Abstract
Mast cell activation results in the immediate release of proinflammatory mediators prestored in cytoplasmic granules, as well as initiation of lipid mediator production and cytokine synthesis by these resident tissue leukocytes. Allergen-induced mast cell activation is central to the pathogenesis of asthma and other allergic diseases. Presently, most pharmacological agents for the treatment of allergic disease target receptors for inflammatory mediators. Many of these mediators, such as histamine, are released by mast cells. Targeting pathways that limit antigen-induced mast cell activation may have greater therapeutic efficacy by inhibiting the synthesis and release of many proinflammatory mediators produced in the mast cell. In vitro studies using cultured human and mouse mast cells, and studies of mice lacking A2B receptors, suggest that adenosine receptors, specifically the Gs-coupled A2A and A2B receptors, might provide such a target. Here, using a panel of mice lacking various combinations of adenosine receptors, and mast cells derived from these animals, we show that adenosine receptor agonists provide an effective means of inhibition of mast cell degranulation and induction of cytokine production both in vitro and in vivo. We identify A2B as the primary receptor limiting mast cell degranulation, whereas the combined activity of A2A and A2B is required for the inhibition of cytokine synthesis.
Introduction
Mast cells play a central role in the pathogenesis of allergic rhinitis, asthma, and anaphylaxis. Through degranulation and de novo production of lipid mediators, mast cells are the major cell type responsible for the acute and sometimes life-threatening manifestations of these allergic disorders. Chronic mast cell activation is also believed to contribute to the pathogenesis of asthma (Peachell, 2005; Bradding et al., 2006; Yu et al., 2006). Mast cells are an important source of Th2 cytokines, including interleukin (IL)-4, IL-5, and IL-13 (Bradding et al., 1994; Brightling et al., 2003; Galli et al., 2005). Infiltration of mast cells into airway smooth muscle bundles has been reported in patients with asthma, and this proximity may be important for the development of airway hyper-responsiveness (Brightling et al., 2002; James et al., 2012). In a murine model of asthma, mast cells have been implicated in the development of airway hyper-responsiveness, airway remodeling, and IgE synthesis (Yu et al., 2006). Inhibiting mast cell activation has therapeutic efficacy in asthmatic subjects, further supporting the critical role for this immune cell in disease pathogenesis (Rodrigo et al., 2011). However, drug options for the inhibition of mast cell activation are limited. Currently, two categories of medications are commercially available to suppress the activity of mast cells: mast cell membrane stabilizers, such as cromolyn and nedocromil sodium, and the anti-IgE antibody omalizumab. Sodium cromolyn at very high concentrations inhibits degranulation of human lung mast cells by only 10%, suggesting that these drugs may be inefficient at inhibiting the activity of mast cells in vivo (Church and Hiroi, 1987). The clinical efficacy of omalizumab has been demonstrated in several clinical trials, and it is believed to be a more potent inhibitor of mast cell activation than cromolyn (Boulet et al., 1997; Fahy et al., 1997; Busse et al., 2001; Rodrigo et al., 2011). Although omalizumab inhibits IgE/FεRI-mediated activation by antigen, mast cells can also be activated through FεRI-independent pathways. Chronic mast cell activation via these alternative pathways may contribute to airway remodeling (Peachell, 2005; Okayama et al., 2007; Carter and Bradding, 2011). Therefore, additional means to inhibit mast cell activity are of considerable interest.
Pharmacological agents with cAMP-elevating properties have long been observed to inhibit mast cell function (Torphy, 1998). cAMP is a ubiquitous intracellular second messenger that is produced from AMP after the activation of adenylyl cyclase. We previously showed that cAMP can inhibit store-operated calcium channels in mast cells through a protein kinase A-dependent pathway, which subsequently limits antigen-induced mast cell degranulation (Hua et al., 2007). Different strategies can raise intracellular cAMP levels, including activation of Gs-coupled receptors. For example, β-adrenergic agonists can increase intracellular cAMP through activation of Gs-coupled β-adrenergic receptors, which in turn directly relaxes airway smooth muscle. Previous studies have shown that β-adrenergic agonists can also increase cAMP in mast cells, and can inhibit mast cell activation as a result (Church and Hiroi, 1987; Weston and Peachell, 1998). However, because β-adrenergic receptors on mast cells tend to internalize and desensitize once bound by agonists, the capacity of β-adrenergic receptor agonists to maintain high cAMP levels in mast cells is limited (Church and Hiroi, 1987; Chong et al., 2003; Scola et al., 2004).
In addition to β-adrenergic receptors, mast cells express other Gs-coupled receptors, including the A2 adenosine receptors A2A and A2B (Marquardt et al., 1994; Zhong et al., 2003; Hua et al., 2007). Activation of both A2A and A2B has been shown to increase intracellular cAMP levels in many different cell types, including mast cells (Pierce et al., 1992; Feoktistov and Biaggioni, 1995; Olah, 1997; Fredholm et al., 2000). However, whether activation of Gs-coupled adenosine receptors in mast cells inhibits mast cell activation is still controversial, especially in vivo. In vitro, adenosine has been shown to elicit both pro- and anti-inflammatory effects on mast cells (Peachell et al., 1991; Suzuki et al., 1998; Zhong et al., 2003; Hua et al., 2007); however, in vivo studies have focused largely on adenosine-induced mast cell activation (Tilley et al., 2000, 2003; Zhong et al., 2001; Oldenburg and Mustafa, 2005; Hua et al., 2008). Since adenosine is a potent anti-inflammatory mediator after the initial phase of an immune response (Hershfield, 2005), we hypothesized that adenosine might also inhibit mast cell activation in vivo through activation of Gs-coupled A2A and/or A2B receptors. In this study, we used genetic methods to investigate the function of Gs-coupled adenosine receptors on mast cells both in vivo and in vitro to evaluate their potential as therapeutic targets for mast cell–mediated diseases.
Materials and Methods
Animals.
All studies were conducted in accordance with the Institutional Animal Care and Use Committee guidelines of the University of North Carolina at Chapel Hill. A2A−/−, A2B−/−, and A3−/− mice were generated and genotyped by Southern blot analysis or polymerase chain reaction as previously described (Ledent et al., 1997; Hua et al., 2007; Salvatore et al., 2000). Each mouse line was backcrossed 12 generations to the C57BL/6 genetic background. The double knockout mice, including A2A−/−A2B−/− and A2B−/−A3−/−, were generated by intercrossing the corresponding double heterozygotes. C57BL/6 wild-type control mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in our animal facility. All mice were housed in a pathogen-free facility with 12-hour day and night switch. For all experiments, mice were greater than 8 weeks old and wild-type and receptor-deficient animals were matched for age and sex.
Bone Marrow-Derived Mast Cell Culture.
Bone marrow-derived mast cells (BMMCs) were isolated from the femurs of 8- to 12-week-old mice and grown in RPMI-1640 medium containing 10% fetal calf serum, 20 ng/ml murine IL-3, and 20 ng/ml stem cell factor as described previously (Salvatore et al., 2000). Cell purity was determined by toluidine blue staining. Cell viability was determined by trypan blue staining.
Hexosaminidase Release, Leukotriene B4, and IL-6 Measurements.
Mast cell degranulation was determined by β-hexosaminidase assay as described (Nguyen et al., 2002). For acute treatment, 5′-N-ethylcarboxamidoadenosine (NECA) (or adenosine) was added 20 minutes before the addition of the antigen dinitrophenyl-human serum albumin (DNP-HSA). For chronic treatment, NECA was added 20 hours before the antigen challenge. Hexosaminidase was measured 30 minutes after stimulation by antigen. IL-6 and leukotriene B4 (LTB4) concentrations in supernatants 6 hours after mast cell activation were determined by enzyme-linked immunosorbent assay (Nguyen et al., 2002; Hua et al., 2007). NECA treatment of these experiments was the same as described in the degranulation assay.
Passive Systemic Anaphylaxis.
Passive systemic anaphylaxis (PSA) was performed as previously described (Hua et al., 2007). Mice were injected intraperitoneally with NECA in dimethyl sulfoxide/phosphate-buffered saline (DMSO/PBS) (10 μg/kg). They were then given 2 μg of murine anti-DNP IgE monoclonal antibody (Sigma-Aldrich, St. Louis, MO) via tail vein. A second NECA treatment (10 μg/kg) was given 12 hours after IgE sensitization. Twenty-four hours after the first NECA treatment, 100 μg of DNP-HSA (Sigma-Aldrich) was injected intravenously to induce mast cell activation. Core body temperature was recorded over time for the indicated period of time using a rectal probe. Investigators were blinded to the treatment given to each mouse during all experiments. The control groups were injected intraperitoneally with vehicle only (DMSO/PBS) at each time point. For histamine measurements, mice were sensitized with anti-DNP IgE systemically as described above. They were then treated with NECA by intraperitoneal injection (400 μg/kg). Twenty-four hours later, these animals were injected with DNP-HSA via tail vein to induce systemic anaphylaxis. Serum was collected immediately after antigen injection and histamine levels were measured by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI).
Passive Cutaneous Anaphylaxis.
In the passive cutaneous anaphylaxis (PCA) model, mice were injected intraperitoneally with NECA in DMSO/PBS (10 μg/kg). Their right ears were then injected subcutaneously with 2 ng murine anti-DNP IgE monoclonal antibody (Sigma-Aldrich) in 20 μl of PBS. The left ears were injected with 20 μl of PBS as negative controls. A booster NECA treatment was given 12 hours after IgE sensitization. Twenty-four hours after IgE sensitization, mice were injected with 0.2 ml 0.9% filtered Evans blue dye containing 100 μg of DNP antigen through the tail vein. The mice were euthanized 1 hour after DNP injection. Both the left and right ears were collected and extravasated dye was extracted with formamide. The optical density values at 610 nm of the pinna extracts were measured.
Statistical Analysis.
All data are presented as mean ± S.E.M. The paired Student’s t test was used for comparison before and after treatment in the same subject. The two-tailed, unpaired Student’s t test was used between different groups. Repeated-measures analysis of variance was used to analyze differences between groups over time, from the beginning of DNP-HSA injection through the response period.
Results
Activation of Gs-Coupled Adenosine Receptors Inhibits Antigen-Induced Mast Cell Degranulation In Vivo.
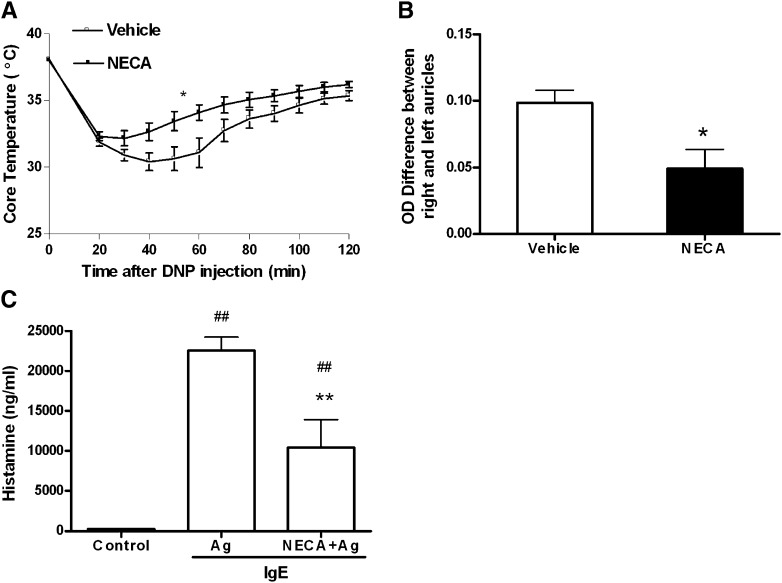
We used PSA and PCA models to evaluate mast cell function in vivo. Previous data indicate that murine mast cells, similar to humans, express A2A, A2B, and A3 receptors, but not A1 receptors (Hua et al., 2007). Since adenosine can activate mast cells through A3 receptors in vivo (Salvatore et al., 2000; Tilley et al., 2003), we used mice lacking A3 receptors (A3−/−) to investigate the effect of activation of the Gs-coupled A2A and A2B receptors in PSA and PCA models. For systemic anaphylaxis, A3−/− mice were given murine anti-DNP IgE by intravenous injection. Mice were then treated with NECA intraperitoneally. Twenty-four hours later, mast cell degranulation was elicited in vivo by intravenous administration of DNP-HSA. Body temperature drop was selected and recorded as a surrogate marker to evaluate mast cell degranulation, as previously described (Hua et al., 2007). Mice treated with NECA demonstrated less drop in body temperature than mice treated with vehicle (n = 7–10 per group; P < 0.05 by repeated-measures analysis of variance), suggesting less mast cell degranulation in the NECA pretreated group (Fig. 1A). Consistent with these findings, PCA experiments also showed that NECA pretreatment could limit antigen-induced mast cell degranulation in vivo. As shown in Fig. 1B, mice treated with NECA prior to antigen challenge demonstrated less plasma extravasation into the ear (n = 7 per group; P < 0.01 by t test). To further establish that our findings were reflective of mast cell degranulation, we measured the levels of histamine in the serum of a separate cohort of A3−/− mice during PSA. Mice pretreated with NECA exhibited significantly lower histamine levels in their serum during PSA compared with the vehicle-treated group (P < 0.01; n = 10, 14, and 15 for the control, DNP, and DNP+NECA groups, respectively). Collectively, these experiments demonstrate that activation of Gs-coupled adenosine receptors can inhibit antigen-induced activation of mast cells in vivo.
Fig. 1.
Effect of NECA on antigen-induced mast cell degranulation in vivo. (A) Passive systemic anaphylaxis (PSA). Mice were injected intraperitoneally with NECA (10 μg/kg) (filled square, n = 7) or DMSO/PBS vehicle (open square, n = 10), and 2 μg murine anti-DNP IgE monoclonal antibody (mAb) (Sigma-Aldrich) was administered via tail vein. A second NECA/vehicle treatment was given 12 hours after IgE sensitization. Twenty-four hours after the first NECA treatment, 100 μg DNP-HSA antigen was injected intravenously. Data represent the mean body temperature ± S.E.M. every 10 minutes postantigen administration. *P < 0.01 by repeated-measures analysis of variance. (B) Passive cutaneous anaphylaxis. Mice were injected intraperitoneally with NECA or vehicle as described for PSA. Right ears were then injected subcutaneously with 2-ng murine anti-DNP IgE mAb in 20 μl of PBS. Left ears were injected with 20 μl of PBS. Twenty-four hours after IgE sensitization, mice were injected with 0.2 ml of 0.9% filtered Evans blue dye containing 100 μg of DNP antigen by tail vein. One hour after DNP injection, ears were collected and extravasated dye was extracted with formamide. Data represent the mean optical density values at 610 nm (right ear–left ear) of the pinna extracts ± S.E.M. *P < 0.05 by Student’s t test between NECA (filled bar, n = 7) versus vehicle (open bar, n = 7) groups. (C) Serum histamine levels during PSA. Mice were subjected to PSA as described in (A), except 400 μg/kg NECA was used. Blood was obtained immediately after antigen (Ag) injection for measurement of serum histamine levels by enzyme-linked immunosorbent assay. Data represent mean histamine level ± S.E.M. **P < 0.001 by Student’s t test between NECA-treated mice (n = 14) versus vehicle-treated mice (n = 15). ##P < 0.001 between both NECA- and vehicle-treated mice versus naïve controls (n = 10).
Activation of Gs-Coupled Adenosine Receptors Inhibits Antigen-Induced Mast Cell Degranulation In Vitro.
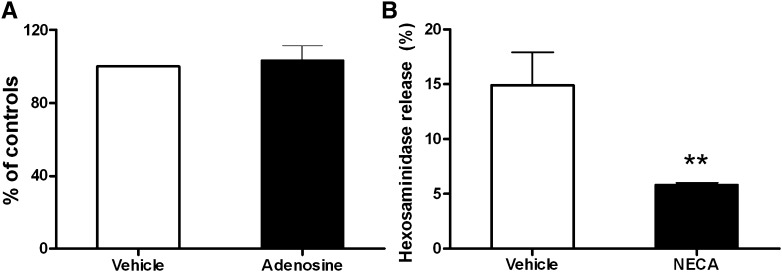
To further investigate the above in vivo findings, we cultured BMMCs and tested the capacity of NECA to inhibit antigen-induced degranulation. BMMCs from A3−/− mice were cultured in vitro for 5 weeks in media containing murine IL-3 and stem cell factor. The expression profile of adenosine receptors on A3−/− BMMCs was measured using real-time polymerase chain reaction. As expected, these cells only expressed Gs-coupled adenosine receptors A2A and A2B, and expression levels were similar to that of wild-type BMMCs (unpublished data). After passive sensitization with murine anti-DNP IgE (100 ng/ml per million cells for 12 hours), the cultured A3−/− BMMCs were treated with adenosine for 20 min followed by antigen challenge. Mast cell degranulation was quantitated by measuring hexosaminidase release. As shown in Fig. 2A, acute activation of Gs-coupled adenosine receptors on A3−/− BMMCs failed to modify antigen-induced degranulation of BMMCs.
Fig. 2.
Effect of acute versus chronic adenosine receptor stimulation on antigen-induced degranulation in vitro. (A) BMMCs from A3−/− mice were sensitized with murine anti-DNP IgE antibody and incubated with adenosine (100 μM) or vehicle for 20 minutes. Mast cell degranulation was triggered by addition of DNP-HSA antigen. Data represent the mean percent change in hexosaminidase release compared to vehicle-treated cells (controls) ± S.E.M. P > 0.05 by the Student’s t test (n = 3 cell lines). (B) Anti-DNP IgE sensitized A3−/− BMMCs were treated with 20 μM NECA or vehicle for 20 hours and hexosaminidase was measured 30 minutes after stimulation with DNP-HSA antigen. **P < 0.01 by the Student’s t test between NECA versus vehicle (n = 3 cell lines per group).
In our in vivo experiments, the inhibitory effects of NECA on mast cell degranulation were observed approximately 20 hours after the initial treatment; we therefore increased the incubation time prior to antigen challenge in the in vitro experiments from 20 minutes to 20 hours. To see whether increased time of incubation with NECA affected cell viability, trypan blue staining was performed. No differences were observed between NECA- and vehicle-treated cells (unpublished data). Antigen-induced hexosaminidase release in A3−/− BMMCs incubated without NECA was 14.9% ± 3%. However, if cells were pretreated with NECA (20 μM) for 20 hours prior to the addition of antigen, the antigen-induced hexosaminidase release from these cells was significantly inhibited (5.8% ± 0.2%; n = 3; P < 0.01) (Fig. 2B). Based on these observations, we conclude that chronic but not acute activation of Gs-coupled adenosine receptors can significantly inhibit antigen-induced mast cell degranulation.
NECA Inhibits Mast Cell Degranulation through Activation of the A2B Receptor.
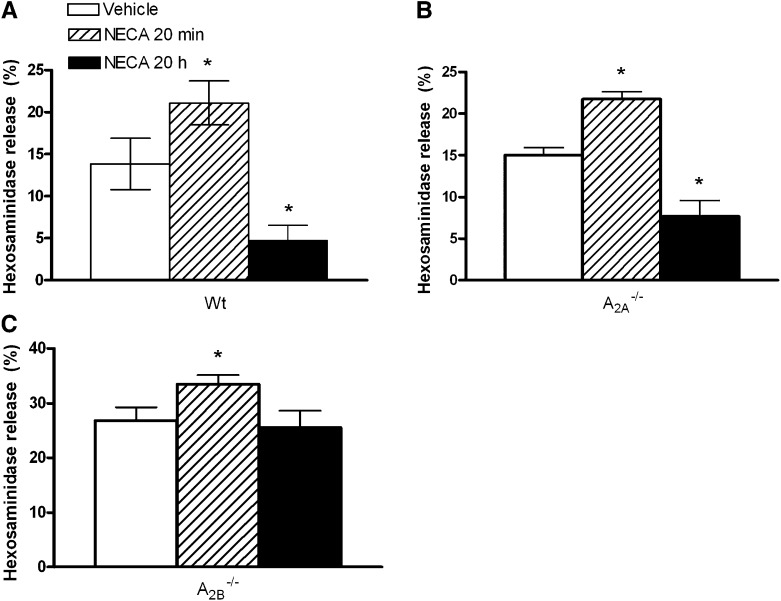
To determine whether the inhibitory effects of NECA on antigen-induced degranulation would still be detectable in cells expressing A3 receptors, we tested the effect of chronic NECA incubation on degranulation of wild-type BMMCs. IgE-loaded BMMCs were incubated with 20 μM NECA for 20 hours. Antigen-induced mast cell degranulation was then evaluated by measuring hexosaminidase release 30 minutes after stimulation by DNP antigen. Similar to our findings in the A3−/− BMMCs, chronic treatment with NECA significantly inhibited antigen-induced degranulation of wild-type mast cells (13.8 ± 3.1% in vehicle-treated cells versus 4.7 ± 1.9% in chronic NECA-treated cells; P < 0.001; n = 4) (Fig. 3A). In addition, the acute effect of NECA on antigen-induced mast cell degranulation was also evaluated to ensure that functional A3 receptors were expressed on these cells, as observed in our previous studies (Salvatore et al., 2000; Tilley et al., 2003). As expected, NECA added 15–20 minutes prior to antigen challenge significantly increased antigen-induced mast cell degranulation (P < 0.05, acute NECA versus controls) (Fig. 3A). These data indicate that adenosine has a biphasic effect on antigen-induced mast cell degranulation. Acutely, adenosine acts as a proinflammatory mediator by potentiating antigen-induced degranulation via A3 receptors; chronically, adenosine inhibits antigen-induced mast cell degranulation via Gs-coupled receptors regardless of the coexpression of Gi/o-coupled A3 receptors.
Fig. 3.
Effect of acute and chronic NECA on antigen-induced degranulation of wild-type, A2A−/−, and A2B−/− mast cells. Cultured wild-type (A), A2A−/− (B), and A2B−/− (C) mast cells were passively sensitized with murine IgE. The cells were then incubated with NECA acutely (20 minutes) or chronically (20 h) prior to the addition of antigen. Controls were treated with aliquot vehicle. Hexosaminidase release was then measured to evaluate mast cell degranulation. *P < 0.05 versus vehicle. Data are from three to four different sets of cells, and are presented as the mean percentage of hexosaminidase release ± S.E.M.
To further substantiate this observation and to determine the receptor subtype responsible for the chronic inhibitory effect of NECA on degranulation, both A2A−/− and A2B−/− BMMCs were treated with NECA acutely and chronically and antigen-induced hexosaminidase release was measured. As shown in Fig. 3B, both the acute potentiation and chronic inhibition of NECA were observed in A2A−/− BMMCs. This finding not only further substantiates the biphasic effect of adenosine on mast cell degranulation, but also indicates that the inhibitory effect of NECA on degranulation is not mediated by A2A receptors. In BMMCs lacking A2B receptors, we first observed an exaggerated mast cell degranulation induced by antigen compared with the wild-type controls (26.8 ± 2.4% versus 13.8 ± 3.1%; P < 0.01 by the Student’s t test), which is consistent with our previous observations (Hua et al., 2007). Second, the acute potentiating effect of NECA was also present in A2B−/− BMMCs, as observed in wild-type and A2A−/− BMMCs (26.8 ± 2.4% in DMSO vehicle-treated A2B−/− cells versus 33.5 ± 1.6% in NECA acutely treated A2B−/− cells; P < 0.05). However, when the A2B−/− BMMCs were chronically treated with NECA, the inhibitory effect on degranulation that was observed in wild-type, A2A−/−, and A3−/− BMMCs was abolished (Fig. 3C). These data indicate that the capacity of NECA to inhibit mast cell degranulation is mediated by the A2B receptor.
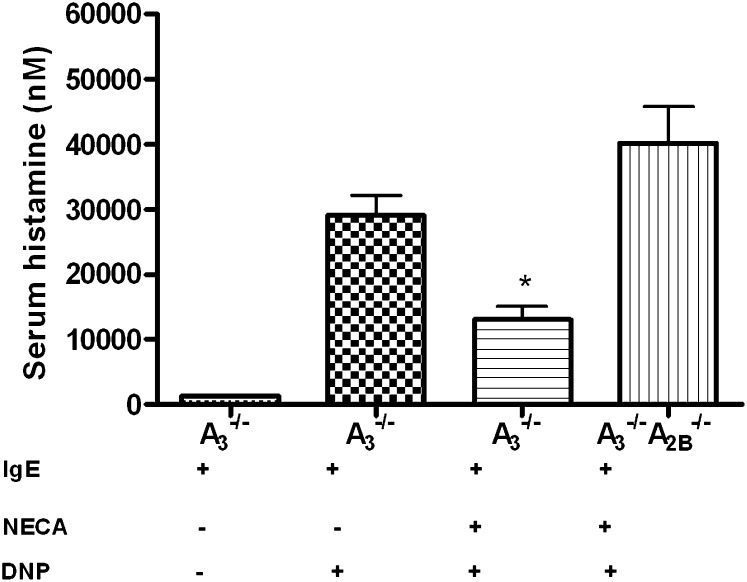
We next conducted experiments to determine whether our above-described in vitro observations implicating the A2B receptor were also present in vivo. Mice lacking the A3 receptor (A3−/−) and mice lacking both A2B and A3 receptors (A2B−/−A3−/−) were sensitized with murine IgE mAb. They were then given an intraperitoneal injection of NECA (400 µg/kg). Antigen-induced mast cell degranulation was evaluated by measuring serum histamine levels the next day immediately after the intravenous administration of DNP antigen. As shown in Fig. 4, treatment with DNP antigen resulted in a marked increase in serum histamine level in A3−/− mice. Antigen-induced histamine levels in the serum were significantly attenuated in A3−/− mice, but not A2B−/−A3−/− mice, pretreated with NECA. These data demonstrate that A2B receptors mediate the inhibitory effect of NECA in systemic anaphylaxis.
Fig. 4.
Effect of NECA on antigen-induced mast cell degranulation in mice lacking both A3 and A2B receptors in vivo. A3−/− and A2B−/−A3−/− mice were sensitized with murine IgE. These mice were then given NECA intraperitoneally (400 μg/kg) as described previously in this study. Blood was obtained immediately after antigen injection the next day for the measurements of serum histamine levels by enzyme-linked immunosorbent assay. Controls include two groups of A3−/− mice treated with IgE/vehicle/PBS and IgE/vehicle/ DNP. Data represent mean histamine level ± S.E.M. (n = 5 in each group). *P < 0.05 by the Student’s t test between NECA-treated A3−/− versus both vehicle-treated A3−/− and NECA-treated A2B−/−A3−/− mice.
A2A Receptors Do Not Influence Antigen-Induced Mast Cell Degranulation.
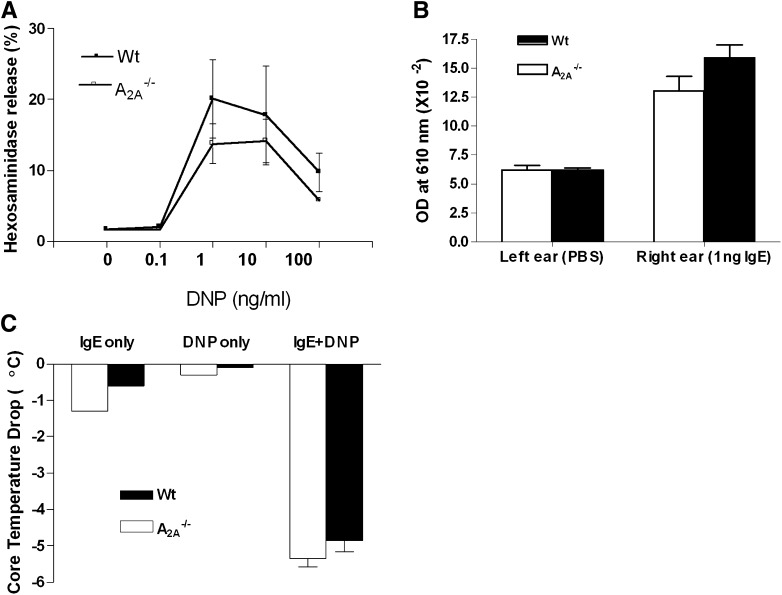
A2A receptors have long been implicated as the major adenosine receptor subtype eliciting inhibitory effects on leukocytes, including macrophages and lymphocytes. Surprisingly, based on the above experiments, activation of A2A receptors had no modulatory effect on mast cell degranulation. To further examine the biologic role of A2A receptors on mast cells, we investigated the impact of genetic deletion of A2A receptors on antigen-induced mast cell degranulation both in vitro and in vivo. BMMCs from wild-type and A2A−/− mice were cultured for 5 weeks, and dose-response studies of antigen-induced mast cell degranulation were conducted. As shown in Fig. 5A, mast cells lacking A2A receptors showed no significant differences in the magnitude of antigen-induced hexosaminidase release compared with wild-type mast cells. To further validate this in vitro observation, we conducted PSA and PCA experiments in mice lacking A2A receptors. Ear plasma protein extravasation as the result of PCA (Fig. 5B) and core temperature drop as a result of PSA (Fig. 5C) were not different between A2A−/− and wild-type mice. Collectively, these data indicate that activation of A2A receptors does not modify antigen-induced mast cell degranulation.
Fig. 5.
In vivo and in vitro evaluation of antigen-induced degranulation in A2A−/− mice. (A) Dose-response curve of hexosaminidase release with DNP-HSA antigen in wild-type and A2A−/− BMMCs. P > 0.05 between wild-type and A2A−/− cells by repeated-measures analysis of variance. Data are from BMMCs from three mice of each genotype, and are presented as mean percentage hexosaminidase release ± S.E.M. (B) Passive cutaneous anaphylaxis in wild-type and A2A−/− mice. P > 0.05 by the Student’s t test (n = 5–6 per group). (C) Systemic anaphylaxis in wild-type and A2A−/− mice. Data represent mean temperature drop 20 minutes after DNP antigen ± S.E.M. P > 0.05 by the Student’s t test (n = 5–7 per group).
NECA Inhibits Antigen-Induced IL-6 Synthesis in BMMCs through A2A and A2B Receptors.
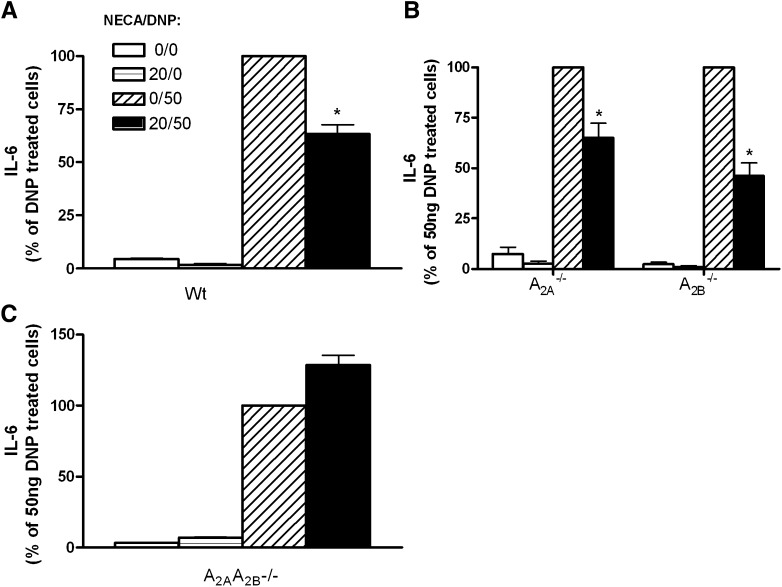
In addition to degranulation, activated mast cells can also synthesize proinflammatory cytokines. To investigate the effect of activation of Gs-coupled adenosine receptors on antigen-induced cytokine synthesis, we treated cultured BMMCs with NECA chronically as described above, and the effects of this treatment on antigen-induced IL-6 synthesis were analyzed. Passively sensitized wild-type BMMCs were incubated with 20 μM NECA. Twenty hours later, cells were treated with DNP for 6 hours and IL-6 levels in the supernatant were measured. Chronic incubation with NECA did not cause significant production of IL-6. In cells chronically pretreated with NECA (20 hours), DNP antigen challenge could still elicit synthesis of IL-6; however, the production of IL-6 in these cells was significantly reduced (27% lower than the vehicle/DNP group, n = 14–15 per group; P < 0.0001) (Fig. 6A). These data indicate that chronic treatment with NECA can inhibit antigen-induced cytokine synthesis in BMMCs. To determine the adenosine receptor subtype(s) mediating this inhibitory effect on cytokine synthesis, both A2A−/− and A2B−/− BMMCs were cultured and chronically treated with NECA prior to DNP antigen challenge, and the capacity of NECA to inhibit antigen-induced IL-6 synthesis was examined. Surprisingly, the deletion of neither A2A nor A2B receptors influenced the inhibitory effects of chronic NECA treatment on IL-6 synthesis (Fig. 6B). These data further substantiate the inhibitory effects of chronic NECA treatment on antigen-induced cytokine synthesis in BMMCs, and also suggest that the inhibition of IL-6 synthesis by NECA is not mediated by either the A2A or A2B receptor. To investigate these observations further, we next cultured BMMCs lacking both A2A and A2B receptors and tested the chronic inhibitory effect of NECA on antigen-induced IL-6 synthesis. As shown in Fig. 6C, the deletion of both A2A and A2B genes completely abolished the chronic inhibitory effect of NECA on IL-6 synthesis in these mast cells. Collectively, these data indicate that activation of Gs-coupled adenosine receptors can also inhibit antigen-induced cytokine synthesis in BMMCs. In contrast to mast cell degranulation in which the inhibitory effect of chronic NECA was only mediated by the A2B receptor, NECA-induced inhibition on cytokine synthesis was cooperatively mediated by both A2A and A2B adenosine receptors.
Fig. 6.
Effect of NECA on antigen-induced IL-6 synthesis. Cultured wild-type (A), A2A−/−, A2B−/− (B), and A2A−/−A2B−/− (C) mast cells were passively sensitized with murine IgE. The cells were then incubated with NECA (20 μM) chronically (20 hours) prior to the addition of antigen. Controls were treated with aliquot vehicle. The IL-6 levels in the supernatants from these cells were then measured 6 hours after the addition of antigen. *P < 0.05 between NECA/DNP- and vehicle/DNP-treated groups by the Student’s t test. Data represent 15 different lines of cells in (A) and 3–4 different lines of cells in both (B) and (C), and are presented as the mean percentage of the IL-6 levels in antigen (DNP)-treated cells ± S.E.M.
Effect of NECA on Antigen-Induced Lipid Mediator Release from BMMCs.
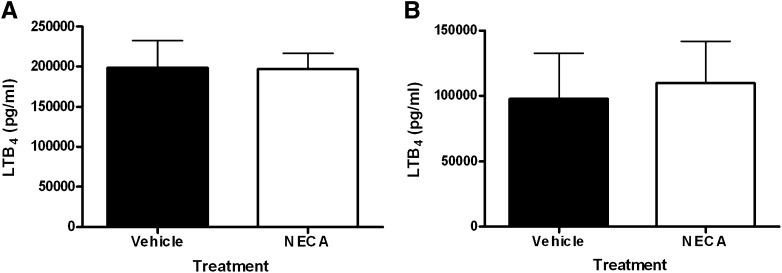
In addition to degranulation and cytokine synthesis, activated mast cells can also release arachidonic acid–derived lipid mediators. To determine whether activation of Gs-coupled adenosine receptors could modify antigen-induced lipid mediator release from mast cells, we measured LTB4 levels in the supernatants of immunologically activated mast cells that were incubated acutely (20 minutes) or chronically (20 hours) with NECA prior to DNP antigen challenge. Unlike the potentiating effect of acute NECA on antigen-induced mast cell degranulation, antigen-induced lipid mediator release was not affected when DNP-stimulated cells were pretreated acutely with NECA (Fig. 7A). Also in contrast to the effects of chronic NECA on degranulation and cytokine synthesis, DNP antigen–induced LTB4 production was no different between mast cells incubated chronically (20 hours) with vehicle or NECA. However, we did observe a 50% decrease DNP-triggered LTB4 release by mast cells incubated for 20 hours with DMSO vehicle (approximately 100,000 pg/ml; Fig. 7A) compared with mast cells incubated for 20 minutes with DMSO vehicle (approximately 200,000 pg/ml; Fig. 7B). Thus, we cannot rule out the possibility that chronic NECA might inhibit DNP-induced LTB4 release and that such an inhibitory effect could not be observed due to potential effects of the DMSO vehicle.
Fig. 7.
Effect of NECA on antigen-induced LTB4 synthesis by BMMCs. Mast cells were sensitized with murine anti-DNP IgE and incubated chronically (20 hours) (A) or acutely (20 minutes) (B) with 20 μM NECA, and LTB4 synthesis was induced with DNP-HSA antigen. LTB4 release in supernatants was measured 6 hours later by enzyme-linked immunosorbent assay. Data for both (A) and (B) were from six different lines of cells, and are presented as mean LTB4 levels ± S.E.M.
Discussion
Previous in vitro studies have shown biphasic effects of adenosine on mast cell activity; however, the receptor subtypes that mediate the inhibitory effects of adenosine are still controversial (Peachell et al., 1991; Yip et al., 2009). Mast cells express two distinct Gs-coupled adenosine receptors; their biologic roles have not been comprehensively defined, especially in vivo. Since activation of Gs-coupled adenosine receptors increases intracellular cAMP, we hypothesized that the inhibitory effects of adenosine on mast cells are mediated by the Gs-coupled adenosine receptors. In this study, we used both genetically modified animal models and mast cell cultures to comprehensively investigate the role of Gs-coupled adenosine receptors on mast cells both in vitro and in vivo. First, our data demonstrate a potent inhibitory effect of the nonhydrolyzable adenosine analog NECA on IgE-induced mast cell degranulation; this inhibitory effect of NECA was abolished by the genetic deletion of the A2B but not the A2A receptor. Second, although A2A receptors also couple to Gs, we were unable to demonstrate a role for this receptor subtype in antigen-induced mast cell degranulation in vitro or in vivo. Third, in addition to mast cell degranulation, activation of Gs-coupled adenosine receptors significantly inhibited antigen-induced synthesis of IL-6 in mast cells; this inhibitory effect was mediated by both A2A and A2B receptors. Last, our in vitro studies revealed a time-dependent biphasic effect of adenosine on mast cell activation. Acutely, adenosine enhances mast cell degranulation but chronically, it limits mast cell activation.
Adenosine levels are elevated in bronchoalveolar lavage fluid from asthmatic subjects (Driver et al., 1993). Adenosine inhalation causes immediate bronchoconstriction in asthmatic subjects but not in normal subjects, an effect that can be inhibited by antagonizing adenosine-induced mast cell activation (Cushley et al., 1983; Phillips et al., 1989). Based on these observations, it has been posited that adenosine activates mast cells and contributes to asthma pathogenesis, and that antagonizing the proinflammatory adenosine receptor subtype may benefit asthmatic subjects (Polosa and Blackburn, 2009). The adenosine receptor subtypes that potentially mediate these proinflammatory effects on mast cells include A1, A2B, and A3 receptors as suggested by previous studies (Ali et al., 1994; Feoktistov and Biaggioni, 1995; Salvatore et al., 2000; Feoktistov et al., 2001, 2003; Tilley et al., 2003; Zhong et al., 2004; Chen et al., 2006; Young et al., 2006; Yip et al., 2009; Hua et al., 2011). However, clinical trials have failed to demonstrate the efficacy of antagonizing these three adenosine receptors in asthmatic subjects, suggesting that the biologic roles of adenosine in asthmatic lungs needs to be re-evaluated (Ball et al., 2003; Pascoe, 2007).
In this study, we observed a time-dependent, biphasic effect of adenosine on mast cell activation. Acutely, adenosine enhanced mast cell degranulation; chronically, adenosine limited mast cell activation. Adenosine is produced by the metabolism of ATP released by cells during hypoxia and other pathophysiological conditions resulting in cellular stress. ATP and its metabolites adenosine and uric acid are major danger-associated molecular patterns, the biologic role of which is to help maintain and restore in vivo homeostasis after cellular stress (Willart and Lambrecht, 2009). The time-dependent, biphasic effects that we and others have observed with adenosine may confer this molecule a unique role in maintaining homeostasis after tissue injury. Acutely, adenosine functions as a danger-associated molecular pattern, signaling the in vivo “alarm system” by upregulating the inflammatory response. Chronically, adenosine through preconditioning and inhibition of the inflammatory response, may act to prevent excessive tissue injury and to help eventually restore homeostasis (Fredholm, 2007). Human studies from our center have shown increased adenosine levels in the induced sputum from asthmatic subjects with mild, moderate, and severe persistent asthma. These subjects were stable and were not in the midst of an asthma exacerbation, suggesting that adenosine levels are chronically elevated in the lungs of asthmatic subjects, particularly those with more severe disease. Therefore, in combination with previous studies, this report suggests that the chronically accumulated adenosine in asthmatic lungs may be anti-inflammatory rather than proinflammatory.
Our observations of the inhibitory effects of adenosine on antigen-induced mast cell degranulation in vitro are consistent with several previous reports on human lung fragments, dispersed human lung mast cells and human umbilical cord blood–derived mast cells (HUCBMCs) (Hughes et al., 1984; Peachell et al., 1988, 1991; Suzuki et al., 1998). At least three independent studies have shown that adenosine can inhibit antigen-induced histamine release from human lung fragments and dispersed human lung mast cells (Hughes et al., 1984; Peachell et al., 1988, 1991). Although these investigators showed that the inhibitory effects of adenosine could be blocked by inhibiting cell-surface adenosine receptors, the tools to identify and isolate specific receptors were not available at the time of these investigations. Peachell et al. (1988) found that low concentrations of adenosine potentiated (<1 μM), and high concentrations inhibited (100 μM), antigen-induced degranulation of human lung mast cells. Given the low affinity of the A2B receptor for adenosine, these observations suggest that the A2B receptor might mediate these inhibitory effects on human lung mast cells. Yip et al. (2011) also observed a similar biphasic effect of adenosine on anti-IgE–induced human mast cell activation. By using pharmacological antagonism, this group reported that the potentiating and inhibitory effect of adenosine was mediated by A1 and A2B receptors, respectively. In contrast, using in vitro cultured HUCBMCs, Suzuki et al. (1998) found that IgE crosslink-induced mast cell degranulation could be inhibited by the A2A receptor agonist 2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS-21680), and that this inhibition could be effectively prevented by 4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385), a potent A2A receptor antagonist, suggesting a role for the A2A receptor in adenosine-mediated inhibition of degranulation. In addition, Rork et al. (2008) found that activation of A2A receptors can limit mast cell degranulation in the murine heart, decreasing reperfusion injury. A study using cheek pouch of the golden hamster also revealed an inhibitory effect of activation of A2A receptors on inosine-induced periarteriolar mast cell degranulation (Fenster et al., 2000). In contrast, our mouse studies both in vitro and in vivo do not support a role for A2A receptors in antigen-induced mast cell degranulation.
Mast cells are a highly heterogeneous cell type. They originate in bone marrow but mature in peripheral organs where they acquire different phenotypic properties after differentiation in the local milieu, which may differ between tissues. It is possible that the environment in which the mast cell resides influences adenosine receptor expression and function, accounting for the differences in receptor effects observed between these studies. Although several studies have suggested that A2A receptors mediate anti-inflammatory effects (Antonioli et al., 2010; Fenster et al., 2000; Harada et al., 2000; Kreckler et al., 2006; Rork et al., 2008; Trevethick et al., 2008), a clinical trial failed to demonstrate efficacy of an A2A agonist in attenuating allergen-induced early and late reactions, sputum total cell counts and EG2+ cell numbers, eosinophil cationic protein levels, or inflammatory cytokine production in asthmatic subjects (Luijk et al., 2008).
Investigations on the biologic role of the Gs-coupled A2B receptor have yielded conflicting results. Studies on HMC-1 cells have revealed a proinflammatory role for A2B receptors (Feoktistov and Biaggioni, 1995; Meade et al., 2002; Ryzhov et al., 2004, 2006). Similarly, our study using HUCBMCs showed that IgE-crosslinking-induced mast cell activation was augmented by A2B receptor activation, further suggesting a proinflammatory role for the A2B receptor on mast cells in vitro (Hua et al., 2011). However, these in vitro observations have not held up in vivo. For example, in a placebo-controlled, double-blinded, randomized, two-way crossover phase Ib clinical trial, the compound QAF805, which antagonizes both A2B and A3 receptors, failed to demonstrate efficacy in attenuating AMP-induced bronchial hyper-responsiveness in asthmatic subjects (Pascoe, 2007).
Previous studies by our group and others have ascribed an anti-inflammatory phenotype to the A2B receptor. We previously reported that murine mast cells lacking A2B receptors exhibited an exaggerated response to antigen (Hua et al., 2007), suggesting an anti-inflammatory role for A2B receptors in mice. In addition, activation of A2B receptors have been reported to reduce the severity of inflammation in lipopolysaccharide-induced murine macrophage activation, murine sepsis, and a mouse model of ventilation-induced lung injury (Kreckler et al., 2006; Yang et al., 2006; Eckle et al., 2008; Csóka et al., 2010). In this study, we also found that the chronic inhibitory effect of adenosine on mast cell activation was abolished by genetic deletion of the A2B receptors both in vivo and in vitro, further supporting an anti-inflammatory role for A2B receptors on mast cells.
In addition to inhibiting degranulation, we also found that chronic stimulation of Gs-coupled adenosine receptors by NECA could inhibit antigen-induced cytokine synthesis. Surprisingly, we were unable to attribute this inhibition to a single adenosine receptor subtype. Mast cells lacking either A2A or A2B receptors responded similarly to wild-type cells, suggesting that the presence of an A2A-Gs or A2B-Gs signal was sufficient to mediate the inhibitory effects of NECA. Interestingly, activation of both Gs-coupled receptors on wild-type mast cells was no more effective than activation of either receptor in isolation on mast cells from A2A- or A2B-deficient mice. When mast cells devoid of both Gs-coupled receptors were examined, the inhibitory effect of NECA on antigen-induced IL-6 synthesis was abolished.
The capacity of adenosine to potently regulate inflammation has made the G-protein– coupled adenosine receptors attractive pharmacological targets. Our findings of an inhibitory role for the A2B receptor, both constitutively (Hua et al., 2007) and when chronically stimulated, may have implications for A2B antagonists under development for the treatment of asthma. A more detailed understanding of the mechanisms by which adenosine regulates the immune response in a biphasic fashion will be critical to the successful introduction of adenosine receptor ligands for the treatment of asthma and other inflammatory diseases.
Acknowledgments
The authors thank the Keck family for generous support of the Keck Animal Models Facility at the University of North Carolina, Yvonne Brooks for assistance with mouse genotyping, and Warren Naselsky for critical review of the manuscript.
Abbreviations
- BMMC
bone marrow-derived mast cell
- CGS-21680
2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine
- DMSO
dimethyl sulfoxide
- DNP-HSA
dinitrophenyl-human serum albumin
- HUCBMC
human umbilical cord blood-derived mast cell
- IL
interleukin
- LTB4
leukotriene B4
- NECA
5′-N-ethylcarboxamidoadenosine
- PBS
phosphate-buffered saline
- PCA
passive cutaneous anaphylaxis
- PSA
passive systemic anaphylaxis
- ZM 241385
4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
Author Contributions
Participated in research design: Hua, Tilley.
Conducted experiments: Hua, Chason, Jania, Acosta, Tilley.
Contributed new reagents or analytic tools: Ledent.
Performed data analysis: Hua, Chason, Jania, Tilley.
Wrote or contributed to the writing of the manuscript: Hua, Tilley.
Footnotes
This work was supported by the National Institutes of Health [Grants HL071802 and AI096139]; the American Academy of Otolaryngology–Head and Neck Surgery/American Academy of Otolaryngic Allergy [Grant 92997]; and the North Carolina Translational and Clinical Sciences Institute [Grant R20926].
References
- Ali S, Mustafa SJ, Metzger WJ. (1994) Adenosine receptor-mediated bronchoconstriction and bronchial hyperresponsiveness in allergic rabbit model. Am J Physiol 266:L271–L277 [DOI] [PubMed] [Google Scholar]
- Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Da Settimo F, La Motta C, Natale G, Duranti E, et al. (2010) The blockade of adenosine deaminase ameliorates chronic experimental colitis through the recruitment of adenosine A2A and A3 receptors. J Pharmacol Exp Ther 335:434–442 [DOI] [PubMed] [Google Scholar]
- Ball HA, Sandrasagra A, Tang L, Van Scott M, Wild J, Nyce JW. (2003) Clinical potential of respirable antisense oligonucleotides (RASONs) in asthma. Am J Pharmacogenomics 3:97–106 [DOI] [PubMed] [Google Scholar]
- Boulet LP, Chapman KR, Côté J, Kalra S, Bhagat R, Swystun VA, Laviolette M, Cleland LD, Deschesnes F, Su JQet al. (1997) Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med 155:1835–1840 [DOI] [PubMed] [Google Scholar]
- Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. (1994) Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol 10:471–480 [DOI] [PubMed] [Google Scholar]
- Bradding P, Walls AF, Holgate ST. (2006) The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol 117:1277–1284 [DOI] [PubMed] [Google Scholar]
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. (2002) Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 346:1699–1705 [DOI] [PubMed] [Google Scholar]
- Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. (2003) Interleukin-4 and -13 expression is co-localized to mast cells within the airway smooth muscle in asthma. Clin Exp Allergy 33:1711–1716 [DOI] [PubMed] [Google Scholar]
- Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. (2001) Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 108:184–190 [DOI] [PubMed] [Google Scholar]
- Carter RJ, Bradding P. (2011) The role of mast cells in the structural alterations of the airways as a potential mechanism in the pathogenesis of severe asthma. Curr Pharm Des 17:685–698 [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795 [DOI] [PubMed] [Google Scholar]
- Chong LK, Suvarna K, Chess-Williams R, Peachell PT. (2003) Desensitization of β2-adrenoceptor-mediated responses by short-acting β2-adrenoceptor agonists in human lung mast cells. Br J Pharmacol 138:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MK, Hiroi J. (1987) Inhibition of IgE-dependent histamine release from human dispersed lung mast cells by anti-allergic drugs and salbutamol. Br J Pharmacol 90:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csóka B, Németh ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscsó B, Himer L, Vizi ES, et al. (2010) A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol 185:542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushley MJ, Tattersfield AE, Holgate ST. (1983) Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol 15:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Ali S, Mustafa SJ. (1993) Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 148:91–97 [DOI] [PubMed] [Google Scholar]
- Eckle T, Grenz A, Laucher S, Eltzschig HK. (2008) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118:3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, Fick RB, Jr, Boushey HA. (1997) The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med 155:1828–1834 [DOI] [PubMed] [Google Scholar]
- Fenster MS, Shepherd RK, Linden J, Duling BR. (2000) Activation of adenosine A2 alpha receptors inhibits mast cell degranulation and mast cell-dependent vasoconstriction. Microcirculation 7:129–135 [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. (1995) Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest 96:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Garland EM, Goldstein AE, Zeng D, Belardinelli L, Wells JN, Biaggioni I. (2001) Inhibition of human mast cell activation with the novel selective adenosine A(2B) receptor antagonist 3-isobutyl-8-pyrrolidinoxanthine (IPDX)(2). Biochem Pharmacol 62:1163–1173 [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. (2003) Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res 92:485–492 [DOI] [PubMed] [Google Scholar]
- Fredholm BB. (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14:1315–1323 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. (2000) Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol 362:364–374 [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. (2005) Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23:749–786 [DOI] [PubMed] [Google Scholar]
- Harada N, Okajima K, Murakami K, Usune S, Sato C, Ohshima K, Katsuragi T. (2000) Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J Pharmacol Exp Ther 294:1034–1042 [PubMed] [Google Scholar]
- Hershfield MS. (2005) New insights into adenosine-receptor-mediated immunosuppression and the role of adenosine in causing the immunodeficiency associated with adenosine deaminase deficiency. Eur J Immunol 35:25–30 [DOI] [PubMed] [Google Scholar]
- Hua X, Chason KD, Fredholm BB, Deshpande DA, Penn RB, and Tilley SL (2008) Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J Allergy Clin Immunol 122:107–113, 113.e101–107. [DOI] [PMC free article] [PubMed]
- Hua X, Chason KD, Patel JY, Naselsky WC, Tilley SL. (2011) IL-4 amplifies the pro-inflammatory effect of adenosine in human mast cells by changing expression levels of adenosine receptors. PLoS ONE 6:e24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. (2007) Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med 204:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PJ, Holgate ST, Church MK. (1984) Adenosine inhibits and potentiates IgE-dependent histamine release from human lung mast cells by an A2-purinoceptor mediated mechanism. Biochem Pharmacol 33:3847–3852 [DOI] [PubMed] [Google Scholar]
- James A, Gyllfors P, Henriksson E, Dahlén SE, Adner M, Nilsson G, Dahlén B. (2012) Corticosteroid treatment selectively decreases mast cells in the smooth muscle and epithelium of asthmatic bronchi. Allergy 67:958–961 [DOI] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. (2006) Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther 317:172–180 [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388:674–678 [DOI] [PubMed] [Google Scholar]
- Luijk B, van den Berge M, Kerstjens HA, Postma DS, Cass L, Sabin A, Lammers JW. (2008) Effect of an inhaled adenosine A2A agonist on the allergen-induced late asthmatic response. Allergy 63:75–80 [DOI] [PubMed] [Google Scholar]
- Marquardt DL, Walker LL, Heinemann S. (1994) Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol 152:4508–4515 [PubMed] [Google Scholar]
- Meade CJ, Worrall L, Hayes D, Protin U. (2002) Induction of interleukin 8 release from the HMC-1 mast cell line: synergy between stem cell factor and activators of the adenosine A(2b) receptor. Biochem Pharmacol 64:317–325 [DOI] [PubMed] [Google Scholar]
- Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, Coffman TM, Dombrowicz D, Koller BH. (2002) Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol 169:4586–4593 [DOI] [PubMed] [Google Scholar]
- Okayama Y, Ra C, Saito H. (2007) Role of mast cells in airway remodeling. Curr Opin Immunol 19:687–693 [DOI] [PubMed] [Google Scholar]
- Olah ME. (1997) Identification of A2a adenosine receptor domains involved in selective coupling to Gs. Analysis of chimeric A1/A2a adenosine receptors. J Biol Chem 272:337–344 [DOI] [PubMed] [Google Scholar]
- Oldenburg PJ, Mustafa SJ. (2005) Involvement of mast cells in adenosine-mediated bronchoconstriction and inflammation in an allergic mouse model. J Pharmacol Exp Ther 313:319–324 [DOI] [PubMed] [Google Scholar]
- Pascoe SJ, Knight H, Woessner R (2007) QAF805, an a2b/A3 adenosine receptor antagonist, does not attenuate AMP challenge in subjects with asthma (Abstract). Am J Resp Crit Care Med 175:A682.
- Peachell P. (2005) Targeting the mast cell in asthma. Curr Opin Pharmacol 5:251–256 [DOI] [PubMed] [Google Scholar]
- Peachell PT, Columbo M, Kagey-Sobotka A, Lichtenstein LM, Marone G. (1988) Adenosine potentiates mediator release from human lung mast cells. Am Rev Respir Dis 138:1143–1151 [DOI] [PubMed] [Google Scholar]
- Peachell PT, Lichtenstein LM, Schleimer RP. (1991) Differential regulation of human basophil and lung mast cell function by adenosine. J Pharmacol Exp Ther 256:717–726 [PubMed] [Google Scholar]
- Phillips GD, Scott VL, Richards R, Holgate ST. (1989) Effect of nedocromil sodium and sodium cromoglycate against bronchoconstriction induced by inhaled adenosine 5′-monophosphate. Eur Respir J 2:210–217 [PubMed] [Google Scholar]
- Pierce KD, Furlong TJ, Selbie LA, Shine J. (1992) Molecular cloning and expression of an adenosine A2b receptor from human brain. Biochem Biophys Res Commun 187:86–93 [DOI] [PubMed] [Google Scholar]
- Polosa R, Blackburn MR. (2009) Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol Sci 30:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo GJ, Neffen H, Castro-Rodriguez JA. (2011) Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 139:28–35 [DOI] [PubMed] [Google Scholar]
- Rork TH, Wallace KL, Kennedy DP, Marshall MA, Lankford AR, Linden J. (2008) Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am J Physiol Heart Circ Physiol 295:H1825–H1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I. (2006) Cross-talk between G(s)- and G(q)-coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol 70:727–735 [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. (2004) Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol 172:7726–7733 [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. (2000) Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 275:4429–4434 [DOI] [PubMed] [Google Scholar]
- Scola A-M, Chong LK, Suvarna SK, Chess-Williams R, Peachell PT. (2004) Desensitisation of mast cell β2-adrenoceptor-mediated responses by salmeterol and formoterol. Br J Pharmacol 141:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takei M, Nakahata T, Fukamachi H. (1998) Inhibitory effect of adenosine on degranulation of human cultured mast cells upon cross-linking of Fc epsilon RI. Biochem Biophys Res Commun 242:697–702 [DOI] [PubMed] [Google Scholar]
- Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, Koller BH. (2003) Identification of A3 receptor- and mast cell-dependent and -independent components of adenosine-mediated airway responsiveness in mice. J Immunol 171:331–337 [DOI] [PubMed] [Google Scholar]
- Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. (2000) Adenosine and inosine increase cutaneous vasopermeability by activating A(3) receptors on mast cells. J Clin Invest 105:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy TJ. (1998) Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 157:351–370 [DOI] [PubMed] [Google Scholar]
- Trevethick MA, Mantell SJ, Stuart EF, Barnard A, Wright KN, Yeadon M. (2008) Treating lung inflammation with agonists of the adenosine A2A receptor: promises, problems and potential solutions. Br J Pharmacol 155:463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Peachell PT. (1998) Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol 31:715–719 [DOI] [PubMed] [Google Scholar]
- Willart MAM, Lambrecht BN. (2009) The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy 39:12–19 [DOI] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli Pet al. (2006) The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KH, Wong LL, Lau HY. (2009) Adenosine: roles of different receptor subtypes in mediating histamine release from human and rodent mast cells. Inflamm Res 58 (Suppl 1):17–19 [DOI] [PubMed] [Google Scholar]
- Yip KH, Lau HY, Wise H. (2011) Reciprocal modulation of anti-IgE induced histamine release from human mast cells by A₁ and A(2B) adenosine receptors. Br J Pharmacol 164 (2b):807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. (2006) A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol 35:549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. (2006) Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest 116:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D. (2004) A(2B) adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol 30:118–125 [DOI] [PubMed] [Google Scholar]
- Zhong H, Chunn JL, Volmer JB, Fozard JR, Blackburn MR. (2001) Adenosine-mediated mast cell degranulation in adenosine deaminase-deficient mice. J Pharmacol Exp Ther 298:433–440 [PubMed] [Google Scholar]
- Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. (2003) Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol 171:338–345 [DOI] [PubMed] [Google Scholar]