Abstract
Nitroxyl (HNO) donors have potential benefit in the treatment of heart failure and other cardiovascular diseases. 1-Nitrosocyclohexyl acetate (NCA), a new HNO donor, in contrast to the classic HNO donors Angeli’s salt and isopropylamine NONOate, predominantly releases HNO and has a longer half-life. This study investigated the vasodilatative properties of NCA in isolated aortic rings and human platelets and its mechanism of action. NCA was applied on aortic rings isolated from wild-type mice and apolipoprotein E–deficient mice and in endothelial-denuded aortae. The mechanism of action of HNO was examined by applying NCA in the absence and presence of the HNO scavenger glutathione (GSH) and inhibitors of soluble guanylyl cyclase (sGC), adenylyl cyclase (AC), calcitonin gene-related peptide receptor (CGRP), and K+ channels. NCA induced a concentration-dependent relaxation (EC50, 4.4 µM). This response did not differ between all groups, indicating an endothelium-independent relaxation effect. The concentration-response was markedly decreased in the presence of excess GSH; the nitric oxide scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide had no effect. Inhibitors of sGC, CGRP, and voltage-dependent K+ channels each significantly impaired the vasodilator response to NCA. In contrast, inhibitors of AC, ATP-sensitive K+ channels, or high-conductance Ca2+-activated K+ channels did not change the effects of NCA. NCA significantly reduced contractile response and platelet aggregation mediated by the thromboxane A2 mimetic 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α in a cGMP-dependent manner. In summary, NCA shows vasoprotective effects and may have a promising profile as a therapeutic agent in vascular dysfunction, warranting further evaluation.
Introduction
Cardiovascular diseases are the leading cause of death worldwide. Hallmarks of these syndromes are reduced contractile force of the heart and increased peripheral resistance due to the activation of neurohumoral systems. Despite extensive efforts to develop therapeutic strategies for this disease, the results are not yet satisfying, leaving a need for further development of new pharmacological treatments (El-Armouche and Eschenhagen, 2009). A potential therapeutic alternative are compounds that generate nitroxyl (HNO), the one-electron reduction product of nitric oxide (NO). HNO has been shown to improve myocardial contractility in normal and failing hearts (Paolocci et al., 2003, 2007) and to have vasodilator actions (De Witt et al., 2001; Wanstall et al., 2001; Irvine et al., 2003, 2007; Favaloro and Kemp-Harper, 2007, 2009; Andrews et al., 2009; Bullen et al., 2011a,b). Moreover, HNO possesses antithrombotic properties (Mondoro et al., 2001; Bermejo et al., 2005), is resistant to scavenging by superoxide (Miranda et al., 2002, 2003b; Switzer et al., 2009), and causes a reduction of blood pressure in vivo (Ma et al., 1999; Choe et al., 2009).
The mechanisms of HNO-induced increases in myocardial contractility have been well investigated (Cheong et al., 2005; Dai et al., 2007; Tocchetti et al., 2007, 2011; Froehlich et al., 2008; Gao et al., 2012) as has the mechanism of HNO-induced vasorelaxation in small resistance arteries (Irvine et al., 2003; Coleman et al., 2006; Andrews et al., 2009; Favaloro and Kemp-Harper, 2009; Bullen et al., 2011a,b). In contrast, the mechanism of how HNO elicits vasorelaxation in large arteries needs further investigation. Thus, the question arises as to whether HNO elicits vasorelaxation in different vascular beds using the same or different channels and messengers.
The dimerization of HNO precludes its storage; thus, the use of an HNO donor is necessary in chemical and, especially, in biologic studies. Many HNO donors are currently available (Miranda et al., 2005b), but not all of them are amenable for in vivo or even in vitro studies. Angeli's salt (AS) and isopropylamine NONOate (IPA/NO) were the most widely used donors to examine HNO chemical biology. However, their relatively fast decomposition [half-life (t1/2) = 2–5 minutes] (Miranda et al., 2005a) results in a high initial concentration of HNO that facilitates self-consumption, thus decreasing the overall amount of HNO available to react with biologic targets. To overcome this limitation, longer-acting HNO donor compounds have been developed. A potential candidate is 1-nitrosocyclohexyl acetate (NCA), which hydrolyzes slowly at neutral pH (t1/2 = 800–890 minutes), producing a low-level steady-state concentration of HNO over hours (Sha et al., 2006; Shoman et al., 2011). NCA has been shown to produce only minimal amounts of NO (<1%) and total nitrate/nitrite (3–4%). Furthermore, NCA can be derivatized by changing the appending organic groups to alter the release rate of HNO. Thus, the slow release of HNO from NCA, its predominant release of HNO, and its structural flexibility (in contrast to AS and IPA/NO) may prove to be advantageous in the future development of drugs with proper pharmacokinetics and distribution profiles. Previously, we used NCA to investigate the role of HNO in normal and β-adrenergically desensitized ventricular cardiomyocytes (El-Armouche et al., 2010). In the current study, we aim to fully explore the underlying mechanisms of HNO-induced vasorelaxant actions in large arteries and its antiaggregatory properties in terms of translational usage of NCA-class HNO donors in vascular disease.
Materials and Methods
NCA.
NCA belongs to the acyloxy nitroso compounds that generate HNO and the corresponding ketone upon hydrolysis in aqueous solutions in a pH-dependent manner, as previously described (Sha et al., 2006; Shoman et al., 2011). NCA is a bright blue liquid at room temperature with strong UV-visible absorption at 667 nm (ε = 20.7 M−1 cm−1) and infrared absorption for the carbonyl (vc=o = 1750 cm−1) and nitroso groups (vN=o = 1561 cm−1). NCA was dissolved in dimethylsulfoxide (DMSO), and stock solutions were kept frozen until use and protected from light. The structure and purity of NCA were always checked after its synthesis. The generation of NO as well as the total nitrate/nitrite release (markers of NO) from NCA have been measured in vitro (neat and in buffer) by chemiluminescence analysis, with reported release of <1% NO and 3%–4% total nitrate/nitrite (Sha et al., 2006). The kinetics, products, and mechanism of HNO release from NCA hydrolysis have been recently reported (Shoman et al., 2011). NCA is a relatively slow HNO donor. The parent NCA compound has a t1/2 of 800–890 minutes in neutral buffer conditions (releasing half of its HNO over this time period). Every molecule of NCA does not break down to HNO simultaneously; rather, hydrolysis of the NCA molecules within the collection follows a predictable probability of hydrolysis and HNO release. The concentrations of HNO under these conditions are expected to be very small; however, the exact concentration has not been calculated. The controls for NCA used in this study are the structurally similar t-butyl ester of NCA, which does not produce any HNO during hydrolysis (Shoman et al., 2011), and DMSO, the vehicle carrier of NCA.
Other Chemicals and Solution Preparation.
Acetylcholine (ACh); 1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one (ODQ); 4-aminopyridine (4-AP); tetraethylammonium (TEA); calcitonin gene-related peptide receptor antagonist fragment 8–37 (CGRP8-37); tetrahydro-2-furanyl-9H-purin-6-amine (SQ22536); sodium nitroprusside; glibenclamide; iberiotoxin; 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO); isoprenaline; glutathione (GSH); ascorbic acid (AA); and spermine NONOate (Sper/NO) were purchased from Sigma-Aldrich (St. Louis, MO). Diethylenetriamine pentaacetic acid (DTPA) was from Merck KGaA (Darmstadt, Germany), heparin was from Ratiopharm (Ulm, Germany), and 9α,11α-dideoxy-11,9-epoxymethanoprostaglandin F2α (U-46619) was from Cayman Europe (Tallinn, Estonia). The soluble guanylyl cyclase (sGC) activity was measured by an enzyme immunoassay kit from cGMP direct Biotrak EIA, GE Healthcare Europe GmbH (Munich, Germany). Modified Krebs solution for the organ bath contained (mM): NaCl, 99; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.0; NaHCO3, 25; glucose, 11.1 (pH 7.4). The solution was kept in a bath at 37°C and prepared daily. The metal chelator DTPA (50 µM) was present in all buffer solutions.
Animal Testing.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the government review board for studies in animals in Hamburg (G21/1-ORG-370/03). Male wild-type (WT) and atherosclerotic apolipoprotein E–deficient (ApoE−/−) mice on the C57Bl/6J background (13–15 weeks; Jackson Laboratory, Bar Harbor, ME) were maintained on a normal diet and light cycle. ApoE−/− mice develop elevated plasma cholesterol levels leading to premature atherosclerosis (Zhang et al., 1992; Coleman et al., 2006). Mice were anesthetized with CO2 and euthanized by decapitation. The thorax was opened and the aorta was prepared with an intracardial injection of heparin solution (500 IU) prior to excision.
Preparation of Isolated Thoracic Aortic Rings and Measurement of Tension.
The thoracic aorta isolated from WT and ApoE−/− mice was removed immediately, carefully cleaned of fat and connective tissue, and cut into 3- to 4-mm-wide transverse rings. Rings were placed in a bath of Krebs solution at pH 7.4, 37°C, under a resting optimal tension of 1.1 g while bubbled with a mixture of 95% O2 and 5% CO2. Isometric tension was recorded with a force displacement transducer (Model TM50; Ing. Jaeckel, Hanau, Germany). The aortic rings in bath tubes were allowed to equilibrate for 45 minutes. Dose-response curves with prostaglandin F2α (PGF2α) were made to check viability and establish the maximum constriction of the vessel preparations (data not shown). From the obtained curves, PGF2α 5 µM resulted in approximately 65–70% of maximal PGF2α constriction. Impairment in the endothelium of the ApoE−/− mice was checked by measuring their dilatory response to ACh (Zhang et al., 1992; Coleman et al., 2006).
Vasodilator Effects of HNO by NCA on Isolated Aortic Rings Using PGF2α.
To study the vasodilator effects of HNO, isolated mouse aortic rings were preconstricted with PGF2α 5 µM. When the vasoconstriction curve of the rings reached the plateau phase of the submaximal tension, the cumulative concentration-response curve of NCA from 80 pM to 80 µM was constructed and the tension was recorded. Percentage of dilation was calculated when the vasodilator curve reached the plateau phase of the minimum tension. To distinguish the action of HNO from that of NO, either GSH (1 mM) or cPTIO (200 µM), respectively, was added to the media with isolated aortic rings.
Pretreatment of Aortic Rings with Inhibitors of sGC, Adenylyl Cyclase, and K+ Channels.
To examine whether sGC, AC, CGRP receptor, and K+ channels were the target of HNO vascular actions, aortic rings with intact endothelium were preincubated for 30 minutes with inhibitors after being preconstricted with PGF2α. ODQ (5 µM) was used to inhibit sGC, SQ22536 (100 µM) to inhibit AC, CGRP8-37 (1 µM) to inhibit CGRP receptor, TEA (1 mM) to nonselectively inhibit K+ channels, 4-AP (10 µM) to inhibit voltage-dependent K+ channels, iberiotoxin (200 nM) to inhibit high-conductance Ca2+-activated K+ channels, and glibenclamide (1 µM) to inhibit ATP-sensitive K+ channels. Only one inhibitor was tested in each experiment, and control responses were compared with the responses obtained without the inhibitor. Because two consecutive concentration-response curves to NCA were generated in each aortic ring preparation, the appropriate time control experiment was performed (i.e., two consecutive concentration-response curves to NCA in the absence of inhibitor). The metal chelator DTPA (50 µM) was always present in the buffer solution to limit the conversion of HNO to NO (Irvine et al., 2003).
Vasodilator Effects of HNO by NCA on Isolated Aortic Rings Using U-46619.
To study the effect of HNO on the thromboxane A2 (TxA2) receptor, a cumulative concentration-response curve in the presence of the Tx mimetic U-46619 was made in isolated mouse aortic rings. Rings were exposed to NCA (300 µM) for 30 minutes before generating a second concentration-response curve for U-46619. Since two consecutive concentration-response curves for U-46619 were constructed in isolated aortae with the addition of NCA between the first and second curves, the appropriate time control experiment was performed (i.e., two consecutive concentration-response curves for U-46619 without NCA after 70 and 250 minutes).
cGMP Evaluation of NCA-Treated Rings.
The integrity of the endothelium was confirmed by the ability of the vessel to relax in presence of ACh. Aortic rings preparations were exposed either to NCA or Sper/NO for 30 minutes in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (500 µM). Either AA or cPTIO was added into the organ bath 10 minutes prior to NCA or Sper/NO incubation. cGMP was assayed by enzyme immunoassay.
Human Platelet Aggregation.
Platelet-rich plasma was obtained from healthy subjects by venipuncture, with sodium citrate (3.8%, 1:14) added as anticoagulant and centrifugation for 10 minutes at 90g. platelet-rich plasma was preincubated 5 minutes with DTPA and DMSO and then 15 minutes with various concentrations of NCA while rotating at 1200 rpm. U-46619 (10 µM) was used as aggregation inducer.
Data Analysis.
All values are given as the mean ± S.E.M., with n indicating the number of animals for each experiment or the number of times that the experiments were repeated. Statistical differences were determined by repeated-measurement analysis of variance followed by the Bonferroni post-test. P < 0.05 was considered to be significant. Analysis of the data and plotting of the figures were aided by GraphPad Prism 4.0 software (GraphPad Software, Inc., La Jolla, CA).
Results
Vasodilatative Properties of NCA.
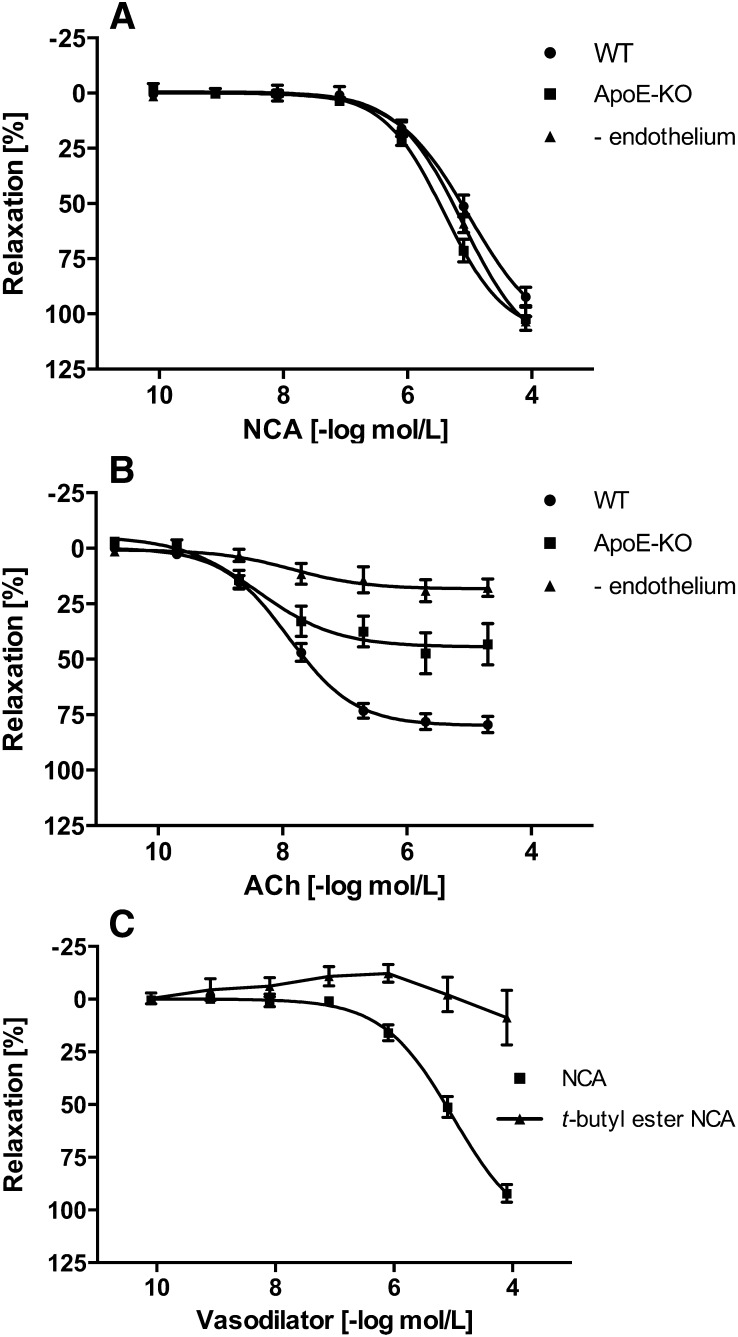
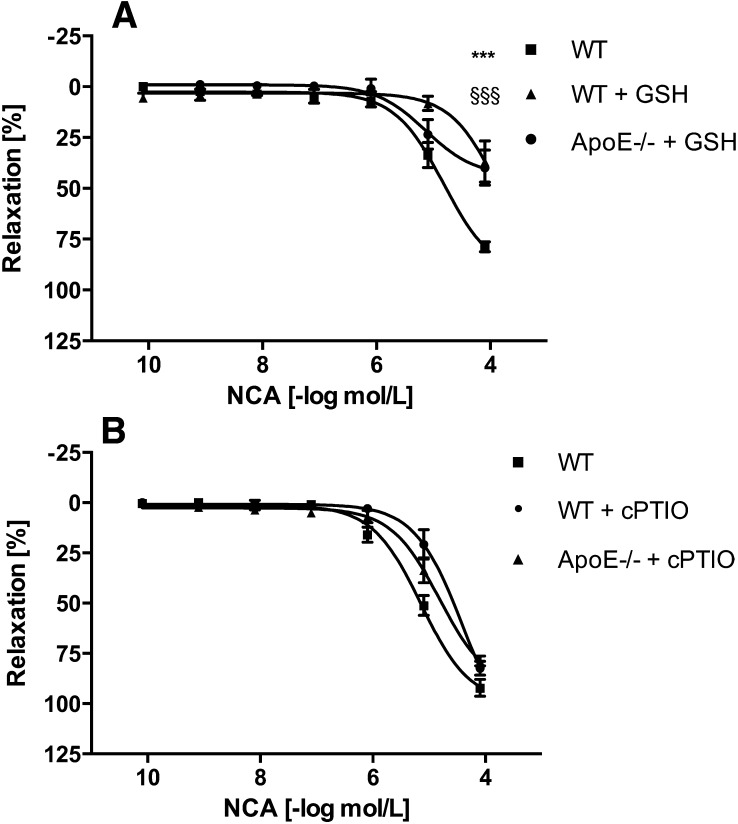
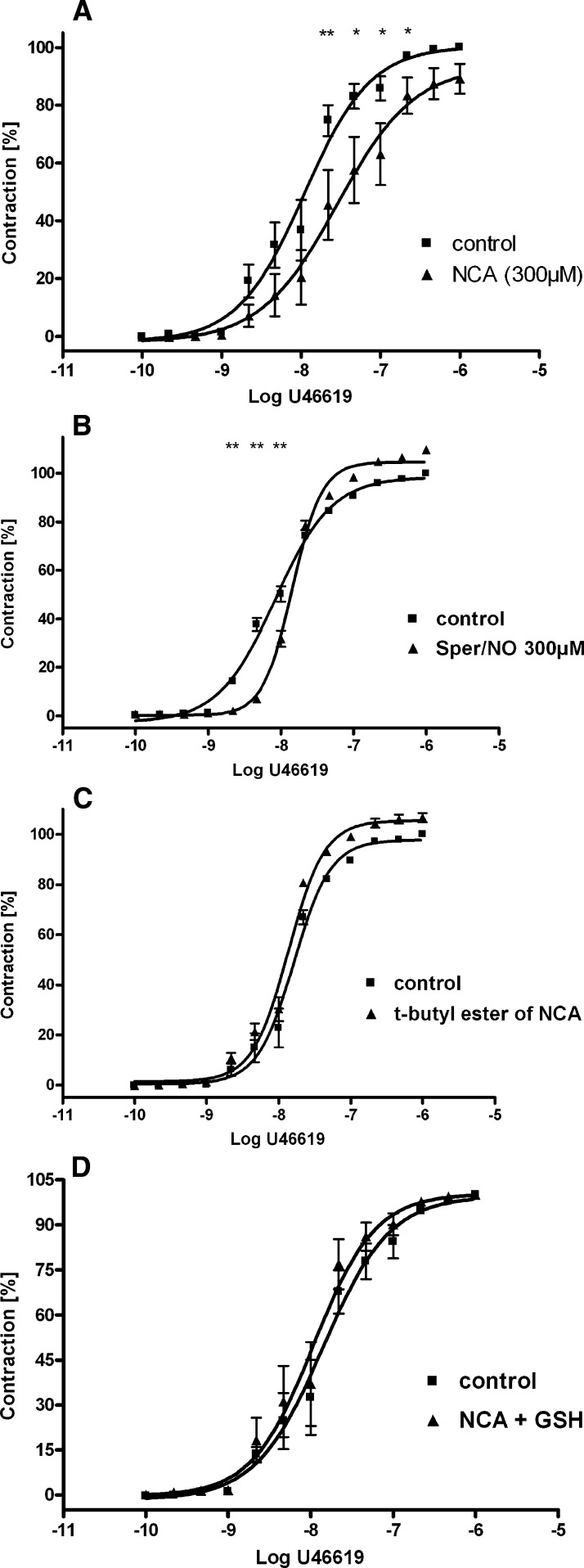
Vasodilatative properties of NCA were examined on aortic rings isolated from WT and ApoE−/− mice and in endothelial-denuded aortae. NCA (80 pM to 80 µM) caused a concentration-dependent vasorelaxation in either experimental group to a similar degree, with an EC50 of 4.4 µM (n = 4–6) (Fig. 1A). Damage of the endothelium in the ApoE−/− mice and in the endothelial-denuded aortae was confirmed by the inability of ACh to induce vasorelaxation in these rings, either partly (ApoE−/− mice) or completely (endothelial-denuded aortae) (each n = 4–6) (Fig. 1B). The dependence of NCA-induced vasorelaxation on HNO was supported by the lack of effect observed with a structurally similar t-butyl ester of NCA, which does not hydrolyze to HNO (Fig. 1C), and DMSO, the vehicle for NCA (data not shown). HNO action was distinguished from NO with inclusion of excess thiol, which acts as a potent scavenger of HNO with little effect on NO (Pino and Feelisch, 1994; Ellis et al., 2000). Addition of GSH (1 mM) to the extracellular medium resulted in ≈50% decrease in NCA (80 µM)-induced relaxation of aortic rings isolated from either WT or ApoE−/− mice (n = 4–6) (Fig. 2A). GSH itself caused no relaxation (data not shown). Conversely, the NO scavenger cPTIO (200 µM) (Irvine et al., 2003) had no effect on NCA-induced vasorelaxation in either group (n = 4–6) (Fig. 2B), suggesting that the effector species released by NCA hydrolysis is HNO rather than NO.
Fig. 1.
Vasodilator effects of NCA in healthy and damaged vasculature. (A) NCA at increasing micromolar concentrations causes a potent and reproducible vasodilatation of intact endothelium (WT mice) as well as damaged endothelium [ApoE−/− mice and mechanically endothelial-denuded aortae (- endothelium)] (n = 4–6), indicating an endothelium-independent mechanism. (B) Verification of the endothelial damage in ApoE−/− mice and in mechanically endothelial-denuded aortae was confirmed by failure of the vessel to relax, either partly or completely, respectively, in the presence of ACh (n = 4–6). (C) The dependence of NCA-induced vasorelaxation on HNO was supported by the lack of effect observed with a structurally similar t-butyl ester of NCA, which does not hydrolyze to HNO (n = 4–6). The rings were precontracted with PGF2α (5 µM). The relaxation effect was expressed as a percentage of decrement to the submaximal tension caused by PGF2α.
Fig. 2.
Dose-dependent effects of NCA in the absence or presence of GSH and cPTIO in wild-type and ApoE−/− mice aortic rings. The HNO scavenger GSH (1 mM) reduced the NCA-induced vasorelaxation in both groups (A), whereas the NO scavenger cPTIO (200 µM) was without effect (B) (n = 4). The rings were precontracted with PGF2α (5 µM). The relaxation effects were expressed as a percentage of decrement to the submaximal tension caused by PGF2α. *** WT vs WT + GSH p<0.001, §§§ WT vs ApoE −/− + GSH p<0.001.
Mechanisms Underlying the Vasodilatative Properties of NCA in Isolated Aortic Rings from WT Mice.
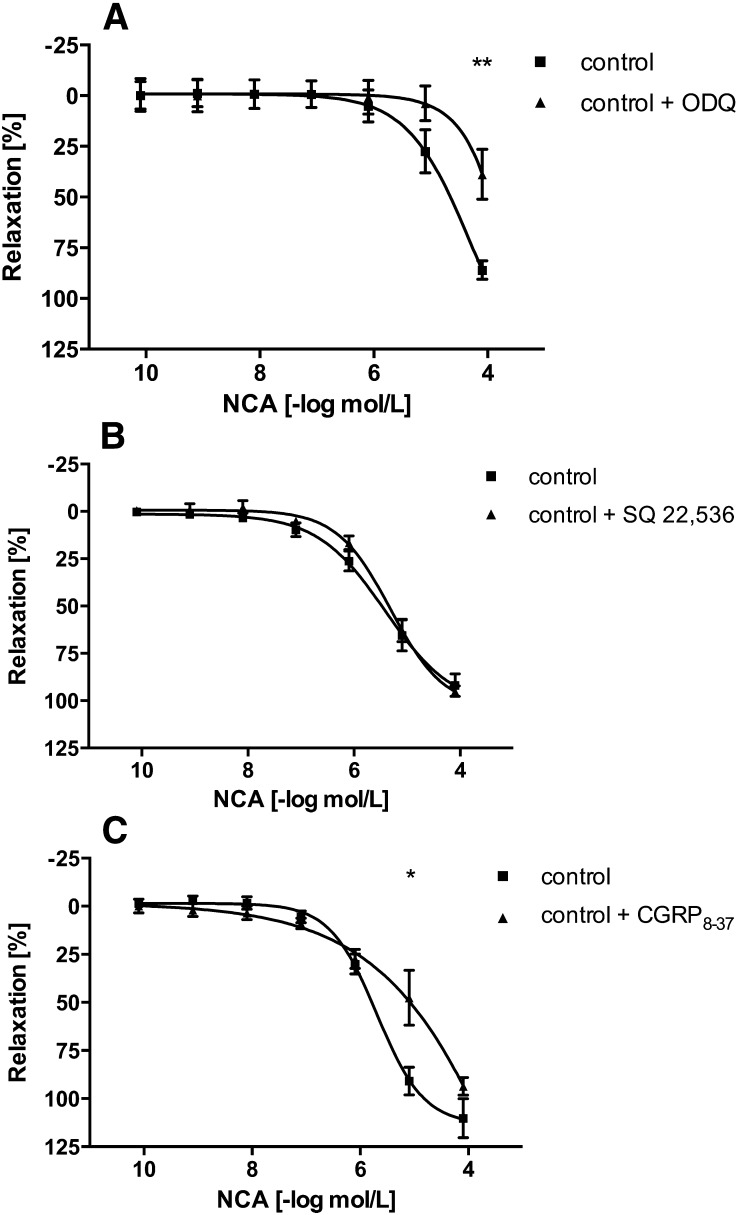
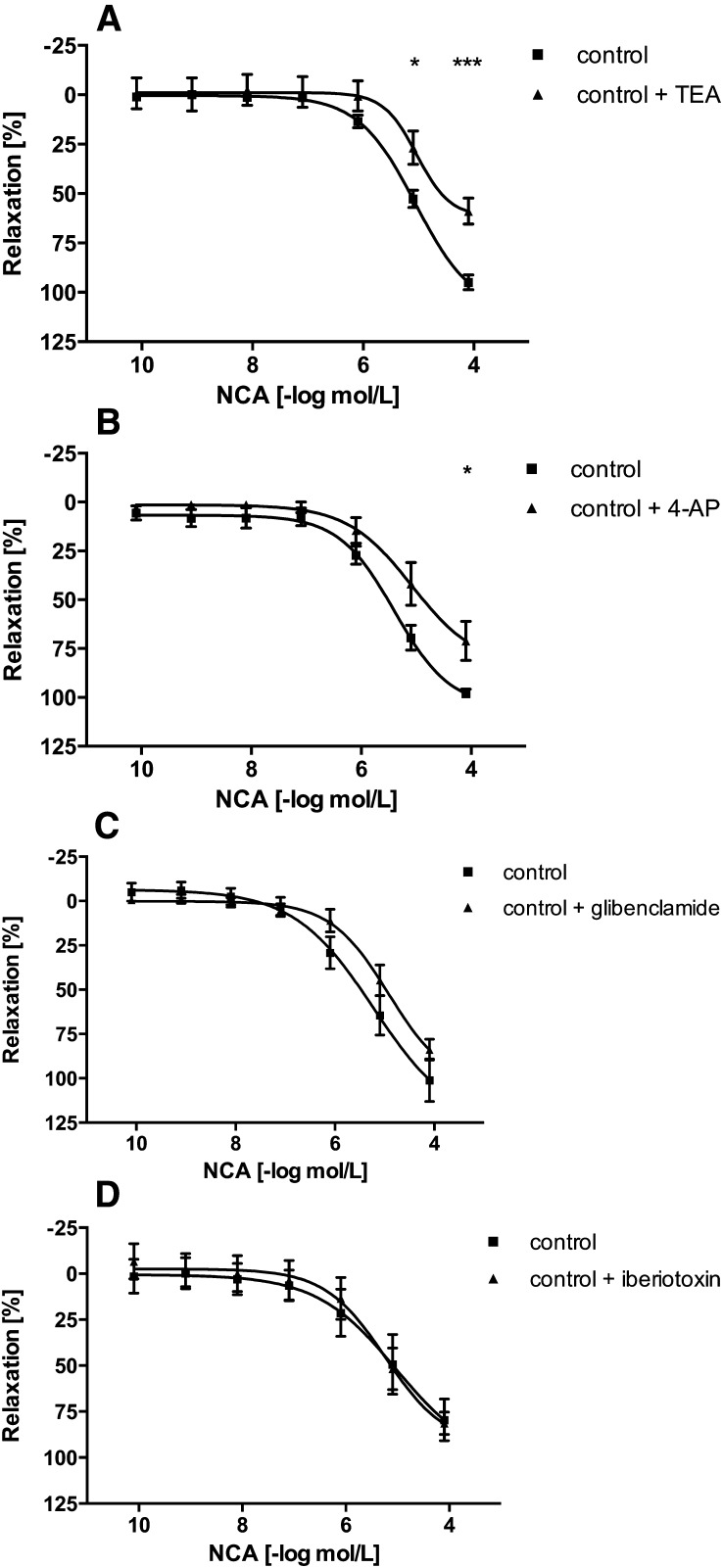
The mechanisms of vasorelaxation induced by HNO released during decomposition of NCA were deduced with a panel of pharmacologic inhibitors in aortic rings isolated from WT mice. ODQ, a compound that inhibits sGC, shifted the concentration-response curve of NCA to the right by approximately half an order of magnitude, thereby reducing vasodilation effects (5 µM, n = 6) (Fig. 3A), consistent with prior findings (Wanstall et al., 2001; Irvine et al., 2003; Favaloro and Kemp-Harper, 2009). Since a previous study in mouse isolated aortae indicated that ODQ causes a concentration-dependent inhibition of HNO-mediated vasorelaxation (Wanstall et al., 2001), a higher concentration of ODQ was tested. ODQ (10 µM) (Wanstall et al., 2001) failed to abolish NCA-induced vasorelaxation (data not shown). Formal changes in EC50 values could not be obtained in the case of ODQ, because in its presence the NCA response did not reach its maximum. As ODQ failed to abolish NCA-induced vasorelaxation, cGMP-independent mechanisms were examined. In contrast to the sGC-cGMP pathway, AC regulates vascular tone by increasing intracellular cAMP levels. Interestingly, the AC inhibitor SQ22536 (100 µM, n = 6) (Morello et al., 2006) (Fig. 3B) had no influence on the vasorelaxation induced by NCA. Control experiments confirmed that SQ22536 completely blocked aortic ring relaxation induced by activation of AC via isoprenaline (data not shown). CGRP8-37 reduced the vasodilator response to NCA (1 µM, n = 6) (Hu et al., 2003) (Fig. 3C), suggesting that the vasodilator effect in isolated aortic rings may be mediated in part by CGRP. At least four distinct types of K+ channels are expressed in vascular smooth muscle cells. The vasodilator response to NCA (80 µM) was significantly inhibited, by 38% and 28%, respectively, by the nonselective K+ channel blocker TEA (1 mM, n = 6) (Lunardi et al., 2007) (Fig. 4A) and the selective voltage-dependent K+ channel blocker 4-AP (10 µM, n = 6) (Jiang et al., 1999) (Fig. 4B). In contrast, the ATP-sensitive K+ channel blocker glibenclamide (1 µM) (Haba et al., 2009) or the high-conductance Ca2+-activated K+ channel blocker iberiotoxin (200 nM) (Cordaillat et al., 2007) had no effect on the NCA-induced vasodilation (each n = 6) (Fig. 4, C and D, respectively). However, the only use of iberiotoxin in the present study may be not sufficient to rule out a major involvement of these Ca2+-activated K+ channels in residual, NO-independent, NCA-induced vasorelaxation. At the completion of the concentration-response curves to NCA, isoprenaline (10 µM) (Shafiei and Mahmoudian, 1999) was added to the medium to check the ability of the artery segments to relax. Isoprenaline induced vasorelaxation of the aortic rings (unpublished data). Furthermore, as controls, two consecutive concentration-response curves to NCA in the absence of inhibitor were performed. A time-dependent change in the potency of NCA was not observed (unpublished data). Thus, inhibitors of sGC, CGRP, and voltage-dependent K+ channels each significantly impaired the vasodilator response to NCA in aortic rings isolated from WT mice (Scheme 1). However, the signaling pathways in arteries from ApoE−/− mice may not match entirely with those operating in WT mice.
Fig. 3.
Signal transduction pathways in NCA-induced vasorelaxation. Concentration-dependent vasorelaxation responses to NCA in isolated aortic rings from WT mice in the absence (control) or presence of ODQ, 5 µM (A); SQ22536, 100 µM (B); or CGRP8-37, 1 µM (C). Values are expressed as a percentage of decrement to the maximum tension caused by PGF2α (5 µM) and given as mean ± S.E.M. (triplicate, n = 4–6 mice). *P < 0.05, **P < 0.01 vs. control.
Fig. 4.
Potassium channel selectivity in NCA-induced vasorelaxation. Concentration-dependent vasorelaxation responses to NCA in isolated aortic rings from WT mice in the absence (control) or presence of TEA, 1 mM (A); 4-AP, 10 µM (B); glibenclamide, 1 µM (C); or iberiotoxin, 200 µM (D). Values are expressed as a percentage of decrement to the maximum tension caused by PGF2α (5 µM) and given as mean ± S.E.M. (triplicate, n = 4–6 mice). *P < 0.05, ***P < 0.001 vs. control.
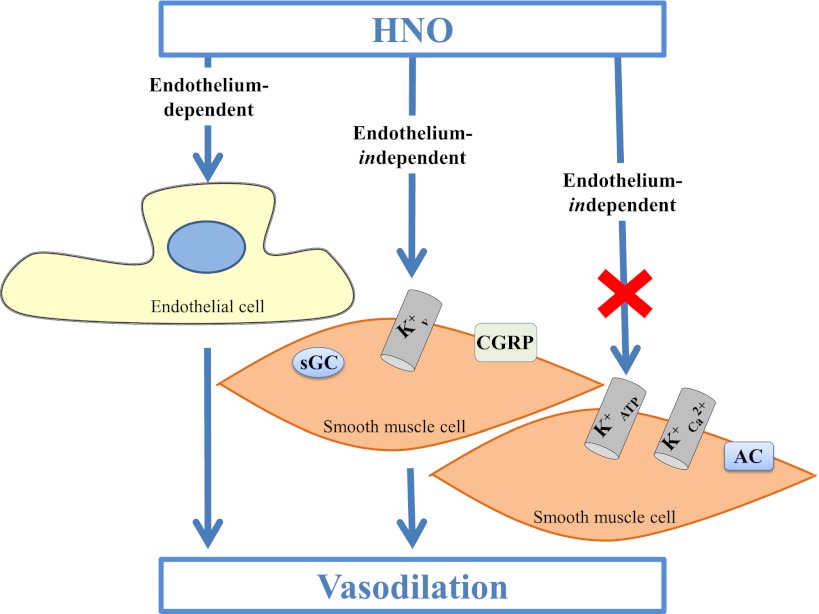
Scheme 1.
The endothelial-dependent and endothelial-independent manner of HNO’s vasorelaxation action. HNO can exert its vasorelaxing effect in both endothelial-dependent and -independent manners. sGC, voltage-dependent K+ channels, and CGRP receptors are probably involved in the vasorelaxation induced by HNO. In contrast, AC, ATP-sensitive K+ channels, and high-conductance Ca2+-activated K+ channels are not involved in the effects of HNO.
Antiaggregatory Actions of NCA.
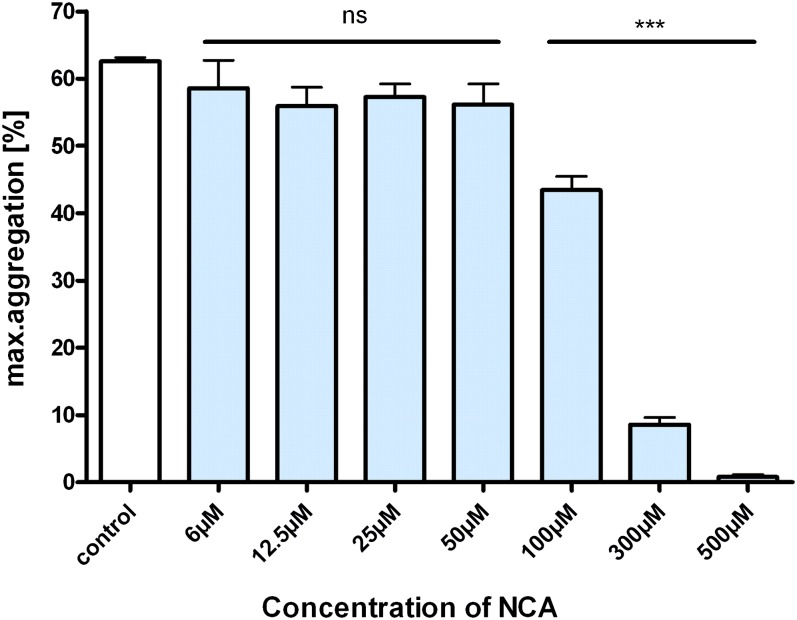
TxA2 has extremely potent vasoconstrictor effects that may become overt and dysregulated in cardiovascular disease (Moncada and Higgs, 1987). To investigate the potential for NCA to counteract pathogenic TxA2 activities, isolated aortic rings were precontracted with the Tx mimetic U-46619. As expected, cumulative concentrations of U-46619 (100 pM to 1 µM) caused a stepwise increase in contraction on isolated aortic rings. NCA (300 µM) shifted the dose-response curve of U-46619 to the right in a parallel fashion (n = 4) (Fig. 5A), indicating an ability of HNO to abrogate the action of TxA2. In contrast, the NO donor Sper/NO (300 µM) did not induce vasorelaxation to the aortic rings precontracted with U-46619 (n = 4) (Fig. 5B). To further corroborate the results obtained with NCA, control experiments with the structurally similar t-butyl ester of NCA and in presence of GSH were made. The t-butyl ester of NCA did not have any effect (n = 4) (Fig. 5C), whereas the addition of GSH (1 mM) to the medium scavenged the effect of HNO on TxA2 exposure (n = 4) (Fig. 5D). A similar result was observed in the ability of NCA to block U-46619 (10 µM)-induced aggregation of human platelets in a concentration-dependent manner (n = 3) (Fig. 6).
Fig. 5.
Attenuation of TxA2 signaling by NCA. Effect of NCA (A), Sper/NO (B), and t-butyl ester of NCA (C) on the U-46619 vasoconstriction activity in isolated aortic rings from WT mice. (D) action of GSH on the vasoconstriction activity of NCA on U-46619. All values are given as the mean ± S.E.M. (triplicate, n = 4 mice). *P < 0.05, **P < 0.01 vs. control.
Fig. 6.
Dose-dependent effects of NCA on platelet aggregation. Effect of NCA on human platelet aggregation induced with 10 µM U-46619 in platelet-rich plasma (PRP) anticoagulated with sodium citrate. Values represent mean in relation to control (100%) ± S.E.M. of four or five experiments.
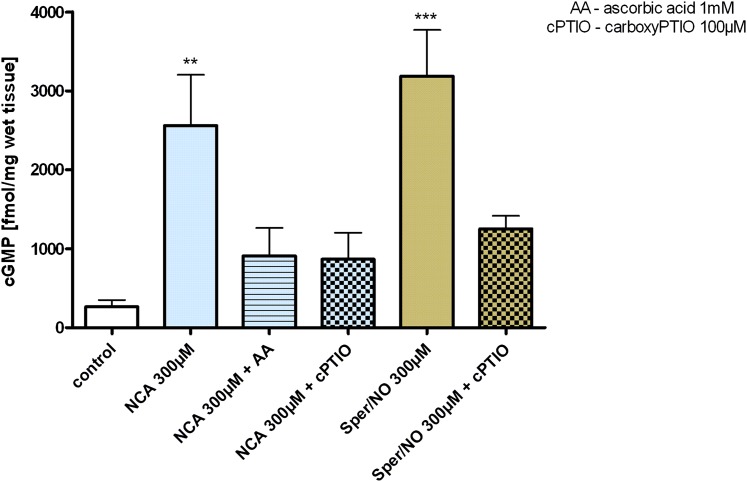
NO attenuates TxA2 signaling through activation of the sGC-cGMP–dependent protein kinase pathway, which targets phosphorylation of the TxA2 receptor (Wang et al., 1998). Isolated aortic rings in the presence of the nonspecific phosphodiesterase inhibitor isobutylmethylxanthine (500 µM) and NCA (300 µM) resulted in a 12-fold increase in cGMP accumulation relative to untreated control. In comparison, an equal concentration of the NO donor Sper/NO was a 24% more effective activator of sGC relative to NCA under the same conditions. To differentiate between HNO and NO in mediating NCA-induced sGC activation, experiments were conducted in the presence of either AA or cPTIO as respective scavengers. The addition of excess AA (1 mM) attenuated sGC activation induced by NCA by 65% (300 µM). An unexpected finding was that cPTIO (100 µM) resulted in a similar decrease in cGMP concentration of 66% with NCA exposure to aortic rings. Control experiments with Sper/NO (300 µM) showed that cPTIO decreased cGMP formation to approximately the same extent (n = 3) (Fig. 7).
Fig. 7.
Effect of NCA and Sper/NO on accumulation of cGMP in isolated aortic rings. Aortic rings isolated from WT mice were incubated for 30 minutes with NCA or Sper/NO (300 µM), and cGMP accumulation was quantified in the absence (control) or presence of AA (1 mM) and cPTIO (100 µM). isobutylmethylxanthine (IBMX) (0.5 mM) was always present in these experiments. All values are given as the mean ± S.E.M. (triplicate, n = 3 mice). **P < 0.01, ***P < 0.001 vs. control.
Discussion
NCA was employed in the present study to explore the underlying mechanisms of HNO-induced vasorelaxant actions in large arteries and its antiaggregatory properties. It has been proposed that HNO donors may be useful in the treatment of vascular diseases (Bullen et al., 2011b; Leo et al., 2012; Wynne et al., 2012). Because endothelial dysfunction appears to be the first step in the chain of events that lead to vascular diseases (Vanhoutte et al., 2009), we sought to investigate the vasodilator effect of NCA in mice with a deficit of apolipoprotein E (ApoE−/−), an established animal model of atherosclerosis (Coleman et al., 2006). Although increased oxidative stress is present in atherosclerosis, HNO-mediated relaxation should be preserved. In support of this hypothesis, Kemp-Harper’s group reported that pretreatment of carotid arteries from WT and ApoE−/− mice with an HNO donor had no effect on their subsequent vasorelaxation. In contrast, an NO donor caused a decrease in sensitivity under the same conditions (Bullen et al., 2011b). Moreover, diabetes-induced oxidant stress was shown to affect NO-mediated vasorelaxation, whereas HNO’s effects were shown to be preserved (Leo et al., 2012). The current results are consistent with these findings and earlier studies examining the vasodilatative properties of NCA (Fig. 1A). Furthermore, AS has been shown to suppress NADPH oxidase (upon both Nox2 expression and superoxide generation) as well as p38 mitogen-activated protein kinase (Lin et al., 2012). To that end, NCA may further rectify redox imbalance as a result of its longer-lasting HNO release profile.
Because of the relatively slower kinetic profile of NCA (lower rate, but sustained release), the observed biologic activities may be attributed to the direct actions of NCA itself. However, the use of the structurally similar t-butyl ester of NCA, which does not hydrolyze to HNO (Sha et al., 2006; Shoman et al., 2011), failed to elicit a vasorelaxant response, suggesting that the observed actions are not due to NCA itself (Fig. 1C).
The addition of excess exogenous reductants was used to discern between the effects of HNO and NO elicited by NCA decomposition. Thiols selectively scavenge HNO with little effect on NO (Pino and Feelisch, 1994; Ellis et al., 2000). Consistent with these reports, we found that GSH markedly inhibited the vasodilator response to NCA in WT and ApoE−/− mice (Fig. 2A). Conversely, the NO scavenger cPTIO did not impact the vasorelaxation induced by NCA (Fig. 2B), suggesting a distinct HNO-mediated mechanism relative to NO in this model system. Earlier results with NCA contradict these results and suggest an NO-mediated mechanism of vasorelaxation. At present, these discrepancies have not been resolved (Shoman et al., 2011). Differences may arise from the relatively slow HNO release rate from NCA that limits HNO concentrations per unit time, which may facilitate discrete conversion to NO depending on the biologic milieu. NCA clearly relaxed vessels with modest potency (IC50 = 4.4 µM), but these experiments do not clearly define an exact nitrogen oxide molecular mediator. As exogenously added GSH peptide remains external to the tissue, these data would indicate that NCA decomposition forms HNO in the medium, which subsequently diffuses into the smooth muscle cell layers (Espey et al., 2002). HNO has been shown to be remarkably reactive toward selected thiol proteins without altering free thiol concentration. One of these proteins is sGC, as reported previously (Miller et al., 2009). Furthermore, it has been shown that HNO stimulates sGC to suppress cardiomyocyte hypertrophy (Lin et al., 2012). In contrast, Zeller’s group reported an intracellular oxidation of HNO to NO following the activation of sGC (Zeller et al., 2009). Our data do not clarify whether HNO itself directly activated sGC (Fukuto et al., 1992) or went through the intermediary of NO following intracellular oxidation (Zeller et al., 2009). The authors believe that NCA releases HNO and that HNO can activate sGC directly, yet intracellular oxidation of HNO to NO cannot be excluded.
In large conduit arteries, mechanisms in addition to the sGC-cGMP pathway are unknown (Fukuto et al., 1992). The vasodilator effects of NCA on the aorta were partially decreased by ODQ, indicating a contribution of the sGC-cGMP pathway, consistent with prior reports (Wanstall et al., 2001; Irvine et al., 2003). The nonexclusivity suggested by the ODQ results led us to examine several alternate pathways. AC is known to regulate vascular tone by increasing intracellular cAMP levels. In our experimental model, the AC inhibitor SQ22536 did not influence the vasorelaxation induced by NCA, but completely blocked the effect of isoprenaline. Thus, the AC pathway appears not to be involved in the NCA-induced vasorelaxation of large arteries. CGRP, a potent relaxation-promoting peptide, has been implicated in HNO-mediated cardiovascular effect. Previous studies have shown that the CGRP receptor antagonist suppressed the inotropic effect of HNO in the heart (Paolocci et al., 2001) and that infusion of HNO donors directly enhanced the release of CGRP in the cardiovascular system (Paolocci et al., 2003). Interestingly, many cardiovascular effects of CGRP itself are distinct from those of HNO. For example, CGRP exclusively enhances arterial dilation indirectly by stimulation of sympathetic innervations and β-adrenergic receptor signaling (Katori et al., 2005), whereas HNO donors in vivo tend to influence venous contractility (Paolocci et al., 2003). Perfusion of antagonists of CGRP receptor, ATP-sensitive K+ channels, or sGC in the Langendorff model significantly blunted the maximum vasodilator response to HNO (Favaloro and Kemp-Harper, 2007). These data are consistent with our observation that ODQ and CGRP8-37 inhibited NCA-induced vasorelaxation of aortic ring preparations (Fig. 3).
K+ channels are a diverse and ubiquitous family of membrane proteins that play key roles in cellular signaling processes such as smooth muscle contraction (Shieh et al., 2000). The exclusive involvement of voltage-dependent K+ channels shown in the present study is consistent with similar findings in rat mesenteric arteries (Irvine et al., 2003; Andrews et al., 2009) but in contrast to findings in the coronary vasculature, where an involvement of ATP-sensitive K+ channels was shown (Favaloro and Kemp-Harper, 2007). Whether HNO activation of K+ channels is direct or proceeds via cGMP-dependent means remains to be elucidated; however, HNO-thiol interactions could enable direct modulation of voltage-dependent K+ channels independently of cGMP (Irvine et al., 2003).
TxA2 elicits diverse pathophysiological actions (Saldeen et al., 1983; Mehta et al., 1988; Eikelboom et al., 2002). Therapeutics aimed at TxA2 function may provide substantial clinical benefit. NCA was effective in reversing the contractile action of the potent TxA2 mimetic U-46619 on mice aortic rings (Fig. 5A). In addition, a similar effect of NCA in abrogating U-46619-induced platelet aggregation was observed (Fig. 6). The mechanism of action in both aorta and platelets seems analogous to that of NO, where an inhibition of G protein receptor signaling is triggered by an increase of cGMP levels and activation of G protein kinase, which in turn phosphorylates the TxA2 receptor, resulting in feedback inhibition (Wang et al., 1998).
This study has shown that the intermediate biochemistry of HNO may proceed through a variety of pathways. Illustrative of this point was the observation that both excess AA and cPTIO, respective scavengers of HNO and NO, impeded cGMP formation in aortic rings exposed to NCA to the same extent (Fig. 7). In contrast, cPTIO was ineffective overall in blocking NCA-induced vasodilation (Fig. 2B). These data reinforce the notion that multiple modes of action are likely in play during NCA hydrolysis, governed by HNO release kinetics and the biologic milieu (Espey et al., 2002; Shoman et al., 2011).
Pharmacological Implications and Conclusions.
Recently Kemp-Harper’s group pointed out: “pure, longer-acting HNO donors are urgently required for experimental evaluation in order to advance the field through the study of long-term vascular effects of HNO” (Bullen et al., 2011a). A potential candidate is NCA. NCA has been shown to 1) hydrolyze slowly at neutral pH (t1/2 = 800–890 minutes) compared with AS and IPA/NO (t1/2 = 2.5 minutes) (Miranda et al., 2003a; Irvine et al., 2008), producing a low-level steady-state concentration of HNO over hours (Sha et al., 2006; Shoman et al., 2011); 2) produce only minimal amounts of NO (<1%) and total nitrate/nitrite (3–4%), factors that may cause nonspecific effects; and 3) alter the release rate of HNO by changing the appending organic groups. In a biologic milieu, NCA has been shown to increase contractile force in normal and β-adrenergically desensitized ventricular myocytes (El-Armouche et al., 2010), to inhibit oxidative stress and the induction of downstream inflammatory genes (Zgheib et al., 2012), and as shown in the present study, to maintain its vasorelaxant effect in a diseased vascular system. However, although experiments in the ApoE−/− mice were limited, these data open a potentially new avenue for investigation in HNO’s translational potential for relief in atherosclerosis, warranting further study.
Abbreviations
- AA
ascorbic acid
- AC
adenylyl cyclase
- ACh
acetylcholine
- 4-AP
4-aminopyridine
- AS
Angeli's salt
- CGRP8-37
calcitonin gene-related peptide receptor antagonist fragment 8–37
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DMSO
dimethylsulfoxide
- DTPA
diethylenetriamine pentaacetic acid
- GSH
glutathione
- HNO
nitroxyl
- IBMX
isobutylmethylxanthine
- IPA/NO
isopropylamine NONOate
- NCA
1-nitrosocyclohexyl acetate
- ODQ
1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one
- NO
nitric oxide
- PGF2α
prostaglandin F2α,
- sGC
soluble guanylyl cyclase
- Sper/NO
spermine NONOate
- SQ22536
tetrahydro-2-furanyl-9H-purin-6-amine
- t1/2
half-life
- TEA
tetraethylammonium
- TxA2
thromboxane A2
- U-46619
9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α
- WT
wild-type
Authorship Contributions
Participated in research design: Donzelli, Fischer, King, Baldus, Gerloff, Böger, Eschenhagen, Espey.
Conducted experiments: Donzelli, Fischer, Niemann, Wieboldt.
Contributed new reagents or analytic tools: DuMond, Fischer, Heeren.
Performed data analysis: Donzelli, Fischer, Espey.
Wrote or contributed to the writing of the manuscript: Donzelli, Fischer, King, Gerloff, Eschenhagen, Böger, Carrier, Espey.
Footnotes
This work was supported by the Marie Curie Intra European Fellowship within the 7th European Community Framework Programme [PIEF-GA-2008-221666]; the sixth Framework Program of the European Union [Marie Curie EXT-014051]; and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant HL62198]. This research was also supported in part by the Intramural Research Program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases.
References
- Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. (2009) A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol 157:540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo E, Sáenz DA, Alberto F, Rosenstein RE, Bari SE, Lazzari MA. (2005) Effect of nitroxyl on human platelets function. Thromb Haemost 94:578–584 [DOI] [PubMed] [Google Scholar]
- Bullen ML, Miller AA, Andrews KL, Irvine JC, Ritchie RH, Sobey CG, Kemp-Harper BK. (2011. a) Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid Redox Signal 14:1675–1686 [DOI] [PubMed] [Google Scholar]
- Bullen ML, Miller AA, Dharmarajah J, Drummond GR, Sobey CG, Kemp-Harper BK. (2011. b) Vasorelaxant and antiaggregatory actions of the nitroxyl donor isopropylamine NONOate are maintained in hypercholesterolemia. Am J Physiol Heart Circ Physiol 301:H1405–H1414 [DOI] [PubMed] [Google Scholar]
- Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. (2005) Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium 37:87–96 [DOI] [PubMed] [Google Scholar]
- Choe CU, Lewerenz J, Fischer G, Uliasz TF, Espey MG, Hummel FC, King SB, Schwedhelm E, Böger RH, Gerloff C, et al. (2009) Nitroxyl exacerbates ischemic cerebral injury and oxidative neurotoxicity. J Neurochem 110:1766–1773 [DOI] [PubMed] [Google Scholar]
- Coleman R, Hayek T, Keidar S, Aviram M. (2006) A mouse model for human atherosclerosis: long-term histopathological study of lesion development in the aortic arch of apolipoprotein E-deficient (E0) mice. Acta Histochem 108:415–424 [DOI] [PubMed] [Google Scholar]
- Cordaillat M, Fort A, Virsolvy A, Elghozi JL, Richard S, Jover B. (2007) Nitric oxide pathway counteracts enhanced contraction to membrane depolarization in aortic rings of rats on high-sodium diet. Am J Physiol Regul Integr Comp Physiol 292:R1557–R1562 [DOI] [PubMed] [Google Scholar]
- Dai T, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. (2007) Nitroxyl increases force development in rat cardiac muscle. J Physiol 580:951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witt BJ, Marrone JR, Kaye AD, Keefer LK, Kadowitz PJ. (2001) Comparison of responses to novel nitric oxide donors in the feline pulmonary vascular bed. Eur J Pharmacol 430:311–315 [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. (2002) Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105:1650–1655 [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Eschenhagen T. (2009) Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev 14:225–241 [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Wahab A, Wittköpper K, Schulze T, Böttcher F, Pohlmann L, King SB, DuMond JF, Gerloff C, Böger RH, et al. (2010) The new HNO donor, 1-nitrosocyclohexyl acetate, increases contractile force in normal and β-adrenergically desensitized ventricular myocytes. Biochem Biophys Res Commun 402:340–344 [DOI] [PubMed] [Google Scholar]
- Ellis A, Li CG, Rand MJ. (2000) Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br J Pharmacol 129:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG, Miranda KM, Thomas DD, Wink DA. (2002) Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic Biol Med 33:827–834 [DOI] [PubMed] [Google Scholar]
- Favaloro JL, Kemp-Harper BK. (2007) The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res 73:587–596 [DOI] [PubMed] [Google Scholar]
- Favaloro JL, Kemp-Harper BK. (2009) Redox variants of NO (NO(.) and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am J Physiol Heart Circ Physiol 296:H1274–H1280 [DOI] [PubMed] [Google Scholar]
- Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, et al. (2008) Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry 47:13150–13152 [DOI] [PubMed] [Google Scholar]
- Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhuri G. (1992) The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther 263:546–551 [PubMed] [Google Scholar]
- Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, et al. (2012) Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res 111:1002–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haba M, Kinoshita H, Matsuda N, Azma T, Hama-Tomioka K, Hatakeyama N, Yamazaki M, Hatano Y. (2009) Beneficial effect of propofol on arterial adenosine triphosphate-sensitive K+ channel function impaired by thromboxane. Anesthesiology 111:279–286 [DOI] [PubMed] [Google Scholar]
- Hu CP, Xiao L, Deng HW, Li YJ. (2003) The depressor and vasodilator effects of rutaecarpine are mediated by calcitonin gene-related peptide. Planta Med 69:125–129 [DOI] [PubMed] [Google Scholar]
- Irvine JC, Favaloro JL, Kemp-Harper BK. (2003) NO- activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 41:1301–1307 [DOI] [PubMed] [Google Scholar]
- Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. (2007) Nitroxyl anion donor, Angeli’s salt, does not develop tolerance in rat isolated aortae. Hypertension 49:885–892 [DOI] [PubMed] [Google Scholar]
- Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. (2008) Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends Pharmacol Sci 29:601–608 [DOI] [PubMed] [Google Scholar]
- Jiang J, Thorén P, Caligiuri G, Hansson GK, Pernow J. (1999) Enhanced phenylephrine-induced rhythmic activity in the atherosclerotic mouse aorta via an increase in opening of KCa channels: relation to Kv channels and nitric oxide. Br J Pharmacol 128:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katori T, Hoover DB, Ardell JL, Helm RH, Belardi DF, Tocchetti CG, Forfia PR, Kass DA, Paolocci N. (2005) Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res 96:234–243 [DOI] [PubMed] [Google Scholar]
- Leo CH, Joshi A, Hart JL, Woodman OL. (2012) Endothelium-dependent nitroxyl-mediated relaxation is resistant to superoxide anion scavenging and preserved in diabetic rat aorta. Pharmacol Res 66:383–391 [DOI] [PubMed] [Google Scholar]
- Lin EQ, Irvine JC, Cao AH, Alexander AE, Love JE, Patel R, McMullen JR, Kaye DM, Kemp-Harper BK, Ritchie RH. (2012) Nitroxyl (HNO) stimulates soluble guanylyl cyclase to suppress cardiomyocyte hypertrophy and superoxide generation. PLoS ONE 7:e34892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunardi CN, Vercesi JA, da Silva RS, Bendhack LM. (2007) Vasorelaxation induced by the new nitric oxide donor cis-[Ru(Cl)(bpy)(2)(NO)](PF(6)) is due to activation of K(Ca) by a cGMP-dependent pathway. Vascul Pharmacol 47:139–144 [DOI] [PubMed] [Google Scholar]
- Ma XL, Gao F, Liu GL, Lopez BL, Christopher TA, Fukuto JM, Wink DA, Feelisch M. (1999) Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proc Natl Acad Sci USA 96:14617–14622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta JL, Lawson D, Mehta P, Saldeen T. (1988) Increased prostacyclin and thromboxane A2 biosynthesis in atherosclerosis. Proc Natl Acad Sci USA 85:4511–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Cherney MM, Lee AJ, Francoleon NE, Farmer PJ, King SB, Hobbs AJ, Miranda KM, Burstyn JN, Fukuto JM. (2009) The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols. J Biol Chem 284:21788–21796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda KM, Katori T, Torres de Holding CL, Thomas L, Ridnour LA, McLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, et al. (2005. a) Comparison of the NO and HNO donating properties of diazeniumdiolates: primary amine adducts release HNO in vivo. J Med Chem 48:8220–8228 [DOI] [PubMed] [Google Scholar]
- Miranda KM, Nagasawa HT, Toscano JP. (2005. b) Donors of HNO. Curr Top Med Chem 5:649–664 [DOI] [PubMed] [Google Scholar]
- Miranda KM, Nims RW, Thomas DD, Espey MG, Citrin D, Bartberger MD, Paolocci N, Fukuto JM, Feelisch M, Wink DA. (2003. a) Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins. A chemical discussion of the differential biological effects of these redox related products of NOS. J Inorg Biochem 93:52–60 [DOI] [PubMed] [Google Scholar]
- Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, et al. (2003. b) A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA 100:9196–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda KM, Yamada K, Espey MG, Thomas DD, DeGraff W, Mitchell JB, Krishna MC, Colton CA, Wink DA. (2002) Further evidence for distinct reactive intermediates from nitroxyl and peroxynitrite: effects of buffer composition on the chemistry of Angeli’s salt and synthetic peroxynitrite. Arch Biochem Biophys 401:134–144 [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. (1987) Prostaglandins in the pathogenesis and prevention of vascular disease. Blood Rev 1:141–145 [DOI] [PubMed] [Google Scholar]
- Mondoro TH, Ryan BB, Hrinczenko BW, Schechter AN, Vostal JG, Alayash AI. (2001) Biological action of nitric oxide donor compounds on platelets from patients with sickle cell disease. Br J Haematol 112:1048–1054 [DOI] [PubMed] [Google Scholar]
- Morello S, Vellecco V, Alfieri A, Mascolo N, Cicala C. (2006) Vasorelaxant effect of the flavonoid galangin on isolated rat thoracic aorta. Life Sci 78:825–830 [DOI] [PubMed] [Google Scholar]
- Paolocci N, Jackson MI, Lopez BE, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM. (2007) The pharmacology of nitroxyl (HNO) and its therapeutic potential: not just the Janus face of NO. Pharmacol Ther 113:442–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA. (2003) Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proc Natl Acad Sci USA 100:5537–5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, et al. (2001) Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci USA 98:10463–10468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino RZ, Feelisch M. (1994) Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO-) using L-cysteine. Biochem Biophys Res Commun 201:54–62 [DOI] [PubMed] [Google Scholar]
- Saldeen P, Nilsson IM, Saldeen T. (1983) Increased synthesis of thromboxane B2 and 6-keto-PGF1 alpha in hand veins from patients with deep venous thrombosis. Thromb Res 32:461–467 [DOI] [PubMed] [Google Scholar]
- Sha X, Isbell TS, Patel RP, Day CS, King SB. (2006) Hydrolysis of acyloxy nitroso compounds yields nitroxyl (HNO). J Am Chem Soc 128:9687–9692 [DOI] [PubMed] [Google Scholar]
- Shafiei M, Mahmoudian M. (1999) Atypical beta-adrenoceptors of rat thoracic aorta. Gen Pharmacol 32:557–562 [DOI] [PubMed] [Google Scholar]
- Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. (2000) Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev 52:557–594 [PubMed] [Google Scholar]
- Shoman ME, DuMond JF, Isbell TS, Crawford JH, Brandon A, Honovar J, Vitturi DA, White CR, Patel RP, King SB. (2011) Acyloxy nitroso compounds as nitroxyl (HNO) donors: kinetics, reactions with thiols, and vasodilation properties. J Med Chem 54:1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer CH, Flores-Santana W, Mancardi D, Donzelli S, Basudhar D, Ridnour LA, Miranda KM, Fukuto JM, Paolocci N, Wink DA. (2009) The emergence of nitroxyl (HNO) as a pharmacological agent. Biochim Biophys Acta 1787:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchetti CG, Stanley BA, Murray CI, Sivakumaran V, Donzelli S, Mancardi D, Pagliaro P, Gao WD, van Eyk J, Kass DA, et al. (2011) Playing with cardiac “redox switches”: the “HNO way” to modulate cardiac function. Antioxid Redox Signal 14:1687–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O’Rourke B, Gao WD, Wink DA, et al. (2007) Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res 100:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. (2009) Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196:193–222 [DOI] [PubMed] [Google Scholar]
- Wang GR, Zhu Y, Halushka PV, Lincoln TM, Mendelsohn ME. (1998) Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc Natl Acad Sci USA 95:4888–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR. (2001) Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol 134:463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne BM, Labazi H, Tostes RC, Webb RC. (2012) Aorta from angiotensin II hypertensive mice exhibit preserved nitroxyl anion mediated relaxation responses. Pharmacol Res 65:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller A, Wenzl MV, Beretta M, Stessel H, Russwurm M, Koesling D, Schmidt K, Mayer B. (2009) Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli’s salt. Mol Pharmacol 76:1115–1122 [DOI] [PubMed] [Google Scholar]
- Zgheib C, Kurdi M, Zouein FA, Gunter BW, Stanley BA, Zgheib J, Romero DG, King SB, Paolocci N, Booz GW. (2012) Acyloxy nitroso compounds inhibit LIF signaling in endothelial cells and cardiac myocytes: evidence that STAT3 signaling is redox-sensitive. PLoS ONE 7:e43313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. (1992) Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258:468–471 [DOI] [PubMed] [Google Scholar]