Abstract
Candesartan is an angiotensin II type 1 receptor blocker (ARB) that has been to shown to limit ischemic stroke and improve stroke outcome. In experimental stroke, candesartan induces a proangiogenic effect that is partly attributable to vascular endothelial growth factor. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family that has been reported to have angiogenic effects and play an important role in recovery after stroke. The purpose of this investigation was to determine the role of BDNF in the proangiogenic effect of candesartan in the brain under hypertensive conditions. Accordingly, spontaneously hypertensive rats were treated with candesartan, and brain tissue samples were collected for quantification of BDNF expression. In addition, human cerebromicrovascular endothelial cells were treated with either low-dose (1 ƒM) or high-dose (1 µM) angiotensin II alone or in combination with candesartan (0.16 µM) to assess the effect of candesartan treatment and BDNF involvement in the behavior of endothelial cells. Candesartan significantly increased the expression of BDNF in the SHR (P < 0.05). In addition, candesartan reversed the antiangiogenic effect of the 1-µM dose of AngII (P = 0.0001). The observed effects of candesartan were ablated by neutralizing the effects of BDNF. Treatment with the AT2 antagonist PD-123319 significantly reduced tube-like formation in endothelial cells. AT2 stimulation induced the BDNF expression and migration (P < 0.05). In conclusion, candesartan exerts a proangiogenic effect on brain microvascular endothelial cells treated with angiotensin II. This response is attributable to increased BDNF expression and is mediated through stimulation of the AT2 receptor.
Introduction
Angiotensin II type 1 receptor blockers (ARBs) have been shown to be vascular protective and seem to have a particularly robust effect in reducing the incidence of cerebrovascular events (Dahlöf et al., 2002; Engelhorn et al., 2004; Kozak et al., 2009). Acutely, ARBs have been shown to improve outcome in experimental stroke (Fagan et al., 2006), and the long-term functional benefit was accompanied by an augmented proangiogenic state (Kozak et al., 2009). This angiogenic effect was only partially attributed to an increase in vascular endothelial growth factor (VEGF) expression (Kozak et al., 2009). Of interest, the angiogenic response of candesartan was maintained even in nonstroked rats (Kozak et al., 2009). Subsequently, it was demonstrated that candesartan increased the expression of a number of genes for proangiogenic growth factors, including brain-derived neurotrophic factor (BDNF), after experimental cerebral ischemia (Guan et al., 2011). BDNF is a member of the neurotrophin family that is expressed in a number of cell types and has been shown to have potent neurogenic, neuroprotective, and angiogenic effects (Caporali and Emanueli, 2009; Greenberg et al., 2009). The proangiogenic effects of ARBs are hotly debated, however, (Schieffer et al., 1994; Herr et al., 2008; Kozak et al., 2009) and may be tissue and situation dependent (Willis et al., 2011). In the brain, it is unclear whether the effects of ARBs are attributable to blood pressure lowering or a direct effect of candesartan on endothelial cells.
Glycogen synthase kinase–3β (GSK-3β) is a serine threonine kinase that plays a key role in gene expression regulation (Wada, 2009; Hur and Zhou, 2010). Recently, Li et al. demonstrated the involvement of GSK-3β in the recovery after CNS ischemic insults (Li et al., 2011). They demonstrated the involvement of GSK inhibition in regulating neural stem cells–endothelial cells cross talk (Li et al., 2011). This cross talk was found to be mediated through soluble growth factors, such as BDNF (Madri, 2009).
The purpose of this investigation was to determine whether candesartan-mediated blood pressure lowering increased BDNF protein expression in the brain in vivo and whether BDNF is involved in the proangiogenic effect of candesartan in vitro. In addition, the involvement of GSK-3β in candesartan-mediated effects was assessed.
Materials and Methods
Animals
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Charlie Norwood Veterans Affairs Medical Center (09-04-008). Male spontaneously hypertensive rats (SHRs; 280–300 g; 4–6 per group) were subjected to middle cerebral artery occlusion sham surgery and randomized to receive a single intravenous dose of candesartan (0.3 mg/kg), hydralazine (1 mg/kg), or saline. In addition, male wistar rats (280–300 g; 4 per group) were subjected to the same surgery and randomized to receive either a single intravenous dose of candesartan (1 mg/kg) or saline. Twenty-four hours later, the rats were euthanized and the brains were harvested and flash-frozen in liquid nitrogen.
Western Blotting
To assess BDNF expression, the right and left hemispheres were separated and processed as described previously (Guan et al., 2011), and the blots were probed with anti-BDNF (1:250; Abcam, Cambridge, MA) and β-actin (1:10000; Sigma-Aldrich, St. Louis, MO). For the in vitro experiments, human cerebrovascular endothelial cells (hCMECs) were cultured to confluence and serum starved for 10 hours, followed by incubations with either 1 ƒM or 1 µM angiotensin II (AngII). After 6 hours, candesartan (0.16 µM) was added to the cells and incubated for 16 hours and then homogenized and processed for immunoblotting. To assess the involvement of AT2 receptor in BDNF expression, hCMECs were serum starved for 16 hours and pretreated with PD-123319 (0.1 µM) 30 minutes before being incubated with AngII (1 ƒM or 1 µM) for 6 hours. Candesartan or vehicle was introduced in the media for 10 hours. To further confirm AT2 involvement, cells were serum starved for 16 hours, followed by treatment with either the AT2 agonist CGP-121141A (0.1 µM) or vehicle for 16 hours. The expression of AT1 and AT2 and the phosphorylation status of GSK-3β were assessed using the same aforementioned treatment paradigm. Blots were probed with mouse monoclonal AT1 antibody (1:1000; Abcam), rabbit monoclonal AT2 antibody (1:1000; Abcam), p-S9GSK-3β (1:1000; Cell Signaling Technology, Danvers, MA), and total GSK-3β (1:1000; cell signaling). Protein expression was quantified as the relative optic density of the protein band normalized to actin using National Institutes of Health image J software.
Cell Culture
hCMECs were provided as a generous gift from Dr. J. Zastre (UGA College of Pharmacy). hCMECs were cultured in minimum essential media (American Type Culture Collection) supplemented with EGM-2 SingleQuot Kit Suppl & Growth Factors (Lonza, Allendale, NJ), and 10% fetal-bovine serum (Atlanta Biologicals, Atlanta, GA) and p30-34 were used in the experiments.
Treatments
Candesartan was provided as a generous gift from Astra-Zeneca Pharmaceuticals LP, Wilmington, DE. Hydralazine was purchased from Sigma-Aldrich and was reconstituted with 0.9% normal saline. AngII was purchased from Sigma-Aldrich and was reconstituted and diluted to the desired concentration using serum-free media. BDNF neutralization was achieved using 100 nM K252a (Trk receptor inhibitor) dissolved in 25% dimethylsulfoxide (DMSO), both purchased from Sigma-Aldrich 10 ng/ml anti-BDNF neutralizing antibody (Abcam); and 0.4 μg/ml TrkB-Fc (soluble BDNF receptor chimera; R&D systems, Minneapolis, MN). The involvement of AT2 receptor was assessed using the AT2 antagonist PD-123319 (0.1 µM) and the AT2 receptor agonist CGP-42112A (0.1 µM), both purchased from Sigma-Aldrich. All the inhibitors were added to the media 30 minutes before AngII treatment.
Dose and Time Study
hCMECs were cultured until confluence and serum starved for 10 hours. Cells were incubated with six different concentrations of AngII (0–1 µM) for 2, 6, and 8 hours, and the cells were homogenized and processed for immunoblotting. BDNF expression was quantified as the relative density of the BDNF band to the corresponding β-actin or glyceraldehyde-3-phosphate dehydrogenase bands. The calculated relative density was normalized to the relative density of the control band and reported as fold change.
Proliferation Assay
Proliferation was assessed using BrdU incorporation (Cell Proliferation ELISA, BrdU [colorimetric]; Roche Applied Science, Indianapolis, IN) according to the manufacturer recommendations. In brief, 5000 hCMECs were seeded into each well of a 96-well plate and left to attach for 24 hours. Cells were serum starved for 10 hours and then treated with AngII (1 ƒM or 1 µM) for 6 hours. After 6 hours, cells were treated with different combinations of candesartan, anti-BDNF, TrkB-Fc, K252a, DMSO, or IgG and incubated for 18 hours. The cells were then labeled with BrdU for 4 hours and processed to quantify BrdU incorporation.
Angiogenesis Assays
Cell Migration.
Wound recovery assay was used to assess cell migration in which hCMECs were cultured in a 12-well plate to confluence and then serum starved for 10 hours, followed by 6 hours of AngII (1 ƒM or 1 µM) treatment. A wound was introduced in the monolayer of endothelial cells, and the cells were treated with AngII (1 ƒM or 1 µM) with different combinations of candesartan, anti-BDNF, TrkB-Fc, K252a, DMSO, or IgG. In some experiments, cells were pretreated with PD-123319 (0.1 µM) 30 minutes before AngII treatment. In another set of studies, cells were treated with CGP-42112A (0.1 µM), candesartan (0.16 µM), or their combination. Scratch recovery was assessed by taking images of the scratch at baseline and at 16 hours after scratch introduction, and the width of the scratch was measured at both time points using National Institutes of Health image J software. The percentage wound recovery was calculated as the percentage decrease in scratch width at 16 hours, and the data were presented as percentage scratch recovery, compared with the control.
Tube Formation.
A total of 2 × 104 hCMECs were suspended in serum-free media, mixed with matrigel (BD Biosciences, San Jose, CA) in a 60:30 ratio, plated in a 96-well plate, and treated with different combinations of AngII (1 ƒM or 1 µM; Sigma-Aldrich), candesartan, anti-BDNF, TrkB-Fc, K252a, DMSO, IgG, or PD-123319. Tube-like structure formation was assessed using a digital camera attached to an Olympus microscope. Three images from each well were photographed at 24 hours, and the number of tube-like structures was quantified.
Statistical Analysis
All experiments were repeated three times in triplicate, and data were quantified in a blinded manner. Statistical significance was detected using one-way analysis of variance for in vitro data, followed by post-hoc Tukey test. Unpaired t test was used to determine the significance of BDNF expression in the right and left brain hemispheres of candesartan-treated SHRs, compared with the corresponding hemisphere in saline-treated animals. Statistical analyses were performed using GraphPad prism (version 5.1; GraphPad Software, La Jolla, California). P < 0.05 was considered to be statistically significant.
Results
Candesartan Increases the Expression of BDNF in SHR Brain
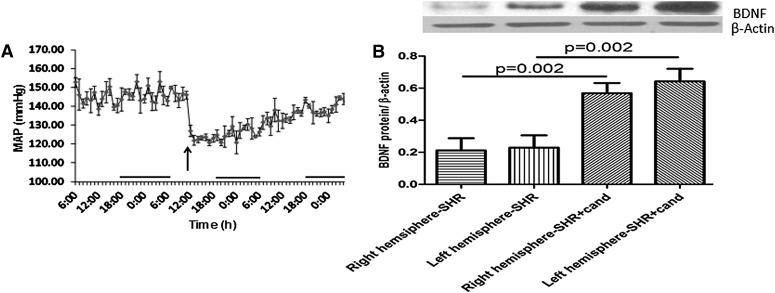
BDNF has been shown to exert a beneficial effect in a variety of CNS pathologies (Greenberg et al., 2009); the direct use of BDNF in therapy is limited by its pharmacokinetic profile (Greenberg et al., 2009). A plausible alternative approach is to use either synthetic BDNF mimetics or agents that can induce BDNF expression in the brain. Treatment with a single dose of candesartan (0.3 mg/kg) dramatically reduced the blood pressure from 150 mm Hg at baseline to 120 mm Hg after treatment (Fig. 1A). Candesartan treatment significantly increased the expression of BDNF in both right and left hemispheres of SHR animals 24 hours after sham surgery (Fig. 1B). The effect of candesartan on BDNF expression was maintained in wistar rats (Supplemental Data S3). In contrast, hydralazine treatment did not affect BDNF expression in SHRs (Supplemental Data S4).
Fig. 1.
Hypertension and AT1 blockade affects the expression of BDNF. Blood pressure transmitters were implanted intraperitoneally in SHRs. Animals had sham surgery and received a single dose of candesartan (0.3 mg/kg), and the mean arterial blood pressure was monitored (A). Arrow indicates time of candesartan administration; n = 6. SHRs underwent sham surgery and were randomized to receive either candesartan (0.3 mg/kg) or saline intravenously (6 per group); 24 hours later, the animals were sacrificed and the brains were extracted. Right and left hemispheres were separated and processed for immunoblotting (B).
Angiotensin II Modulates the Expression of BDNF in hCMECs
Angiotensin II has been reported to affect the expression of BDNF in the adrenals (Szekeres et al., 2010) and brain (Chan et al., 2010), but its effect on BDNF expression in endothelial cells has never been reported. In hCMECs, treatment with AngII was found to induce a dose- and time-dependent modulation of BDNF expression that was maximal at 6 hours after incubation with AngII at either 1 ƒM or 1 µM (Fig. 2A). These two concentrations were used in the following experiments.
Fig. 2.
Angiotensin II and AT1 blockade affects the expression of BDNF and the angiogenic potential in hCMECs. hCMECs were cultured to confluence, followed by serum starvation for 16 hours. Cells were treated with a concentration range of AngII for different periods. The expression of BDNF was assessed using immunoblotting (A) n = 3–5. hCMECs were treated with AngII (1ƒM or 1µM) for 6 hours, followed by treatment with candesartan (0.16 µM) for 10 hours, and the expression of BDNF was assessed (B) n = 3. The ability of candesartan to modulate the angiogenic response of hCMECs was evaluated using in vitro matrigel tube formation assay (C) and wound recovery assay (D) in response to treatment with a concentration range of candesartan (0–1.6 µM) n = 3. (A and B) Data presented as mean ± S.E.M., * P < 0.05. (C and D) *Significantly different from control; $Significantly different from candesartan (0.16 µM). Overall, P = 0.0014, F=8.19 (C) and P < 0.0001, F=22.44 (D).
Candesartan Increases BDNF Expression in hCMECs
After establishing the effect of ARBs on the expression of BDNF in brain tissue in vivo, the effect of candesartan on the expression of BDNF in AngII-treated cells in vitro was investigated. Candesartan significantly increased the expression of BDNF in hCMECs treated with AngII (1 ƒM), compared with AngII (1 ƒM) alone, and in hCMECs treated with both concentrations of AngII, compared with the control, as assessed after 16 hours of incubation with candesartan (Fig. 2B).
Candesartan Has a Dose-Dependent Modulatory Effect on the Angiogenic Potential of hCMECs
Data from clinical studies in stroke presented conflicting results on whether the hypotensive effect of ARBs is an essential requirement for its reported benefit (Schrader et al., 2003; Schrader et al., 2005). Consequently, we were interested in assessing whether a therapeutically relevant concentration of candesartan would have an in vitro effect on hCMECs in the absence of AngII. We calculated the amount of candesartan that can give a concentration similar to that achieved in patients receiving the drug (0.16 µM). The angiogenic effect of a range of candesartan concentrations, including the concentration under consideration, was assessed using an in vitro matrigel tube formation assay (Fig. 2C). The observed response was further confirmed using the wound recovery migration assay (Fig. 2B). Candesartan induced a dose-dependent, bell-shaped modulatory effect on the tube formation rate in hCMECs. The 0.16 µM candesartan concentration (therapeutically relevant) significantly increased the tube formation rate, whereas the other concentrations did not affect the rate of tube formation (Fig. 2C). A similar effect was observed in the wound recovery migration assay, except for an increased migration rate in hCMECs treated with candesartan 1.6 µM concentration (Fig. 2D).This differential effect of the 1.6 µM candesartan concentration might be attributed to dose-dependent effects of candesartan on the different processes involved in migration and tube formation.
Candesartan Modulates the Proliferation of hCMECs
AngII has been reported to induce the proliferation of cells in vitro (Kou et al., 2007; Herr et al., 2008). This proliferative effect has been shown to be blocked with ARBs (Kou et al., 2007; Herr et al., 2008), but the reported concentration of ARBs in these studies was supratherpaeutic (Herr et al., 2008). Consequently, we attempted to assess the effect of the therapeutically relevant concentration on the proliferative effect of AngII in hCMECs. Both low and high concentrations of AngII significantly increased the proliferation of hCMECs (Fig. 3, A and D). Treatment with candesartan maintained and further enhanced this proliferative effect (Fig. 3, A and D).
Fig. 3.
AngII modulates the proliferation of hCMECs. Proliferative response of hCMECs was evaluated using BrdU incorporation assay. hCMECs were treated with AngII (1ƒM or 1µM) for 6 hours, followed by candesartan (0.16 µM) alone or in combination with other treatments. The plates were then processed according to the manufacturer recommendations. Data are presented as mean ± S.E.M. of three different experiments each in triplicate. *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P = 0.005, F=8.85 for AngII 10−9 and P < 0.0001, F=34.97 for AngII 1µM
Candesartan Modulates the Migration Rate of hCMECs
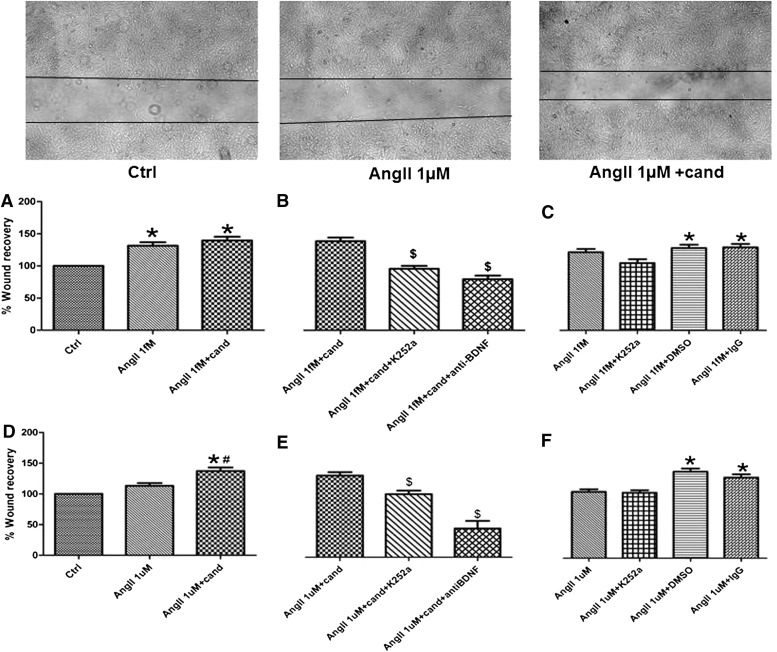
Similar to its proliferative effect, AngII has been reported to affect the migration of cells in vitro (Buharalioglu et al., 2011). Because we have found a proliferative effect of candesartan on AngII-treated hCMECs, we were interested in assessing the effect of this dose of candesartan on two critical steps of angiogenesis: migration and tube formation in AngII-treated hCMECs. We observed a significant increase in the migration of hCMECs in response to treatment with AngII 1 ƒM (Fig. 4A). Of interest, the higher AngII concentration did not have an effect on the migration of hCMECs (Fig. 4D) in our model. Although candesartan maintained the increased hCMECs migration in the low-dose AngII group (Fig. 4A), candesartan increased hCMECs migration in the high-dose AngII-treated cells (Fig. 4D). Cell migration was not affected by K252a, DMSO, or IgG (Fig. 4, C and F).
Fig. 4.
AngII modulates the migration of hCMECs. Insert; representative image of control, AngII 1 µM and AngII+candesartan showing candesartan induced migration of hCMECs. hCMECs were cultured to confluence, followed by 10 hours serum starvation. Cells were treated with either AngII 1ƒM or 1µM for 6 hours, and then a scratch was introduced in the monolayer. Cells were then incubated with AngII 1ƒM or 1µM alone or with candesartan (A and D). The involvement of BDNF was assessed using a number of inhibitors for BDNF functions (B and E), which were added to the media 30 minutes before AngII treatment. Data are presented as mean ± S.E.M. of three different experiments each in triplicate. *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P < 0.0001, F=16.08 for AngII 1 ƒM and P < 0.0001, F=22.08 for AngII 1 µM.
Candesartan Has a Proangiogenic Effect in hCMECs
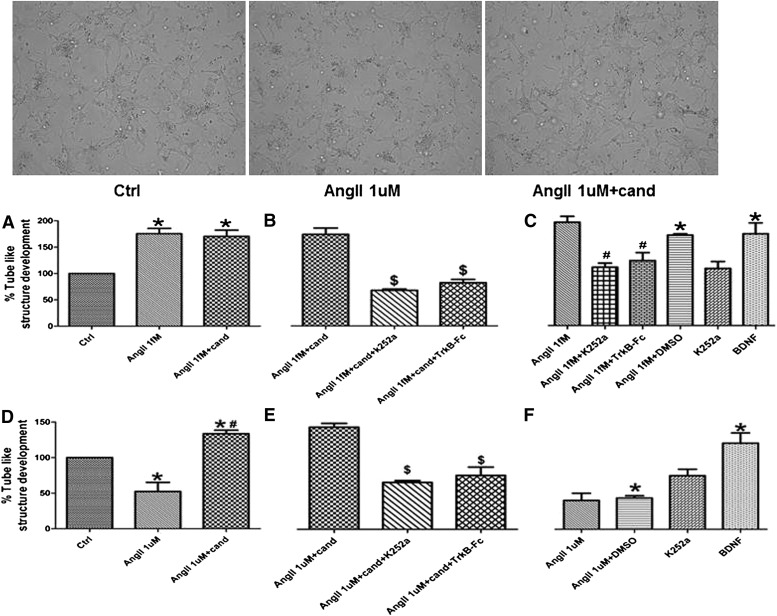
The proangiogenic effect of AngII has been reported previously (Hu et al., 2007; Buharalioglu et al., 2011). In hCMECs, there was a significantly increased rate of tube formation in response to AngII 1 ƒM treatment (Fig. 5A) and a reduction in tube formation in AngII 1 µM–treated cells (Fig. 5D). Of interest, candesartan maintained AngII 1 ƒM–induced tube formation and reversed the antiangiogenic effect of AngII 1 µM (Fig. 5, A and D).
Fig. 5.
AngII modulates the angiogenic potential of hCMECs. Insert: representative images of in vitro tube formation (arrows) showing reduced tube formation rate in AngII 1 µM treated hCMECs and the reversal of AngII 1 µM antiangiogenic effect by candesartan. hCMECS (2 × 104 cells/well)were suspended in a 30:60 solution of matrigel and serum-free media. Angiogenic response of hCMECs to AngII 1 ƒM (A) and 1 µM (D) in the presence and absence of candesartan was evaluated 24 hours after treatment. The involvement of BDNF was assessed through using K252a (Trk inhbitor) or TrkB-Fc (soluble chimeric receptor) (B and E). Data are presented as mean ± S.E.M. of three different experiments each in triplicate. *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P < 0.0001, F=12.74 for AngII 1ƒM and P < 0.0001, F=9.54 for AngII 1µM.
BDNF Mediates the Effects of Candesartan on hCMECs
Our in vivo results demonstrated the ability of candesartan to increase the expression of BDNF in the brain of SHR. Previously, BDNF has been demonstrated to have an angiogenic effect, which shifted our interest to assess whether BDNF is involved in the observed effects of candesartan. After neutralizing BDNF using three different methods of BDNF neutralization, the observed effects of candesartan were consistently ablated (Fig. 3, B and E; Fig. 4, B and E; Fig. 5, B and E), a finding that identifies BDNF as an essential mediator of candesartan effects in hCMECs. Of interest, the angiogenic effect of AngII 1 ƒM was significantly inhibited after BDNF neutralization (Fig. 5C), which suggests the involvement of BDNF in AngII-induced angiogenesis, which is confirmed by the robust angiogenic response to BDNF treatment (Fig. 5, C and F).
AT2 Receptor Mediates the Angiogenic Response to Angiotensin II in hCMECs
Candesartan mediates its effects through AT1 blockade (Engelhorn et al., 2004; Willis et al., 2011), which induces an unopposed stimulation of AT2 receptor (Hashikawa-Hobara et al., 2012). Findings in this investigation suggest a possible involvement of AT2 receptor in mediating candesartan effects.
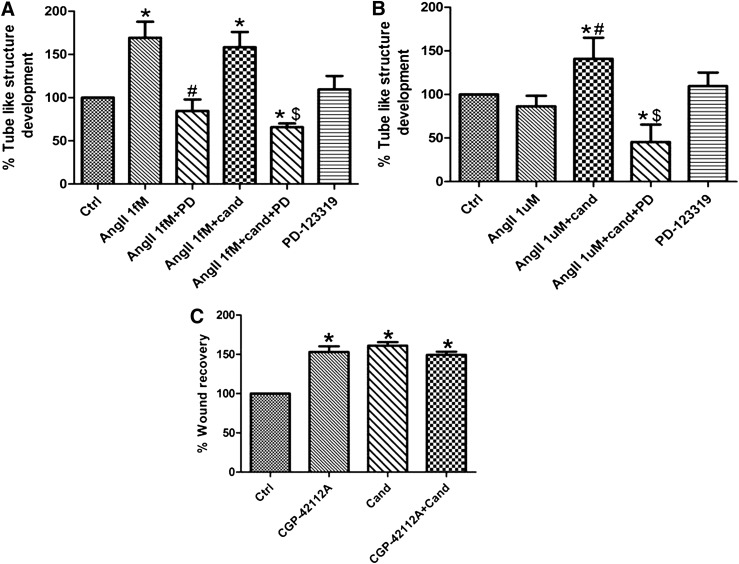
AT2 blockade using PD-123319 significantly inhibited the angiogenic response induced by either angiotensin II or the combination of angiotensin II and candesartan (Fig. 6, A and B). In addition the AT2 agonist, CGP-24112A significantly increased the migration of hCMECs. This migratory response was not affected by the concomitant treatment with candesartan (Fig. 6C). These findings demonstrate an essential role of AT2 in the angiogenic process of hCMECs.
Fig. 6.
AT2 receptor mediates the angiogenic response in hCMECs. The involvement of AT2 receptor in the angiogenic response to AngII 1 ƒM alone or in combination with candesartan (A) and to the combination of AngII 1 µM and candesartan (B) was assessed using the AT2 antagonist PD-123319 (0.1 µM). The angiogenic response was evaluated using matrigel tube formation assay as described in the methods section. Data are presented as mean ± S.E.M. of three different experiments each in triplicate. *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P < 0.0001, F=8.779 for both AngII 1ƒM and AngII 1µM.
Candesartan-Induced BDNF Expression Is Mediated through the AT2 Receptor
Findings in this study demonstrated the ability of candesartan to increase the expression of BDNF both in vivo and in vitro; in addition, the in vitro effects of candesartan were shown to be mediated through the AT2 receptor. Accordingly, the involvement of AT2 receptor in BDNF expression was evaluated. As has been demonstrated earlier, candesartan significantly increased the expression of BDNF in AngII-treated cells (Fig. 2B; Fig. 7A). Pretreatment with the AT2 antagonist PD-123319 significantly inhibited candesartan- induced BDNF expression, which suggests the involvement of AT2 receptor. To confirm this finding, hCMECs were treated with the AT2 agonist CGP-42112A (0.1 µM), and the expression of BDNF was assessed. CGP-42112A significantly increased the expression of BDNF in hCMECs by about one-fold, compared with vehicle-treated cells (Fig. 7B).
Fig. 7.
Candesartan induced BDNF expression is mediated through AT2 receptor. To evaluate the involvement of AT2 receptor in candesartan-induced BDNF expression, hCMECs were pretreated with PD-123319 or vehicle, followed by AngII (1 ƒM or 1 µM) in the presence or absence of candesartan (0.16 µM) (A). To further confirm the role of AT2, hCMECs were treated with CGP-42112A (0.1 µM) or vehicle for 16 hours (B). *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P = 0.02, F=3.086, n = 4–6.
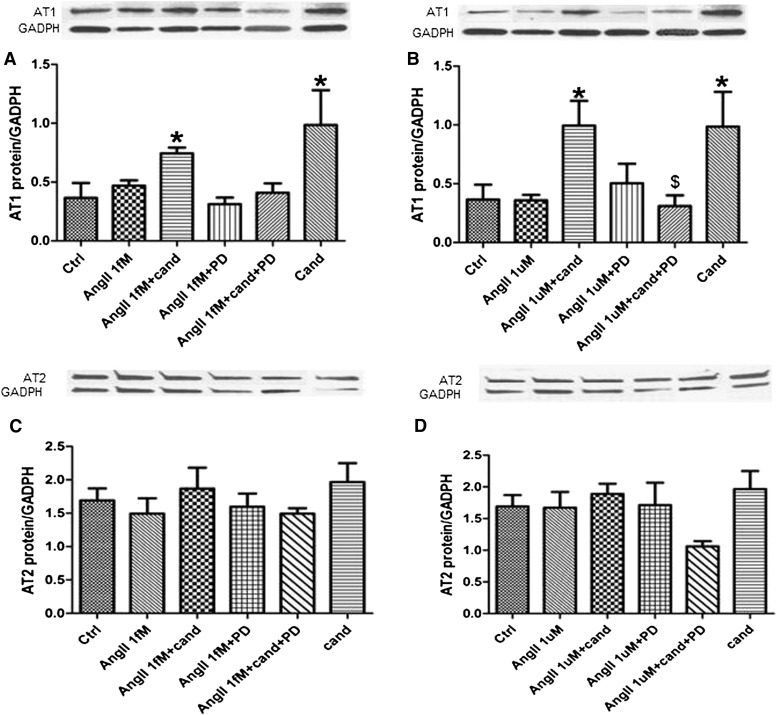
AT1 Antagonism Regulates the Expression of AT1 in an AT2-Dependent Manner
Candesartan treatment significantly increased the expression of AT1 receptor in cells treated with high dose of AngII, compared with both control and AngII alone. This response was ablated when the AT2 receptor was blocked using PD-123319 (Fig. 7B). In addition, the AT2 agonist CGP-24112A induced a significant four-fold increase in AT1 receptor expression in hCMECs (Supplementary Data). In cells treated with low-dose AngII, candesartan significantly increased the expression of AT1, compared with control alone (Fig. 7A). The expression of AT2 was not changed under the different treatments used (Fig. 7, C and D).
AT1 Antagonism Modulates the Activity of GSK-3β in an AT2-Dependent Manner
GSK-3β was found to modulate BDNF expression (Hur and Zhou, 2010; Li et al., 2011). Our results demonstrated the ability of candesartan to increase the expression of BDNF in both in vivo and in vitro settings. Accordingly, we were interested in assessing the activity of GSK-3β under the different treatment conditions that we were using. Candesartan treatment significantly increased the phosphorylation of GSK-3β at the inhibitory serine 9 residue in cells treated with high-dose AngII (Fig. 8A). The higher phosphorylation was reversed by the concomitant treatment with AT2 blocker PD-123319 (Fig. 8A; Fig.9; Fig.10).
Fig. 8.
AT1 antagonism affects the expression of AT1 receptor in an AT2 receptor–mediated manner. To assess the expression of AT1 (A and B) and AT2 (C and D) receptors in response to the different treatments used. cells were incubated with PD-123319 (0.1 µM) or vehicle for 30 minutes, followed by 6 hours of AngII 1 ƒM (A and C) or 1 µM (B and D). After 6 hours of AngII treatment, cells were coincubated with candesartan or vehicle for 10 hours. Receptor expression was assessed using immunoblotting. Data are presented as mean ± S.E.M.; n = 3–5. *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P < 0.0042, F=8.742 for AngII 1 µM.
Fig. 9.
AT1 antagonism modulates the phosphorylation of GSK-3β in an AT2 receptor–mediated manner. To assess the phosphorylation of GSK-3β at the inhibitory serine 9 residue, hCMECs were treated as described for AngII receptors expression evaluation. (A) Response to AngII 1 ƒM. (B) AngII 1 µM response. Data are presented as mean ± S.E.M.; n = 3–5. *Significantly different from control, #significantly different from AngII in the same group, $significantly different from AngII+cand in the same group; overall, P = 0.0038, F=6.94 for AngII 1 µM.
Fig. 10.
A schematic representation of the results. By blocking AT1 receptors ARBs induce an unopposed stimulation of AT2 receptors. AT2 stimulation induces the expression of BDNF, which will bind to its TrkB receptor to promote a proangiogenic state in hCMECs.
Discussion
Our results demonstrate, for the first time, the ability of candesartan to promote a proangiogenic state in hCMECs in an AngII concentration-independent manner, in which candesartan was able to maintain the angiogenic effect of AngII at the low dose and reverse the antiangiogenic effect of the high dose. The proangiogenic effect of candesartan was found to be mainly dependent on BDNF and is mediated through AngII stimulation of the AT2 receptor. In addition, we demonstrated the ability of candesartan to induce an angiogenic response in endothelial cells, even in the absence of exogenously added AngII.
After an ischemic insult in the brain, induction of angiogenesis has been shown to ameliorate the damage and is coupled to neurogenesis, leading to enhanced recovery and better outcome (Navaratna et al., 2009; Xiong et al., 2010). ARB administration was found to increase vascular density in heart and brain after myocardial infarction (Sladek et al., 1996) and stroke (Kozak et al., 2009; Guan et al., 2011); this was attributed to an increase in VEGF expression in stroke (Guan et al., 2011). In contrast to in vivo data, in vitro angiogenesis studies demonstrated an antiangiogenic effect of ARBs in endothelial cells treated with AngII (Hu et al., 2007; Herr et al., 2008). This antiangiogenic effect was mediated mainly through inhibiting AngII-induced increase in VEGF signaling (Fujiyama et al., 2001; Herr et al., 2008). The majority of these studies were conducted in either human umblical vein endothelial cells (Kou et al., 2007; Buharalioglu et al., 2011) or coronary artery endothelial cells (Fujiyama et al., 2001; Hu et al., 2007), and none of them evaluated the effect of AngII or AngII and ARBs in hCMECs, which are phenotypically different from other endothelial cells (Feletou, 2011). In addition, the majority of the studies focused on the involvement of VEGF (Buharalioglu et al., 2011), angiopoietins (Herr et al., 2008), and EGFR transactivation (Fujiyama et al., 2001) in the angiogenic response to AngII. This ignores the possible role of other angiogenic mediators, such as BDNF, which has been shown to induce VEGF expression (Caporali and Emanueli, 2009) and produce an angiogenic response comparable to that of VEGF in endothelial cells (Li et al., 2006). Results from this study demonstrate a dose-dependent effect of AngII on brain angiogenesis, where low concentrations of AngII induce angiogenesis and higher doses inhibit it. This is consistent with that shown in other vascular beds (Kou et al., 2007; Buharalioglu et al., 2011).
The design of this study is unique in many aspects. In a typical in vitro study, cells are initially treated with candesartan, followed by AngII treatment. This precludes detection of a possible AngII-independent interaction between candesartan and endothelial cells. This design has limited clinical relevance, because candesartan will be introduced to the system in response to the effects of AngII or other circulating vasoactive mediators. In our design, we attempted to model the temporal relationship of treatment introduction. Data on the effect of AngII on BDNF expression in endothelial cells were lacking, and the only reports addressing the effect of AngII on BDNF expression were in the adrenal cortex (Szekeres et al., 2010) and the brain (Chan et al., 2010). Accordingly, we did a time and dose response study in hCMECs to determine the incubation time and AngII dose to be used in the in vitro studies. This dose and time response study was followed by assessing the effect of those AngII on the viability of hCMECs (Supplementary Data S1). In addition, the concentration of candesartan used in the in vitro studies was calculated to produce the midpoint therapeutic steady state concentration in humans (Schulz and Schmoldt, 2003). The calculated concentration was tested for its angiogenic and migratory effect and compared with other candesartan doses in a dose-response curve (Fig. 2, C and D). The in vivo dose was determined on the basis of previously reported data demonstrating neurovascular protection in SHRs (Kozak et al., 2008).
Our findings support and expand our previously reported data on the ability of cerebral spinal fluid from candesartan-treated nonstroked animals to induce angiogenesis in endothelial cells (Kozak et al., 2009). This proangiogenic effect of candesartan was only partially attributed to VEGF (Fagan et al., 2006), suggesting the involvement of other angiogenic factors. In this study, we identified BDNF as another important mediator of the angiogenic effect of candesartan. BDNF neutralization using receptor inhibitor K252a, antiBDNF antibody, or TrkB-Fc almost ablated the effects of candesartan in all reported assays.
The proliferative and angiogenic effects of AngII have been largely attributed to AT1-mediated signaling (Otani et al., 1998; Fujiyama et al., 2001; Hu et al., 2007; Herr et al., 2008; Buharalioglu et al., 2011), and AT2-mediated signaling was thought to either counteract AT1-induced angiogenesis (Fujiyama et al., 2001; Javier Carbajo-Lozoya et al., 2012) or not have an effect on AngII-induced angiogenesis (Otani et al., 1998; Hu et al., 2007). In contrast to this notion, AT2-mediated signaling has been demonstrated to promote angiogenesis in ischemic myocardium (Munk et al., 2007) and retinal endothelial cells (Sarlos et al., 2003). In this study, AT2-mediated signaling was found to be largely responsible for the angiogenic response in hCMECs. AT2 involvement in angiogenesis was initially suggested by the lack of effect on AngII-mediated angiogenesis when AT1 was blocked using candesartan and was further confirmed when the angiogenic response was totally prevented after AT2 blockade using PD-123319. This finding suggests the importance of unopposed AT2 stimulation after stroke (McCarthy et al., 2009; Oprisiu-Fournier et al., 2009) and may explain the lack of protective effect of ARBs in the absence of AT2 signaling after cerebral ischemia (Iwai et al., 2004; Li et al., 2005; Lu et al., 2005; Faure et al., 2008) . Our data demonstrate the indispensible role of AT2 for angiogenesis in hCMECs, which has been linked to neurogenesis and improving recovery after CNS ischemic insults (Madri, 2009; Navaratna et al., 2009; Xiong et al., 2010).
The activity of GSK-3β has been shown to be involved in the expression of neurotrophins in the brain (Wada, 2009) and in the cross talk between endothelial and neural stem cells through regulating BDNF and VEGF expression (Li et al., 2011). In addition, GSK-3β has been recently demonstrated to regulate the angiogenic response in endothelial cells (Flugel et al., 2012). Data from this study highlight a possible involvement of GSK-3β in mediating the effects of AT1 antagonism in hCMECs in an Ang II dose-dependent manner. Candesartan increased phosphorylation of GSK-3β at the inhibitory serine 9 residue, which will inhibit the activity of GSK-3β when used alone or in combination with 1 µM AngII the concentration that induced antiangiogenic effects in hCMECs (Fig. 9B). Concomitantly, this increased inhibition of GSK-3β was associated with increased BDNF expression and angiogenic response in hCMECs. These findings are consistent with previously published data about the interaction between GSK-3β and BDNF expression (Wada, 2009; Li et al., 2011).
In this study, candesartan demonstrated the ability to modulate the angiogenic response of hCMECs in the absence of exogenously added AngII. This effect was found to be dose dependent. The therapeutically relevant candesartan concentration induces a proangiogenic effect, whereas the other two concentrations did not affect the angiogenic potential of the cells. This finding may help explain the controversial angiogenic effect of ARBs between the in vivo and the in vitro data (Willis et al., 2011). Previously published reports on the in vitro antiangiogenic effect of ARBs used supra-therapeutic concentrations (Hu et al., 2007; Herr et al., 2008). As demonstrated by the findings of this study, the supra-therapeutic doses of ARBs might have direct antiangiogenic effects on endothelial cells.
Another unique aspect of this work is the demonstration of candesartan’s ability to increase the expression of BDNF in both hCMECs and the brain tissue of hypertensive animals. Hashikawa-Hobara et al. recently demonstrated the ability of candesartan to stimulate neurite growth in an AT2-dependent manner through Akt signaling (Hashikawa-Hobara et al., 2012). This is consistent with our findings, highlighting the involvement of the AT2 receptor in BDNF expression. which is known to stimulate neurite growth (Parrish et al., 2007) and Akt signaling (Caporali and Emanueli, 2009). Of interest, the effect of candesartan on BDNF expression appears to be independent of blood pressure lowering. Candesartan increased BDNF expression in wistar rats (Supplemental Data S3), whereas hydralazine had no effect on BDNF expression in SHRs (Supplemental Data S4).
An interesting finding in this study is the involvement of AT2 in AT1 expression. Cross-talk between AngII receptors have been previously reported at both expression and functional levels (De Paolis et al., 1999; Saavedra, 1999; Miura et al., 2010). These reports consistently demonstrated the involvement of AT1 in AT2 expression (De Paolis et al., 1999). In addition, it has been shown that AT2-mediated signaling antagonizes some aspects of AT1-mediated effects (Miura et al., 2010). Our results demonstrate for the first time that AT1 expression in hCMECs is positively regulated by AT2-mediated signaling.
In this study, all efforts were made to confirm each result with use of different methods, but the following limitations can be identified; the in vitro studies were conducted in an endothelial cell line, rather than primary endothelial cells. In addition, BDNF neutralization studies were performed using mainly pharmacologic methods, although a genetic approach using RNA interference would provide more power to the study. In addition, in our study, we used human derived cell line in our in vitro study and a murine model as an in vivo model. In our research, the main goal is to model and understand the changes that accompany ischemic stroke in humans. Because of the inability to directly probe human brain samples, we are using rats to study the in vivo changes in response to candesartan or any pharmacologic agent of interest. In addition, when we had the chance to use human-derived tissues or cells, we used them to give a better understanding of what changes are taking place. In this investigation, we used human cerebromicrovascular endothelial cells as an in vitro system and we used SHRs as an in vivo system.
In conclusion, our findings demonstrate the ability of candesartan to modulate the behavior of endothelial cells to promote a proangiogenic state. In hCMECs, this modulatory effect of candesartan can be largely attributed to BDNF and is mediated through the AT2 receptor (Fig. 10). In addition, the dose-dependent proangiogenic effect of candesartan and even the mechanism involved may help explain the disparate findings on the angiogenic potential of ARBs that prevails in the biomedical literature.
Supplementary Material
Abbreviations
- AngII
angiotensin II
- ARB
angiotensin II receptor blocker
- BDNF
brain-derived neurotrophic factor
- cand
candesartan
- CGP-42112A
Nα-Nicotinoyl-Tyr-(Nα-Cbz-Arg)-Lys-His-Pro-Ile
- DMSO
dimethylsulfoxide
- GSK-3β
glycogen synthase kinase–3β
- hCMEC
human cerebromicrovascular endothelial cell
- K252a
(9S,10R,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid methyl ester
- PD
PD-123319 (S-(+)-1-[(4-(Dimethylamino)-3-methylphenyl)methyl]-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid di(trifluoroacetate) salt hydrate)
- SHR
spontaneously hypertensive rats
- VEGF
vascular endothelial growth factor
Authorship Contributions
Participated in research design: Alhusban, Ergul, Fagan.
Conducted experiments: Alhusban, Kozak.
Performed data analysis: Alhusban, Ergul, Fagan.
Wrote or contributed to the writing of the manuscript: Alhusban, Kozak, Ergul, Fagan.
Footnotes
This work was supported by the Veterans Affairs Merit Review [Grants BX000347and BX000891 to S.C.F.], National Institutes of Health [Grants NS070239 and NS063965], and Jordan University of Science and Technology predoctoral fellowship.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group. (2002) Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359:995–1002. [DOI] [PubMed] [Google Scholar]
- Buharalioglu CK, Song CY, Yaghini FA, Ghafoor HU, Motiwala M, Adris T, Estes AM, Malik KU. (2011) Angiotensin II-induced process of angiogenesis is mediated by spleen tyrosine kinase via VEGF receptor-1 phosphorylation. Am J Physiol Heart Circ Physiol 301:H1043–H1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali A, Emanueli C. (2009) Cardiovascular actions of neurotrophins. Physiol Rev 89:279–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Wu CW, Chang AY, Hsu KS, Chan JY. (2010) Transcriptional upregulation of brain-derived neurotrophic factor in rostral ventrolateral medulla by angiotensin II: significance in superoxide homeostasis and neural regulation of arterial pressure. Circ Res 107:1127–1139 [DOI] [PubMed] [Google Scholar]
- De Paolis P, Porcellini A, Gigante B, Giliberti R, Lombardi A, Savoia C, Rubattu S, Volpe M. (1999) Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J Hypertens 17:1873–1877 [DOI] [PubMed] [Google Scholar]
- Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, Schulz R. (2004) The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab 24:467–474 [DOI] [PubMed] [Google Scholar]
- Fagan SC, Kozak A, Hill WD, Pollock DM, Xu L, Johnson MH, Ergul A, Hess DC. (2006) Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J Hypertens 24:535–539 [DOI] [PubMed] [Google Scholar]
- Faure S, Bureau A, Oudart N, Javellaud J, Fournier A, Achard JM. (2008) Protective effect of candesartan in experimental ischemic stroke in the rat mediated by AT2 and AT4 receptors. J Hypertens 26:2008–2015 [DOI] [PubMed] [Google Scholar]
- Feletou M. (2011) Series on Integrated Systems Physiology: From Molecule to Function to Disease, in The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators (Granger DNGJ, ed) Morgan & Claypool Life Sciences, San Rafael, CA: [PubMed] [Google Scholar]
- Flügel D, Görlach A, Kietzmann T. (2012) GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1α. Blood 119:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, Masaki H, Uchiyama Y, Koyama Y, Nose A, et al. (2001) Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res 88:22–29 [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 29:12764–12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, Johnson MH, Alhusban A, Soliman S, Fagan SC. (2011) Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS ONE 6:e24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa-Hobara N, Hashikawa N, Inoue Y, Sanda H, Zamami Y, Takatori S, Kawasaki H. (2012) Candesartan cilexetil improves angiotensin II type 2 receptor-mediated neurite outgrowth via the PI3K-Akt pathway in fructose-induced insulin-resistant rats. Diabetes 61:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr D, Rodewald M, Fraser HM, Hack G, Konrad R, Kreienberg R, Wulff C. (2008) Regulation of endothelial proliferation by the renin-angiotensin system in human umbilical vein endothelial cells. Reproduction 136:125–130 [DOI] [PubMed] [Google Scholar]
- Hu C, Dandapat A, Mehta JL. (2007) Angiotensin II induces capillary formation from endothelial cells via the LOX-1 dependent redox-sensitive pathway. Hypertension 50:952–957 [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11:539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, Sakanaka M, Shiuchi T, Horiuchi M. (2004) Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 110:843–848 [DOI] [PubMed] [Google Scholar]
- Carbajo-Lozoya J, Lutz S, Feng Y, Kroll J, Hammes HP, Wieland T. (2012) Angiotensin II modulates VEGF-driven angiogenesis by opposing effects of type 1 and type 2 receptor stimulation in the microvascular endothelium. Cell Signal 24:1261–1269 [DOI] [PubMed] [Google Scholar]
- Kou B, Vatish M, Singer DR. (2007) Effects of angiotensin II on human endothelial cells survival signalling pathways and its angiogenic response. Vascul Pharmacol 47:199–208 [DOI] [PubMed] [Google Scholar]
- Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, Abdelsaid M, Wiley DC, Fagan SC. (2009) Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke 40:1870–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Kozak A, Johnson MH, Elewa HF, Fagan SC. (2008) Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. J Pharmacol Exp Ther 326:773–782 [DOI] [PubMed] [Google Scholar]
- Li J, Culman J, Hörtnagl H, Zhao Y, Gerova N, Timm M, Blume A, Zimmermann M, Seidel K, Dirnagl U, et al. (2005) Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J 19:617–619 [DOI] [PubMed] [Google Scholar]
- Li Q, Ford MC, Lavik EB, Madri JA. (2006) Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res 84:1656–1668 [DOI] [PubMed] [Google Scholar]
- Li Q, Michaud M, Canosa S, Kuo A, Madri JA. (2011) GSK-3β: a signaling pathway node modulating neural stem cell and endothelial cell interactions. Angiogenesis 14:173–185 [DOI] [PubMed] [Google Scholar]
- Lu Q, Zhu YZ, Wong PT. (2005) Neuroprotective effects of candesartan against cerebral ischemia in spontaneously hypertensive rats. Neuroreport 16:1963–1967 [DOI] [PubMed] [Google Scholar]
- Madri JA. (2009) Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol 60 (Suppl 4):95–104 [PubMed] [Google Scholar]
- McCarthy CA, Vinh A, Callaway JK, Widdop RE. (2009) Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke 40:1482–1489 [DOI] [PubMed] [Google Scholar]
- Miura S, Matsuo Y, Kiya Y, Karnik SS, Saku K. (2010) Molecular mechanisms of the antagonistic action between AT1 and AT2 receptors. Biochem Biophys Res Commun 391:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk VC, Sanchez de Miguel L, Petrimpol M, Butz N, Banfi A, Eriksson U, Hein L, Humar R, Battegay EJ. (2007) Angiotensin II induces angiogenesis in the hypoxic adult mouse heart in vitro through an AT2-B2 receptor pathway. Hypertension 49:1178–1185 [DOI] [PubMed] [Google Scholar]
- Navaratna D, Guo S, Arai K, Lo EH. (2009) Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes Migr 3:216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprisiu-Fournier RFS, Mazouz H, Boutitie F, Serot JM, Achard JM, Godefroy O, Hanon O, Temmar M, Albu A, Strandgaard S, et al. (2009) Angiotensin AT1-receptor blockers and cerebrovascular protection: do they actually have a cutting edge over angiotensin-converting enzyme inhibitors? Expert Rev Neurother9:1289–1305. [DOI] [PubMed] [Google Scholar]
- Otani A, Takagi H, Suzuma K, Honda Y. (1998) Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res 82:619–628 [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. (2007) Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 30:399–423 [DOI] [PubMed] [Google Scholar]
- Saavedra JM. (1999) Emerging features of brain angiotensin receptors. Regul Pept 85:31–45 [DOI] [PubMed] [Google Scholar]
- Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. (2003) Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol 163:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. (1994) Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation 89:2273–2282 [DOI] [PubMed] [Google Scholar]
- Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhäupl K, Diener HC, Dominiak P, Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group (2003) The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke 34:1699–1703 [DOI] [PubMed] [Google Scholar]
- Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, Zidek W, Dominiak P, Diener HC, MOSES Study Group (2005) Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES). Stroke 36:1218–1226 [DOI] [PubMed] [Google Scholar]
- Schulz M, Schmoldt A. (2003) Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 58:447–474 [PubMed] [Google Scholar]
- Sladek T, Sladkova J, Kolar F, Papousek F, Cicutti N, Korecky B, Rakusan K. (1996) The effect of AT1 receptor antagonist on chronic cardiac response to coronary artery ligation in rats. Cardiovasc Res 31:568–576 [PubMed] [Google Scholar]
- Szekeres M, Nádasy GL, Turu G, Süpeki K, Szidonya L, Buday L, Chaplin T, Clark AJ, Hunyady L. (2010) Angiotensin II-induced expression of brain-derived neurotrophic factor in human and rat adrenocortical cells. Endocrinology 151:1695–1703 [DOI] [PubMed] [Google Scholar]
- Wada A. (2009) Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J Pharmacol Sci 110:14–28 [DOI] [PubMed] [Google Scholar]
- Willis LM, El-Remessy AB, Somanath PR, Deremer DL, Fagan SC. (2011) Angiotensin receptor blockers and angiogenesis: clinical and experimental evidence. Clin Sci (Lond) 120:307–319 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. (2010) Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 11:298–308 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.