Abstract
Dopamine D2/D3 receptor partial agonists have been suggested as medications for cocaine dependence. The present experiments examined the effect of acute and repeated administration of drugs with varying intrinsic efficacy at D2/D3 receptors on the relative reinforcing strength of cocaine. Use of socially housed cynomolgus monkeys permitted the assessment of whether social status, known to alter D2/D3 receptor availability, influenced the behavioral effects of D2/D3 receptor compounds. The high-efficacy agonist R(−)−norpropylapomorphine [(−)−NPA], low-efficacy agonist aripiprazole (ARI), and antagonist eticlopride (ETIC) were administered acutely to monkeys self-administering cocaine under a food-cocaine choice procedure in which a cocaine self-administration dose-effect curve was determined daily. The effects of 5-day treatment with ARI and (−)−NPA were characterized under conditions in which monkeys did (ARI) or did not [ARI and (−)−NPA] self-administer cocaine during treatment. When administered acutely, ARI and ETIC increased the choice of low cocaine doses, and only (−)−NPA decreased the choice of higher cocaine doses and cocaine intake; effects were similar across social ranks. When administered repeatedly while self administration occurred only on days 1 and 5 of treatment, ARI, but not (−)−NPA, decreased cocaine choice in dominant monkeys, whereas (−)−NPA, but not ARI, did so in subordinates. When dominant monkeys self-administered cocaine on all five days of ARI treatment, however, these effects were not observed. The results indicate that the behavioral effects of D2/D3 receptor agonists can differ according to intrinsic efficacy and subject characteristics. Moreover, these results suggest that exposure to cocaine during treatment can counteract treatment-induced reductions in the reinforcing effects of cocaine.
Introduction
Cocaine abuse persists as a major public health problem for which there is no Food and Drug Administration–approved pharmacotherapy (Vocci and Elkashef, 2005). Considering the prominent involvement of dopamine (DA) in the abuse-related behavioral effects of cocaine (e.g., Ritz et al., 1987; Bradberry, 2000; Kimmel et al., 2012), medication development has focused on drugs that interact with brain DA systems (Carroll et al., 1999; Platt et al., 2002). DA D2/D3 receptors have been implicated in mediating the reinforcing effects of cocaine (Woolverton et al., 1984; Bergman et al., 1990; Ranaldi et al., 2001), although practical concerns limit the clinical use of D2/D3 receptor agonists and antagonists. For example, direct-acting D2/D3 receptor agonists are self-administered by laboratory animals, and thus possess abuse liability (e.g., Koffarnus et al., 2012). Conversely, D2/D3 receptor antagonists decrease cocaine self-administration in laboratory animals (e.g., Bergman et al., 1989), but their clinical use is limited by dysphoric, cardiovascular, and motoric side effects (e.g., Hollister, 1992; Newcomer, 2005).
D2/D3 receptor partial agonists may circumvent these obstacles (Platt et al., 2002; Pulvirenti and Koob, 2002). It has been hypothesized that during cocaine self-administration, when extracellular DA levels are high, a partial agonist would compete with DA for binding to the receptor, blunting the abuse-related effects of cocaine. When extracellular DA levels are low, such as during withdrawal, a partial agonist could compensate for reduced DA tone, normalizing DA function and reducing craving (Childress and O’Brien, 2000). Furthermore, partial agonists may have lower abuse liability than full agonists and fewer side effects than antagonists (Newman et al., 2005).
Aripiprazole (ARI) is a D2/D3 receptor partial agonist currently approved to treat schizophrenia. In rodents, ARI can decrease cocaine self-administration and attenuate reinstatement (Sorensen et al., 2008; Feltenstein et al., 2009). Moreover, in rats self-administering cocaine under a food-drug choice procedure, ARI shifted the cocaine dose-effect curve downward (Thomsen et al., 2008). In that study, however, tolerance developed to the effect on cocaine choice after five days of ARI treatment, and other discouraging data have been reported in monkeys (Bergman, 2008). Although mixed results have emerged from preclinical studies in humans (Stoops et al., 2007; Lile et al., 2008, 2011; Haney et al., 2011), a recent phase II study using chronic treatment with lower doses reported that ARI decreased cocaine craving (Meini et al., 2011). Thus, the primary goal of the present study was to assess the effects of ARI on cocaine self-administration in monkeys using a food-drug choice procedure.
The effects of partial agonists can vary substantially within an assay and according to a number of variables, including receptor density (Burris et al., 2002; Gay et al., 2004). In general, however, a relatively consistent rank ordering of effects emerges across studies, such that certain drugs are considered to have relatively high [e.g., quinpirole, R(−)−norpropylapomorphine ([−]−NPA)], intermediate (apomorphine), or low efficacy (e.g., terguride). ARI has been identified as a relatively low-efficacy agonist in vitro and in vivo (e.g., Semba et al., 1995; Urban et al., 2007; Tadori et al., 2011). A second goal of the present study was to examine the relationship between intrinsic efficacy and ability to alter the reinforcing effects of cocaine. To address this question, the effects of ARI were compared with those of the high-efficacy D2/D3 receptor agonist (−)−NPA and the D2/D3 receptor antagonist eticlopride (ETIC).
In addition to pharmacological factors, effects of D2/D3 receptor agonists can be modulated by subject factors, including position in the social dominance hierarchy. Several studies have shown that the effects of cocaine and d-amphetamine on social behavior differ according to social rank (e.g., Miczek and Gold, 1983). We have demonstrated that becoming dominant, but not subordinate, increases D2/D3 receptor availability as measured with positron emission tomography, and that dominant and subordinate male monkeys are differentially sensitive to cocaine (Morgan et al., 2002; Czoty et al., 2005). Thus, another objective was to assess whether monkeys were differentially sensitive to these D2/D3 drugs according to social rank. The effects of treatment drugs were assessed on the relative reinforcing strength of cocaine using a food-drug choice procedure in which responding by monkeys was contingent on food pellets or injections of increasing doses of cocaine within a daily session (Negus, 2003). One advantage of choice procedures is that, because the dependent variable is a measure of response allocation independent of response rates, measures of relative reinforcing strength are less sensitive to disruptive effects of treatment drugs. In addition, choice procedures may more closely resemble the clinical condition in which drug use occurs in the context of forfeiting access to another reinforcer (e.g., food or money; Katz, 1990).
Materials and Methods
Subjects.
Twenty-one adult male cynomolgus monkeys (Macaca fascicularis), ranging in age from 8.5 to 17.5 years, served as subjects. Seven of these monkeys were used to characterize acute effects of ARI, (−)−NPA, and ETIC on food-maintained responding. Five of these monkeys were treated for 17 days with ARI approximately 2–3 months prior to the start of this study (unpublished observation). The remaining 14 monkeys were trained to self-administer cocaine under a concurrent schedule (see following paragraphs); they had not been tested with any drug pretreatment, but were previously exposed to various environmental manipulations (Czoty and Nader, 2012). Each monkey was fitted with a nylon collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products). Monkeys were weighed weekly and fed enough food daily [Purina Monkey Chow (Nestlé Purina PetCare Company, St. Louis, MO) and fresh fruit and vegetables] to maintain body weights at approximately 95% of free-feeding levels. Body weights did not change significantly during this study and were not different between dominant and subordinate monkeys. Water was available ad libitum in the home cage.
Monkeys lived in stainless steel cages (0.71 × 1.73 × 1.83 m; Allentown Caging Equipment, Co., Allentown, NJ) with removable wire mesh partitions that separated monkeys into quadrants (0.71 × 0.84 × 0.84 m). Monkeys were separated daily for several hours during operant behavioral sessions and feeding. Social status had previously been determined for each monkey according to the outcomes of agonistic encounters using procedures similar to those described previously (see Kaplan et al., 1982; Czoty et al., 2005, 2009). Briefly, two observers separately conducted several 15-minute observation sessions per pen. Aggressive, submissive, and affiliative behaviors were recorded according to an ethogram described previously (see Table 1 in Morgan et al., 2000) using Noldus Observer software (Noldus Information Technology, Wageningen, The Netherlands). In these focal group sessions, both initiators and recipients of behaviors were recorded. The monkey in each pen aggressing toward all others and submitting to none was ranked number 1 (most dominant). The number 2–ranked monkey aggressed toward all but the number 1–ranked monkey; rather, the number 2 monkey submitted to the number 1 monkey. The number 3–ranked monkey aggressed toward only the number 4–ranked monkey and submitted toward the number 1– and number 2–ranked pen-mates. The monkey designated most subordinate (number 4) displayed a low frequency of aggressive behaviors and submitted to all other monkeys in the pen. Thus, these hierarchies were linear and transitive, and they did not change during the time these experiments were conducted. For the present study, number 1– and number 2–ranked monkeys were considered dominant, and number 3– and number 4–ranked monkeys were considered to be subordinate. Animal housing and handling and all experimental procedures were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, and were approved by the Animal Care and Use Committee of Wake Forest University. Environmental enrichment was provided as outlined in the Animal Care and Use Committee of Wake Forest University Non-Human Primate Environmental Enrichment Plan.
Catheter Implantation.
Each monkey had been prepared with an indwelling venous catheter and subcutaneous vascular access port (VAP; Access Technologies, Skokie, IL) under sterile surgical conditions. An antibiotic (30 mg/kg of kefzol, i.m.; cefazolin sodium; Marsam Pharmaceuticals, Inc., Cherry Hill, NJ) was administered 1 hour prior to surgery. Anesthesia was induced with ketamine (15 mg/kg, i.m.) and maintained with ketamine supplements. A catheter was inserted into a major vein (femoral or internal or external jugular) to the level of the posterior vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was attached to a VAP, which was placed in a pocket formed by blunt dissection.
Apparatus and General Behavioral Procedures.
Five days per week, monkeys were separated by partitioning the living space into quadrants. Next, each monkey was seated in a primate chair and placed in a ventilated, sound-attenuating chamber (1.5 × 0.74 × 0.76 m; Med Associates, St. Albans, VT). The back of the animal was cleaned with 95% ethanol and chlorhexidine, and the VAP was connected to an infusion pump (Cole-Parmer Instrument Co., Niles, IL) located outside the chamber via a 20-gauge Huber Point Needle (Access Technologies) and tubing. The pump was operated for approximately 3 seconds to fill the port and catheter with the concentration of cocaine available for the session. Two photo-optic switches (model 117-1007; Stewart Ergonomics, Inc., Furlong, PA) were located on one side of the chamber with a horizontal row of three stimulus lights positioned 14 cm above each switch. The switches were positioned to be easily within reach of the monkey seated in the primate chair. A food receptacle, above which was a single white stimulus light, was located between the switches and connected with a Tygon tube to a pellet dispenser (Med Associates) located on the top of the chamber for delivery of 1-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ). At the conclusion of each behavioral session, monkeys were returned to their home cages. Partitions were left in place for 60–90 minutes, during which time the monkeys were fed.
Food-Maintained Responding.
Effects of ARI, (−)−NPA, and ETIC on food-maintained responding were studied in seven monkeys (one dominant, two subordinate, and four individually housed monkeys who did not participate in any other experiments described in this manuscript). Monkeys had previously been trained to respond under a 50-response fixed-ratio (FR 50) schedule of food pellet presentation. Sessions lasted until 30 reinforcers had been received or 1 hour had elapsed, whichever came first. An i.v. injection of vehicle, ARI (0.01–0.1 mg/kg, n = 6), (−)−NPA (0.001–0.01 mg/kg, n = 6), or ETIC (0.001–0.1 mg/kg, n = 5) was administered 30 minutes [ARI, (−)−NPA, and their vehicles) or 3 minutes (ETIC and its vehicle) prior to the start of a session. Pretreatment times were based on previous studies in monkeys (Nader et al., 1999; Bergman, 2008). Drugs were administered on Tuesdays and Fridays, and vehicle (control) experiments were conducted on Thursdays. Each dose was at least double determined, except in cases in which the highest tested doses resulted in prominent reductions in response rate. Additional tests were conducted in which 0.1 mg/kg ARI was administered once at pretreatment times ranging from 5 minutes to 18 hours (n = 5).
Food-Cocaine Choice.
Monkeys were trained to self administer cocaine under a concurrent FR schedule of food and cocaine availability using a procedure similar to that described by Negus (2003). Monkeys were initially trained to respond using food reinforcement. To make a response, the monkey inserted his finger into a 2.5-cm opening in the photo-optic switch, which broke a photobeam, recorded a response, and activated a relay that provided auditory feedback to the monkey. It was necessary for the monkey to completely withdraw his finger before another response could be counted. Following initial exposure to FR contingencies on each switch, training under the choice procedure began under a concurrent FR 30 schedule. Responding on one switch (henceforth termed the “food switch”) always resulted in delivery of a single food pellet; the yellow light above this switch was illuminated during pellet availability. Responding on the other switch (henceforth termed the “drug switch”) resulted in activation of the infusion pump and an injection of cocaine (0.003–0.1 mg/kg per injection). Availability of each cocaine dose was associated with illumination of a different set of stimulus lights above the switch; different cocaine doses were studied by varying the duration of pump activation (see Czoty and Nader, 2012). If a response was emitted on the alternate switch before an FR was completed, the response requirement on the first switch was reset. Assignment of food or drug to a switch was counterbalanced across monkeys. Delivery of either reinforcer was accompanied by illumination of the red light above the corresponding switch (for 5 seconds after a pellet delivery or during an injection) and a subsequent period during which all lights remained off and responding had no scheduled consequences. The total time-out duration was 30 seconds. Initial training sessions consisted of one component in which monkeys chose between food and a single dose of cocaine in the presence of the appropriate discriminative stimuli. These sessions ended after 60 minutes had elapsed or 30 total reinforcers were earned, whichever occurred first. Once monkeys had experienced approximately 10 such sessions per cocaine dose, terminal schedule conditions were enacted for subsequent sessions.
Each daily session consisted of five components in which monkeys chose between food pellets and ascending doses of cocaine (i.e., no injection, 0.003, 0.01, 0.03, and 0.1 mg/kg per injection cocaine in components 1–5, respectively). Each component ended when 10 total reinforcers had been earned or 20 minutes had elapsed, whichever came first; a 120-second time-out followed each component. Ratio requirements for food and cocaine were adjusted for each monkey such that allocation of responding to the drug switch increased during the session as the available dose of cocaine increased. Responding was considered stable when ≤20% of reinforcers were earned on the drug switch when the alternative to food was no injection (component 1) or 0.003 mg/kg per injection cocaine (component 2), and when ≥80% of reinforcers were earned on the drug switch when the alternative to food was 0.1 mg/kg per injection cocaine (component 5). An additional criterion was observation of a dose-related increase in drug choice. A complete dose-effect curve was determined for each monkey each day, typically 5 days per week.
Once responding was stable, drug treatments were started in dominant (dom) and subordinate (sub) monkeys under three dosing regimens. First, ARI, (−)−NPA, and ETIC were administered acutely (0.01–0.056 mg/kg ARI in five doms and five subs; 0.001–0.0056 mg/kg (−)−NPA in four doms and four subs; and 0.01–0.1 mg/kg ETIC in four doms and three subs). Effects of most doses were at least double determined, except for the lowest doses that were ineffective and in cases when the highest doses were observed to disrupt behavior in several monkeys. Second, ARI (0.01–0.1 mg/kg in four doms and five subs) and (−)−NPA (0.001–0.0056 mg/kg in four doms and 5 subs) were administered for 5 consecutive days at the same time in the morning. Cocaine self-administration sessions were only conducted on days 1 and 5 of treatment 30 minutes after drug administration. Finally, to examine whether the effects of 0.056 mg/kg of ARI differed under a different regimen of cocaine exposure, the latter experiment was repeated in the four dominant monkeys, except that cocaine self-administration sessions occurred every day during treatment.
Data Analysis.
Data for acute drug pretreatments on food-maintained responding and cocaine choice were analyzed using repeated-measures one- or two-way analyses of variance (ANOVAs) with post hoc Dunnett’s or Fisher’s least significant difference multiple comparisons tests, respectively. Data on number of reinforcers earned and cocaine intake during the studies of acute drug treatment were analyzed using paired t tests between drug and baseline conditions. In all cases, differences were considered significant when P < 0.05. Daily ED50 values were also determined using the linear portion of the curve that crossed 50% choice. ED50 values were compared using independent or paired t tests, as appropriate. For studies involving acute pretreatment with ARI, NPA, and ETIC, the magnitude of change in ED50 (“shift”) was expressed in log units in Table 2.
TABLE 2.
ED50 values (mg/kg) for cocaine choice at baseline and after acute administration of ARI, (−)−NPA, and ETIC Magnitude of change in ED50 shift of curve is expressed in log units.
| BL | ARI | Shift | BL | (−)−NPA | Shift | BL | ETIC | Shift | |
|---|---|---|---|---|---|---|---|---|---|
| Dominant | |||||||||
| C-7079 | 0.017 | 0.010 | −0.23 | 0.012 | 0.173 | 1.16 | 0.015 | 0.005 | −0.48 |
| C-6527 | 0.014 | 0.073 | 0.72 | 0.014 | 0.017 | 0.08 | 0.015 | 0.173 | 1.06 |
| C-6526 | 0.013 | 0.003 | −0.72 | Not tested | 0.012 | 0.002 | −0.76 | ||
| C-7081 | 0.010 | 0.007 | −0.15 | 0.006 | 0.009 | 0.18 | Not tested | ||
| C-6214 | 0.015 | 0.015 | 0.00 | Not tested | Not tested | ||||
| C-7426 | Not tested | 0.023 | 0.015 | −0.19 | 0.025 | 0.036 | 0.16 | ||
| Mean | −0.08 | 0.31 | −0.00 | ||||||
| S.E.M. | 0.18 | 0.21 | 0.28 | ||||||
| Subordinate | |||||||||
| C-6529 | 0.01 | 0.003 | −0.52 | 0.008 | 0.041 | 0.71 | 0.013 | 0.003 | −0.72 |
| C-6625 | 0.017 | 0.008 | −0.33 | 0.015 | 0.002 | −0.88 | 0.010 | 0.011 | 0.04 |
| C-7424 | 0.025 | 0.003 | −1.00 | 0.014 | 0.042 | 0.48 | 0.008 | 0.005 | −0.20 |
| C-7083 | 0.017 | 0.003 | −0.75 | 0.007 | 0.004 | −0.24 | Not tested | ||
| C-7425 | 0.012 | 0.003 | −0.60 | Not tested | Not tested | ||||
| Mean | −0.64 | 0.02 | −0.29 | ||||||
| S.E.M. | 0.13 | 0.36 | 0.14 |
ARI, aripiprazole; BL, baseline; ETIC, eticlopride; (−)−NPA, R(−)−norpropylapomorphine.
Drugs.
(−)−Cocaine was supplied by the National Institutes of Health National Institute on Drug Abuse (Bethesda, MD), and was dissolved in sterile 0.9% saline. ARI was obtained from Toronto Research Chemicals, Inc. (Ontario, Canada) and was dissolved in a vehicle of 4% Tween 80 in sterile water. (−)−NPA and ETIC were obtained from Sigma-Aldrich (St. Louis, MO). (−)−NPA was dissolved in a vehicle consisting of 1.0 mg/ml ascorbic acid in sterile water. ETIC was dissolved in sterile 0.9% saline.
Results
Effects of Acute Drug Treatment on Food-Maintained Responding.
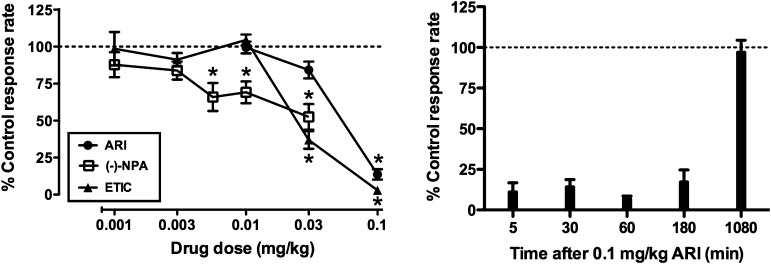
Mean response rates under the FR 50 schedule of food pellet presentation ranged from 1.0 to 3.3 responses per second. ARI, (−)−NPA, and ETIC produced dose-related decreases in response rates when behavior was maintained by food pellet delivery (Fig. 1, left). ANOVA performed on raw data indicated a main effect of dose for each drug [ARI: F(3,15) = 66.03; (−)−NPA: F(5,25) = 8.92; ETIC: F(5,20) = 79.26; P < 0.001 for all). Figure 1 indicates specific doses at which response rates were significantly less than those observed after saline/vehicle administration (P < 0.05). ARI was the least potent of the three drugs tested. Suppression of response rates by the highest dose of ARI lasted at least 3 hours, but was absent when ARI was administered 18 hours prior to the behavioral session (Fig. 1, right).
Fig. 1.
Effects of ARI (n = 6), (−)−NPA (n = 6), and ETIC (n = 5) on food-maintained responding. (Left) Ordinate: percentage of response rate measures after vehicle administration. Abscissa: drug dose (mg/kg). Data represent the mean ± S.E.M. *P < 0.05 compared with baseline response rates. (Right) Same as the left graph, except n = 5 and the abscissa represents the time after administration of 0.1 mg/kg ARI.
Effects of Acute Drug Treatment on Food-Cocaine Choice.
Monkeys showed a certain degree of individual differences in sensitivity to the effects of ARI, (−)−NPA, and ETIC. Therefore, a “best dose” approach was used, whereby a dose that produced the greatest effect on cocaine choice without disrupting responding (defined as a 33% decrease in total reinforcers earned in a session) was selected for each monkey. Table 1 lists the selected doses for the analysis of group-averaged data. Table 2 lists ED50 values for cocaine choice at baseline and after administration of the selected dose for all subjects according to social rank. Social rank did not have an orderly effect on sensitivity to acute administration of ARI, (−)−NPA, or ETIC; thus, data for this experiment were collapsed across social ranks. One qualitative exception to this lack of effect of social rank is that the effects of ARI were more consistent in subordinate monkeys, in that all subordinates showed leftward shits in the dose-effect curve compared with only three of five dominant monkeys (Table 2).
TABLE 1.
Highest doses (mg/kg) of ARI, (−)−NPA, and ETIC that did not disrupt responding in each monkey
| ARI | (−)−NPA | ETIC | |
|---|---|---|---|
| Dominant | |||
| C-7079 | 0.1 | 0.0056 | 0.03 |
| C-6527 | 0.1 | 0.0056 | 0.03 |
| C-6526 | 0.056 | Not tested | 0.03 |
| C-7081 | 0.056 | 0.0056 | Not tested |
| C-6214 | 0.056 | Not tested | Not tested |
| C-7426 | Not tested | 0.0056 | 0.03 |
| Subordinate | |||
| C-6529 | 0.056 | 0.0056 | 0.1 |
| C-6625 | 0.056 | 0.0056 | 0.03 |
| C-7424 | 0.056 | 0.0056 | 0.03 |
| C-7083 | 0.056 | 0.003 | Not tested |
| C-7425 | 0.056 | Not tested | Not tested |
ARI, aripiprazole; ETIC, eticlopride; (−)−NPA, R(−)−norpropylapomorphine.
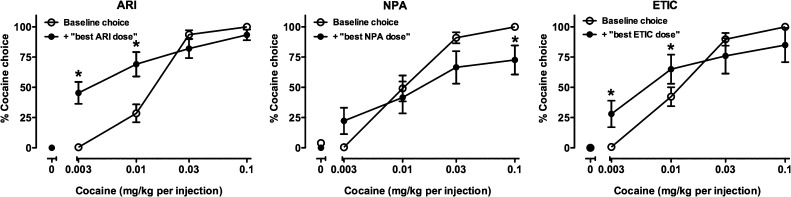
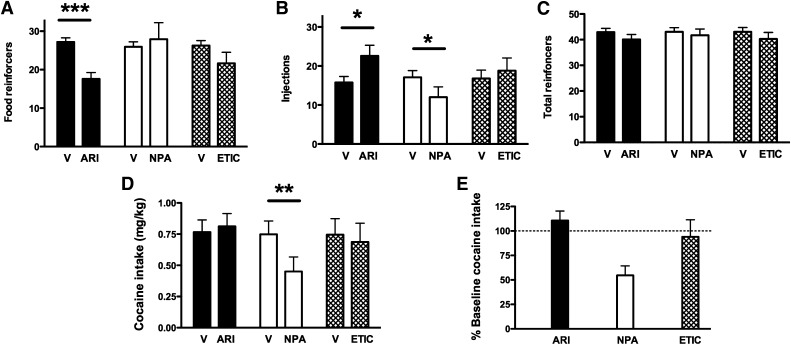
Acute administration of ARI produced an increase in cocaine choice when a low and intermediate dose was available, but did not alter choice of higher doses of cocaine (Fig. 2, left). Two-way ANOVA revealed a significant main effect of ARI treatment [F(1,9) = 5.50, P < 0.05] and cocaine dose [F(4,36) = 147.12, P < 0.001] and a significant interaction [F(4,36) = 17.235, P < 0.001]. ARI treatment significantly increased choice of 0.003 and 0.01 mg/kg per injection cocaine relative to baseline (both P < 0.001). In addition, paired t tests revealed that ARI treatment produced a significant decrease in food reinforcers earned (Fig. 3A; P < 0.001) and a significant increase in injections received (Fig. 3B; P < 0.05), which resulted in no significant change in total reinforcers earned (Fig. 3C). Because the increase in injections occurred when low doses of cocaine were available, total session intake was not affected by ARI treatment (Fig. 3, D and E). In contrast to the low-efficacy agonist ARI, the high-efficacy agonist (−)−NPA did not significantly alter choice of lower cocaine doses, but significantly decreased choice of the highest dose of cocaine (P < 0.05; Fig. 2, center). There was a main effect of cocaine dose [F(4,36) = 21.3, P < 0.001] and a significant interaction [F(4,36) = 5.01, P < 0.01], but no main effect of (−)−NPA treatment. (−)−NPA treatment significantly decreased the number of injections received (P < 0.05) without altering food pellets or total reinforcers earned (Fig. 3, A–C), and significantly decreased overall session intake (P < 0.01; Fig. 3, D and E). When the D2/D3 receptor antagonist ETIC was administered acutely (Fig. 2C), choice of lower cocaine doses was significantly increased (P < 0.05; Fig. 2, right). Overall, there was a main effect of cocaine dose [F(4,36) = 17.45, P < 0.001] and a significant interaction [F(4,36) = 7.92, P < 0.001], but no main effect of ETIC treatment. ETIC treatment did not significantly alter the number of reinforcers earned or intake (Fig. 3, D and E).
Fig. 2.
Effects of acute administration of ARI (n = 10), (−)−NPA (n = 8), and ETIC (n = 7) on cocaine choice. Ordinates: percentage of reinforcers earned on the cocaine-associated switch. Abscissa: dose of cocaine (mg/kg) available as an alternative to a food pellet. Data represent the mean ± S.E.M. *P < 0.05 compared with baseline responding.
Fig. 3.
Effects of acute administration of ARI, (−)−NPA, and ETIC on choice behavior. Effects of treatment drugs on number of food pellets (A), injections (B), and total reinforcers earned (C), as well as total session cocaine intake in raw numbers (D) and expressed as a percentage of baseline cocaine intake (E). V, vehicle. *P < 0.05; **P < 0.01; ***P < 0.001.
Effects of Repeated ARI Treatment on Food-Cocaine Choice.
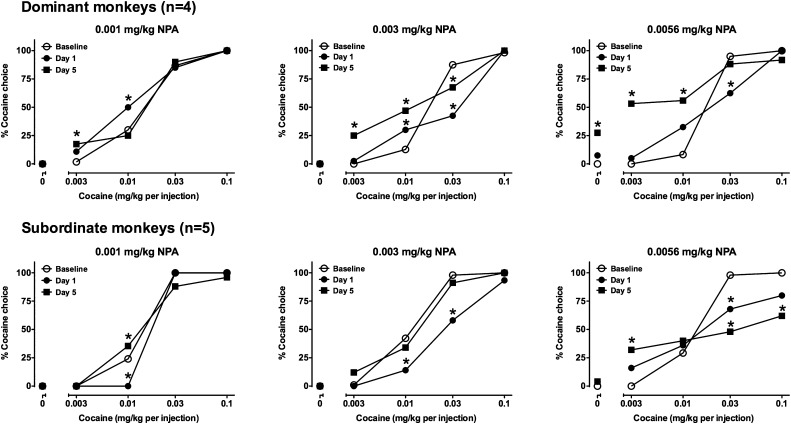
ARI (0.01–0.056 mg/kg; Fig. 4) and (−)−NPA (0.001–0.0056 mg/kg; Fig. 5) were administered to dominant and subordinate monkeys for 5 consecutive days as i.v. injections 30 minutes prior to the self-administration session on days 1 and 5, and at a comparable time on days 2–4 in the absence of a subsequent self-administration session. Data were analyzed using a two-way repeated-measures ANOVA with treatment day (baseline, day 1, day 5) and cocaine dose as factors. Because cocaine choice always increased in a dose-related manner, a main effect of cocaine dose was observed in every analysis [F(4,28) = 2.28–9.08, P < 0.05]. Because effects on cocaine choice tended to be observed at either higher or lower cocaine doses, but not both, no main effects of treatment day were observed (with one exception noted below), and a significant interaction [F(8,56) = 2.49–7.57, P < 0.001 for all] was observed in each analysis.
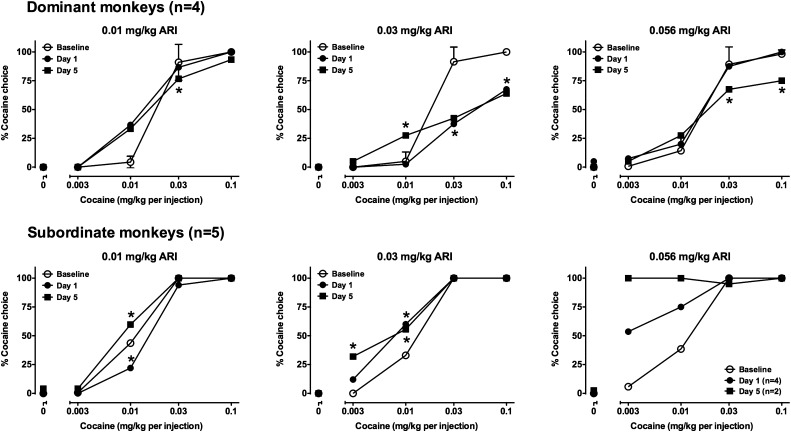
Fig. 4.
Effects of 5 days of treatment with ARI on cocaine choice in dominant (top row, n = 4) and subordinate monkeys (bottom row, n = 5). Some error bars have been removed for clarity. Graphs from left to right in each row show data from experiments using ascending ARI doses. Ordinates: percentage of reinforcers earned on the cocaine-associated switch. Abscissa: dose of cocaine (mg/kg) available as an alternative to a food pellet. Data represent the mean ± S.E.M. *P < 0.05 compared with baseline responding.
Fig. 5.
Effects of 5 days of treatment with (−)−NPA on cocaine choice in dominant (top row, n = 4) and subordinate monkeys (bottom row, n = 5). Graphs from left to right in each row show data from experiments using ascending ARI doses. Ordinates: percentage of reinforcers earned on the cocaine-associated switch. Abscissa: dose of cocaine (mg/kg) available as an alternative to a food pellet. Data represent the mean ± S.E.M. *P < 0.05 compared with baseline responding.
In dominant monkeys (Fig. 4, top row), on average, 0.01 mg/kg ARI produced an increase in choice of 0.01 mg/kg cocaine in some animals that was maintained when monkeys were tested on day 5, and a slight but significant decrease in choice of 0.03 mg/kg cocaine. An intermediate dose of ARI (0.03 mg/kg) significantly (P < 0.01) decreased choice of higher cocaine doses on day 1. Although average cocaine choice was similar on day 5, the effect was no longer statistically significant. In addition, on day 5, a significant (P < 0.01) increase in choice of 0.01 mg/kg cocaine was observed. A higher dose of ARI (0.056 mg/kg) was without effect on the first day of treatment, but when monkeys were tested on day 5, the cocaine choice curve was displaced downward at the upper end; post hoc tests indicated that choice of 0.03 and 0.1 mg/kg cocaine was significantly decreased compared with baseline (P < 0.01). Effects of ARI on cocaine choice were qualitatively different in subordinate monkeys (Fig. 4, bottom row). In subordinates, 0.01 mg/kg produced modest, opposite effects on days 1 and 5, and administration of 0.03 mg/kg ARI significantly (P < 0.01) increased choice of lower doses of cocaine without affecting choice of higher cocaine doses; a main effect of 0.03 mg/kg ARI treatment was found [F(2,14) = 3.879, P < 0.05]. Treatment with 0.056 mg/kg ARI produced behaviorally disruptive effects in one monkey on day 1 and in that same monkey and two additional subjects on day 5. Because these response rate–decreasing effects resulted in monkeys making no responses in three or more components, their data are excluded from presentation in Fig. 4, and for this reason, these data were not analyzed with ANOVA. In the remaining monkeys, however, choice of lower cocaine doses was substantially increased on day 1, and increased further (in the two subjects whose behavior was not disrupted) on day 5.
Effects of Repeated (−)−NPA Treatment on Food-Cocaine Choice.
As observed for ARI treatment, in all repeated (−)−NPA experiments, there was a main effect of cocaine dose [F(4,32) = 5.81–9.71, P ≤ 0.001] but not treatment, and a significant interaction [F(8,64) = 2.63–7.90, P ≤ 0.001 for all]. When 0.001 mg/kg (−)−NPA was administered to either dominant or subordinate monkeys, no substantial, lasting effects were observed (Fig. 5, left panels). In dominant monkeys, a higher dose (0.003 mg/kg) significantly increased choice of 0.01 mg/kg cocaine (P < 0.01) but decreased choice of a higher dose on day 1 (P < 0.01). On day 5, the increase in choice of 0.01 mg/kg cocaine was more pronounced, and choice of the lowest dose was significantly increased (P < 0.001 and P < 0.01, respectively), whereas the decrease in choice of 0.03 mg/kg cocaine was smaller on day 5 (but still significant; P < 0.05). At the highest (−)−NPA dose tested (0.0056 mg/kg), dominant monkeys chose the two lower doses more frequently on day 5 compared with baseline (P < 0.001 for both), but choice of higher cocaine doses was not affected. This dose of (−)−NPA also resulted in a significant (P < 0.05) amount of responding on the drug switch during the first component in which the choice was between food and no injection. Effects of 0.003 and 0.056 mg/kg (−)−NPA differed qualitatively in subordinate monkeys compared with dominants. The intermediate dose produced a downward shift of the choice curve on day 1, but tolerance developed by day 5. Treatment with 0.0056 mg/kg (−)−NPA, however, produced a progressively greater decrease in choice of higher cocaine doses, whereas choice of the lowest cocaine dose increased on day 5 [P < 0.05 for all except 0.03 mg/kg cocaine on day 5 (P = 0.001)].
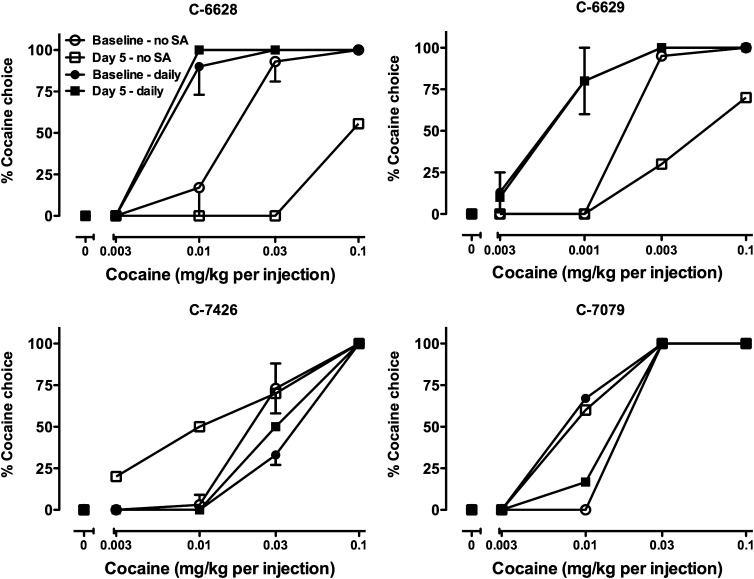
Effects of Daily Self-Administration on Repeated ARI Treatment.
In a final experiment in the four dominant monkeys, 0.056 mg/kg ARI was again administered for 5 consecutive days, but unlike the previous experiment in which self-administration did not occur on days 2–4 (open symbols in Fig. 6), a cocaine choice session was conducted on each day of treatment (filled “daily” symbols in Fig. 6). As shown in Fig. 6, the effects of 5 days of ARI treatment were eliminated in three of the four monkeys (C-6628, C-6629, and C-7426) and reversed in C-7079 when cocaine was self-administered every day.
Fig. 6.
Effects of 5 days of treatment with 0.056 mg/kg ARI on cocaine choice in the presence or absence of daily cocaine self-administration during treatment. Each graph depicts data from an individual subject at baseline and on day 5 of ARI treatment. Open symbols indicate data collected when monkeys did not self-administer cocaine during treatment (no SA), whereas filled symbols indicate data when monkeys self-administered cocaine every day during treatment (daily). Ordinates: percentage of reinforcers earned on the cocaine-associated switch. Abscissa: dose of cocaine (mg/kg) available as an alternative to a food pellet. Data represent the mean ± S.D.
Discussion
The primary objective of the present study was to examine the effects of the dopamine D2/D3 receptor partial agonist ARI on cocaine choice. The effects of ARI were compared with those of the high-efficacy agonist (−)−NPA and the antagonist eticlopride to gain a clearer understanding of the relationship between intrinsic efficacy and behavioral effects. Initial experiments involving food-maintained responding noted that all three drugs decreased response rates in a dose-dependent manner, with ARI being the least potent. When administered acutely to monkeys choosing between cocaine and food, ARI and ETIC increased choice of low doses of cocaine, whereas (−)−NPA had a more desirable preclinical profile: it decreased the reinforcing strength of higher cocaine doses as well as cocaine intake. Although effects of acute treatment did not differ between dominant- and subordinate-ranked monkeys, when ARI and (−)−NPA were administered for 5 days, divergent effects were observed across social ranks. ARI tended to decrease choice of higher cocaine doses in dominant monkeys, but disrupted subordinates’ behavior or increased choice of lower cocaine doses. In contrast, (−)−NPA increased low-dose cocaine choice in dominant monkeys but decreased choice of higher doses in subordinates. The results suggest that the effects of repeated treatment on the reinforcing effects of cocaine with D2/D3 receptor agonists differ according to the duration of treatment, intrinsic efficacy, and subject factors associated with social dominance or subordination. A final experiment indicated that these effects on cocaine self-administration can also be influenced by whether monkeys self-administer cocaine during treatment.
Effects of Acute Administration of ARI, (−)−NPA, and ETIC on Food-Maintained Responding and Food-Cocaine Choice.
Initial experiments in monkeys responding under an FR schedule of food presentation indicated that the behavioral effects of ARI were present for more than 3 hours but dissipated by 18 hours after administration. This places the half-life of ARI in cynomolgus monkeys clearly between its half-life in humans (∼60 hours; McGavin and Goa, 2002; Mallikaarjun et al., 2004) and rodents (∼2 hours; Shimokawa et al., 2005). Although all three drugs only decreased food-maintained responding under an FR contingency, when tested in a food-cocaine choice paradigm, acute administration of drugs revealed individual-subject variability in sensitivity to the effects of ARI, (−)−NPA, and ETIC, with increases in behavior noted. For group comparisons, we used a “best dose” approach, using the highest tested dose, for each monkey, that did not produce behavioral disruption, which was defined as a ≥33% decrease in total reinforcers (Table 1). In these experiments, ARI and ETIC significantly increased choice of lower cocaine doses without affecting choice of higher doses or overall session cocaine intake. This profile of effects on cocaine choice is similar to that observed previously for D2/D3 receptor antagonists (Woolverton and Balster, 1981; Negus, 2003). Thus, although ARI has been shown to have agonist activity in some assays, its acute effects on cocaine choice were indistinguishable from those of D2/D3 receptor antagonists. In contrast, the high-efficacy agonist (−)−NPA produced more promising effects on cocaine choice, significantly decreasing choice of the highest cocaine dose without affecting other doses. These effects are similar to those of the DA releaser and indirect DA agonist d-amphetamine (Negus, 2003), although the magnitude of effect with (−)−NPA that was observed in the absence of marked disruption of responding was substantially less. Moreover, these results are similar to those of clinical studies that suggest that DA agonists have more promise as medications for cocaine dependence than antagonists (e.g., Grabowski et al., 2000, 2001; Haney et al., 2001, 2011). It is worth noting that ARI and (−)−NPA appeared more potent when behavior was maintained under a concurrent schedule compared with a simple FR schedule of reinforcement, supporting the long-known powerful role of environmental context in drug action (Barrett and Katz, 1981).
Effects of Chronic Administration of ARI and (−)−NPA on Cocaine Choice.
Characterizing the acute effects of drugs on behavior is instructive, but understanding a drug’s effects when administered chronically is more relevant to its ultimate clinical use. For example, development of tolerance or sensitization to the therapeutic effects of a drug could change its pharmacological profile compared with what is observed upon acute administration. Moreover, the emergence of behaviorally disruptive side effects could render the drug less useful in humans. Thus, ARI and (−)−NPA were administered to dominant and subordinate monkeys for 5 consecutive days with cocaine choice sessions conducted on days 1 and 5. ARI produced qualitatively different effects in dominant monkeys and subordinates. In dominants, ARI decreased choice of higher cocaine doses, a desirable effect for a candidate medication. In subordinates, however, ARI either increased choice of lower cocaine doses without affecting high doses or disrupted behavior. We had hypothesized that partial agonists, such as ARI, would be more effective under conditions in which extracellular DA concentrations were low. Using positron emission tomography, differences have been noted in D2/D3 receptor availability in dominant and subordinate monkeys, which led to differential sensitivity to the reinforcing effects of cocaine (Morgan et al., 2002; Czoty et al., 2010; Czoty and Nader, 2012; Nader et al., 2012). Dominant monkeys have higher D2/D3 receptor availability, which may be a result of lower extracellular concentrations of DA, making them more likely to show positive effects with a D2/D3 partial agonist.
Similar to repeated ARI administration, 5-day (−)−NPA treatment also produced divergent effects across social ranks, but with a more encouraging preclinical profile in subordinate monkeys. In dominants, (−)−NPA increased choice of lower doses and had fewer pronounced effects on higher cocaine doses. Moreover, on day 5 of treatment with 0.0056 mg/kg (−)−NPA, dominant monkeys earned approximately 30% of reinforcers on the injection-associated switch when responding resulted in no injections. Such responding may indicate that, on day 5, (−)−NPA produced cocaine-like discriminative stimulus effects and functioned like a “priming” dose of cocaine. Regardless of the mechanism, this result and the increase in choice of low cocaine doses in the absence of any sustained decrease in choice is not a desirable characteristic of a putative pharmacotherapy. In contrast, much like the effects of ARI in dominant monkeys, (−)−NPA was effective in significantly decreasing cocaine choice in subordinates when high cocaine doses were available. It may be that the different discriminative stimuli used in this choice paradigm differentially affect extracellular DA concentrations in dominant and subordinate monkeys, making some monkeys more sensitive to partial agonists/antagonists and others to full agonists (Bradberry, 2000).
Importance of Self-Administering Cocaine During Treatment.
ARI was effective in decreasing cocaine choice in dominant monkeys on the fifth day of treatment. This result differed from a study in rats with a similar experimental design in which tolerance developed by day 5 (Thomsen et al., 2008). Although species differences in the intrinsic efficacy of DA receptor agonists have been reported (c.f. Weed and Woolverton, 1995), 30-day ARI treatment in squirrel monkeys was also found to lack an effect on cocaine choice (Bergman, 2008). One procedural difference that may resolve this apparent inconsistency is that, unlike the study in rats, the monkeys did not self-administer cocaine daily during ARI treatment in the present experiment. To examine this possibility, the 5-day treatment with 0.056 mg/kg ARI was repeated in the four dominant monkeys in whom, on average, 0.056 mg/kg ARI decreased choice of higher cocaine doses. When animals had the opportunity to self-administer cocaine daily during ARI treatment, the effects of ARI were substantially attenuated. Taken together, it appeared that daily exposure to cocaine during exposure to the low-efficacy agonist mitigated its effects on day 5.
One mechanism for this effect could be that manifestation of behavioral effects of a chronically administered antagonist, or in this case, a very low-efficacy agonist, requires prolonged antagonism of D2/D3 receptors. However, this effect would be counteracted by increases in extracellular DA resulting from daily cocaine self-administration, resulting in a reduced net effect of treatment with the antagonist. Interestingly, this hypothesis—that chronically administered antagonists/low-efficacy agonists would be more effective in reducing the reinforcing effects of cocaine in the absence of daily cocaine self-administration—appears to be the opposite of what would be expected with indirect agonists, which would presumably have effects more similar to high-efficacy agonists. For example, chronic d-amphetamine administration decreases cocaine self-administration under several schedules of reinforcement in rats and monkeys, but only when cocaine self-administration occurs during d-amphetamine treatment (e.g., Negus and Mello, 2003; Chiodo et al., 2008; Czoty et al., 2011). Further studies are needed to fully understand the neuropharmacological and behavioral mechanisms that contribute to these divergent effects on cocaine reinforcement according to the intrinsic efficacy of potential pharmacotherapies. Regardless, these relationships suggest that a substitute agonist approach may be more effective when frequent cocaine use might be anticipated during treatment, whereas treatment with an antagonist or low-efficacy agonist might be more efficacious in preventing relapse during abstinence.
In summary, ARI was more effective in decreasing cocaine choice in dominant monkeys, whereas (−)−NPA was more effective in subordinate monkeys. It is noteworthy that these clear group differences were not apparent when the drugs were administered acutely. These data suggest that the effects of DA receptor direct agonists and antagonists, similar to cocaine, differ as a function of rank in the social dominance hierarchy. Thus, it is possible that pre-existing differences in D2/D3 receptor availability may underlie differential responses to drugs that target these receptors, as is clearly true for stimulants in monkeys and humans (e.g., Volkow et al., 1999; Morgan et al., 2002). These findings underscore the conclusion that a given drug treatment should not be expected to have the same effectiveness in all patients, but that different pharmacotherapeutic strategies may be optimal for different individuals.
Acknowledgments
The authors acknowledge the technical assistance of Michael Coller and Michelle Icenhower Bell.
Abbreviations
- ANOVA
analysis of variance
- ARI
aripiprazole
- DA
dopamine
- dom
dominant
- ETIC
eticlopride
- FR 50
50-response fixed ratio
- (−)−NPA
R(−)−norpropylapomorphine
- sub
subordinate
- VAP
vascular access port
Authorship Contributions
Participated in research design: Czoty, Nader.
Conducted experiments: Czoty, Nader.
Performed data analysis: Czoty.
Wrote or contributed to the writing of the manuscript: Czoty, Nader.
Footnotes
The research described in this manuscript was supported by grants from the National Institutes of Health National Institute on Drug Abuse [DA 10584 and DA 12460].
References
- Barrett JE, Katz JL. (1981) Drug effects on behaviors maintained by different events, in Advances in Behavioral Pharmacology, Vol. 3 (Thompson T, Dews PB, McKim WA, eds, ed) pp 119–168, Academic Press, New York, NY [Google Scholar]
- Bergman J. (2008) Medications for stimulant abuse: agonist-based strategies and preclinical evaluation of the mixed-action D-sub-2 partial agonist aripiprazole (Abilify). Exp Clin Psychopharmacol 16:475–483 [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. (1990) Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol 1:355–363 [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. (1989) Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther 251:150–155 [PubMed] [Google Scholar]
- Bradberry CW. (2000) Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci 20:7109–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. (2002) Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302:381–389 [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. (1999) Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem 42:2721–2736 [DOI] [PubMed] [Google Scholar]
- Childress AR, O’Brien CP. (2000) Dopamine receptor partial agonists could address the duality of cocaine craving. Trends Pharmacol Sci 21:6–9 [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Läck CM, Roberts DC. (2008) Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 200:465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. (2010) Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology (Berl) 208:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. (2011) Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology 36:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. (2009) Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J Neuroendocrinol 21:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. (2005) Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther 312:96–102 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. (2012) Individual differences in the effects of environmental stimuli on cocaine choice in socially housed male cynomolgus monkeys. Psychopharmacology (Berl) 224:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Do PH, See RE. (2009) Repeated aripiprazole administration attenuates cocaine seeking in a rat model of relapse. Psychopharmacology (Berl) 207:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. (2004) Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol 66:97–105 [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. (2001) Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol 21:522–526 [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. (2000) Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol 20:305–310 [DOI] [PubMed] [Google Scholar]
- Haney M, Rubin E, Foltin RW. (2011) Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology (Berl) 216:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. (2001) Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 155:330–337 [DOI] [PubMed] [Google Scholar]
- Hollister LE. (1992) Neuroleptic dysphoria: so what’s new? Biol Psychiatry 31:531–532 [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. (1982) Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis 2:359–368 [DOI] [PubMed] [Google Scholar]
- Katz JL. (1990) Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol 1:283–301 [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Nye JA, Voll R, Mun J, Stehouwer J, Goodman MM, Votaw JR, Carroll FI, Howell LL. (2012) Simultaneous measurement of extracellular dopamine and dopamine transporter occupancy by cocaine analogs in squirrel monkeys. Synapse 66:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Collins GT, Rice KC, Chen J, Woods JH, Winger G. (2012) Self-administration of agonists selective for dopamine D2, D3, and D4 receptors by rhesus monkeys. Behav Pharmacol 23:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. (2011) Discriminative stimulus, subject-rated and cardiovascular effects of cocaine alone and in combination with aripiprazole in humans. J Psychopharmacol 25:1469–1479 [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Hays LR, Rush CR. (2008) The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance II: increased aripipirazole dose and maintenance period. Am J Drug Alcohol Abuse 34:721–729 e [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin JK, Goa KL. (2002) Aripiprazole. CNS Drugs 16:779–786, discussion 787–788 [DOI] [PubMed] [Google Scholar]
- Mallikaarjun S, Salazar DE, Bramer SL. (2004) Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol 44:179–187 [DOI] [PubMed] [Google Scholar]
- Meini M, Moncini M, Cecconi D, Cellesi V, Biasci L, Simoni G, Ameglio M, Pellegrini M, Forgione RN, Rucci P. (2011) Safety, tolerability, and self-rated effects of aripiprazole and ropinirole treatment for cocaine dependence: a pilot study. Am J Addict 20:179–180 [DOI] [PubMed] [Google Scholar]
- Miczek KA, Gold LH. (1983) d-Amphetamine in squirrel monkeys of different social status: effects on social and agonistic behavior, locomotion, and stereotypies. Psychopharmacology (Berl) 81:183–190 [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5:169–174 [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. (2000) Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol 52:115–131 [DOI] [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. (1999) The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 147:143–152 [DOI] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HM, et al. (2012) Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry 72:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. (2003) Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology 28:919–931 [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. (2003) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 167:324–332 [DOI] [PubMed] [Google Scholar]
- Newcomer JW. (2005) Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19 (Suppl 1):1–93 [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. (2005) Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutics. J Med Chem 48:3663–3679 [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163:265–282 [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. (2002) Being partial to psychostimulant addiction therapy. Trends Pharmacol Sci 23:151–153 [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wang Z, Woolverton WL. (2001) Reinforcing effects of D2 dopamine receptor agonists and partial agonists in rhesus monkeys. Drug Alcohol Depend 64:209–217 [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223 [DOI] [PubMed] [Google Scholar]
- Semba J, Watanabe A, Kito S, Toru M. (1995) Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology 34:785–791 [DOI] [PubMed] [Google Scholar]
- Shimokawa Y, Akiyama H, Kashiyama E, Koga T, Miyamoto G. (2005) High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: application to the pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 821:8–14 [DOI] [PubMed] [Google Scholar]
- Sørensen G, Sager TN, Petersen JH, Brennum LT, Thøgersen P, Hee Bengtsen C, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DP. (2008) Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology (Berl) 199:37–46 [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Lofwall MR, Rush CR. (2007) The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. Am J Drug Alcohol Abuse 33:769–776 [DOI] [PubMed] [Google Scholar]
- Tadori Y, Forbes RA, McQuade RD, Kikuchi T. (2011) In vitro pharmacology of aripiprazole, its metabolite and experimental dopamine partial agonists at human dopamine D2 and D3 receptors. Eur J Pharmacol 668:355–365 [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DP, Wörtwein G, Sager TN, Holm R, Pepe LM, Caine SB. (2008) Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology (Berl) 201:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Vargas GA, von Zastrow M, Mailman RB. (2007) Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology 32:67–77 [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. (2005) Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry 18:265–270 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. (1999) Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 156:1440–1443 [DOI] [PubMed] [Google Scholar]
- Weed MR, Woolverton WL. (1995) The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 275:1367–1374 [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. (1981) Effects of antipsychotic compounds in rhesus monkeys given a choice between cocaine and food. Drug Alcohol Depend 8:69–78 [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. (1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230:678–683 [PubMed] [Google Scholar]