Abstract
Population studies, preclinical, and clinical trials suggest a role for cyclooxygenase-2 (COX-2, PTGS2) in tumor formation and progression. The downstream prostanoid receptor signaling pathways involved in tumorigenesis are poorly understood, although prostaglandin E2 (PGE2), a major COX-2 metabolite which is usually upregulated in the involved tissues, presumably plays important roles in tumor biology. Taking advantage of our recently identified novel selective antagonist for the EP2 (PTGER2) subtype of PGE2 receptor, we demonstrated that EP2 receptor activation could promote prostate cancer cell growth and invasion in vitro, accompanied by upregulation of the tumor-promoting inflammatory cytokines, such as IL-1β and IL-6. Our results suggest the involvement of prostaglandin receptor EP2 in cancer cell proliferation and invasion possibly via its inflammatory actions, and indicate that selective blockade of the PGE2-EP2 signaling pathway via small molecule antagonists might represent a novel therapy for tumorigenesis.
Introduction
As the inducible isozyme of cyclooxygenase (COX), COX-2 is often upregulated in damaged tissue (Nakayama et al., 1998; Steinauer et al., 2000) and thereby contributes to inflammation-related secondary injury (Nogawa et al., 1997; Nakayama et al., 1998; Hawkey, 1999; Iadecola et al., 2001; Minghetti, 2004; Serrano et al., 2011). Over the past decade, COX-2 and its prostanoid products have attracted substantial attention for their possible roles in progression of tumors including those of lung, head and neck, prostate and colon, ovary, breast, and liver (Williams et al., 1999; Soslow et al., 2000; Bae et al., 2001; Grivennikov et al., 2010). Genetic ablation of COX-2 reduces colorectal polyp formation by 86% in a model of human familial adenomatous polyposis, which can be recapitulated by administration of COX inhibitors (Oshima et al., 1996). Epidemiologic studies, preclinical, and clinical trials suggest that taking COX-2 inhibitor drugs regularly might reduce the rates of certain cancers and cancer-related deaths (Pentland et al., 1999; Masferrer et al., 2000; Subbaramaiah and Dannenberg, 2003; Menter et al., 2010), although COX-2 inhibitors are not FDA approved for these indications. Which downstream prostanoid receptor signaling pathway is involved in tumorigenesis has not been fully uncovered; however, upregulation of COX-2 in tumor tissues is accompanied by high levels of prostaglandin E2 (PGE2) (Gupta and Dubois, 2001; Loh et al., 2002; Murakami and Kudo, 2006). Moreover, administration of PGE2 can enhance colon carcinogenesis in an azoxymethane (AOM)-induced colon tumor model (Kawamori et al., 2003).
As a major COX-2 product, PGE2 acts on four G protein-coupled receptors: EP1, EP2, EP3, and EP4. Among these, EP2 and EP4 receptors are positively coupled through Gs to cyclic AMP (cAMP) production (Hirata and Narumiya, 2011). Elevated cAMP in turn initiates multiple downstream events via protein kinase A (PKA) or exchange protein activated by cAMP. For example, PGE2 via PKA-mediated epidermal growth factor receptor (EGFR) activation can promote cancer cell growth by activating inducible nitric oxide synthase (iNOS)/guanylate cyclase (GC) and mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase 1/2 (ERK1/2) (Donnini et al., 2007). Genetic ablation of the EP2 receptor attenuates tumor growth and prolongs survival in syngeneic mouse tumor models, accompanied by cancer-associated immunodeficiency and defective dendritic-cell differentiation (Yang et al., 2003), decelerates the progression of intestinal polyposis with decreased COX-2 expression in polyp tissues (Sonoshita et al., 2001) and suppresses skin tumor development with reduced blood vessels and increased apoptotic cells in a skin cancer model (Sung et al., 2005). Global ablation of the EP2 gene suppressed hyperplasia of mammary glands that was induced by COX-2 overexpression (Chang et al., 2005a), and EP2 receptor expression is correlated with vascular endothelial growth factor induction by PGE2 in mouse mammary tumor cells (Chang et al., 2005b). In addition, EP2 signaling was proposed to regulate tumor angiogenesis in endothelium by enhancing endothelial cell motility and cell survival, to mediate epidermal hypertrophy and tumor aggression in response to UV-irradiation, and to induce skin carcinogenesis (Hawkey, 1999; Steinauer et al., 2000; Majima et al., 2003; Kamiyama et al., 2006; Sung et al., 2006; Brouxhon et al., 2007).
The pathologic functions of EP2 signaling in tumorigenesis described above were derived from studies employing either a selective EP2 agonist (e.g., butaprost) or EP2-deficient mice. Studies based on direct pharmacological inhibition of EP2 receptor are absent, because in contrast to all other prostaglandin receptors no selective antagonist for the EP2 receptor had been available until recently (af Forselles et al., 2011; Jiang et al., 2012). Utilizing our newly discovered EP2 antagonist, we demonstrate here that EP2 receptor activation can promote in vitro cancer cell proliferation, invasion, and the induction of tumor-promoting cytokines. Our results indicate that pharmacological block of PGE2-EP2 signaling might provide an alternative strategy to suppress tumor formation and progression possibly via an anti-inflammatory mechanism.
Materials and Methods
Cell Culture.
Rat C6 glioma (C6G) and human embryonic kidney 293 (HEK) cells stably expressing the human prostanoid receptors were established in the laboratory. Human prostate cancer cell lines DU145, LNCap, and PC3 were purchased from the American Type Culture Collection. Cells were grown in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Grand Island, NY), supplemented with 10% (v/v) fetal bovine serum (Invitrogen), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen).
Chemicals and Drugs.
PGE2 and butaprost were purchased from Cayman Chemical (Ann Arbor, MI). Rolipram was purchased from Sigma-Aldrich (St. Louis, MO). Compound TG4-155 (PubChem SID 17,515,129) was either purchased from ChemDiv (#C281-0155, San Diego, CA) or synthesized by Dr. Thota Ganesh in the laboratory (Jiang et al., 2012).
TR-FRET cAMP Assay.
Cytosol cAMP was measured with a homogeneous time-resolved fluorescence resonance energy transfer (TR-FRET) method (Cisbio Bioassay) (Jiang et al., 2010). Cells were seeded into 384-well plates in 30 µl complete medium (4,000 cells/well) and grown overnight. The medium was thoroughly withdrawn and 10 µl Hank's buffered salt solution (Mediatech, Manassas, VA) plus 20 µM rolipram was added into the wells to block phosphodiesterase. The cells were incubated at room temperature for 0.5–1 hour and then treated with vehicle or test compound for 5–10 minutes before incubation with increasing concentrations of the EP2 agonist butaprost for 40 minutes. The cells were lysed in 10 µl lysis buffer containing the FRET acceptor cAMP-d2 and 1 minute later another 10 µl lysis buffer with anti-cAMP-cryptate was added. After 60 minutes of incubation at room temperature, the FRET signal was measured by an Envision 2103 Multilabel Plate Reader (PerkinElmer Life Sciences, Waltham, MA) with a laser excitation at 337 nm and dual emissions at 665 nm and 590 nm for d2 and cryptate, respectively. The FRET signal is expressed as: F665/F590 × 104.
Schild Regression Analysis.
Schild regression was characterized by the equation log (dr − 1) = log XB − log KB, where dose ratio (dr) = fold shift in EC50, XB = [antagonist], KB = equilibrium dissociation constant for the antagonist-receptor complex. A linear regression of log (dr − 1) on log XB with a slope of unity characterizes a competitive antagonism and the KB value indicates the antagonist concentration required for twofold rightward shift in the dose-response curve. Thus, a smaller KB value indicates a higher inhibitory potency (Jiang et al., 2012).
Potency of TG4-155 on Prostanoid Receptors.
Inhibition by TG4-155 of Gs-coupled prostanoid receptors DP1, EP2, EP4, and IP was assessed by TR-FRET cAMP assay in C6G or HEK cells stably expressing the human receptors. Inhibition by TG4-155 of prostanoid receptors EP1, EP3, FP, and TP was carried out by Cerep (www.cerep.com) and the Schild KB values were estimated from their cell-based assays.
Off-Target Activity Assays.
The effects of TG4-155 on a panel of 40 enzymes, ion channels, and receptors were assessed by either enzyme assays or radioligand binding assays at SRI International (www.sri.com).
Western Blot Analysis.
DU145, LNCap, and PC3 cells were grown in 60-mm dishes for 3 days and lysed on ice with 350 µl of RIPA buffer (25 mM Tris HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing a mixture of protease and phosphatase inhibitors (Roche Applied Science, Penzberg, Upper Bavaria, Germany). The homogenates were centrifuged (15,000 rpm, 15 minutes, 4°C) and protein concentration in the supernate was measured by Bradford assay (Thermo Fisher Scientific, Waltham, MA). Proteins (20 µg) were resolved by 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto PVDF membranes (Millipore, Billerica, MA). Membranes were blocked for 2 hours at room temperature with 5% nonfat milk, then incubated overnight at 4°C with primary antibodies: COX-2 (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA) or EP2 (1:1,000, Cayman Chemical), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:3,000, Santa Cruz Biotechnology). The blots were developed by enhanced chemiluminescence (ECL) (Thermo Fisher Scientific).
Cell Proliferation Assay.
In vitro cell proliferation was quantified by Vybrant MTT cell proliferation assay (Molecular Probes, Eugene, OR). In brief, PC3 cells were seeded in 96-well plates (2,000 cells/well) in complete DMEM medium with test compound. After incubation for 48 hours, medium was removed and cells were incubated for 4 hours with 100 µl fresh phenol red-free medium in the presence of 10 µl 12 mM MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. Living cells convert MTT to insoluble formazan. After solubilization of formazan in 50 µl DMSO, absorbance of the formazan was measured by a microplate reader (Molecular Devices, Sunnyvale, CA) at 540 nm.
Cell Invasion Assay.
Twenty-four-well cell culture inserts (BD Biosciences, San Jose, CA) were coated with 250 µl/ml Matrigel (Invitrogen). PC3 cells (10,000 cells/well) in 0.5 ml DMEM medium were added to the culture inserts. Butaprost with vehicle or test compound in 0.5 ml medium were added to the lower wells. Invasion was measured in triplicate by counting the cells that moved across the membrane coated with Matrigel for 24 hours. Cells were stained with HEMA 3 stain set (Fisher Diagnostics, Middletown, VA) and counted in 5 random fields/well at a magnification of 200 × . Data were reported as the total number of cells counted/well.
Cytokine Measurement.
Cytokines secreted from cultured cancer cells were measured using the Meso Scale Discovery (MSD; Rockville, MD) multiplex cytokine/chemokine kit according to the manufacturer’s instruction. The MSD plates were read using the MSD SECTOR Imager 2400.
Cytotoxicity Assay.
Cytotoxicity of TG4-155 was examined with the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Fitchburg, WI) by measuring cellular ATP level, which correlates with cell viability. Briefly, C6G cells were plated in 384-well plates at 2,000 cells/well in 25 μl DMEM plus test compound and incubated for 2 days. CellTiter-Glo reagent (25 μl) was then added to each well. The contents were mixed for 2 minutes on an orbital shaker to induce cell lysis and incubated at room temperature for 10 minutes. Relative viability is proportional to luminescence intensity as measured by a microplate reader (Molecular Devices) with an integration time of 1 second.
Data Mining.
Gene expression levels in human cancer cells were obtained from the Agilent mRNA database of the National Cancer Institute (NCI) CellMiner (http://discover.nci.nih.gov). Data on all mRNA probes were normalized using GeneSpring GX software (Agilent Technologies, Santa Clara, CA) according to Liu et al. (2010). The average was calculated if multiple probes were used for one gene. Data were transformed to linear scale and plotted appropriately.
Statistical Analysis.
Data were plotted with Origin software (Northampton, MA). Statistical analyses were performed using GraphPad Prizm software (La Jolla, CA) with one-way analysis of variance (ANOVA) and post hoc Bonferroni test. P < 0.05 was considered to be statistically significant. All data are presented as mean ± S.E.M.
Results
Pharmacological Inhibition of Prostaglandin Receptor EP2 in Cancer Cells.
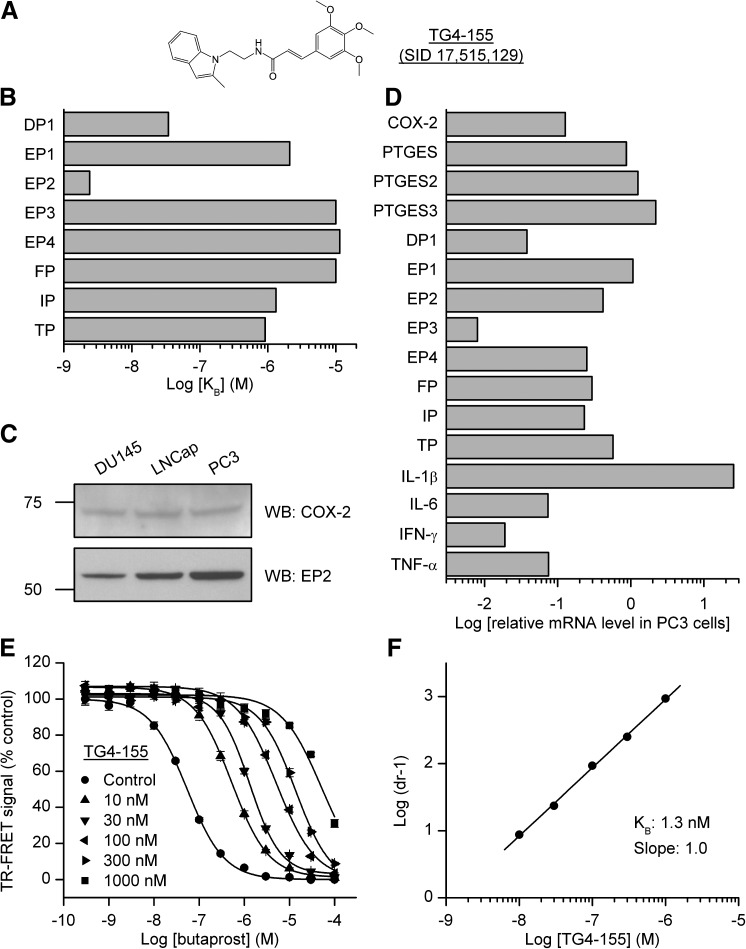
PGE2 is markedly elevated in some tumors (Loh et al., 2002; Murakami and Kudo, 2006). Furthermore, PGE2-EP2 signaling has been suggested to mediate tumor progression and tumor-associated angiogenesis from studies using EP2 agonists (Chang et al., 2005b; Kamiyama et al., 2006), EP2 deficient mice (Sonoshita et al., 2001; Yang et al., 2003; Chang et al., 2005a; Sung et al., 2005; Kamiyama et al., 2006; Brouxhon et al., 2007), and EP2 overexpressing mice (Sung et al., 2006). However, the effect of direct EP2 inhibition on tumor progression has not been evaluated yet. Taking advantage of our newly-identified EP2 antagonist TG4-155 (PubChem SID 17,515,129) (Fig. 1A), we wanted to study the pharmacological effect of selective EP2 inhibition in the prostate cancer cells. First, we evaluated the selectivity of TG4-155 for EP2 receptor against other prostanoid receptors in cell-based functional assays. In a comparison of Schild KB values, TG4-155 displayed at least 1000-fold selectivity for the EP2 receptor over human EP3, EP4, and FP receptors; at least 500-fold selectivity against human EP1 and IP receptors; at least 300-fold selectivity against human TP receptor; and approximately 14-fold selectivity against human DP1 receptor (Fig. 1B). These results indicate that of the eight canonical prostanoid receptors, TG4-155 shows low nanomolar antagonist activity against only EP2 and DP1, the receptor activated by prostaglandin D2 (PGD2). Interestingly, the EP2 and DP1 genes are oriented head to head in close proximity to each other in both human and mouse genomes. In the mouse genome, the DP1 gene is located on chromosome 14: 44.85–44.86 Mb and the EP2 gene is located on chromosome 14: 44.99–45.00 Mb; in human genome, the DP1 gene is located on chromosome 14: 52.73–52.74 Mb and the EP2 gene is located on chromosome 14: 52.78–52.80 Mb. This information indicates that they might be the result of a recent gene duplication. Indeed, of the eight prostanoid receptors, EP2 and DP1 share the closest sequence homology (Hirata and Narumiya, 2011). Thus it is unsurprising that EP2 and DP1 receptors share ligand-binding properties. In addition, other off-target activity assays showed that TG4-155 had negligible effect on a panel of 40 human enzymes, ion channels, and receptors (IC50 values > 10 µM) except that TG4-155 weakly inhibited the serotonin 5-HT2B receptor with IC50 = 2.6 µM (Table 1). At high concentration (10 µM) TG4-155 had little or no effect on the enzymatic activity of COX-1 (3% inhibition) and of COX-2 (–4% inhibition), and inhibited leukotriene B4 (LTB4) receptor BLT1 by 15% (Table 1).
Fig. 1.
COX-2 and prostaglandin receptor EP2 signaling in cancer cells. (A) Chemical structure of EP2 antagonist TG4-155 (SID 17,515,129). (B) The Schild KB values of TG4-155 were compared for inhibition of eight human prostanoid receptors. Schild KB values: 34.5, 2100, 2.4, 10,000, 11,400, 10,000, 1320, and 910 nM for DP1, EP1, EP2, EP3, EP4, FP, IP, and TP receptors, respectively. (C) The expression of COX-2 and EP2 receptor in three human prostate cancer cell lines, DU145, LNCap, and PC3, was examined by Western blot analysis. A low basal level of COX-2 (72 kDa) was detected in all three types of cancer cells and the PC3 cells have a relatively high level of EP2 (52 kDa) expressed. (D) Data mining from NCI CellMiner (http://discover.nci.nih.gov). Relative mRNA levels in PC3 cells of COX-2; prostaglandin E synthase; prostanoid receptors DP1, EP1, EP2, EP3, EP4, FP, IP, and TP; and cytokines IL-1β, IL-6, IFN-γ, and TNF-α, as measured by Agilent Whole Human Genome Oligo Microarray kit (Agilent-mRNA, Agilent Technologies). (E) Butaprost induced cAMP production in PC3 cells with an EC50 = 54 nM, which was substantially blocked by EP2 antagonist TG4-155 in a concentration-dependent manner. Data were normalized as percent maximum response; points represent mean ± S.E.M. (n = 4 independent experiments). (F) Schild regression analysis was performed to evaluate the potency of TG4-155 in PC3 cells. TG4-155 displayed a competitive antagonism mode of action on EP2 receptor shown by Schild plot with a KB value 1.3 nM and a slope of 1.0.
TABLE 1.
Off-target activity of EP2 antagonist TG4-155
TG4-155 inhibited the serotonin 5-HT2B receptor with IC50 = 2.6 µM and hERG (human Ether-à-go-go-Related Gene) with IC50 = 12 µM (n = 2).
| Target | % Inhibition at 10 µM |
|---|---|
| Acetylcholinesterase (AChE) | −2 |
| Adenosine A2A | 11 |
| Adrenergic α1B | 3 |
| Adrenergic α1D | −7 |
| Adrenergic α2A | 43 |
| Adrenergic α2C | 1 |
| Adrenergic β1 | 7 |
| Androgen AR | 13 |
| Cannabinoid CB1 | 12 |
| Cyclooxygenase-1 (COX-1) | 3 |
| Cyclooxygenase-2 (COX-2) | −4 |
| Cytochrome P450 1A2 (CYP1A2) | 8 |
| Cytochrome P450 2B6 (CYP2B6) | 18 |
| Cytochrome P450 2C9 (CYP2C9) | 34 |
| Cytochrome P450 2C19 (CYP2C19) | 26 |
| Cytochrome P450 2D6 (CYP2D6) | 13 |
| Cytochrome P450 3A4 (CYP3A4) | 30 |
| Dopamine D1 | 2 |
| Dopamine D2L | 12 |
| Estrogen ERα | 4 |
| Histamine H1 | 5 |
| Histamine H2 | −10 |
| Leukotriene, BLT (LTB4) | 15 |
| Monoamine oxidase (MAO-A) | 31 |
| Monoamine oxidase (MAO-B) | 10 |
| Nicotinic acetylcholine α, bungarotoxin | −3 |
| Nicotinic acetylcholine αβ, cytisine | −13 |
| Opiate κ (OP2, KOP) | 2 |
| Opiate µ (OP3, MOP) | 12 |
| Phosphodiesterase PDE3 | 12 |
| Phosphodiesterase PDE4 | −2 |
| Potassium channel Kv11.1 (hERG) | 43 |
| Progesterone PR-B | 27 |
| Serotonin 5-HT1B | 10 |
| Serotonin 5-HT2A | 15 |
| Serotonin 5-HT2B | 82 |
| Serotonin 5-HT4 | 31 |
| Transporter, dopamine (DAT) | 4 |
| Transporter, norepinephrine (NET) | −19 |
| Transporter, serotonin (SERT) | 7 |
Next, we examined the protein levels of COX-2 and EP2 in three human prostate cancer cell lines: DU145, LNCap, and PC3, by Western blot analysis. All three cell types express a low basal level of COX-2; the PC3 cell line has a relatively high EP2 expression (Fig. 1C), thus was selected for further studies. The NCI-60 panel consists of 60 human cancer cell lines derived from nine types of tumors: breast, central nervous system, colon, kidney, leukemia, lung, melanoma, ovarian, and prostate. Among these are two cell lines with prostate origin—DU145 and PC3. The mRNA and microRNA expression profiles in these cancer cell lines have been extensively studied by microarray and the data are available on NCI CellMiner database (http://discover.nci.nih.gov). We examined the mRNA expression data of PGE2 signaling-related genes and several proinflammatory cytokine genes in PC3 cells generated by the Agilent whole human genome oligo microarray kit (Agilent-mRNA, Agilent Technologies) (Liu et al., 2010). Among all four Gs-coupled prostanoid receptors, EP2 has the highest mRNA level in PC3 cells, approximately 11-fold higher than DP1 (Fig. 1D).
EP2 activation stimulates adenylate cyclase activity resulting in elevated cytoplasmic cAMP level. We used a cell-based time-resolved fluorescence resonance energy transfer (TR-FRET) assay to monitor cAMP accumulation in PC3 cells induced by butaprost, a selective EP2 agonist. The assay is based on generation of a strong FRET signal upon the interaction of two molecules: an anti-cAMP antibody coupled to a FRET donor (cryptate) and cAMP coupled to a FRET acceptor (d2). Endogenous cAMP produced by cells competes with labeled cAMP for binding to the cAMP antibody and thus reduces the FRET signal (Jiang et al., 2010). To evaluate the potency of EP2 antagonist TG4-155 in cancer cells, PC3 cells were incubated first with vehicle or TG4-155 for 5–10 minutes, then with increasing concentrations of butaprost for 40 minutes to activate EP2 receptors. Butaprost induced cAMP production in PC3 cells with an EC50 54 nM, which was blocked by the EP2 antagonist TG4-155 in a concentration-dependent manner (Fig. 1E). Schild regression analysis was performed to evaluate the potency of EP2 antagonist TG4-155 in PC3 cells. TG4-155 displayed a competitive antagonism mode of action on the EP2 receptor as shown by Schild plot with a KB value of 1.3 nM (Fig. 1F), which is similar to that measured in human EP2 overexpressing cell lines (Fig. 1B) (Jiang et al., 2012). These results indicate a strong COX-2-PGE2-EP2-cAMP signaling pathway in cancer cells and confirm TG4-155 as a highly potent antagonist at the endogenous EP2 receptor in prostate cancer cells.
EP2 Receptor Activation and Inhibition of Cancer Cell Proliferation and Invasion.
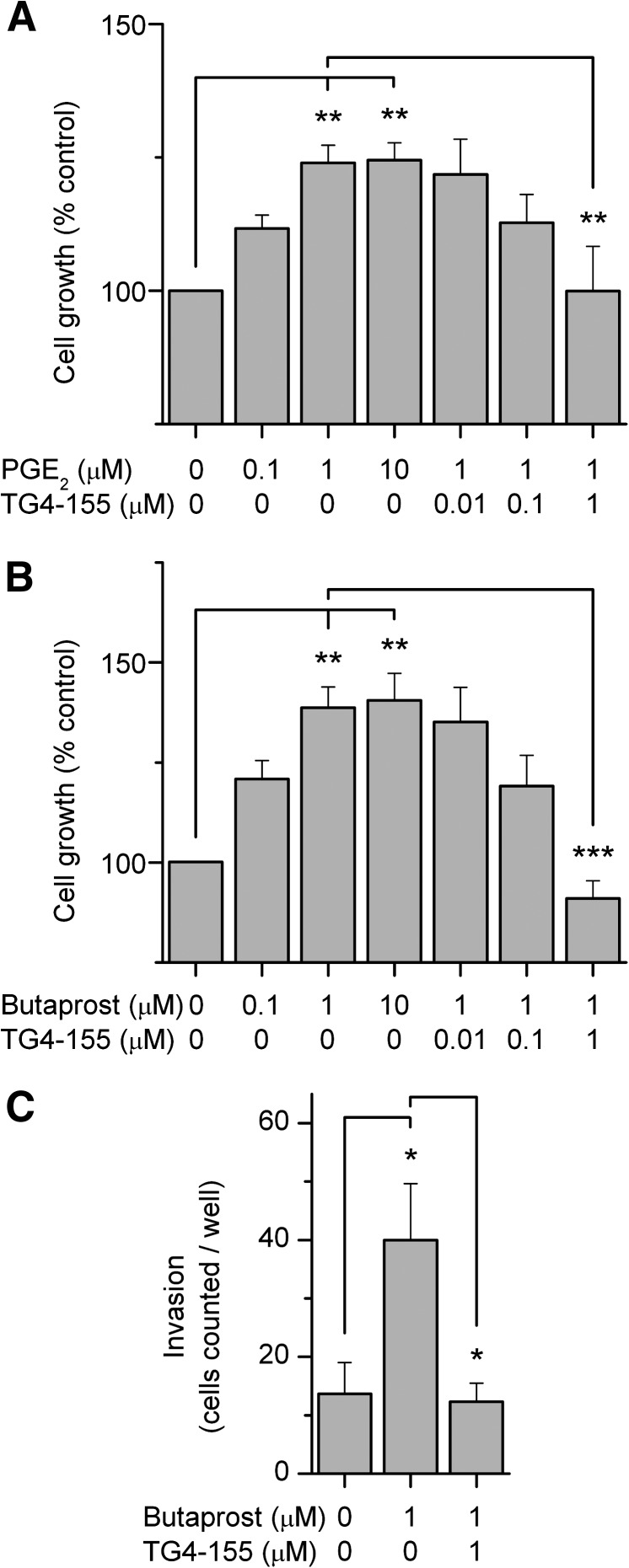
Upregulation of COX-2 in tumor tissues typically yields high levels of PGE2 (Gupta and Dubois, 2001; Loh et al., 2002; Murakami and Kudo, 2006), which can promote cancer cell growth and invasion through PKA-mediated EGFR activation (Donnini et al., 2007). Next, we investigated whether PGE2 receptor subtype EP2 is involved in the process of cancer cell growth and invasion using the EP2 antagonist TG4-155. First, PC3 cells were treated with the EP2 natural ligand PGE2 to mimic the elevated COX-2 and PGE2 levels in the tumor tissues in the presence of vehicle or TG4-155. After 48 hours of cell proliferation were quantified by MTT assay. PGE2 stimulation significantly enhanced PC3 cell growth in a concentration-dependent manner with a maximal response being obtained at approximately 1 µM (Fig. 2A). This PGE2-induced cancer cell proliferation was significantly suppressed by TG4-155 also in a concentration-dependent manner (Fig. 2A). These results were recapitulated by using an EP2 selective agonist, butaprost, in a parallel experiment (Fig. 2B) and suggest that among all four PGE2 receptor subtypes EP2 is prominently involved in cancer cell growth.
Fig. 2.
Effects of EP2 receptor activation and inhibition on cancer cell proliferation and invasion. (A) EP2 activation by its natural ligand, PGE2, promoted PC3 cell growth, measured by MTT cell proliferation assay; growth was attenuated by treatment with compound TG4-155 in a concentration-dependent manner. (B) EP2 selective agonist butaprost promoted PC3 cell growth, which was also attenuated by TG4-155. (C) TG4-155 blocked the PC3 cell invasion triggered by EP2 activation. Butaprost (1 µM) treatment significantly increased the number of cells crossing the filter coated with Matrigel, which was blocked by cotreatment with EP2 antagonist TG4-155 (1 µM). Bars represent the mean ± S.E.M. (n = 4 independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001 by one-way ANOVA and post hoc Bonferroni test with selected pairs.
To further investigate the effect of EP2 inhibition on cancer cell invasion, PC3 cells were seeded into culture inserts that were precoated with Matrigel. Butaprost (1 µM) as chemoattractant molecule was added into the lower wells in the presence of vehicle or TG4-155 (1 µM). After 24 hours, the cells that moved across the membrane coated with Matrigel were stained and counted. Butaprost (1 µM) significantly promoted PC3 cell invasion, which was completely prevented by cotreatment with the TG4-155 (Fig. 2C). These results together with the cell proliferation data demonstrate that PGE2 mediates tumor activities at least partially through the EP2 receptor and suggest a potential value of EP2 antagonist in cancer therapy.
EP2 Receptor Activation Induces Tumor-Promoting Cytokines in Cancer Cells.
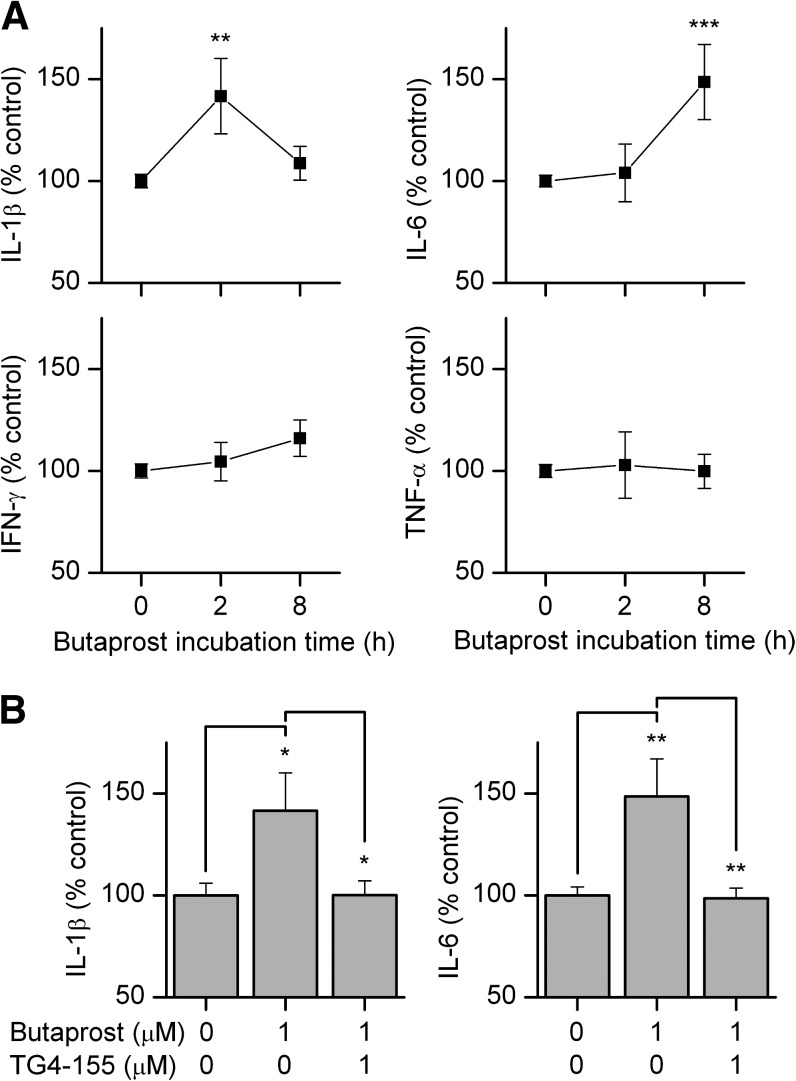
PGE2 is emerging as the primary inflammatory prostaglandin in a variety of involved tissues via its receptor EP2 subtype, while inflammation is now recognized as a critical component of tumor progression (Aggarwal et al., 2006; Grivennikov et al., 2010). To examine the effect of EP2 receptor activation on inflammation in cancer cells, PC3 cells were treated with butaprost in the presence of vehicle or EP2 antagonist TG4-155 and secretion of four proinflammatory cytokines into the culture medium was measured by the Meso Scale Discovery (MSD) multiplex cytokine/chemokine kit. EP2 activation by butaprost significantly elevated tumor-promoting cytokines IL-1β at 2 hours and IL-6 at 8 hours after butaprost stimulation, but expression of the antitumor cytokines IFN-γ and TNF-α was unaffected (Fig. 3A). EP2-dependent cytokine induction was substantially mitigated by TG4-155 (Fig. 3B). Among these four cytokines, IL-1β has a high basal mRNA level in PC3 cells (Fig. 1D).
Fig. 3.
EP2 receptor activation induces inflammatory cytokines in cancer cells. (A) PC3 cells were incubated with 1 µM butaprost for 0, 2, and 8 hours, and the levels of four proinflammatory cytokines, IL-1β, IL-6, IFN-γ, and TNF-α in the medium were measured. EP2 activation by butaprost significantly elevated IL-1β at 2 hours and IL-6 at 8 hours after butaprost stimulation, but not IFN-γ and TNF-α. (B) Cytokine induction following EP2 activation was mitigated by treatment with EP2 antagonist TG4-155 (1 µM), assessed at 2 hours and 8 hours after treatment of IL-1β and IL-6, respectively. Bars represent the mean ± S.E.M. (n = 5 independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001 by one-way ANOVA and post hoc Bonferroni test with selected pairs.
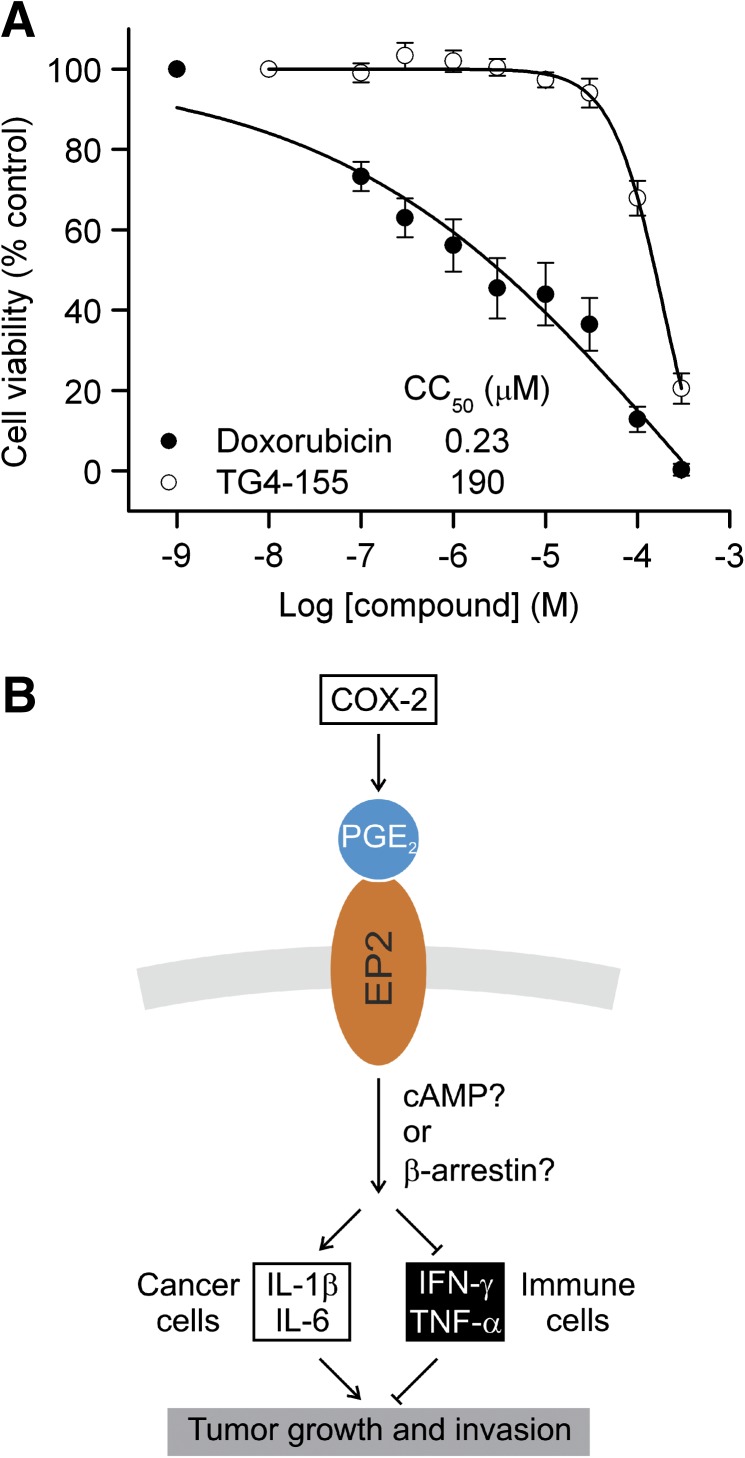
Interestingly, TG4-155 did not show significant cytotoxicity, indicating that its antitumor action is unlikely caused via a cytotoxic mechanism like doxorubicin (Fig. 4A). Taken together, these results suggest the involvement of a prostaglandin signaling pathway in the inflammatory cascade in cancer cells and that inhibition of prostaglandin receptor EP2 by selective small molecule antagonists might provide a novel strategy to repress cancer cell activities via reducing inflammation (Fig. 4B).
Fig. 4.
EP2 receptor mediates cancer cell activities possibly via an inflammatory mechanism. (A) Low cytotoxicity of EP2 antagonist TG4-155. Cytotoxicity of TG4-155 was tested in C6G cells with the CellTiter-Glo luminescent cell viability assay. TG4-155 did not show significant cytotoxicity with CC50 = 190 µM; doxorubicin as positive control with CC50 = 0.23 µM. Data are shown as mean ± S.E.M. (n ≥ 6 independent experiments). (B) Model proposed for the inflammatory action of EP2 signaling in cancer cell activities. COX-2 increases PGE2 level in tumor tissues. PGE2 signaling through the EP2 receptor upregulates tumor-promoting cytokines, including IL-1β and IL-6 in cancer cells, while downregulating antitumor cytokines such as IFN-γ and TNF-α in immune cells (Yamane et al., 2000; Walker and Rotondo, 2004; Li et al., 2006; Oxford et al., 2010); therefore, tumor growth and invasion are facilitated. A selective EP2 antagonist might reduce inflammation in tumor tissues, thus repress tumor growth.
Discussion
Recent studies using EP2 receptor-deficient mice implicated PGE2-EP2 signaling in tumor progression and tumor-associated angiogenesis (Sonoshita et al., 2001; Yang et al., 2003; Chang et al., 2005a; Sung et al., 2005; Kamiyama et al., 2006; Brouxhon et al., 2007). Genetic ablation of prostaglandin receptors has been useful but complicated by the possibility of developmental and other homeostatic adjustments (Narumiya and FitzGerald, 2001; Hirata and Narumiya, 2011). Therefore, selective small molecule modulators for prostaglandin receptors would be a valuable complement to genetic strategies. In this report, we revealed a PGE2-EP2 signaling pathway in human prostate cancer cells (Fig. 1). Using a novel potent EP2 receptor antagonist TG4-155, we further demonstrated the involvement of EP2 receptor in cancer cell proliferation and invasion (Fig. 2) and an inflammatory cascade among these cancer cells (Fig. 3). TG4-155 is 14-fold more potent on the EP2 than the DP1 receptor, and the expression level of DP1 mRNA in PC3 cells is 11-fold lower than that of EP2 (Fig. 1, B and D). Thus most of the effects of TG4-155 on prostanoid receptors can be attributed to inhibition of EP2, although DP1 can potentially contribute. Our results and data mining suggest that pharmacological inhibition of prostaglandin receptors might represent a novel strategy to repress tumorigenesis likely via an anti-inflammatory mechanism (Fig. 4B), although the effect of EP2 antagonists on other tumor activities such as angiogenesis and metastasis in tumor models awaits study.
The mechanism underlying the regulation of tumor development by EP2 is not clear, but could involve downstream β-arrestin signaling (Chun et al., 2009; Chun et al., 2010; Yun et al., 2011). The chronic inflammation mediated by EP2 signaling might also play a critical role in tumorigenesis given that inflammation has been widely recognized as a prominent risk factor for cancer (Dannenberg and Subbaramaiah, 2003; Aggarwal et al., 2006). Inflammatory events create a local microenvironment that fosters genomic alterations and promotes the neoplastic processes involving proliferation, survival, and migration. Tumor cells often release many cytokines and chemokines to attract monocytes and macrophages. The infiltrating macrophages in turn secrete growth factors that promote tumor progression and recruit secondary leukocytes to enhance and maintain this mutual promotion between inflammation and tumor. As the major inflammatory mediator derived from COX-2, PGE2 via EP2 receptor can induce a host of proinflammatory mediators including cytokines, chemokines, iNOS, and COX-2 itself, which in turn facilitates cell proliferation, cell survival, angiogenesis, invasion, and metastasis, thereby promoting tumorigenesis (Aggarwal et al., 2006).
EP2 activation can significantly induce proinflammatory cytokines, such as IL-1β and IL-6, in cancer cells (Fig. 3A). IL-1β has been recognized for its capability of promoting tumor growth, invasiveness, and angiogenesis (Saijo et al., 2002; Voronov et al., 2003; Grivennikov et al., 2010). Cancer patients usually have elevated levels of IL-6, which is related to progression of many types of cancer, including prostate, colorectal, breast, and ovarian cancers (Plante et al., 1994; Smith et al., 2001; Chung and Chang, 2003; Knupfer and Preiss, 2007; Grivennikov et al., 2010). On the other hand, EP2 receptor activation failed to induce the two antitumor cytokines in cancer cells, IFN-γ and TNF-α (Fig. 3A). Interestingly, EP2 receptor activation by butaprost suppresses IFN-γ synthesis in natural killer T cells (Walker and Rotondo, 2004; Oxford et al., 2010); similarly, EP2 activation downregulates TNF-α expression in immune cells such as neutrophils and macrophages (Yamane et al., 2000; Li et al., 2006; Oxford et al., 2010). Both INF-γ and TNF-α are well-recognized for their roles in inducing apoptotic cell death and inhibiting tumorigenesis. EP2 receptor activation by butaprost did not reduce these two antitumor cytokines in PC3 cells, probably because of their low basal levels in the in vitro culture setting (Fig. 3A). Nonetheless, blockade of EP2 signaling by small molecule antagonists can mitigate chronic inflammation in damaged tissues and might provide a novel therapeutic strategy for cancer treatment with interest waning in the use of COX-2 inhibitors in recognition of their detrimental cardiovascular and cerebrovascular side effects (Fig. 4B) (Abraham et al., 2007).
Abbreviations
- ANOVA
analysis of variance
- AOM
azoxymethane
- cAMP
cyclic AMP
- CNS
central nervous system
- COX
cyclooxygenase
- DMEM
Dulbecco’s modified Eagle medium
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- GC
guanylate cyclase
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- MSD
Meso Scale Discovery
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
- PGE2
prostaglandin E2
- PKA
protein kinase A
- SID
PubChem substance identifier
- TNF-α
tumor necrosis factor-α
- TR-FRET
time-resolved fluorescence resonance energy transfer
Authorship Contributions
Participated in research design: Jiang, Dingledine.
Conducted experiments: Jiang.
Performed data analysis: Jiang.
Wrote or contributed to the writing of the manuscript: Jiang, Dingledine.
Footnotes
This work was supported by the Epilepsy Foundation (to J.J.); the CounterACT Program, National Institutes of Health Office of the Director; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant U01NS058158] (to R.D.); and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R21NS074169] (to R.D.).
National Institutes of Health Office of the Director (OD) and the National Institute of Neurologic Disorders and Stroke (NINDS), Grant Number [U01NS058158]; and National Institute of Neurologic Disorders and Stroke (NINDS), Grant Number [R21NS074169].
References
- Abraham NS, El-Serag HB, Hartman C, Richardson P, Deswal A. (2007) Cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction and cerebrovascular accident. Aliment Pharmacol Ther 25:913–924 [DOI] [PubMed] [Google Scholar]
- af Forselles KJ, Root J, Clarke T, Davey D, Aughton K, Dack K, Pullen N. (2011) In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP₂ receptor antagonist. Br J Pharmacol 164:1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. (2006) Inflammation and cancer: how hot is the link? Biochem Pharmacol 72:1605–1621 [DOI] [PubMed] [Google Scholar]
- Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. (2001) Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res 7:1410–1418 [PubMed] [Google Scholar]
- Brouxhon S, Konger RL, VanBuskirk J, Sheu TJ, Ryan J, Erdle B, Almudevar A, Breyer RM, Scott G, Pentland AP. (2007) Deletion of prostaglandin E2 EP2 receptor protects against ultraviolet-induced carcinogenesis, but increases tumor aggressiveness. J Invest Dermatol 127:439–446 [DOI] [PubMed] [Google Scholar]
- Chang SH, Ai Y, Breyer RM, Lane TF, Hla T. (2005a) The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res 65:4496–4499 [DOI] [PubMed] [Google Scholar]
- Chang SH, Liu CH, Wu MT, Hla T. (2005b) Regulation of vascular endothelial cell growth factor expression in mouse mammary tumor cells by the EP2 subtype of the prostaglandin E2 receptor. Prostaglandins Other Lipid Mediat 76:48–58 [DOI] [PubMed] [Google Scholar]
- Chun KS, Lao HC, Langenbach R. (2010) The prostaglandin E2 receptor, EP2, stimulates keratinocyte proliferation in mouse skin by G protein-dependent and beta-arrestin1-dependent signaling pathways. J Biol Chem 285:39672–39681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun KS, Lao HC, Trempus CS, Okada M, Langenbach R. (2009) The prostaglandin receptor EP2 activates multiple signaling pathways and beta-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis 30:1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Chang YF. (2003) Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol 83:222–226 [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, Subbaramaiah K. (2003) Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 4:431–436 [DOI] [PubMed] [Google Scholar]
- Donnini S, Finetti F, Solito R, Terzuoli E, Sacchetti A, Morbidelli L, Patrignani P, Ziche M. (2007) EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J 21:2418–2430 [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. (2010) Immunity, inflammation, and cancer. Cell 140:883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN. (2001) Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 1:11–21 [DOI] [PubMed] [Google Scholar]
- Hawkey CJ. (1999) COX-2 inhibitors. Lancet 353:307–314 [DOI] [PubMed] [Google Scholar]
- Hirata T, Narumiya S. (2011) Prostanoid receptors. Chem Rev 111:6209–6230 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. (2001) Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA 98:1294–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, Qui M, Speigel I, Rojas A, Lelutiu N, Dingledine R. (2012) Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci USA 109:3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ganesh T, Du Y, Thepchatri P, Rojas A, Lewis I, Kurtkaya S, Li L, Qui M, Serrano G, et al. (2010) Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci USA 107:2307–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. (2006) EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene 25:7019–7028 [DOI] [PubMed] [Google Scholar]
- Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. (2003) Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis 24:985–990 [DOI] [PubMed] [Google Scholar]
- Knüpfer H, Preiss R. (2007) Significance of interleukin-6 (IL-6) in breast cancer (review). review Breast Cancer Res Treat 102:129–135 [DOI] [PubMed] [Google Scholar]
- Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. (2006) Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res 98:642–650 [DOI] [PubMed] [Google Scholar]
- Liu H, D’Andrade P, Fulmer-Smentek S, Lorenzi P, Kohn KW, Weinstein JN, Pommier Y, Reinhold WC. (2010) mRNA and microRNA expression profiles of the NCI-60 integrated with drug activities. Mol Cancer Ther 9:1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh JK, Hwang SL, Lieu AS, Huang TY, Howng SL. (2002) The alteration of prostaglandin E2 levels in patients with brain tumors before and after tumor removal. J Neurooncol 57:147–150 [DOI] [PubMed] [Google Scholar]
- Majima M, Amano H, Hayashi I. (2003) Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends Pharmacol Sci 24:524–529 [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60:1306–1311 [PubMed] [Google Scholar]
- Menter DG, Schilsky RL, DuBois RN. (2010) Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin Cancer Res 16:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L. (2004) Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63:901–910 [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. (2006) Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des 12:943–954 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. (1998) Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA 95:10954–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, FitzGerald GA. (2001) Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest 108:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. (1997) Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci 17:2746–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803–809 [DOI] [PubMed] [Google Scholar]
- Oxford AW, Davis RJ, Coleman RA, Clark KL, Harris NV, Fenton G, Hynd G, Stuttle KAJ, Sutton JM, Ashton MR, et al.(2010) EP2 receptor agonists. US Patent 7,803,841. Asterand UK Limited.
- Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. (1999) Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis 20:1939–1944 [DOI] [PubMed] [Google Scholar]
- Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. (1994) Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer 73:1882–1888 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, Hong X, Tazawa R, Kikuchi T, Matsushima K, et al. (2002) Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol 169:469–475 [DOI] [PubMed] [Google Scholar]
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R. (2011) Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci 31:14850–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. (2001) Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev 12:33–40 [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM. (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 7:1048–1051 [DOI] [PubMed] [Google Scholar]
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. (2000) COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89:2637–2645 [DOI] [PubMed] [Google Scholar]
- Steinauer KK, Gibbs I, Ning S, French JN, Armstrong J, Knox SJ. (2000) Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int J Radiat Oncol Biol Phys 48:325–328 [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Dannenberg AJ. (2003) Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci 24:96–102 [DOI] [PubMed] [Google Scholar]
- Sung YM, He G, Fischer SM. (2005) Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res 65:9304–9311 [DOI] [PubMed] [Google Scholar]
- Sung YM, He G, Hwang DH, Fischer SM. (2006) Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene 25:5507–5516 [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. (2003) IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA 100:2645–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W, Rotondo D. (2004) Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology 111:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CS, Mann M, DuBois RN. (1999) The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18:7908–7916 [DOI] [PubMed] [Google Scholar]
- Yamane H, Sugimoto Y, Tanaka S, Ichikawa A. (2000) Prostaglandin E(2) receptors, EP2 and EP4, differentially modulate TNF-alpha and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem Biophys Res Commun 278:224–228 [DOI] [PubMed] [Google Scholar]
- Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, et al. (2003) Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest 111:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Ryu JM, Jang MW, Han HJ. (2011) Interaction of profilin-1 and F-actin via a β-arrestin-1/JNK signaling pathway involved in prostaglandin E(2)-induced human mesenchymal stem cells migration and proliferation. J Cell Physiol 226:559–571 [DOI] [PubMed] [Google Scholar]