists. J Neurosci 32:4827–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet N, Galés C, Archer-Lahlou E, Vallières M, Schiller PW, Bouvier M, Pineyro G. (2008) Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins. J Biol Chem 283:15078–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet N, Piñeyro G. (2011) Using BRET to detect ligand-specific conformational changes in preformed signalling complexes. Methods Mol Biol 756:149–163 [DOI] [PubMed] [Google Scholar]
- Berchiche YA, Gravel S, Pelletier ME, St-Onge G, Heveker N. (2011) Different effects of the different natural CC chemokine receptor 2b ligands on beta-arrestin recruitment, Gαi signaling, and receptor internalization. Mol Pharmacol 79:488–498 [DOI] [PubMed] [Google Scholar]
- Berlin S, Keren-Raifman T, Castel R, Rubinstein M, Dessauer CW, Ivanina T, Dascal N. (2010) G alpha(i) and G betagamma jointly regulate the conformations of a G betagamma effector, the neuronal G protein-activated K+ channel (GIRK). J Biol Chem 285:6179–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin S, Tsemakhovich VA, Castel R, Ivanina T, Dessauer CW, Keren-Raifman T, Dascal N. (2011) Two distinct aspects of coupling between Gα(i) protein and G protein-activated K+ channel (GIRK) revealed by fluorescently labeled Gα(i3) protein subunits. J Biol Chem 286:33223–33235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92:7297–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit A, Gagnidze K, Devi LA, Lagacé M, Bouvier M. (2006) Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol Pharmacol 70:686–696 [DOI] [PubMed] [Google Scholar]
- Clancy SM, Fowler CE, Finley M, Suen KF, Arrabit C, Berton F, Kosaza T, Casey PJ, Slesinger PA. (2005) Pertussis-toxin-sensitive Galpha subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol Cell Neurosci 28:375–389 [DOI] [PubMed] [Google Scholar]
- David M, Richer M, Mamarbachi AM, Villeneuve LR, Dupré DJ, Hebert TE. (2006) Interactions between GABA-B1 receptors and Kir 3 inwardly rectifying potassium channels. Cell Signal 18:2172–2181 [DOI] [PubMed] [Google Scholar]
- Finley M, Arrabit C, Fowler C, Suen KF, Slesinger PA. (2004) betaL-betaM loop in the C-terminal domain of G protein-activated inwardly rectifying K(+) channels is important for G(betagamma) subunit activation. J Physiol 555:643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Skiba NP, Bae H, et al. (1998) Molecular basis for interactions of G protein betagamma subunits with effectors. Science 280:1271–1274 [DOI] [PubMed] [Google Scholar]
- Fowler CE, Aryal P, Suen KF, Slesinger PA. (2007) Evidence for association of GABA(B) receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J Physiol 580:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galés C, Rebois RV, Hogue M, Trieu P, Breit A, Hébert TE, Bouvier M. (2005) Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods 2:177–184 [DOI] [PubMed] [Google Scholar]
- He C, Yan X, Zhang H, Mirshahi T, Jin T, Huang A, Logothetis DE. (2002) Identification of critical residues controlling G protein-gated inwardly rectifying K(+) channel activity through interactions with the beta gamma subunits of G proteins. J Biol Chem 277:6088–6096 [DOI] [PubMed] [Google Scholar]
- He C, Zhang H, Mirshahi T, Logothetis DE. (1999) Identification of a potassium channel site that interacts with G protein betagamma subunits to mediate agonist-induced signaling. J Biol Chem 274:12517–12524 [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366 [DOI] [PubMed] [Google Scholar]
- Huang CL, Jan YN, Jan LY. (1997) Binding of the G protein betagamma subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett 405:291–298 [DOI] [PubMed] [Google Scholar]
- Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. (1995) Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron 15:1133–1143 [DOI] [PubMed] [Google Scholar]
- Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, Schreibmayer W, Dessauer CW, Dascal N. (2003) Mapping the Gbetagamma-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem 278:29174–29183 [DOI] [PubMed] [Google Scholar]
- Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. (2002) G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem 277:46010–46019 [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. (1987) The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325:321–326 [DOI] [PubMed] [Google Scholar]
- Lüscher C, Slesinger PA. (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11:301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. (2002) Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron 33:715–729 [DOI] [PubMed] [Google Scholar]
- Marker CL, Cintora SC, Roman MI, Stoffel M, Wickman K. (2002) Hyperalgesia and blunted morphine analgesia in G protein-gated potassium channel subunit knockout mice. Neuroreport 13:2509–2513 [DOI] [PubMed] [Google Scholar]
- Mase Y, Yokogawa M, Osawa M, Shimada I. (2012) Structural basis for modulation of gating property of G protein-gated inwardly rectifying potassium ion channel (GIRK) by i/o-family G protein α subunit (Gαi/o). J Biol Chem 287:19537–19549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. (2002) Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem 277:44925–44931 [DOI] [PubMed] [Google Scholar]
- Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. (2002) G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron 33:87–99 [DOI] [PubMed] [Google Scholar]
- Piñeyro G. (2009) Membrane signalling complexes: implications for development of functionally selective ligands modulating heptahelical receptor signalling. Cell Signal 21:179–185 [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Azzi M, De Léan A, Schiller P, Bouvier M. (2001) Short-term inverse-agonist treatment induces reciprocal changes in delta-opioid agonist and inverse-agonist binding capacity. Mol Pharmacol 60:816–827 [PubMed] [Google Scholar]
- Piñeyro G, Azzi M, deLéan A, Schiller PW, Bouvier M. (2005) Reciprocal regulation of agonist and inverse agonist signaling efficacy upon short-term treatment of the human delta-opioid receptor with an inverse agonist. Mol Pharmacol 67:336–348 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE. (2006) Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci 119:2807–2818 [DOI] [PubMed] [Google Scholar]
- Riven I, Iwanir S, Reuveny E. (2006) GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron 51:561–573 [DOI] [PubMed] [Google Scholar]
- Robitaille M, Ramakrishnan N, Baragli A, Hébert TE. (2009) Intracellular trafficking and assembly of specific Kir3 channel/G protein complexes. Cell Signal 21:488–501 [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Peleg S, Berlin S, Brass D, Keren-Raifman T, Dessauer CW, Ivanina T, Dascal N. (2009) Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GalphaiGDP and Gbetagamma. J Physiol 587:3473–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW, Weltrowska G, Berezowska I, Nguyen TM, Wilkes BC, Lemieux C, Chung NN. (1999) The TIPP opioid peptide family: development of delta antagonists, delta agonists, and mixed mu agonist/delta antagonists. Biopolymers 51:411–425 [DOI] [PubMed] [Google Scholar]
- Smrcka AV, Lehmann DM, Dessal AL. (2008) G protein betagamma subunits as targets for small molecule therapeutic development. Comb Chem High Throughput Screen 11:382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, et al. (2008) Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides 29:93–103 [DOI] [PubMed] [Google Scholar]
- Westfield GH, Rasmussen SG, Su M, et al. (2011) Structural flexibility of the G alpha s alpha-helical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci USA 108:16086–16091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa M, Osawa M, Takeuchi K, Mase Y, Shimada I. (2011) NMR analyses of the Gbetagamma binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1). J Biol Chem 286:2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. (1999) A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 4:67–73 [DOI] [PubMed] [Google Scholar]