Abstract
Behavioral studies of chronic CB1 receptor activation may provide a pharmacological approach to understanding efficacy-related differences among CB1 ligands as well as mechanistic commonalities between cannabinoid and noncannabinoid drugs. In the present studies, the effects of CB1 agonists [(6aR,10aR)-3-(1-adamantyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol (AM411), 9β-(hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol (AM4054), R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212.2), Δ9-tetrahydrocannabinol (Δ9-THC), (R)-(+)-arachidonyl-1'-hydroxy-2'-propylamide (methanandamide)], CB1 antagonists [5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (SR141716A), 5-(4-alkylphenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (AM4113)], and dopamine (DA)–related [methamphetamine, (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide (SKF82958), (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390), (6aR)-5,6,6a,7-tetrahydro-6-propyl-4H-dibenzo[de,g]quinoline-10,11-diol (R-(−)-NPA), haloperidol] and opioid (morphine, naltrexone) drugs on scheduled-controlled responding under a 30-response fixed ratio schedule of stimulus-shock termination in squirrel monkeys were compared before and during chronic treatment with the long-acting CB1 agonist AM411 (1.0 mg/kg per day, i.m.). Prechronic treatment with all drugs except naltrexone (1–10 mg/kg) produced dose-related decreases in responses rates. Dose-response re-determinations during chronic treatment revealed the following: 1) >250-fold (AM411, methanandamide) and >45-fold (AM4054, WIN55,212.2, Δ9-THC) rightward shifts in the ED50 values for CB1 agonists; 2) >100-fold and >20-fold leftward shifts in the ED50 values for SR141716A and AM4113, respectively; and 3) approximately 4.8-fold and 10-fold rightward shifts in the ED50 values for methamphetamine and the DA D2 agonist R-(−)-NPA, respectively. Dose-response relationships for other DA-related and opioid drugs were unchanged by chronic CB1 agonist treatment. Differences in the magnitude of tolerance among CB1 agonists during chronic treatment may be indicative of differences in their pharmacological efficacy, whereas the enhanced sensitivity to behaviorally disruptive effects of CB1 antagonists may provide evidence for CB1-related behavioral and/or physical dependence. Finally, the development of cross-tolerance to methamphetamine and R-(−)-NPA bolsters previous evidence of interplay between CB1 and DA D2 signaling mechanisms.
Introduction
The frequent use of CB1 receptor agonists may lead to pharmacological tolerance and dependence, especially in view of the long-lasting effects of commonly used drugs such as Δ9-THC in marijuana and Marinol or, more recently, synthetic cannabinoids such as JWH-018 or JWH-073 in K2 and Spice (Panagis et al., 2008; Atwood et al., 2011; Benford and Caplan, 2011). However, tolerance does not appear to develop uniformly across studies or ligands. Thus, rapid (within 3–7 days) tolerance may occur to some effects (e.g., antinociception) of Δ9-THC and other CB1 agonists in rodents; however, sensitivity to other effects (e.g., memory impairment) remains unaltered—even after weeks of chronic treatment with large doses of CB1 agonists (Panagis et al., 2008). Furthermore, cross-tolerance among CB1 agonists, such as Δ9-THC and the endogenous CB1 ligand anandamide or its analogs is not consistent across assay endpoints, i.e., it is evident in studies of antinociception but not in studies of hypothermia or the disruption of operant behavior [reviewed in Panagis et al. (2008) and Singh et al. (2011)]. Based on current understanding, differential tolerance across CB1-related endpoints may reflect regional differences in CB1 receptor down-regulation/desensitization in the central nervous system, whereas differential cross-tolerance across CB1 ligands may reflect differences in pharmacological efficacy (Panagis et al., 2008; McMahon, 2011).
Recent studies in human subjects and laboratory animals suggest that frequent exposure to CB1 agonists also leads to physical dependence (Haney et al., 1999; Budney et al., 2004; Panagis et al., 2008). Classical physical dependence may be defined by physiologic adaptation to effects of a drug that subsequently necessitates the presence of the drug for normative function (Alford et al., 2006). Physical dependence is identified by the appearance of an abstinence syndrome after either discontinuation of chronic drug treatment (spontaneous withdrawal) or administration of a receptor antagonist during chronic treatment (antagonist-precipitated withdrawal). Disturbed sleep is a recognized effect of discontinuing Δ9-THC in humans, but prominent signs of a CB1-related abstinence syndrome have been difficult to identify in laboratory animals (Budney et al., 2004; Panagis et al., 2008). Early studies with Δ9-THC by Beardsley et al. (1986) provided evidence for behavioral dependence (i.e., the disruption of learned behavior by discontinuation of chronic treatment) in nonhuman primates; however, the relationship between behavioral dependence and physical dependence was not clarified. Other studies in chronically treated monkeys have suggested that relatively small changes in overt and operant behavior after abrupt discontinuation may be indicative of a Δ9-THC–induced abstinence syndrome (Fredericks and Benowitz, 1980; Stewart and McMahon, 2010). The relatively slow clearance of Δ9-THC after discontinuation possibly prevents a full expression of signs of withdrawal—similar to the limited withdrawal observed after discontinuation of long-acting benzodiazepines or gradual dose reduction of chronically administered opioids (Bergman and Schuster, 1985; Landry et al., 1992).
The development of CB1 antagonists such as rimonabant (SR141716A) has enabled studies of antagonist-precipitated cannabinoid withdrawal, as previously done with naltrexone in opioid-dependent subjects (Gellert and Holtzman, 1979; Woods et al., 1982). For example, i.v. doses of 0.32–1.0 mg/kg of rimonabant recently were shown to produce discriminative stimulus or rate-decreasing effects in monkeys that were chronically treated with Δ9-THC, whereas a dose of 3.2 mg/kg was required to produce behavioral effects in untreated subjects (Stewart and McMahon, 2010; McMahon, 2006; McMahon, 2011). Such findings encourage the idea that antagonist-precipitated withdrawal may be a useful means for studying CB1 dependence.
In the present work, CB1-related tolerance and dependence was further characterized by evaluating changes in sensitivity to effects of CB1 receptor agonists and antagonists on schedule-controlled behavior, a reliable and sensitive assay for studying cannabinoid tolerance and dependence (Beardsley et al., 1986; McMahon, 2011). Subjects were treated daily with the CB1 agonist AM411, a recently developed and long-acting adamantyl analog of Δ8-THC (Lu et al., 2005), using doses that disrupt operant behavior for 24–48 h when given acutely (0.3–1.0 mg/kg) (M.S. Delatte et al., submitted manuscript). Next, changes in sensitivity to effects of AM411 and other CB1 receptor agonists and antagonists during chronic treatment were studied by analyzing dose-response functions for rightward (tolerance) or leftward (sensitization) movement. Based on reported interactions with dopaminergic and opioid systems (Maldonado, 2002; Tanda and Goldberg, 2003), similar evaluations were conducted with selected monoaminergic and opioid drugs. Overall, the present results show differential magnitudes of tolerance among CB1 agonists that may be related to efficacy at CB1 receptors, enhanced sensitivity to behaviorally disruptive effects of CB1 antagonists, and cross-tolerance to effects of methamphetamine and the D2 receptor agonist R-(−)-NPA. These data provide further evidence for CB1-related behavioral and/or physical dependence and strengthen previous findings of interplay between CB1 and dopamine (DA) D2 signaling mechanisms.
Materials and Methods
Subjects.
Experimentally naïve adult male squirrel monkeys (Saimiri sciureus; n = 4), weighing 650–900 g were individually housed in stainless steel cages in a temperature- and humidity-controlled vivarium under a 12-h light/dark cycle. All monkeys had unlimited access to water (except during the test session) and were fed a daily allotment of high-protein monkey chow (Purina Monkey Chow; Purina, St. Louis, MO), supplemented with fruit and multivitamins, in the home cage. Behavioral testing was conducted between 08:00 AM and 06:00 PM, under protocols that were approved by the Institutional Animal Care and Use Committee at McLean Hospital. This facility is licensed by the U.S. Department of Agriculture. Subjects were maintained in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, National Institutes of Health.
Apparatus.
During experimental sessions, subjects sat in customized Plexiglas chairs (based on Kelleher and Morse, 1968) in ventilated chambers that were provided with white noise to mask extraneous sounds. While seated, monkeys faced a panel containing colored stimulus lights that could be illuminated to serve as visual stimuli and a response lever that protruded through the front panel of the chair and was easily accessible to the subject. Each lever-press with a force >0.2 N produced an audible click and was recorded as a response. A shaved portion of the seated monkey’s tail was comfortably secured under brass electrodes by a small stock and was coated with electrode paste to ensure a low-resistance electrical contact between electrodes and tail. Brief, low-intensity current (200 ms; 3 mA) could be programmed for delivery to the tail from a 50-Hz transformer. Stimulus events were programmed and data were recorded using a commercially available interface and computer program (MED-PC, MedState Notation; Med Associates Inc., St. Albans, VT).
Behavioral Procedure.
Subjects responded under a 30-response fixed ratio (FR30) schedule of stimulus-shock termination (Morse and Kelleher, 1966; Bergman et al., 1995). Under terminal contingencies, daily sessions began with illumination of the visual stimuli on the front panel of the chair; current was scheduled for delivery to the monkey’s tail every 20-seconds during the time that stimulus lights were illuminated. Under this schedule, the completion of 30 consecutive responses within the 20-s interval extinguished stimulus lights and the associated program of current delivery, and initiated a 10-second timeout (TO10”) period during which all lights in the chamber were off and lever-presses had no scheduled consequences. After the TO10, the visual stimuli were re-illuminated and the schedule contingencies were again in effect. Daily sessions comprised five successive components. Each component consisted of a 10-minute timeout period during which no stimuli were presented and lever-press responses had no scheduled consequences followed by a 3-minute response period during which the FR30, 10” schedule of stimulus-shock termination was in effect. Under these conditions, each component terminated after the 3-minute response period or the fourth delivery of current to the monkey’s tail, whichever occurred first.
Drugs.
CB1 ligands, except Δ9-THC and rimonabant, were synthesized at the Center for Drug Discovery (Northeastern University, Boston, MA). These included the novel CB1 agonists AM411 (Lu et al., 2005) and AM4054 (Delatte et al., 2008; G. A. Thakur and A. Makriyannis, manuscript in preparation), the stable analog of the endocannabinoid anandamide, methanandamide, and the CB1 neutral antagonist AM4113 (Bergman et al., 2008; Sink et al., 2008). The CB1 agonist Δ9-THC and the inverse agonist/antagonist rimonabant (SR141716A) were obtained from the National Institute of Drug Abuse (Bethesda, MD). WIN55,212.2, methamphetamine, SKF82958, SCH23390, R-(−)-NPA, haloperidol, morphine, and naltrexone were obtained from Sigma-Aldrich (St. Louis, MO). Vehicles were either 0.9% saline (methamphetamine, morphine, naltrexone, R-(−)-NPA, SCH23390, SKF82958) or a 20:60:20 mixture of emulphor (Alkamuls EL-620; Rhone-Poulenc, Cranbury, NJ), 0.9% saline, and 95% ethanol (AM411, AM4054, methanandamide, AM4113, Δ9-THC, rimonabant, haloperidol, WIN55,212.2). With the exception of CB1 antagonists, all drug solutions were injected i.m. (calf or thigh muscle) in volumes ranging from 0.2 to 0.4 ml/kg body weight; control injections were similar volumes of drug vehicles. The effects of the two CB1 antagonists, rimonabant and AM4113, were studied during sessions that followed i.v. injections via the tail vein.
Drug Testing.
Drug studies were initiated after stable responding was achieved in all subjects. Stable responding was defined by less than 10% variability in overall response rates during the session over 10 successive sessions. Typically, test sessions were conducted once or twice per week (Tuesdays and/or Fridays), and training sessions were conducted on intervening days. During test sessions, all schedule parameters and contingencies were identical to those in the training sessions, with the exceptions that the program of current delivery was not in effect and that either 30 consecutive lever responses or the passage of 20-seconds resulted in the termination of stimulus lights followed by a short timeout period. Whenever possible, full dose-response functions for each drug were established in all subjects by determining the effects of a range of doses up to those that decreased response rates to below 50% of control values. The effects of CB1 agonists and antagonists were determined using single-dosing procedures, whereas the effects of all other drugs were determined using cumulative-dosing procedures previously employed in our laboratory (Bergman and Spealman, 1988). Briefly, under single-dosing procedures, a dose of the test drug was administered prior to the start of the first component, and injections of saline (approximately 0.3 ml) were administered at the beginning of each subsequent component of the test session. Under cumulative-dosing procedures, graded doses of the drug were administered i.m. at the beginning of sequential components of the test session (i.e., at the onset of the 10-minute timeout periods that initiated session components). The latter procedure allowed for determination of up to five cumulative doses in a single test session; overlapping ranges of cumulative doses were administered in separate test sessions when necessary to determine the effects of more than five drug doses. Test sessions always were preceded by either vehicle- or noninjection-training sessions in which the subject consistently terminated visual stimuli and the program of current delivery within the 20-second limited hold throughout the five components of the daily session with response rates that were within control values (3–4 responses per second across monkeys). Experiments with one drug were generally completed prior to determining the effects of another drug, and drugs were tested in an irregular order among subjects. For cannabinoids, doses of each drug also were studied in an irregular order in individual subjects using single-dosing procedures described above. Based on the results of preliminary experiments, AM411 was studied by administering single i.m. doses 90-minutes presession, whereas other CB1 agonists including AM4054, methanandamide, Δ9-THC, and WIN55,212.2 were administered i.m. 30-minutes prior to the session. CB1 receptor antagonists including the inverse agonist/antagonist SR141716A and the neutral antagonist AM4113 were studied by administering i.v. doses 10-minutes presession. The effects of all noncannabinoid drugs were determined using the above-described cumulative-dosing procedures.
Upon completion of prechronic studies, chronic treatment with the CB1 receptor full agonist AM411 began with daily i.m. injections 90-minutes prior to the session. Injections were given between 10:00 AM and 2:00 PM on weekend days when behavioral sessions were not conducted. The daily dosage of AM411 was selected on the basis of prechronic dose-response data; treatment began with a single i.m. injection of 0.32 mg/kg per day. After 4 days, the daily dosage was increased to 1.0 mg/kg, where it remained for the rest of the chronic regimen (460 days). After tolerance to the behaviorally disruptive effects of 1.0 mg/kg AM411 was evident (see Results) and stable baseline performance was regained, dose-response functions for all drugs were re-determined in each subject, using the injection procedures described above. On test days, presession injections of AM411 were suspended and, instead, the chronic dosage of AM411 was administered when the subject was returned to the vivarium after the test session. After the effects of all drugs were re-determined during the chronic regimen, the dosage of AM411 was reduced to 0.32 mg/kg per day for approximately 7 days before terminating daily drug injections. This was done as a precaution to minimize untoward effects that might have resulted from more abrupt discontinuation of chronic treatment with AM411.
Data Analysis.
Rates of responding under the FR30 stimulus-shock termination schedule were calculated separately in each component of the session by dividing the total number of responses during the time stimulus lights were illuminated by the total time of illumination. Mean control rates of responding in each component were calculated by averaging data from all training sessions that immediately preceded drug test sessions. The effects of each treatment administered as a single injection were calculated by averaging data across all components and expressing the mean value as a percentage of the mean control rate of responding throughout the session. The effect of each cumulative dose was calculated as a percentage of the mean control response rate during the session component in which it was administered.
Data from drug testing sessions were calculated for each subject and averaged for the group of monkeys to provide mean (± S.E.M.) values for the construction of dose-response functions for each drug during prechronic and chronic phases of study. When appropriate, averaged dose-effect data were analyzed using standard linear regression techniques using Bioassay software (Bioassay version Beta 6.2; MED Associates Inc.). ED50 values and their 95% confidence limits were determined from points on the linear portions of the dose-response functions (Snedecor and Cochran, 1967). ED50 values from prechronic and chronic dose-effect curves were compared for each drug, and were considered to be significantly different if their 95% confidence limits did not overlap. The magnitude of shifts that occurred in dose-response functions are expressed in terms of relative potency estimates that were calculated by standard parallel-line bioassay techniques described by Finney (1964). Briefly, when slopes do not significantly deviate from parallelism, a significant difference between two dose-response functions is indicated when the 95% confidence limits for the relative potency ratio do not include the value 1.0. Time course data shown in Fig. 1 were analyzed using a repeated-measures analysis of variance followed by the Bonferroni t test for post hoc analysis. In all cases, significance was defined at the 95% level of confidence (P < 0.05).
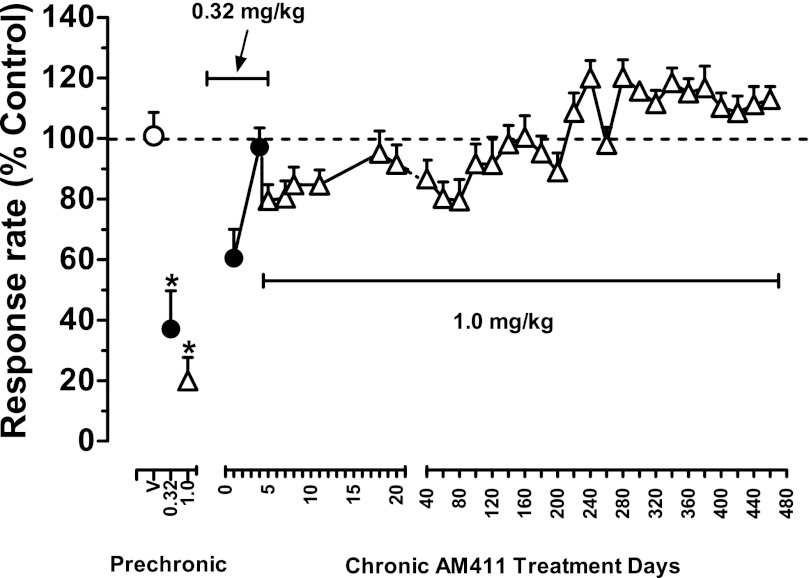
Fig. 1.
Effects of the cannabinoid CB1 agonist AM411 in squirrel monkeys responding under a FR30 schedule of stimulus-shock termination before and during chronic treatment with AM411 (1.0 mg/kg per day, i.m.). Abscissa: prechronic vehicle, 0.32, and 1.0 mg/kg AM411, and days of chronic treatment with AM411 (0.32 mg/kg: filled circles; 1.0 mg/kg: open triangles); ordinates: rate of responding as a percentage of control rates. Points are means (± S.E.M.) based on data from three or four monkeys. Open circle above prechronic “V” represents mean control values (100% ± average S.D.) over the five sequential components of the session. Dotted line represents mean 100% averaged across session components. Capped solid lines indicate treatment dose of AM411 in mg/kg per day. Between days 40 and 460, if data for 1.0 mg/kg AM411 were not available for a subject on a particular day (e.g., due to test sessions with other doses of drugs or weekends), data for that subject from the next 1.0 mg/kg AM411 control session were used to calculate the plotted group response rate. *Indicates a significant difference from vehicle control value.
Results
Control Performance.
Under control conditions, subjects reliably terminated visual stimuli and the program of current delivery within 20-seconds after illumination throughout the five components of the daily session. Stable control rates and patterns of responding were evident across all five successive components of the session throughout the present studies and, averaged for the group of monkeys, ranged from 3.22 (±0.07) to 4.40 (±0.09) responses per second. Injections of vehicle had no systematic effect on responding in individual subjects and control rates of responding also did not vary significantly across injection procedures (i.e., pretreatment time: 10, 30, 60, 90 minutes; route of administration: i.m. or i.v.; session: vehicle or nonvehicle). Hence, mean overall values (± S.E.M.) were obtained by averaging response rates from all control sessions during the three phases of study (i.e., before, during, and after discontinuation of chronic exposure to AM411; 18–26 control sessions per condition). Mean control rates of responding exhibited an upward trend over the course of the present studies but did not differ significantly across the three phases of study (3.53±0.08, 4.08±0.12, and 4.40±0.09 responses per second before, during, and after chronic treatment with AM411).
Figure 1 shows the effects of the AM411 in squirrel monkeys responding under a FR30 schedule of stimulus-shock termination before and during chronic treatment with AM411 (1.0 mg/kg per day i.m.). (Note that because marked changes in the effects of AM411 across sessions were not evident, data averaged for the group of monkeys from day 20 to day 480 were plotted in increments of 20 sessions.) On the first day of chronic treatment, 0.32 mg/kg AM411 reduced fixed ratio (FR) responding to approximately 60% of previous control values, an effect that dissipated over the next four sessions (Fig. 1). Subsequently, the daily i.m. treatment dose of AM411 was increased to 1.0 mg/kg per day, which decreased response rates to approximately 80% of previous control values (Fig. 1). This slight depression of response rate was still evident after 7–10 days of treatment and slowly dissipated through the first half of the chronic regimen (approximately 220 days; Fig. 1). Thereafter, from days 220 to 460, response rates slightly increased to approximately 110–120% of previous control values and remained at that level for the remainder of the chronic treatment regimen. A repeated-measures analysis of variance confirms a significant effect of treatment [F(37,111) = 7.61; P < 0.001] prior to chronic treatment. Post hoc analysis (Bonferroni t test) revealed a significant difference between control response rates and response rates after 0.32 and 1.0 mg/kg AM411 (P values < 0.001). However, the effects of 0.32 and 1.0 mg/kg AM411 on response rates no longer differed significantly from control values during chronic treatment (P values > 0.05).
Effects of CB1 Receptor Agonists.
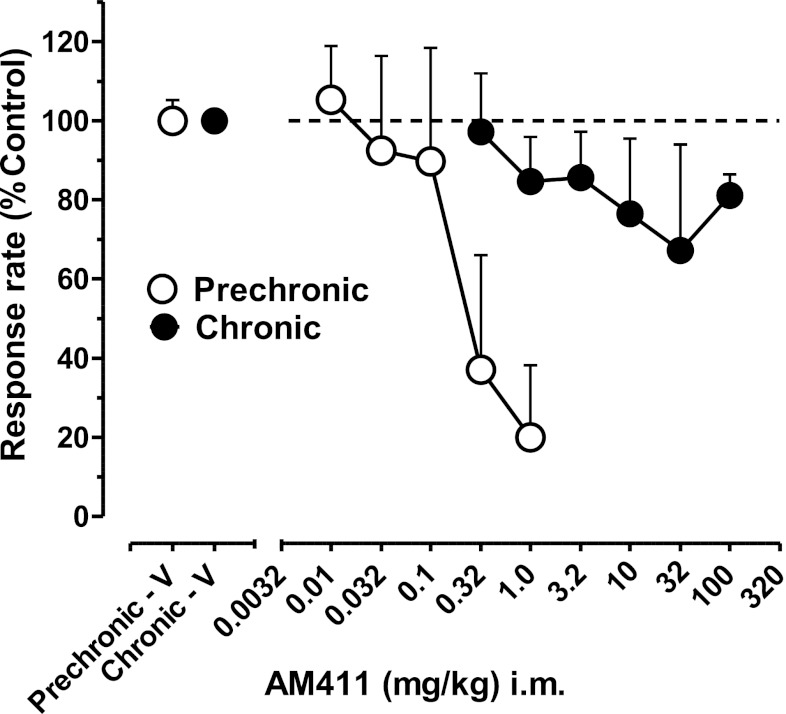
The effects of AM411 before and during chronic exposure are compared for the group of subjects in Fig. 2. Prior to chronic treatment, AM411 (0.01–1.0 mg/kg i.m.) decreased response rates in a dose-related manner to 20% of control values after the highest dose (1.0 mg/kg; Fig. 2). The group ED50 value (i.e., the dose of AM 411 calculated to decrease response rates to 50% of control values) was 0.26 mg/kg (95% confidence limits, 0.08–5.24; Table 1). After chronic treatment, a profound tolerance to the rate-decreasing effects of AM411 was observed in all monkeys; the highest i.m. doses of AM411 that could be administered (100 mg/kg) failed to produce >35% decreases in FR responding, precluding the determination of a mean ED50 value (Fig. 2).
Fig. 2.
Effects of the cannabinoid CB1 agonist AM411 in squirrel monkeys responding under a FR30 schedule of stimulus-shock termination before (open circles) and during (solid circles) chronic treatment with AM411 (1.0 mg/kg per day, i.m.). Abscissa: single AM411 dose, log scale; ordinate (left and right panel): rate of responding as a percentage of control rates. Points are means (± S.E.M.) based on data from three or four monkeys. Open and closed circles above prechronic “V” and chronic “V”, respectively, represent mean control values (100% ± average S.D.) under the FR30 stimulus-shock termination schedule in the five sequential components of the session. Control data for prechronic AM411 are re-plotted from Fig. 1. Dotted line represents mean 100% averaged across session components.
TABLE 1.
Effective doses, their 95% confidence limits, and relative potencies with which cannabinoid CB1 receptor agonists, cannabinoid CB1 receptor antagonists, and dopaminergic and opioid drugs decreased schedule-controlled behavior in squirrel monkeys before and during repeated exposure to the cannabinoid CB1 receptor agonist AM411 (1.0 mg/kg per day)
Italicized data represent significant difference in relative potency values between prechronic versus chronic effects of drugs.
| Drug | Prechronic Doses | Prechronic ED50 (95% CL) | Chronic Doses | Chronic ED50 (95% CL) | Relative Potency: Prechronic versus Chronic (95% CL) | |
|---|---|---|---|---|---|---|
| mg/kg | mg/kg | mg/kg | mg/kg | |||
| CB1 agonists | AM411 | 0.01–1.0 | 0.26 (0.08–5.24) | 0.32–100 | ND | ND |
| AM4054 | 0.003–0.1 | 0.02 (0.01–0.07) | 0.1–3.2 | 0.95 (0.32–11.29) | 0.02 (0.01–0.04) | |
| WIN55,212.2 | 0.032–1.0 | 0.26 (0.10–1.20) | 3.2–32 | 23.05 (11.81–24.38) | 0.01 (0.003–0.028) | |
| Δ9-THC | 0.1–3.2 | 0.74 (0.33–2.11) | 3.2–100 | 57.27 (22.73–1479.82) | 0.02 (0.006–0.065) | |
| Methanandamide | 1–10 | NS | 10–320 | ND | ND | |
| CB1 antagonists | SR141716A | 3.2–18 | 14.27 (10.85–22.01) | 0.01–0.32 | 0.13 (0.04–1273.51) | 221 (83.1−715) |
| AM4113 | 1.0–10 | 5.97 (2.98–53.22) | 0.032–1.0 | 0.25 (0.08–1.72) | 31.54 (12.17–102.35) | |
| Indirect DA agonist | Methamphetamine | 0.032–0.32 | 0.20 (0.09–4.80) | 0.1–3.2 | 0.96 (0.44–3.57) | 0.26 (0.09–0.90) |
| DA D1 agonist | SKF82958 | 0.032–1.0 | 0.18 (0.09–0.37) | 0.032–1.0 | 0.11 (0.06–0.17) | 1.58 (0.83–3.19) |
| DA D1 antagonist | SCH23390 | 0.01–0.032 | ND | 0.01–0.1 | ND | ND |
| DA D2 agonist | R-(−)-NPA | 0.003–0.01 | 0.006 (0.004–0.01)a | 0.003–0.1 | 0.05 (0.03–0.15) | 0.18 (0.05–0.49) |
| DA D2 antagonist | Haloperidol | 0.003–0.1 | 0.04 (0.02–0.07) | 0.003–0.32 | 0.03 (0.02–0.06) | 1.19 (0.61–2.49) |
| Opioid agonist | Morphine | 0.32–3.2 | 1.39 (0.75–3.37) | 0.32–10 | 2.99 (1.36–11.56) | 0.52 (0.23–1.12) |
| Opioid antagonist | Naltrexone | 1.0–10 | ND | 1.0–10 | ND | ND |
95% CL, 95% confidence limits; DA, dopamine; ND, not determined; NS, nonsignificant regression.
Significant deviation from linearity.
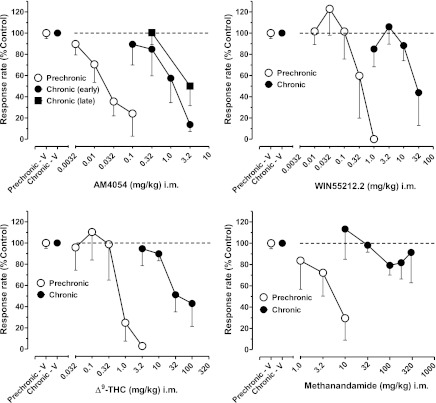
The effects of other CB1 receptor agonists before and during chronic treatment with AM411 are shown in Fig. 3. Like AM411, prechronic administration of AM4054 (0.0032–0.1 mg/kg), WIN55,212.2 (0.01–1.0 mg/kg), Δ9-THC (0.032–3.2 mg/kg), and methanandamide (1.0–10 mg/kg) produced dose-dependent decreases in rates of FR responding. After administration of the highest dose of each CB1 agonist, overall response rates decreased to ≤30% of control values. Based on ED50 values (Table 1), the rank order of potencies with which the CB1 agonists decreased response rates prior to chronic treatment with AM411 were as follows: AM4054 > AM411 ∼ WIN55,212.2 > Δ9-THC > methanandamide (Note: ED50 values for methanadamide were not determined due to nonsignificant regression; however, behaviorally effective doses were higher than those of other drugs). Chronic daily treatment with AM411 resulted in a >1.5-log unit rightward shift in the dose-response function for each CB1 agonist (Fig. 3). As in prechronic determinations, the highest doses of AM4054 (3.2 mg/kg), WIN55,212.2 (32 mg/kg), and Δ9-THC (100 mg/kg) decreased overall rates of FR responding to <50% of control values, and their rank order of potency was unchanged: AM4054 > WIN55,212.2 > Δ9-THC (Fig. 3, top and bottom left panels, respectively; Table 1). Relative potency estimates confirm the significant differences observed between the dose-effect functions for each CB1 agonist determined before and during chronic treatment (Table 1). As with AM411, the highest doses of methanadamide (100–320 mg/kg i.m.) failed to produce >25% decreases in rates of FR responding (Fig. 3, bottom right panel), precluding the determination of ED50 values during the chronic regimen. Studies with higher doses of AM411 and methanandamide during the chronic AM411 regimen were not possible due to limitations in drug solubility.
Fig. 3.
Effects of the cannabinoid CB1 receptor agonists AM4054, WIN55,212.2, Δ9-THC, and methanandamide in squirrel monkeys responding under the FR30 schedule of stimulus-shock termination. Other details are as in Fig. 2.
The effects of AM4054 also were re-determined immediately prior to the conclusion of the chronic regimen to determine whether further changes in CB1 sensitivity might have occurred. In these last determinations, AM4054 continued to produce dose-dependent decreases in rates of FR responding, and overall response rates decreased to <50% of control values for the group of subjects following the highest dose of AM4054 (3.2 mg/kg; Fig. 3, top left panel).
Effects of Cannabinoid CB1 Receptor Antagonists.
Prechronic administration of both rimonabant (3.2–18 mg/kg i.v.), and AM4113 (1–10 mg/kg i.v.) produced dose-related decreases in overall response rates and, after the highest doses, decreased responding to approximately 20–30% of control rates of responding (Fig. 4). During chronic treatment with AM411, the dose-related effects of both drugs were markedly enhanced, as evident in the >2-fold and 1- to 1.5-fold log unit leftward shifts in the dose-effect functions for SR141716A and AM4113, respectively, as well as the corresponding decreases in their ED50 values (Fig. 4; Table 1). All subjects were observed for signs of motoric impairment and easily identifiable changes in home cage behaviors (e.g., grooming, affiliation, vocalization) after doses of SR141716A and AM4113 that significantly disrupted rates of responding. Excepting profuse salivation, easily identifiable or consistent effects on overt behavior that might be indicative of physical dependence (Panagis et al., 2008) were not observed after rate-decreasing doses of the two drugs.
Fig. 4.
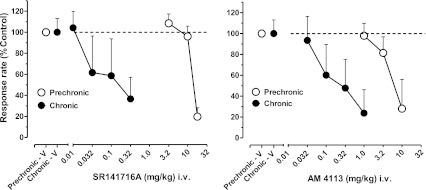
Effects of the cannabinoid CB1 receptor antagonists SR141716A and AM4113 administered i.v. via the tail vein in squirrel monkeys responding under the FR30 schedule of stimulus-shock termination. Other details are as in Fig. 2.
Effects of DA-Related Agonists and Antagonists.
Interactions between cannabinoid and DA systems were examined by comparing the effects of the indirect DA agonist, methamphetamine, the DA D1- and D2-like agonists (SKF82958 and R-(−)-NPA, respectively), and the D1- and D2-like antagonists (SCH23390 and haloperidol, respectively) on FR response rates before and during the chronic regimen. Prior to chronic treatment, all drugs produced dose-related reductions in responding, with substantive (>75%) decreases in overall response rates following the highest dose of each DA agonist or antagonist (Fig. 5; Table 2). Chronic treatment with AM411 led to significant changes in potency for some but not all DA-related drugs. Thus, 0.5- to 1.0-log unit rightward shifts in dose-effect functions, with corresponding and significant increases in ED50 values, were evident for methamphetamine and the D2-like agonist R-(−)-NPA (Fig. 5; Table 1). However, the position of the dose-response function for the D1-like agonist SKF82958 and its ED50 value were unchanged by chronic treatment with AM411 (Table 1). Similarly, despite some attenuation of the rate-decreasing effects of the highest doses of SCH23390 (0.032 mg/kg) and haloperidol (0.32 mg/kg), the dose-response functions and, correspondingly, ED50 values for the D1 and D2 receptor antagonists were not significantly altered by chronic AM411 treatment (Tables 1 and 2).
Fig. 5.
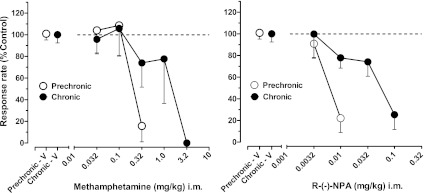
Effects of the indirect DA agonist methamphetamine and the DA D2 receptor agonist R-(−)-NPA in squirrel monkeys responding under the FR30 schedule of stimulus-shock termination. Abscissa: cumulative dose, log scale. Other details are as in Fig. 2.
TABLE 2.
Mean (± S.E.M.) rates of responding under the FR30 stimulus-shock termination schedule (n=3-4) after administration of the DA D1-like agonist (SKF82958) and antagonist (SCH23390), DA D2-like antagonist (haloperidol), and opioid agonist (morphine) and antagonist (naltrexone) before and during chronic exposure to the cannabinoid CB1 receptor agonist AM411 (1.0 mg/kg per day, i.m.)
Control values (± S.D.) before and during chronic treatment are also given for comparison.
| Drug | Dose | Control Response Rate (S.E.M.) |
||
|---|---|---|---|---|
| Prechronic | Chronic | |||
| mg/kg | % | % | ||
| Control | Vehicle | 0 | 100.90 (±5.78 S.D.) | 100.00 (±7.59 S.D.) |
| DA D1 agonist | SKF82958 | 0.032 | 121.41 (±9.48) | 98.22 (±27.18) |
| 0.1 | 51.95 (±26.81) | 35.81 (±17.99) | ||
| 0.32 | 26.95 (±26.95) | 8.99 (±8.99) | ||
| 1.0 | 0 (±0) | 0.70 (±0.70) | ||
| DA D1 antagonist | SCH23390 | 0.01 | 100.37 (±22.18) | 101.86 (±24.42) |
| 0.032 | 9.88 (±7.84) | 36.51 (±18.77) | ||
| 0.1 | – | 28.60 (±28.14) | ||
| DA D2 antagonist | Haloperidol | 0.0032 | 99.55 (±10.03) | 93.18 (±13.07) |
| 0.01 | 91.09 (±10.99) | 77.44 (±7.44) | ||
| 0.032 | 83.23 (±17.91) | 61.16 (±14.31) | ||
| 0.1 | 0.30 (±0.21) | 26.36 (±24.03) | ||
| 0.32 | – | 0.16 (±0.16) | ||
| Opioid agonist | Morphine | 0.032 | 107.78 (±19.57) | – |
| 0.1 | 96.41 (±22.06) | 107.01 (±16.89) | ||
| 0.32 | 100.15 (±23.99) | 94.74 (±14.97) | ||
| 1.0 | 67.81 (±24.53) | 89.33 (±24.36) | ||
| 1.8 | 40.62 (±15.0) | – | ||
| 3.2 | 17.22 (±17.22) | 51.63 (±18.25) | ||
| 10.0 | – | 14.19 (±5.96) | ||
| Opioid antagonist | Naltrexone | 1.0 | 121.93 (±24.24) | 111.09 (±19.82) |
| 3.2 | 100.90 (±39.18) | 96.20 (±23.83) | ||
| 10.0 | 114.97 (±22.79) | 100.78 (±22.43) | ||
DA, dopamine; FR30, 30-response fixed ratio.
Effects of Morphine and Naltrexone.
As shown in Table 2, no evidence for significant cross-tolerance or sensitization was obtained with either the µ-opioid agonist morphine or the nonselective opioid antagonist naltrexone in the present experiments. Prior to chronic treatment, morphine (0.032–3.2 mg/kg i.m.) produced a dose-dependent reduction in response rates, with the highest dose, 3.2 mg/kg, decreasing responding to <20% of control values (Table 2). Although a slight rightward shift in the dose-response function occurred during chronic AM411 exposure, inspection of ED50 values and relative potency analysis of the prechronic and chronic dose-response functions indicate no significant change in morphine sensitivity (Table 1). Similarly, no change in sensitivity to naltrexone (1.0–10 mg/kg i.m.) was apparent; the opioid antagonist failed to significantly alter FR response rates prior to or during chronic AM411 exposure (Tables 1 and 2).
Discussion
In the present study, chronic treatment with AM411, a long-acting CB1 receptor agonist produced marked tolerance and cross-tolerance to the rate-decreasing effects of CB1 receptor agonists on schedule-controlled responding in monkeys. In addition, chronic treatment with AM411 increased the potency with which CB1 receptor antagonists disrupted schedule-controlled behavior. Chronic AM411 treatment attenuated the behaviorally disruptive effects of methamphetamine and the DA D2-family agonist R-(−)-NPA without substantively altering the behavioral effects of other dopaminergic ligands including the D1-family agonist SKF82958 and the DA D1- and D2-family receptor blockers SCH23390 and haloperidol, respectively. Finally, chronic treatment with AM411 did not substantively alter the effects of the µ-opioid agonist morphine or the nonselective opioid antagonist naltrexone. In distinction to previous findings of behavioral disruption upon cessation of chronically administered Δ9-THC in monkeys (Beardsley et al., 1986; McMahon, 2011), no signs of withdrawal-related physical discomfort or alteration in operant performance were evident upon discontinuation of the chronic regimen of AM411. Such differences may reflect, at least partly, a slower offset in action for AM411 than for Δ9-THC (Delatte and Bergman, unpublished data) as a result of a more gradual reduction in plasma and brain levels. This idea is consistent with previous findings showing that the severity of spontaneous withdrawal with other dependence-inducing drugs, such as morphine, may be lessened by gradual reductions in dosage (Bergman and Schuster, 1985).
CB1 Tolerance and Cross-Tolerance.
Tolerance developed to the effects of chronic treatment with AM411 in a manner that is generally consistent with previous findings of tolerance to the effects of Δ9-THC on schedule-controlled behavior in monkeys. Notwithstanding the daily administration of initially high doses of AM411, response rates recovered to within approximately 20% of prechronic control values within 4–8 days of treatment. Tolerance to the effects of a behaviorally comparable dosage of Δ9-THC in monkeys (1.0 mg/kg, administered twice daily) developed somewhat more slowly (i.e., 20–24 days; McMahon, 2011); however, based on ED50 values for Δ9-THC in the two studies, the magnitude of changes in sensitivity is highly comparable, suggesting similar changes in the sensitivity of underlying CB1 receptor mechanisms.
The present findings also are consistent with previous reports of cross-tolerance between Δ9-THC and other CB1 receptor agonists (Panagis et al., 2008; McMahon, 2011; Singh et al., 2011) and extend them to include cross-tolerance to the rate-decreasing effects of the CB1 receptor agonists methanandamide, Δ9-THC, AM4054, and WIN55,212.2 in AM411-treated subjects. The magnitude of cross-tolerance to WIN 55-212.2, Δ9-THC, and AM4054 were generally similar in the present studies, whereas chronic treatment with AM411 produced significantly greater tolerance to its own effects and those of methanandamide. Thus, even doses of AM411 and methanandamide ≥100 mg/kg failed to substantively decrease response rates during the chronic regimen, precluding accurate estimates of the tolerance that developed. The gradation in tolerance to CB1-mediated behavioral effects (i.e., AM4054 ≈ WIN55,212.2 < Δ9-THC < methanandamide, AM411), is consistent with these drugs differing in intrinsic efficacy at the CB1 receptor—even though such differences typically are not apparent in studies of their acute effects. For example, the CB1 putative partial (Δ9-THC) and full (CP55,940 or WIN55,212.2) agonists produce comparable antinociceptive, motoric, or hypothermic effects when administered acutely (Chaperon and Thiebot, 1999) reflecting the low fractional occupancy that appears to be sufficient for such CB1-mediated behavioral effects (McMahon, 2011; Singh et al., 2011). In the present studies, however, the fractional occupancy required for rate-decreasing effects presumably increased during chronic treatment with AM411, perhaps as a result of CB1 receptor desensitization or down-regulation. Consequently, only ligands with sufficient efficacy, (i.e., that did not require high levels of occupancy) still could decrease response rates (Sim et al., 1996; Breivogel et al., 1999).
The proposed role of efficacy in the present studies is consistent with previous findings using other biologic endpoints (e.g., inhibition of adenylyl cyclase or smooth muscle contraction; Pertwee et al., 1993; Selley et al., 2004). From this perspective, the present results suggest that the CB1 agonists AM411 and methanandamide, like Δ9-THC, are best characterized as CB1 partial agonists. In this regard, methanandamide previously has been shown to only partially mimic Δ9-THC–like discriminative stimulus effects in rats (Jarbe et al., 1998), which may be a further indication of its partial agonist activity (Lamb et al., 2000). Yet, this suggestion must be viewed cautiously in light of the limited number of studies that address the in vivo efficacy of these ligands.
Enhanced Sensitivity to CB1 Antagonists.
The increased potency with which both the CB1 inverse agonist, rimonabant, and the CB1 neutral antagonist, AM4113, decreased response rates during chronic AM411 treatment extend previous observations in Δ9-THC–treated subjects (Lamb et al., 2000; McMahon, 2011) and are analogous to the increased behavioral sensitivity to antagonists or inverse agonists observed during chronic treatment with other classes of drugs such as opioids or benzodiazepines (Goldberg and Schuster, 1967; McMahon and France, 2002). Based upon evidence from these pharmacological classes, such increased sensitivity in the present studies may reflect increases in negative efficacy (Liu and Prather, 2001; Liu and Prather, 2002; McMahon and France, 2002). However, this idea remains speculative in the absence of further direct evidence.
Previous studies also have delineated pharmacological distinctions between the CB1 inverse agonist rimonabant and novel cannabinoid CB1 receptor neutral antagonists (e.g., AM4113) in signal transduction and in their physiologic or behavioral actions (McLaughlin et al., 2006; Bergman et al., 2008; Sink et al., 2008). The leftward shift in dose-response function was noticeably greater for rimonabant (>2 log units) than for AM4113 (<1.5 log unit) in the present studies. The extent to which simply displacement of AM411 from its binding site contributes to the observed effects of rimonabant and AM4113 in chronically treated subjects is unknown. It is also unclear whether the difference in increased sensitivity of rimonabant and AM4113 can be attributed to differences in mechanism ascribed to the two drugs (i.e., inverse agonism versus neutral antagonism). Earlier studies with GABAA ligands have not supported the idea that the magnitude of increased sensitization varies in relation to efficacy of antagonists and inverse agonists (Martin et al., 1995; McMahon and France, 2002). Although some tendency to greater increases in potency for neutral compared with inverse agonists were observed in diazepam-treated subjects (McMahon and France, 2002), the effects of GABAA and CB1 ligands are mediated differently (i.e., ion channel versus G-protein-coupled receptors signaling) and the extent to which conclusions can be applied across these types of systems is unknown.
CB1 Drug Dependence.
The association between CB1 agonist-induced tolerance and classical physical dependence generally has been controversial. Signs of physical distress that might indicate an abstinence syndrome have not been easily identified after discontinuation of chronic CB1 agonist treatment (spontaneous withdrawal) or administration of rimonabant (antagonist-precipitated withdrawal; Panagis et al., 2008). Early studies in monkeys showed that the discontinuation of chronic i.v. infusion of Δ9-THC led to the disruption of schedule-controlled responding, which could be attenuated by the resumption of chronic treatment (Beardsley et al., 1986). However, these effects occurred without clear physical distress, and may have reflected only the offset of the effects of Δ9-THC, leading the authors to conservatively describe their results as denoting “behavioral dependence,” which did not imply other changes in underlying substrate. More recently, studies of withdrawal from chronic CB1 agonist treatment have reported signs of physical dependence, such as increased wakefulness in humans and monkeys upon spontaneous withdrawal, or increased frequency of paw tremors and head-shaking in mice and monkeys upon antagonist-precipitated withdrawal (Lichtman et al., 2001; Budney et al., 2004; Stewart and McMahon, 2010). The increased sensitivity to rimonabant and AM4113 in the present studies is consistent with changes in behavioral sensitivity to the effects of rimonabant observed in Δ9-THC–treated rodents and monkeys and, accordingly, may reflect physical dependence (McMahon, 2011; Singh et al., 2011). However, this view should be considered carefully for several reasons. First, the utility of increased sensitivity to the effects of CB1 antagonists during chronic treatment as a marker of physical dependence is not firmly established. Thus, increased sensitivity to the disruptive effects of rimonabant on operant behavior in previous studies of Δ9-THC–treated rats was not maintained over the course of repeated determinations (Lamb et al., 2000). It is difficult to imagine that physical dependence, if present, subsided during chronic treatment in those studies. Second, as suggested above, antagonist-precipitated disruptions in operant behavior in the present studies may be attributable to the abrupt offset of agonist action (Beardsley et al., 1986). Finally, increases in head-shaking after rimonabant, reported in Δ9-THC–treated rhesus monkeys by Stewart and McMahon (2010), were not prominent after rimonabant or AM4113 treatment in the present experiments. Such effects on overt behavior may be species dependent or context dependent; nonetheless, doses of rimonabant that disrupted operant behavior in AM411-treated squirrel monkeys did not reveal easily identifiable or consistent effects on overt behavior aside from profuse salivation. However, profuse salivation can be observed following the repeated administration of rimonabant itself and, thus, cannot be viewed as a sufficient indicator of physical dependence (unpublished observations; including rimonabant-induced scratching in nondependent rats/mice) (Janoyan et al., 2002; Jarbe et al., 2006; Schlosburg et al., 2011). Taken together, the above findings suggest that the term behavioral dependence—which, beyond displacement of the agonist from its binding site, does not presume alterations in substrate that mediate physical dependence—still may be the most appropriate term to describe changes in operant behavior that are precipitated by an antagonist either during chronic CB1 agonist treatment or after its discontinuation.
Effects of μ-Opioid and DA Ligands.
Evidence from other studies suggests that complex neurochemical and behavioral interactions exist between CB1–opioid–DA systems that may contribute importantly to the addiction liability of cannabinoids (Maldonado, 2002; Tanda and Goldberg, 2003). In the present study, chronic AM411 treatment failed to alter the effects of either morphine or naltrexone on response rates, indicating that tolerance to the effects of CB1 agonists is mediated via brain mechanisms that do not prominently involve μ-opioid systems. With regard to CB1–DA interactions, previous studies in nonhuman primates have demonstrated that co-administration of a threshold dose of levonantradol and a DA D2-like receptor agonist (quinelorane) precipitated marked sedation, ptosis, and decreased general activity and locomotor behavior. In contrast, co-administration of levonantradol and the DA D1-like receptor agonist SKF81297 had no effect, highlighting the specificity of the interaction between CB1 and DA D2-like systems (Meschler et al., 2000). In the present studies, the effects of acute AM411 treatment in combination with a D2-like agonist were not studied, precluding the collection of additional evidence for such DA receptor subtype-selective interactions with a CB1 agonist. However, consistent with such selective interactions, chronic AM411 treatment produced cross-tolerance to the effects of the direct DA D2-like receptor agonist R-(−)-NPA and no change in the effects of the direct DA D1-like agonist SKF82958. Cross-tolerance to the rate-decreasing effects of the indirect DA agonist methamphetamine also was observed, implicating the involvement of DA D2-like receptor mechanisms. However, the lack of significant changes in the effects of the DA D2-like antagonist haloperidol indicates that such CB1 and DA D2-like interactions likely do not occur at the receptor level, but may involve common downstream actions.
Acknowledgments
The authors thank Lindsey Wood and Wyatt Traina for expert technical support, and Carol A. Paronis for comments on an earlier version of this manuscript.
Abbreviations
- AM4054
9β-(hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol
- AM411
(6aR,10aR)-3-(1-adamantyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol
- AM4113
5-(4-alkylphenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- DA
dopamine
- FR
fixed ratio
- FR30
30-response fixed ratio
- R-(−)-NPA
(6aR)-5,6,6a,7-tetrahydro-6-propyl-4H-dibenzo[de,g]quinoline-10,11-diol
- SCH 23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- SKF 82958
(±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide
- SR141716A
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- Δ9-THC
Δ9-tetrahydrocannabinol
- TO10
10-second timeout period
- WIN55,212
R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate
Authorship Contributions
Participated in research design: Desai, Bergman.
Conducted experiments: Desai.
Contributed new reagents: Thakur, Vemuri, Bajaj, Makriyannis.
Performed data analysis: Desai, Bergman.
Wrote or contributed to the writing of the manuscript: Desai, Bergman.
Footnotes
This research was supported by grants from National Institutes of Health National Institute on Drug Abuse [Grants DA19205 (J.B., principal investigator) and DA26795 (A.M., principal investigator)], as well as a Ruth L. Kirschstein National Service Award [awarded to R.I.D. (N.K. Mello, principal investigator).
References
- Alford DP, Compton P, Samet JH. (2006) Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med 144:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. (2011) CP47,497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol 659:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311–319 [PubMed] [Google Scholar]
- Benford DM, Caplan JP. (2011) Psychiatric sequelae of Spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics 52:295. [DOI] [PubMed] [Google Scholar]
- Bergman J, Schuster CR. (1985) Behavioral effects of naloxone and nalorphine preceding and following morphine maintenance in the rhesus monkey. Psychopharmacology (Berl) 86:324–327 [DOI] [PubMed] [Google Scholar]
- Bergman J, Spealman RD. (1988) Behavioral effects of histamine H1 antagonists: comparison with other drugs and modification by haloperidol. J Pharmacol Exp Ther 245:471–478 [PubMed] [Google Scholar]
- Bergman J, Rosenzweig-Lipson S, Spealman RD. (1995) Differential effects of dopamine D1 and D2 receptor agonists on schedule-controlled behavior of squirrel monkeys. J Pharmacol Exp Ther 273:40–48 [PubMed] [Google Scholar]
- Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. (2008) Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav 93:666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. (1999) Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem 73:2447–2459 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. (2004) Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 161:1967–1977 [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiébot MH. (1999) Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol 13:243–281 [DOI] [PubMed] [Google Scholar]
- Delatte MS, Thakur G, Connolly J, Zakarian A, Makriyannis A, Bergman J. (2008) Discriminative-stimulus effects of the novel CB1 agonist AM 4054 in squirrel monkeys. FASEB J 22:711 [Google Scholar]
- Finney DJ. (1964) Statistical Method in Biological Assay, 2nd ed, Hafner, New York [Google Scholar]
- Fredericks AB, Benowitz NL. (1980) An abstinence syndrome following chronic administration of delta-9-terahydrocannabinol in rhesus monkeys. Psychopharmacology (Berl) 71:201–202 [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. (1979) Discriminative stimulus effects of naltrexone in the morphine-dependent rat. J Pharmacol Exp Ther 211:596–605 [PubMed] [Google Scholar]
- Goldberg SR, Schuster CR. (1967) Conditioned suppression by a stimulus associated with nalorphine in morphine-dependent monkeys. J Exp Anal Behav 10:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. (1999) Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 141:385–394 [DOI] [PubMed] [Google Scholar]
- Janoyan JJ, Crim JL, Darmani NA. (2002) Reversal of SR 141716A-induced head-twitch and ear-scratch responses in mice by delta 9-THC and other cannabinoids. Pharmacol Biochem Behav 71:155–162 [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. (1998) Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 140:519–522 [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Ross T, DiPatrizio NV, Pandarinathan L, Makriyannis A. (2006) Effects of the CB1R agonist WIN-55,212-2 and the CB1R antagonists SR-141716 and AM-1387: open-field examination in rats. Pharmacol Biochem Behav 85:243–252 [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. (1968) Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol 60:1–56 [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TU, Makriyannis A, Lin S, Goutopoulos A. (2000) Effects of Delta 9-tetrahydrocannabinol, (R)-methanandamide, SR 141716,and d-amphetamine before and during daily Delta 9-tetrahydrocannabinol dosing. Eur J Pharmacol 398:251–258 [DOI] [PubMed] [Google Scholar]
- Landry MJ, Smith DE, McDuff DR, Baughman OL., 3rd (1992) Benzodiazepine dependence and withdrawal: identification and medical management. J Am Board Fam Pract 5:167–175 [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. (2001) Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav 69:181–188 [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. (2001) Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol 60:53–62 [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. (2002) Chronic agonist treatment converts antagonists into inverse agonists at delta-opioid receptors. J Pharmacol Exp Ther 302:1070–1079 [DOI] [PubMed] [Google Scholar]
- Lu D, Meng Z, Thakur GA, Fan P, Steed J, Tartal CL, Hurst DP, Reggio PH, Deschamps JR, Parrish DA, et al. (2005) Adamantyl cannabinoids: a novel class of cannabinergic ligands. J Med Chem 48:4576–4585 [DOI] [PubMed] [Google Scholar]
- Maldonado R. (2002) Study of cannabinoid dependence in animals. Pharmacol Ther 95:153–164 [DOI] [PubMed] [Google Scholar]
- Martin JR, Jenck F, Moreau JL. (1995) Comparison of benzodiazepine receptor ligands with partial agonistic, antagonistic or partial inverse agonistic properties in precipitating withdrawal in squirrel monkeys. J Pharmacol Exp Ther 275:405–411 [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, Ishiwari K, Betz AJ, Pandarinathan L, Xu W, et al. (2006) Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav 83:396–402 [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. (2002) Daily treatment with diazepam differentially modifies sensitivity to the effects of gamma-aminobutyric acid(A) modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther 300:1017–1025 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006) Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 188:306–314 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2011) Chronic Δ⁹-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol 162:1060–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschler JP, Clarkson FA, Mathews PJ, Howlett AC, Madras BK. (2000) D(2), but not D(1) dopamine receptor agonists potentiate cannabinoid-induced sedation in nonhuman primates. J Pharmacol Exp Ther 292:952–959 [PubMed] [Google Scholar]
- Morse WH, Kelleher RT. (1966) Schedules using noxious stimuli. I. Multiple fixed-ratio and fixed-interval termination of schedule complexes. J Exp Anal Behav 9:267–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G, Vlachou S, Nomikos GG. (2008) Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev 1:350–374 [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. (1993) Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol 110:1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, O’Neal ST, Conrad DH, Lichtman AH. (2011) CB1 receptors mediate rimonabant-induced pruritic responses in mice: investigation of locus of action. Psychopharmacology (Berl) 216:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Cassidy MP, Martin BR, Sim-Selley LJ. (2004) Long-term administration of Delta9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol Pharmacol 66:1275–1284 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. (1996) Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:8057–8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. (2011) Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology (Berl) 215:665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, et al. (2008) The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology 33:946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. (1967) Statistical Methods, Ed. 6th Iowa State University Press, Ames, IA. [Google Scholar]
- Stewart JL, McMahon LR. (2010) Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther 334:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. (2003) Cannabinoids: reward, dependence, and underlying neurochemical mechanisms—a review of recent preclinical data. Psychopharmacology (Berl) 169:115–134 [DOI] [PubMed] [Google Scholar]
- Woods JH, Young AM, Herling S. (1982) Classification of narcotics on the basis of their reinforcing, discriminative, and antagonist effects in rhesus monkeys. Fed Proc 41:221–227 [PubMed] [Google Scholar]