Abstract
Sphingosine 1-phosphate receptor 1 (S1P1) is a G protein–coupled receptor that is critical for proper lymphocyte development and recirculation. Agonists to S1P1 are currently in use clinically for the treatment of multiple sclerosis, and these drugs may act on both S1P1 expressed on lymphocytes and S1P1 expressed within the central nervous system. Agonists to S1P1 and deficiency in S1P1 both cause lymphocyte sequestration in the lymph nodes. In the present study, we show that S1P1 antagonism induces lymphocyte sequestration in the lymph nodes similar to that observed with S1P1 agonists while upregulating S1P1 on lymphocytes and endothelial cells. Additionally, we show that S1P1 antagonism reverses experimental autoimmune encephalomyelitis in mice without acting on S1P1 expressed within the central nervous system, demonstrating that lymphocyte sequestration via S1P1 antagonism is sufficient to alleviate autoimmune pathology.
Introduction
Sphingosine 1-phosphate receptor 1 (S1P1) plays an important role in many physiologic systems, including vascular development, lymphocyte development, and lymphocyte recirculation (Liu et al., 2000; Allende et al., 2003, 2004; Matloubian et al., 2004; Cyster and Schwab, 2012). S1P1 is required on developing lymphocytes to mature beyond a semimature CD69hi, CD62Llo state, rendering the blood and lymph of mice lacking S1P1 on developing lymphocytes largely devoid of T cells. When S1P1−/− thymocytes are transferred into recipient mice, they are also retained from blood and lymphatic circulation. S1P1 became a relevant drug target in the treatment of autoimmune disease following the discovery that 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol (FTY720; fingolimod, Gilenya), which was known to inhibit lymphocyte recirculation, is a sphingosine 1-phosphate (S1P) receptor prodrug that is phosphorylated in vivo to yield 2-amino-2[2-(4-octylphenyl)ethyl]-1,3-propanediol, mono dihydrogen phosphate ester (FTY720-P), a potent agonist of S1P1, S1P3, S1P4, and S1P5 (Mandala et al., 2002). S1P1 selective agonists demonstrated that FTY720 acted via S1P1 to induce lymphocyte sequestration (Sanna et al., 2004). The ability of FTY720-P and other S1P1 agonists to induce sustained internalization and/or degradation of S1P1 (Graler and Goetzl, 2004; Gonzalez-Cabrera et al., 2007, 2008), combined with the deficient egress of S1P1-deficient lymphocytes, has led to the hypothesis that S1P1 agonists act as functional antagonists (Graler and Goetzl, 2004). Several S1P1-selective antagonists have also been generated, which inhibit agonist-dependent effects in vitro; stabilize the S1P1 receptor, allowing for its structural determination; and induce pulmonary edema in vivo. In addition, initial antagonists could reverse agonist-induced lymphocyte sequestration while being unable to induce lymphocyte sequestration themselves (Foss et al., 2005; Wei et al., 2005; Sanna et al., 2006; Hanson et al., 2012). Recent work has shown that S1P1 antagonists can indeed induce lymphocyte sequestration at high plasma concentrations (Tarrason et al., 2011) and S1P1 antagonists can alleviate animal models of autoimmune arthritis (Fujii et al., 2012), cardiac allograft rejection (Angst et al., 2012), and multiple sclerosis (Quancard et al., 2012).
S1P receptor agonists have come of age with the Food and Drug Administration’s approval of FTY720 for the treatment of relapsing-remitting multiple sclerosis. The efficacy of FTY720 is not solely dependent on its ability to cause full lymphocyte sequestration via S1P1, as it is effective at doses that maintain ∼50% lymphopenia. This efficacy probably involves both S1P1 and other S1P receptors within the central nervous system (CNS) (Cohen and Chun, 2011; Hla and Brinkmann, 2011). S1P1 agonists that can efficiently penetrate the CNS can induce receptor signaling and degradation of S1P1 expressed on neurons and astrocytes (Gonzalez-Cabrera et al., 2012), and require lymphocyte sequestration for only one-third of a dosing interval to reverse experimental autoimmune encephalomyelitis (EAE) in mice. Additionally, mice lacking S1P1 on astrocytes are refractory to developing EAE, and are suggested to be important targets of FTY720 (Choi et al., 2011). Several other S1P receptors are expressed within the CNS, and the activation and/or degradation of these receptors by FTY720 may also play important roles in reversing the immunopathology of multiple sclerosis (Miron et al., 2008, 2010).
In the present study, we demonstrate that S1P1 antagonism sequesters lymphocytes in the peripheral lymph nodes but not the spleen, similar to that observed with S1P1 agonists. S1P1 antagonism also causes significant upregulation of S1P1 expression on peripheral lymphocytes, mature thymocytes, and lung endothelial cells. Additionally, S1P1 antagonism can alleviate EAE in mice despite the inability of the antagonist to penetrate the CNS. Thus, lymphocyte sequestration induced by S1P1 antagonists is sufficient to ameliorate the autoimmune pathology observed in EAE, and does not require antagonism of S1P1 expressed on neurons or astrocytes within the CNS.
Materials and Methods
Compounds and In Vitro Assays.
Example 26 [Ex26, 1-(5′-((1-(4-chloro-3-methylphenyl)ethyl)amino)-2'-fluoro-3,5-dimethyl-[1,1'-biphenyl]-4-ylcarboxamido)cyclopropanecarboxylic acid] was synthesized as a racemic mixture according to its published synthesis in the patent literature (Angst et al., 2009). RP-001 (3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-inden-1-ylamino)propanoic acid) was synthesized as previously described (Cahalan et al., 2011). FTY720 was purchased from Cayman Chemicals (Ann Arbor, MI). Ex26 and RP-001 were solubilized in 50 mM Na2CO3, while FTY720 was solubilized in H2O. In vitro assays for S1P receptor function were performed using the following cell lines: S1P1, S1P4, and S1P5: Tango Human Osteosarcoma U2OS cells (Invitrogen, Carlsbad, CA) expressing the indicated receptor; S1P2: Chinese hamster ovary cells expressing S1P2 coupled to a cAMP response element coupled to beta-lactamase reporter; and S1P3: Chinese hamster ovary cells expressing S1P3 coupled to an Nuclear factor of activated T-cells promoter coupled to beta-lactamase reporter through G protein α 16. S1P1 internalization and polyubiquitinylation were evaluated using human embryonic kidney cells expressing S1P1 enhanced green fluorescent protein (eGFP) as previously described (Gonzalez-Cabrera et al., 2007), pretreating cells for 1 hour with Ex26.
Evaluation of Lymphocyte Sequestration, Pulmonary Edema.
Eight-week-old male C57Bl/6J mice were purchased from the The Scripps Research Institute mouse breeding facility (La Jolla, CA) for evaluation of lymphocyte sequestration and pulmonary edema. Mice were injected i.p. with Ex26 or 50 mM Na2CO3 vehicle, and blood was removed from the heart following euthanasia. Blood was lysed in 150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA; washed with phosphate-buffered saline (PBS) containing 2% fetal bovine serum, 1 mM EDTA, and 0.1% NaN3; counted using a ViCell-XR counter (Beckman Coulter, Brea, CA); stained with antibodies; and analyzed by flow cytometry. To evaluate pulmonary edema, mice were perfused with 15 ml of PBS through the right ventricle, then the lungs were removed, blotted dry to remove excess PBS, and weighed. All mouse experiments were performed using protocols approved by the Institutional Animal Care and Usage Committee.
Compound Concentrations in Plasma and Tissues.
Ex26 plasma concentrations were determined using methanol extraction as previously described (Cahalan et al., 2011), detecting an m/z value of 495.2 for Ex26 using an Agilent 6410 triple quadrupole mass spectrometer coupled to an Agilent 1100LC system (Agilent Technologies, Santa Clara, CA). Ex26 concentrations in the brain were determined by disruption of brain tissue in water by probe sonication, followed by extraction with acetonitrile and filtration through MultiScreen hydrophilic polytetrafluoroethylene 0.45 μm filters (EMD Millipore, Billerica, MA). Filtrates were analyzed using a API 4000 liquid chromatography-tandem mass spectrometer (AbSciex, Framingham, MA) and quantified using a positive-ion multiple reaction monitoring method (495.1/242.1, m/z).
Continuous Administration of S1P1 Antagonist.
Six-week-old S1P1-eGFP mice were anesthetized with isoflurane, and their backs were shaved, cleaned with 70% ethanol to remove any excess hair, then wiped with povidone iodine. An incision was made on the lower back of the mice, and micro-osmotic pumps (Alzet model 1003D; Alzet, Cupertino, CA) containing either 50 mM Na2CO3 vehicle or 2 mg/ml Ex26 were implanted, yielding a dose of ∼0.1 mg/kg per hour. Mice were given an i.p. dose of 3 mg/kg Ex26 or vehicle immediately following surgery.
Flow Cytometry, S1P1 Expression, and Statistical Analysis.
Fluorescently labeled antibodies specific to CD4 and CD8 were obtained from Biolegend (San Diego, CA). Fluorescently labeled antibodies specific to CD19, CD31, CD45.2, CD62L, and CD69 were obtained from Beckton-Dickinson (San Diego, CA). Data were collected using an LSRII flow cytometer (Beckton-Dickinson) and analyzed using FlowJo (Treestar, Ashland, OR). S1P1 expression by flow cytometry was measured using S1P1-eGFP knockin mice (Cahalan et al., 2011). S1P1 expression in the CNS in EAE experiments was evaluated using a C-terminal–specific S1P1 antibody (H-60, Santa Cruz Biotechnology, Santa Cruz, CA; used at 1:500 dilution). All statistical analyses were performed using GraphPad Prism Software (GraphPad, La Jolla, CA).
EAE Induction and Scoring.
EAE was induced in female 10-week-old C57Bl/6J mice purchased from Jackson Laboratories (Bar Harbor, ME). EAE was induced using a Hooke Laboratories EAE induction kit (Lawrence, MA; EK-0114 for EAE, CK-0114 for control) according to the manufacturer’s instructions. Mice were scored by the following criteria: 0.5 (weak tail), 1 (limp tail), 1.5 (weak tail + weak hind limbs), 2 (limp tail + weak hind limbs), 2.5 (limp tail + unilateral hind limb paralysis), 3 (limp tail + bilateral hind limb paralysis), 4 (limp tail + bilateral hind limb paralysis + partial front limb paralysis), and 5 (moribund or dead). Mice scoring 4 for two consecutive days were euthanized and recorded as 5 for the remaining days of the experiment. Mice were injected i.p. daily with 50 mM Na2CO3 vehicle, 30 mg/kg Ex26, or 10 mg/kg FTY720 in a volume of 10 µl per gram weight of mouse beginning the first day on which clinical signs were observed in that mouse.
Results and Discussion
Ex26 is an S1P1 Antagonist that Inhibits Lymphocyte Egress.
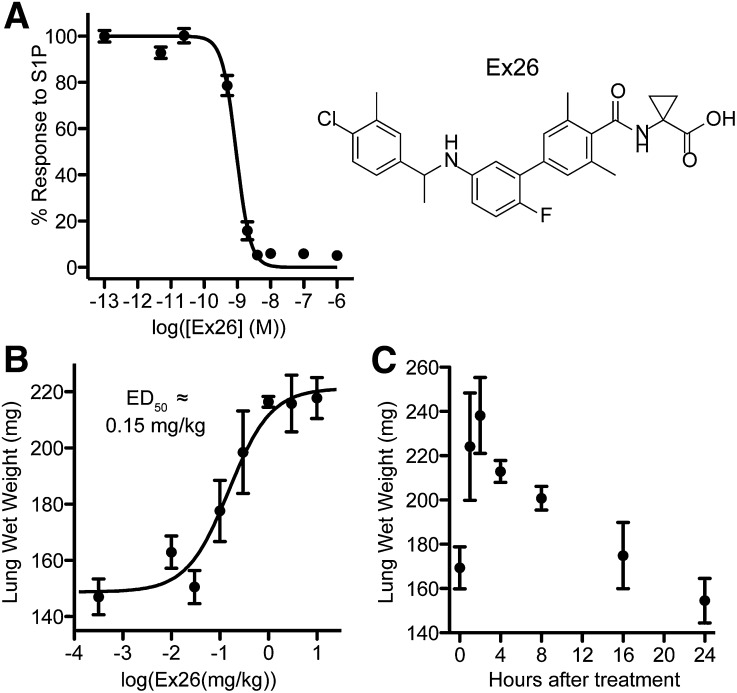
Most existing S1P1 antagonists are S1P analogs with IC50 values in the double-digit nanomolar range that possess relatively short half-lives. Recently, new S1P1 antagonists have been described, including a series of biaryl benzylamines by Novartis (Angst et al. 2009). We synthesized and characterized one of these compounds, Ex26, and confirmed it to be a potent and selective antagonist of S1P1 (Fig. 1A; Table 1), similar to a recently published antagonist (Quancard et al., 2012). Ex26 could inhibit RP-001–induced S1P1 internalization and polyubiquitinylation in vitro (Supplemental Fig. 1). Similar to other previously described S1P1 antagonists, Ex26 induced dose-dependent and time-dependent pulmonary edema in vivo (Fig. 1, B and C), and had a relatively short in vivo half-life of approximately 73.5 minutes (Supplemental Fig. 1C).
Fig. 1.
Ex26 is a potent, selective S1P1 antagonist. (A) Dose response in vitro of Ex26 on S1P1-expressing cells in the presence of 5 nM S1P. The structure of Ex26 is depicted on the right. (B) Ex26 induces dose-dependent pulmonary edema 2 hours following i.p. treatment. (C) Pulmonary edema induced by 3 mg/kg Ex26 i.p. resolves by 16–24 hours following treatment. All data are representative of at least two experiments, with (B) and (C) having four mice per group per experiment. Graphs are plotted as the mean ± S.E.M.
TABLE 1.
Selectivity of Ex26 on S1P receptors
Ex26 displays excellent selectivity for S1P1 over other S1P receptors. It also does not exhibit any detectable agonist activity on any S1P receptor.
| Receptor | Antagonist IC50 | Agonist EC50 |
|---|---|---|
| nM | nM | |
| S1P1 | 0.93 | >10,000 |
| S1P2 | >10,000 | >10,000 |
| S1P3 | >10,000 | >10,000 |
| S1P4 | 4900 | >10,000 |
| S1P5 | 3100 | >10,000 |
Ex26, 1-(5′-((1-(4-chloro-3-methylphenyl)ethyl)amino)-2'-fluoro-3,5-dimethyl-[1,1'-biphenyl]-4-ylcarboxamido)cyclopropanecarboxylic acid; S1P, sphingosine 1-phosphate.
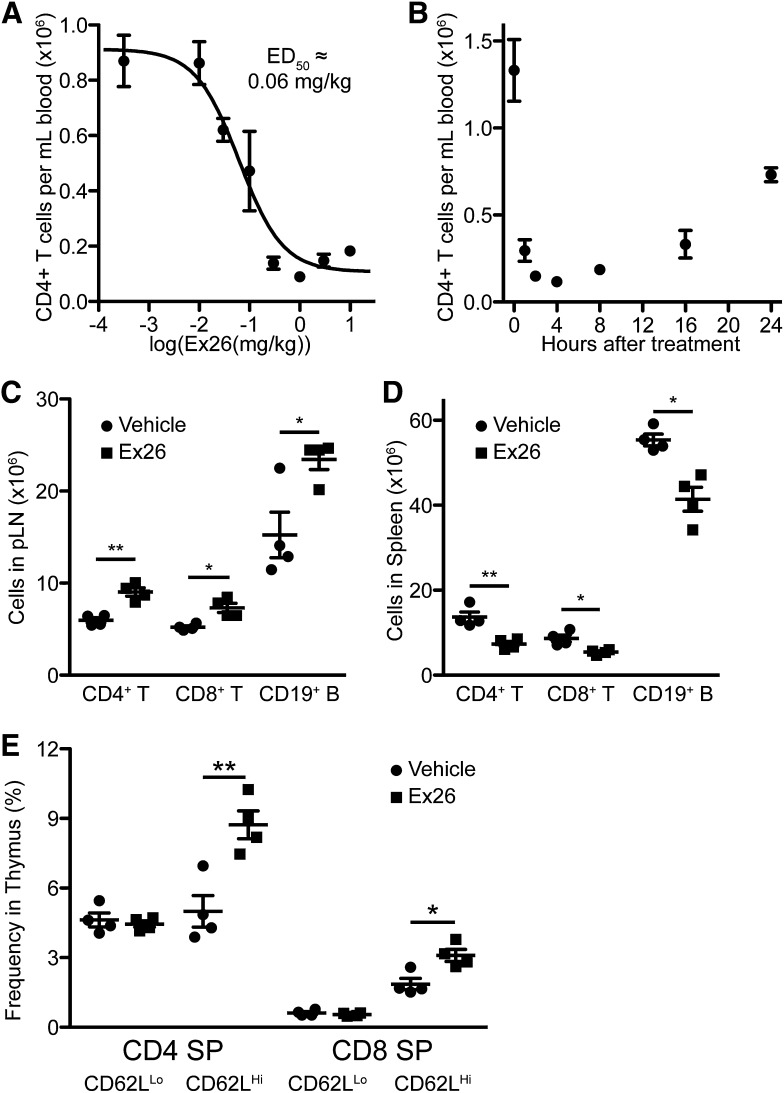
Earlier work showed that the S1P-like S1P1 antagonists W146 and VPC44116 reversed agonist-induced lymphocyte sequestration while not causing lymphocyte sequestration (Sanna et al., 2006; Foss et al., 2007). Recent work has found that W146 induces transient lymphocyte sequestration at high doses (Tarrason et al., 2011), which we replicated (unpublished data). Ex26 induced lymphocyte sequestration at low doses, possessing an ED50 of ∼0.06 mg/kg when examined 2 hours following treatment (Fig. 2A). Lymphocyte sequestration by Ex26 resolved with similar kinetics as did Ex26-evoked pulmonary edema (Fig. 2B). To examine the effects of extended antagonist treatment, we implanted mice with micro-osmotic pumps to continuously deliver Ex26 at a dose of 0.1 mg/kg per hour for 3 days following a loading dose of 3 mg/kg. Extended treatment with Ex26 led to significant retention of T and B cells within the lymph nodes and significant decreases in T and B cells within the spleen, similar to S1P1 agonists (Fig. 2, C and D). Continuous administration of Ex26 also led to thymic retention of mature CD62LHi single-positive thymocytes, also similar to the effects induced by S1P1 agonists (Fig. 2E). These data demonstrate that disruption of S1P1 signaling by S1P1 antagonism leads to the inhibition of lymphocyte and thymocyte egress.
Fig. 2.
S1P1 antagonism by Ex26 induces lymphocyte sequestration in the lymph nodes and thymus. (A) Ex26 induces dose-dependent lymphocyte sequestration 2 hours following i.p. treatment. (B) Lymphopenia induced by 3 mg/kg Ex26 i.p. resolves by 24 hours following treatment. (C and D) Continuous administration of Ex26 in 6-week-old mice by micro-osmotic pumps sequesters T and B cells in the peripheral lymph nodes (pLN) (C), leaving the spleen depleted of lymphocytes (D). pLN cell numbers derive from combined inguinal, axillary, and brachial lymph nodes. (E) Ex26 leads to accumulation of mature CD62LHi, but not immature CD62LLo, SP thymocytes. All graphs are representative of three experiments, with 3–4 mice per group per experiment. Graphs are plotted as the mean ± S.E.M. *P < 0.05, **P < 0.01 as determined by an unpaired, two-tailed t test.
S1P1 Antagonism Upregulates S1P1 Expression.
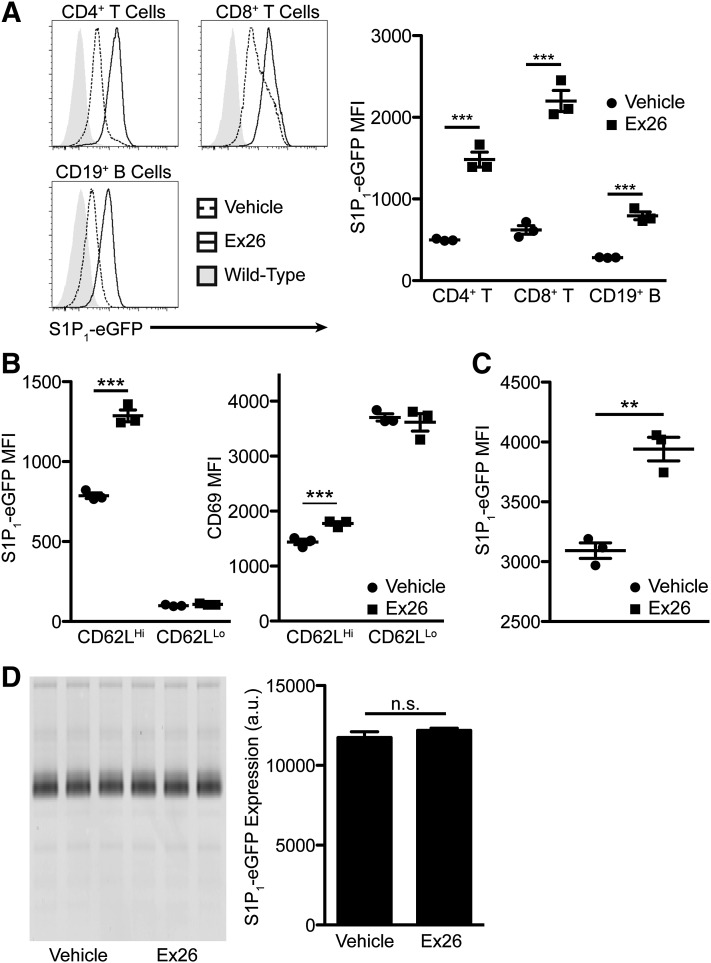
Since S1P1 agonists downregulate S1P1, we wanted to determine whether S1P1 antagonism could conversely upregulate S1P1. Continuous S1P1 antagonism in mice expressing S1P1-eGFP from the S1P1 locus (Cahalan et al., 2011) for 3 days by micro-osmotic pumps caused significant upregulation of S1P1-eGFP on lymphocytes within the lymph node (Fig. 3A). This suggests that the low concentration of S1P within the lymph node (Schwab et al., 2005) under normal physiologic conditions is sufficient to suppress the expression of S1P1. We observed similar upregulation within the spleen (unpublished data) and a modest upregulation of S1P1-eGFP on fully mature CD62Lhi SP thymocytes (Fig. 3B). S1P1 agonists cause a loss of surface expression of CD69 on mature thymocytes (Alfonso et al., 2006). In contrast to the effects seen with agonists, continuous Ex26 treatment led to significant upregulation of CD69 (Fig. 3B), indicating that S1P1 signaling, not only expression of S1P1 (Bankovich et al., 2010), is critical for suppressing the surface expression of CD69; thus, downregulation of CD69 by S1P1 agonists is a measure of agonism, not functional antagonism. Upregulation of S1P1-eGFP was not limited to lymphocytes, as blood endothelial cells within the lung significantly upregulated S1P1-eGFP expression (Fig. 3C). Unlike many S1P1 agonists, including FTY720-P, Ex26 did not cause any changes in the expression of S1P1-eGFP within the brain (Fig. 3D), due to the fact that Ex26 was almost undetectable within the CNS (plasma: 6.8 ± 0.3 µM, brain: 0.01 ± 0.005 µM; 2/3 animals below the level of detection; mean ± S.E.M.).
Fig. 3.
S1P1 antagonism by Ex26 upregulates S1P1 and CD69, but not in the central nervous system. (A) S1P1-eGFP expression on lymphocytes from lymph nodes from S1P1-eGFP knockin mice continuously administered Ex26 by micro-osmotic pump for 3 days. Gray shaded histograms represent background fluorescence in wild-type mice. The graph on the right represents the mean fluorescence intensity of S1P1-eGFP on the indicated cell type. (B) Mean fluorescence intensity of S1P1-eGFP (left) and CD69 (right) on CD4 SP thymocytes following continuous treatment with Ex26. (C) Mean fluorescence intensity of S1P1-eGFP on lung endothelial cells following continuous treatment with Ex26. (D) Fluorescent scan of SDS-PAGE gel from the brains of mice following 3 days of treatment with Ex26. The graph on the right is obtained by densitometric analysis of the gel on the left. All histograms and graphs are representative of three experiments, with 3–4 mice per group per experiment. Graphs are plotted as the mean ± S.E.M. **P < 0.01, ***P < 0.001 as determined by an unpaired, two-tailed t test. a.u., arbitrary units; MFI, mean fluorescence intensity; n.s., not significant.
S1P1 Antagonism Ameliorates EAE.
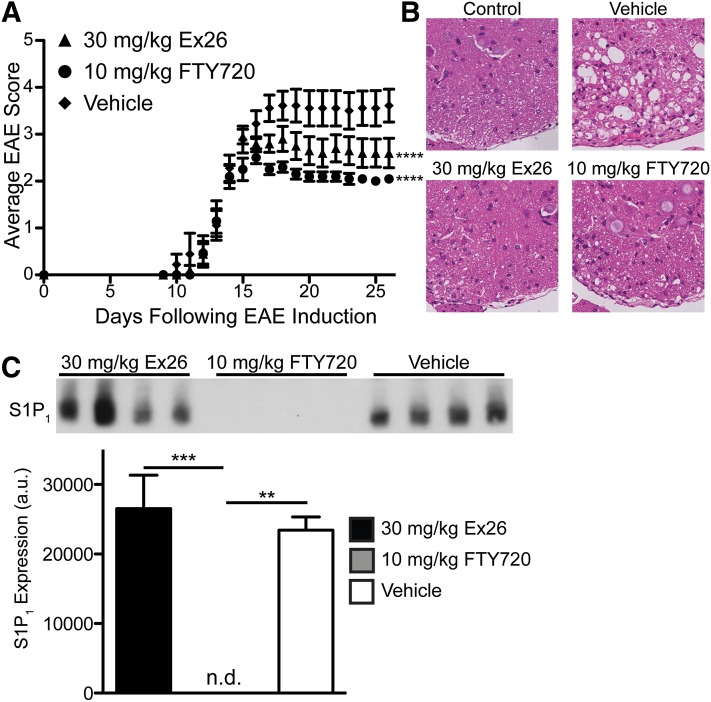
Because Ex26 did not enter the CNS or cause any change in S1P1 expression within the CNS, it allowed us to determine whether lymphocyte sequestration alone was able to reverse EAE. Whereas 3 mg/kg Ex26 induced relatively short-duration lymphocyte sequestration, we found that a single dose of 30 mg/kg caused lymphocyte sequestration and pulmonary edema that lasted 24 hours in naïve mice (Supplemental Fig. 2, A and B). To examine whether S1P1 antagonism could ameliorate EAE similar to S1P1 agonism, we induced disease using the myelin oligodendrocyte glycoprotein residues 33–55 peptide model, and, upon development of clinical signs of disease, treated mice i.p. once daily with 30 mg/kg Ex26, 10 mg/kg FTY720, or 50 mM Na2CO3 vehicle, which we found to be indistinguishable from water, the usual vehicle for FTY720 (unpublished data). We found that treatment of mice with 30 mg/kg Ex26 daily significantly reduced the severity of EAE as assessed by examining clinical signs (Fig. 4A). We observed significant lymphocyte sequestration 3 hours following the last treatment with both 30 mg/kg Ex26 and 10 mg/kg FTY720; however, unlike its effect in naïve mice, 30 mg/kg Ex26 did not cause lymphocyte sequestration that lasted a full 24 hours in mice with EAE, whereas 10 mg/kg FTY720 did (unpublished data), suggesting that treatment with pertussis toxin used in the induction of EAE, or repeated dosing of Ex26, reduced the efficacy of Ex26, potentially by upregulating S1P1 expression on lymphocytes. The reduction in the severity of EAE was seen in the spinal cord, as 30 mg/kg Ex26 inhibited both lymphocyte infiltration and destruction of the white matter in the spinal cord of mice euthanized at the end of the experiment (Fig. 4B). Consistent with the lack of CNS penetration of Ex26, we did not observe any changes in S1P1 expression within the brains of mice euthanized at the end of the experiment that were treated daily with 30 mg/kg Ex26 compared with those treated daily with vehicle, whereas mice treated daily with 10 mg/kg FTY720 exhibited a complete loss in S1P1 within the brain (Fig. 4C). This indicates that antagonism of S1P1 expressed on neurons or astrocytes within the CNS is not required for the amelioration of EAE by S1P1 antagonists, implying that lymphocyte sequestration by S1P1 antagonists is sufficient to reverse the pathology of EAE, in keeping with the efficacy of lymphocyte migration inhibitory agents, such as natalizumab, that successfully treat multiple sclerosis.
Fig. 4.
S1P1 antagonism by Ex26 alleviates EAE. (A) Average EAE scores from myelin oligodendrocyte glycoprotein residues 33-55–induced mice injected daily i.p. with vehicle, 30 mg/kg Ex26, or 10 mg/kg FTY720 following the onset of symptoms. ****P < 0.0001 compared with vehicle as calculated by one-way repeated measures analysis of variance with Bonferroni’s multiple comparison post test. The graph represents two separate experiments as the mean ± S.E.M with 9–10 mice per group. (B) Representative spinal cord sections stained with H&E from control mice without EAE (top left) or mice with EAE that had been treated daily as indicated following the onset of clinical signs. (C) Western blot for S1P1 on the brains of mice with EAE treated daily with vehicle (50 mM Na2CO3), 30 mg/kg Ex26, or 10 mg/kg FTY720 following the onset of symptoms. The graph represents S1P1 expression as determined by densitometry. a.u., arbitrary units; n.d., not detectable.
Supplementary Material
Acknowledgments
The authors thank Bill Webb and Mike Cameron for help with mass spectrometry. The authors thank Margie Chadwell for help with histology.
Abbreviations
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- Ex26
1-(5′-((1-(4-chloro-3-methylphenyl)ethyl)amino)-2'-fluoro-3,5-dimethyl-[1,1'-biphenyl]-4-ylcarboxamido)cyclopropanecarboxylic acid
- FTY720
2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol
- PBS
phosphate-buffered saline
- S1P
sphingosine 1-phosphate
- S1P1–5
sphingosine 1-phosphate receptors 1–5
- S1P1-eGRP
S1P1 enhanced green fluorescent protein
- RP-001
3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-inden-1-ylamino)propanoic acid
Authorship Contributions
Participated in research design: Cahalan, Gonzalez-Cabrera, Rosen.
Conducted experiments: Cahalan, Gonzalez-Cabrera, Nguyen, Cisar, Leaf, Brown.
Contributed new reagents or analytic tools: Guerrero, Roberts.
Performed data analysis: Cahalan, Gonzalez-Cabrera, Cisar, Brown, Rosen.
Wrote or contributed to the writing of the manuscript: Cahalan, Gonzalez-Cabrera, Rosen.
Footnotes
This work was funded by National Institutes of Health [Grants AI05509, AI074564, and MH084812].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Alfonso C, McHeyzer-Williams MG, Rosen H. (2006) CD69 down-modulation and inhibition of thymic egress by short- and long-term selective chemical agonism of sphingosine 1-phosphate receptors. Eur J Immunol 36:149–159 [DOI] [PubMed] [Google Scholar]
- Allende ML, Dreier JL, Mandala S, Proia RL. (2004) Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279:15396–15401 [DOI] [PubMed] [Google Scholar]
- Allende ML, Yamashita T, Proia RL. (2003) G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102:3665–3667 [DOI] [PubMed] [Google Scholar]
- Angst D, Bollbuck P, Janser P, and Quancard J (2009) World Intellectual Property Organization. Biaryl benzylamine derivatives. Patent Number WO 072712 A1. Submission Date 2009 Dec 21.
- Angst D., Janser P., Quancard J., Buehlmayer P., Berst F., Oberer L., Beerli C., Streiff M., Pally C., Hersperger R., et al. (2012). An oral sphingosine 1-phosphate receptor 1 (S1P(1)) antagonist prodrug with efficacy in vivo: discovery, synthesis, and evaluation. J Med Chem 55:9722–9734 [DOI] [PubMed] [Google Scholar]
- Bankovich AJ, Shiow LR, Cyster JG. (2010) CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem 285:22328–22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan SM, Gonzalez-Cabrera PJ, Sarkisyan G, Nguyen N, Schaeffer MT, Huang L, Yeager A, Clemons B, Scott F, Rosen H. (2011) Actions of a picomolar short-acting S1P₁ agonist in S1P₁-eGFP knock-in mice. Nat Chem Biol 7:254–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, et al. (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA 108:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Chun J. (2011) Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol 69:759–777 [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30:69–94 [DOI] [PubMed] [Google Scholar]
- Foss FW, Jr, Clemens JJ, Davis MD, Snyder AH, Zigler MA, Lynch KR, Macdonald TL. (2005) Synthesis, stability, and implications of phosphothioate agonists of sphingosine-1-phosphate receptors. Bioorg Med Chem Lett 15:4470–4474 [DOI] [PubMed] [Google Scholar]
- Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, Macdonald TL. (2007) Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem 15:663–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Hirayama T, Ohtake H, Ono N, Inoue T, Sakurai T, Takayama T, Matsumoto K, Tsukahara N, Hidano S, et al. (2012) Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J Immunol 188:206–215 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Cahalan SM, Nguyen N, Sarkisyan G, Leaf NB, Cameron MD, Kago T, Rosen H. (2012) S1P(1) receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol Pharmacol 81:166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Hla T, Rosen H. (2007) Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem 282:7254–7264 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, Schaeffer MT, Chapman J, Cameron M, Guerrero M, et al. (2008) Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol 74:1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräler MH, Goetzl EJ. (2004) The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J 18:551–553 [DOI] [PubMed] [Google Scholar]
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, et al. (2012) Crystal structure of a lipid G protein-coupled receptor. Science 335:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Brinkmann V. (2011) Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 76(8, Suppl 3)S3–S8 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, et al. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346–349 [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355–360 [DOI] [PubMed] [Google Scholar]
- Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. (2008) FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol 63:61–71 [DOI] [PubMed] [Google Scholar]
- Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. (2010) Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 176:2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quancard J, Bollbuck B, Janser P, Angst D, Berst F, Buehlmayer P, Streiff M, Beerli C, Brinkmann V, Guerini D, et al. (2012) A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem Biol 19:1142–1151 [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, et al. (2004) Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279:13839–13848 [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. (2006) Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2:434–441 [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. (2005) Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309:1735–1739 [DOI] [PubMed] [Google Scholar]
- Tarrasón G, Aulí M, Mustafa S, Dolgachev V, Domènech MT, Prats N, Domínguez M, López R, Aguilar N, Calbet M, et al. (2011) The sphingosine-1-phosphate receptor-1 antagonist, W146, causes early and short-lasting peripheral blood lymphopenia in mice. Int Immunopharmacol 11:1773–1779 [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. (2005) Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol 6:1228–1235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.