Abstract
The neurotoxicity of (±)-3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”) is influenced by temperature and varies according to species. The mechanisms underlying these two features of MDMA neurotoxicity are unknown, but differences in MDMA metabolism have recently been implicated in both. The present study was designed to 1) assess the effect of hypothermia on MDMA metabolism, 2) determine whether the neuroprotective effect of hypothermia is related to inhibition of MDMA metabolism, and 3) determine if different neurotoxicity profiles in mice and rats are related to differences in MDMA metabolism and/or disposition in the two species. Rats and mice received single neurotoxic oral doses of MDMA at 25°C and 4°C, and body temperature, pharmacokinetic parameters, and serotonergic and dopaminergic neuronal markers were measured. Hypothermia did not alter MDMA metabolism in rats and only modestly inhibited MDMA metabolism in mice; however, it afforded complete neuroprotection in both species. Rats and mice metabolized MDMA in a similar pattern, with 3,4-methylenedioxyamphetamine being the major metabolite, followed by 4-hydroxy-3-methoxymethamphetamine and 3,4-dihydroxymethamphetamine, respectively. Differences between MDMA pharmacokinetics in rats and mice, including faster elimination in mice, did not account for the different profile of MDMA neurotoxicity in the two species. Taken together, the results of these studies indicate that inhibition of MDMA metabolism is not responsible for the neuroprotective effect of hypothermia in rodents, and that different neurotoxicity profiles in rats and mice are not readily explained by differences in MDMA metabolism or disposition.
Introduction
Over the last two decades, a large body of data has accrued indicating that the recreational drug (±)-3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”) has neurotoxic potential toward brain monoamine-containing neurons (Steele et al., 1994; Green et al., 2003; Capela et al., 2009; Sarkar and Schmued, 2010). In particular, animals treated with MDMA develop long-lasting depletions of various presynaptic serotonin (5-HT) and/or dopamine (DA) neuronal markers, including 5-HT and DA, their major metabolites [5-hydroxyindoleacetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC)], their rate-limiting biosynthetic enzymes (tryptophan hydroxylase and tyrosine hydroxylase), and their membrane reuptake sites [the 5-HT transporter (SERT) and the DA transporter (DAT)] (Sarkar and Schmued, 2010). Morphologic studies indicate that the loss of presynaptic 5-HT and DA neuronal markers after MDMA exposure is related to axon terminal injury (Commins et al., 1987; O'Hearn et al., 1988), with no lasting effect on serotonergic or dopaminergic nerve cells bodies.
Although the mechanisms underlying MDMA neurotoxicity remain unclear, two factors are firmly established. First, body temperature can markedly influence MDMA neurotoxicity, with high body temperature typically enhancing neurotoxicity and low body temperature generally affording neuroprotection (Broening et al., 1995; Malberg and Seiden, 1998). Second, DAT and SERT play a key role in MDMA neurotoxicity. Evidence for the essential role of transporters in MDMA neurotoxicity comes from studies demonstrating that either pharmacological or genetic alterations of SERT and/or DAT interfere with the development of MDMA-induced monoaminergic neurotoxicity (McCann and Ricaurte, 2004).
Notably, the profile of MDMA neurotoxicity varies according to species. In mice, DA neurons are selectively damaged (O'Callaghan and Miller, 1994), whereas in rats and most other species examined to date (including nonhuman primates), 5-HT neurons are typically selectively affected (Steele et al., 1994; Green et al., 2003). The basis for the different profile of MDMA neurotoxicity in different species is unknown, but it has recently been stated that differences in MDMA disposition and metabolism play a key role (Green et al., 2012).
The mechanism by which temperature influences the expression of MDMA neurotoxicity is not fully understood. However, based on in vitro findings, it has been suggested that temperature modulates substituted amphetamine neurotoxicity by altering transporter function (Xie et al., 2000). More recently, others have proposed that temperature modulates MDMA neurotoxicity by altering MDMA metabolism, with low temperatures decreasing the production of toxic MDMA metabolites (Goni-Allo et al., 2008).
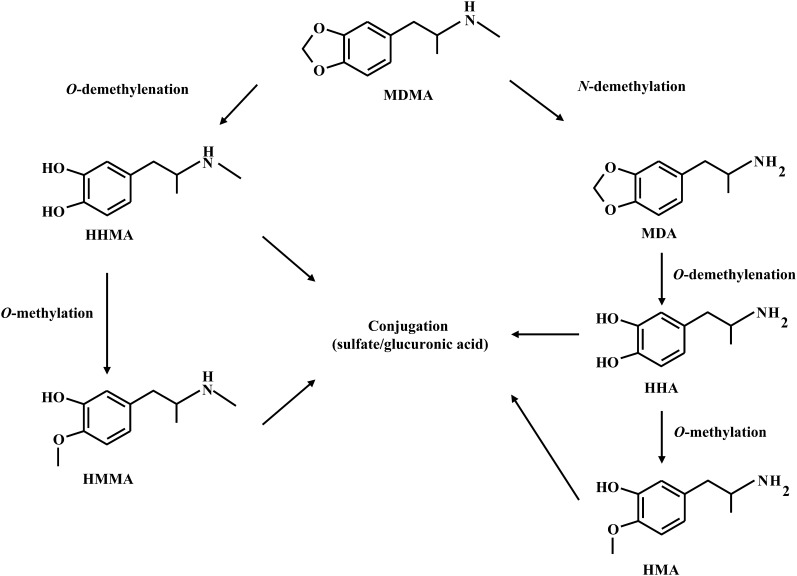
MDMA is metabolized through two different pathways (de la Torre et al., 2004; Meyer et al., 2008) (Fig. 1). The first involves MDMA O-demethylenation to 3,4-dihydroxymethamphetamine (HHMA), which is then O-methylated to 4-hydroxy-3-methoxymethamphetamine (HMMA). Both HHMA and HMMA are subsequently O-conjugated with sulfate or glucuronic acid. The second pathway of MDMA metabolism involves N-demethylation to 3,4-dihydroxyamphetamine (MDA). Like MDMA, MDA undergoes O-demethylenation to 3,4-dihydroxyamphetamine (HHA), which, in turn, is O-methylated to 4-hydroxy-3-methoxyamphetamine (Fig. 1). Notably, HHMA and HMMA can also undergo N-demethylation, resulting in the formation of HHA and 4-hydroxy-3-methoxyamphetamine, respectively. The catechol metabolites of MDMA and MDA (HHMA and HHA) can be further oxidized to corresponding quinones, which can then form adducts with glutathione and other thiol-containing compounds and have been implicated in MDMA neurotoxicity (Hiramatsu et al., 1990; Monks et al., 2004; Perfetti et al., 2009). Metabolism of MDMA along these pathways proceeds at different rates in different species (Meyer et al., 2008).
Fig. 1.
Metabolic pathways of MDMA, along with the associated microsomal enzymes.
The present studies were designed to test two hypotheses: 1) temperature-induced alterations in MDMA metabolism account for the neuroprotective effect of hypothermia, and 2) differences in drug metabolism underlie the different neurotoxic profiles of MDMA in mice and rats.
Materials and Methods
Animals.
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–224 g and male albino Swiss-Webster mice (Taconic Farms Inc., Germantown, NY) weighing 20–22 g were used. When not undergoing drug treatment, rats were housed three per cage in standard polypropylene cages (17 × 10 × 8 inches) at an ambient temperature of 22 ± 2°C. Similarly, when not undergoing treatment, mice were housed five per cage in clear acrylic cages, also at an ambient temperature of 22 ± 2°C. During treatment, animals were housed singly, and ambient temperatures were either 4°C or 25°C. All animals had free access to food and water and were maintained on a 12-hour light/dark cycle. The facilities for housing and care of the animals are accredited by the American Association for the Assessment and Accreditation of Laboratory Animal Care. Animal care and experimental manipulations were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs and Reagents.
Racemic MDMA HCl was obtained from the National Institute on Drug Abuse (Rockville, MD). Racemic HHMA HCl and methanolic solutions (1000 mg/l) of racemic MDMA HCl and racemic MDA HCl were purchased from Lipomed (Cambridge, MA). Methanolic solutions (1000 mg/l) of racemic HMMA and methanolic solutions (100 mg/l) of racemic MDMA-d5 and MDA-d5 were obtained from Cerilliant (Round Rock, TX). 4-Hydroxy-methamphetamine (pholedrine), 4-methylcatechol, EDTA disodium salt dihydrate, and glucuronidase type HP-2 from helix pomatia (glucuronidase activity ≥100.000 units/ml and sulfatase activity <7.500 units/ml) were obtained from Sigma-Aldrich (Saint Louis, MO). Sodium metabisulfite was obtained from E. Merck (Darmstadt, Germany). Perchloric acid was obtained from J.T. Baker (Phillipsburg, NJ). The authenticity of MDMA, HHMA, HMMA, and MDA samples used in the present studies was confirmed using liquid chromatographic–mass spectrometric methods to determine the corresponding pseudomolecular ions and at least one fragment ion for each compound. Analysis was performed in full scan (mass range, 100–1000) to check for presence of possible impurities.
Drug Treatment.
Rats were treated orally (by gavage) with a single dose of 20 mg/kg MDMA (or vehicle) at an ambient temperature of either 25°C or 4°C. The oral route was selected because MDMA is typically taken by mouth (Capela et al., 2009) and previous results indicate that MDMA has excellent oral bioavailability relative to the i.p. route, ranging from 65% to 75% depending on the dose administered [namely, 10 or 20 mg/kg, respectively (Baumann et al., 2009; Mueller et al., 2009a, 2011a)]. A single dose (rather than multiple doses) was used to simplify pharmacokinetic analyses; the 20-mg/kg dose was selected because it has previously been shown to produce neurotoxic effects in rats (Mueller et al., 2009a, 2011a). Except where indicated (see below), mice received a single oral dose of 60 mg/kg of MDMA by gavage, at either 25°C or 4°C. This dose was selected because pilot studies showed that the 60-mg/kg dose reliably produces MDMA neurotoxicity in mice and is generally well tolerated. All animals (rats and mice) remained at the experimental temperature for a total of 40 hours (16 hours prior to treatment and 24 hours after treatment), so that exposure to drug took place while body temperature was altered by ambient temperature. In a second experiment designed to determine whether the ratio of MDMA to metabolites in mice was dose-related, different groups of mice were treated with single oral doses of 20 or 40 mg/kg of MDMA at 25°C (again, with animals remaining at the experimental temperature for 24 hours after treatment). In a third experiment that tested the effect of prolonged exposure to drug and metabolites on the neurotoxic profile of MDMA in mice, mice were administered six subcutaneous doses of 15 mg/kg of MDMA at 4-hour intervals. During treatment sessions, animals had free access to food and water at all times. Previous studies in our laboratory testing the effects of fasting on MDMA blood levels revealed no differences with regard to MDMA pharmacokinetics in fasted animals compared with those with free access to food and water (unpublished data).
Influence of Hypothermia on MDMA Pharmacokinetics.
For evaluation of MDMA disposition and metabolism, blood was collected at various times after MDMA administration using a repeated measures within-subject crossover design. This was done in both rats and mice at 25°C and 4°C. A repeated measures within-subject crossover design was used to minimize interanimal differences between treatment groups at the different temperatures tested. Rats (n = 9) were divided into two groups (n = 4 or 5 per group); one group was given 20 mg/kg MDMA orally by gavage at 25°C, and the second group was given the same dose at 4°C. Approximately 0.2 ml of blood was taken at 1, 3, 6, 8, and 24 hours after MDMA administration by the retro-orbital method. One week later, rats that were previously treated at 4°C were crossed over to receive 20 mg/kg MDMA orally at 25°C, and vice versa. As before, blood was collected at 1, 3, 6, 8, and 24 hours after MDMA administration. Similarly, mice (n = 9) were divided into two groups (n = 4 or 5 per group); one group was given 60 mg/kg MDMA orally by gavage at 25°C, and the second group was treated with the same dose at 4°C. Approximately 0.04 ml of blood was taken by retro-orbital means at 1, 2, 4, and 8 hours after MDMA administration. One week later, mice that were previously treated at 25°C were crossed over to receive 60 mg/kg MDMA orally at 4°C, and vice versa. Again, blood was collected at 1, 2, 4, and 8 hours after MDMA administration for determination of pharmacokinetic parameters. To facilitate blood collection, animals of both species were briefly anesthetized with isoflurane. Plasma samples from both species were processed as previously described (Mueller et al., 2007, 2011a).
Calculation of Pharmacokinetic Parameters.
Peak plasma concentration (Cmax), time of peak plasma concentration (Tmax), area under the concentration-time curve (AUC), and half-life were obtained using the pharmacokinetic functions for Microsoft Excel [developed by Usansky et al., (2012) http://www.boomer.org/pkin/xcel/pkf/pkf.doc]. AUC and area under the temperature-time curve (TAUC) were calculated using the linear trapezoidal rule starting at time 0 and finishing at the last quantifiable point.
Effect of Dose on MDMA/Metabolite Ratio.
To determine if the ratio of MDMA to metabolites in mice varies as a function of dose, separate groups of mice were treated with 20- or 40-mg/kg oral dosages of MDMA at 25°C, and pharmacokinetic parameters for MDMA and metabolites were determined as described above. Of note, the 40 mg/kg dose was selected because it is “equivalent” to the 20 mg/kg dose used in the rat. Equivalent dosages in rats and mice were determined using the standard interspecies dose-scaling equation: Drat = Dmouse(Wrat/Wmouse)0.7, where D = dose in milligrams, W = weight of the animal in kilograms, and 0.7 is a commonly used and empirically derived exponent (Mordenti and Chappell, 1989).
Effect of Prolonged Duration of Drug Action on Neurotoxicity in Mice.
To determine if differences in clearance of MDMA and/or metabolites (namely faster drug clearance in mice than in rats) are responsible for the observed species differences in neurotoxicity profiles, the duration of action of MDMA and metabolites in mice was prolonged via administration of repeated doses of MDMA. A group of mice received six doses of 15 mg/kg of MDMA s.c., at 4-hour intervals, for a total dose of 90 mg/kg. Body temperature was measured and blood was collected to determine pharmacokinetics as described above. One week later, brain indole and catechol levels were determined as indicated below. Drug was given at normal temperature (25°).
Core Temperature Measurements.
Core (rectal) temperature was measured using a Bat-12 thermometer coupled to a RET-3 rectal probe (Physitemp, Inc., Clifton, NJ). Baseline temperatures were determined before animals were placed in experimental ambient temperatures (25°C or 4°C). Body temperature was determined immediately before MDMA treatment and prior to each time point at which blood was collected.
Measurement of Plasma MDMA and Metabolite Concentrations.
Plasma MDMA, MDA, HHMA, and HMMA plasma concentrations were determined as previously described (Mueller et al., 2007, 2011a). Briefly, aliquots of rat (100 µl) and mouse (20 µl) plasma samples were preserved with 20 µl of sodium metabisulfite (250 mM) and 10 µl of EDTA (250 mM). After addition of 100 µl of an aqueous solution of the racemic internal standards MDMA-d5, MDA-d5, and pholedrine (1.0 µg/ml, each) and 10 µl of glucuronidase solution, samples were mixed (15 seconds) on a rotary shaker and left at 50°C for 90 minutes to perform conjugate cleavage. After cooling to room temperature, 20 µl of 4-methylcatechol (1 mg/ml) was added, and samples were briefly vortexed prior to the addition of perchloric acid (10 µl) to the samples. The samples were then mixed again on a rotary shaker for 15 seconds to perform protein precipitation. The samples were centrifuged (16,000g for 5 minutes), and 5 µl of the supernatant was injected into the liquid chromatography–mass spectrometry system. MDMA and its metabolites were quantified by comparison of their peak area ratios (analyte versus internal standard) to calibration curves in which the peak area ratios of spiked calibration standards had been plotted versus their concentrations using a weighted (1/x2) second-order calibration model. Total amounts (conjugated and free) of HHMA and HMMA were determined. The procedure employed for cleavage of conjugates in rat plasma has been optimized and found to be reproducible (Mueller et al., 2009b). The linear range for the method used in the present study was 20–1000 ng/ml for HHMA, HMMA, and MDMA and 10–500 ng/ml for MDA. If, after initial plasma analysis, values were found to be above the calibration range, the corresponding plasma samples were diluted in the same way as samples for the determination of the above-calibration-range quality control samples during the method validation procedure (Mueller et al., 2007) and were reanalyzed. Values below the limit of quantification [20 ng/ml (HHMA, HMMA, and MDMA) or 10 ng/ml (MDA)] were assumed to be 0 and treated as such for calculation of pharmacokinetics.
Determination of Brain 5-HT, 5-HIAA, DA, and DOPAC Concentrations.
Samples of striatum were analyzed for their content of 5-HT and 5-HIAA or of DA and DOPAC 1 week after drug treatment as previously described (Mechan et al., 2006). Briefly, frozen tissue samples were homogenized in 0.4 N perchloric acid for 15 seconds using a Brinkmann Polytron Homogenizer (Brinkmann Instruments, Inc., Westbury, NY) at setting 5. Next, the homogenates were centrifuged for 20 minutes at 25,000g and 50 µl of the supernatant was injected into a high-performance liquid chromatography system coupled with an amperometric L-ECD-6A detector (Shimadzu, Columbia, MD). Separation of monoamines and their metabolites was conducted on a reverse-phase C18 column. The mobile phase was 100% aqueous and contained citric acid (125 mM), sodium phosphate (125 mM), EDTA (0.27 mM), and sodium octyl sulfate (0.12 mM) and had a pH of 2.5 ± 3.0. The ECD contained a glassy carbon working electrode, and a silver/silver chloride reference electrode was used. The fixed potential difference between the reference and working electrodes was +0.70 V.
Statistics.
The significance of differences between means was determined using two-tailed unpaired or paired (where appropriate) Student’s t tests or one-way analyses of variance followed by Tukey's multiple comparison tests. Statistical analyses were performed using Prism, version 3.02 (GraphPad Software, Inc., La Jolla, CA). Differences were considered significant if P < 0.05.
Results
Pharmacokinetics.
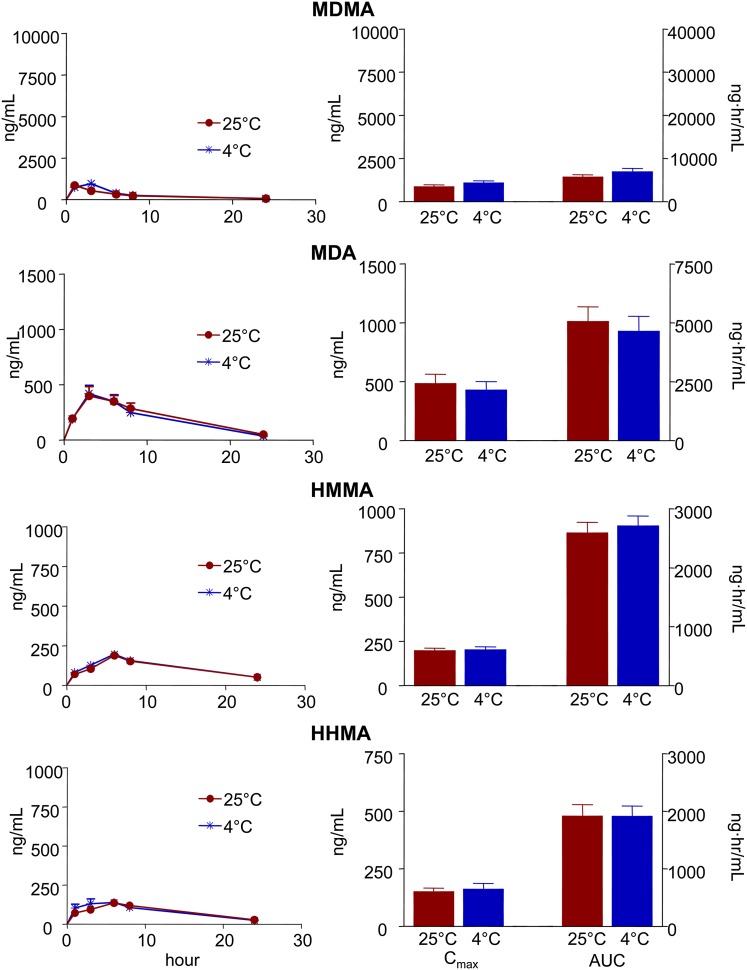
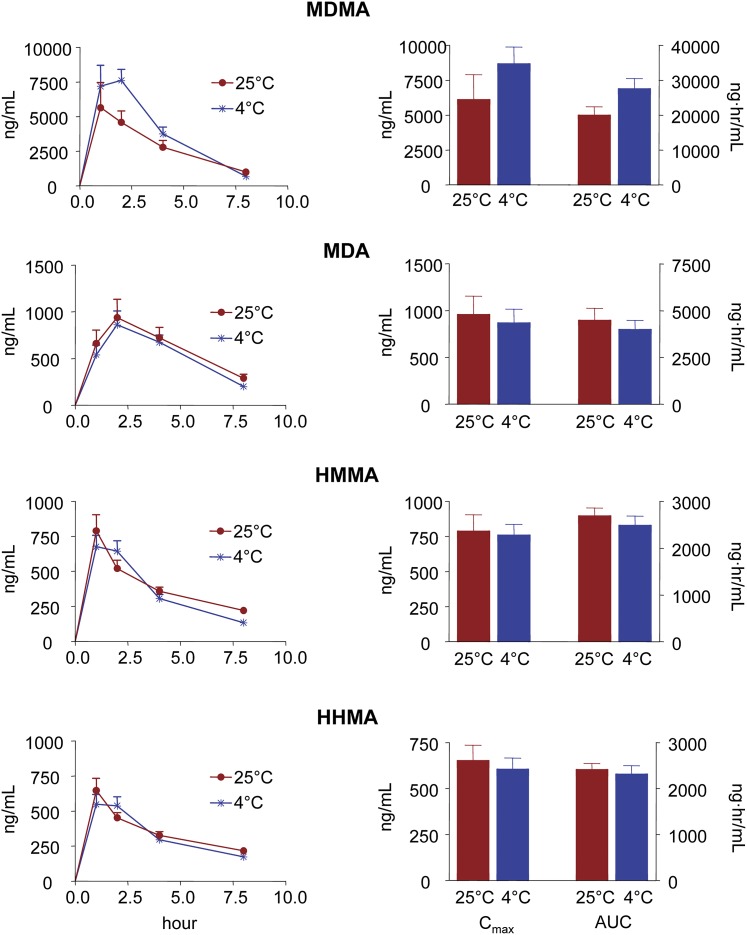
Figures 2 and 3 show plasma time-concentration profiles, along with Cmax and AUC values, for MDMA and each of its metabolites (MDA, HHMA, and HMMA) in rats and mice given single oral neurotoxic doses of MDMA (20 and 60 mg/kg, respectively) at two different ambient temperatures (25°C and 4°C). Comparison of the pharmacokinetic parameters of each of the analytes measured at each temperature failed to reveal a significant effect of hypothermia, except for a delay of Tmax of MDMA in rats and Tmax of HMMA in mice at the lower temperature (Figs. 2 and 3; Tables 1 and 2).
Fig. 2.
Plasma time-concentration profiles and pharmacokinetic parameters of MDMA and its metabolites in rats given single oral doses of MDMA (20 mg/kg) at ambient temperatures of 25°C and 4°C. Concentrations of HMMA and HHMA represent total amounts of free HMMA and HHMA obtained after enzymatic conjugate cleavage. Data represent the mean ± S.E.M. (n = 9). Pharmacokinetic parameters were compared using two-tailed paired Student’s t tests. Differences found at the two ambient temperatures were nonsignificant (i.e., P > 0.05).
Fig. 3.
Plasma time-concentration profiles and pharmacokinetic parameters of MDMA and its metabolites in mice given single oral doses of MDMA (60 mg/kg) at ambient temperatures of 25°C and 4°C. Concentrations of HMMA and HHMA represent total amounts of free HMMA and HHMA obtained after enzymatic conjugate cleavage. Data represent the mean ± S.E.M. (n = 9). Pharmacokinetic parameters were compared using two-tailed paired Student’s t tests. Differences found at the two ambient temperatures were nonsignificant (i.e., P > 0.05).
TABLE 1.
Pharmacokinetics of MDMA and its metabolites in rats at two different temperatures
Peak plasma concentrations (Cmax), areas under the concentration-time curve (AUC), times of peak plasma concentration (Tmax), and elimination half-lives (T1/2) of 3,4-methylenedioxymethamphetamine (MDMA) and its metabolites 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxymethamphetamine (HMMA), and 3,4-dihydroxymethamphetamine (HHMA) in rats given a single oral dose of 20 mg/kg MDMA at two different ambient temperatures (25°C and 4°C). Values represent the mean ± S.E.M. (n = 9).
| Cmax | AUC | Tmax | T1/2 | |||||
|---|---|---|---|---|---|---|---|---|
| 25°C | 4°C | 25°C | 4°C | 25°C | 4°C | 25°C | 4°C | |
| Analyte | ng/ml | ng⋅h/ml | h | h | ||||
| MDMA | 865 ± 124 | 1076 ± 140 | 5692 ± 575 | 6912 ± 761 | 1.0 ± 0 | 2.3 ± 0.3* | 6.3 ± 0.9 | 4.9 ± 0.4 |
| MDA | 484 ± 80 | 430 ± 72 | 5064 ± 617 | 4642 ± 635 | 5.4 ± 0.7 | 4.2 ± 0.6 | 7.0 ± 1.0 | 5.2 ± 0.5 |
| HMMA | 195 ± 16 | 202 ± 18 | 2594 ± 179 | 2711 ± 166 | 6.4 ± 0.3 | 6.1 ± 0.5 | 10.8 ± 2.6 | 8.3 ± 1.0 |
| HHMA | 150 ± 17 | 162 ± 26 | 1916 ± 204 | 1914 ± 177 | 6.6 ± 0.6 | 5.6 ± 0.5 | 5.4 ± 0.2 | 6.6 ± 0.9 |
P < 0.05 vs. 25°C.
TABLE 2.
Pharmacokinetics of MDMA and its metabolites in mice at two different temperatures
Peak plasma concentrations (Cmax), areas under the concentration-time curve (AUC), times of peak plasma concentration (Tmax), and elimination half-lives (T1/2) of 3,4-methylenedioxymethamphetamine (MDMA) and its metabolites 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxymethamphetamine (HMMA), and 3,4-dihydroxymethamphetamine (HHMA) in mice given a single oral dose of 60 mg/kg MDMA at two different ambient temperatures (25°C and 4°C). Values represent the mean ± S.E.M. (n = 9).
| Cmax | AUC | Tmax | T1/2 | |||||
|---|---|---|---|---|---|---|---|---|
| 25°C | 4°C | 25°C | 4°C | 25°C | 4°C | 25°C | 4°C | |
| Analyte Ratio | ng/ml | ng⋅h/ml | h | h | ||||
| MDMA | 6120 ± 1777 | 8682 ± 1196 | 20,050 ± 2358 | 27,595 ± 2904 | 2.0 ± 0.4 | 1.6 ± 0.2 | 4.2 ± 1.3 | 1.8 ± 0.3 |
| MDA | 961 ± 193 | 870 ± 145 | 4495 ± 627 | 3996 ± 489 | 3.1 ± 0.7 | 2.4 ± 0.3 | 4.0 ± 1.0 | 3.4 ± 0.5 |
| HMMA | 791 ± 115 | 761 ± 76 | 2696 ± 167 | 2495 ± 196 | 1.0 ± 0.1 | 1.4 ± 0.2* | 8.1 ± 2.5 | 2.9 ± 0.3 |
| HHMA | 654 ± 83 | 607 ± 59 | 2418 ± 128 | 2321 ± 60 | 1.3 ± 0.2 | 1.4 ± 0.2 | 6.9 ± 1.6 | 3.9 ± 0.5 |
P < 0.05 vs. 25°C.
Rats and mice metabolized MDMA similarly. MDA was the major metabolite in both species, followed by HMMA and HHMA, respectively (Figs. 2 and 3; Tables 1 and 2). The ratio of MDMA to metabolites was significantly higher at the lower temperature in mice but not in rats (Table 3). Of note, although hepatic metabolism is expected to be more extensive after oral administration (compared with parenteral treatment methods), a significant portion of the parent compound remained unaltered following oral administration, confirming excellent oral bioavailability of MDMA in both rats and mice (Tables 1 and 2).
TABLE 3.
Ratio of MDMA to metabolites in rats and mice at two different temperatures
Calculations are based on peak plasma concentrations (Cmax) and areas under the curve (AUC) obtained in rats and mice treated with a single oral dose of MDMA at 25°C and 4°C ambient temperatures. The significance of differences between means at both temperatures was determined using two-tailed paired Student’s t tests, and significance was assumed when P < 0.05. Values represent the mean ± S.E.M. (n = 9). No significant difference was found when MDMA/metabolite ratios were compared between both temperatures in rats.
| Rat | Mouse | |||||||
|---|---|---|---|---|---|---|---|---|
| Cmax Ratio | AUC Ratio | Cmax Ratio | AUC Ratio | |||||
| 25°C | 4°C | 25°C | 4°C | 25°C | 4°C | 25°C | 4°C | |
| MDMA/MDA | 2.2 ± 0.5 | 2.8 ± 0.4 | 1.2 ± 0.1 | 1.6 ± 0.2 | 5.9 ± 0.6 | 10.4 ± 0.8* | 4.6 ± 0.3 | 7.2 ± 0.6* |
| MDMA/HMMA | 4.8 ± 1.0 | 5.7 ± 0.9 | 2.2 ± 0.2 | 2.6 ± 0.3 | 7.1 ± 1.1 | 11.3 ± 0.8* | 7.4 ± 0.7 | 11.2 ± 0.9* |
| MDMA/HHMA | 6.0 ± 0.8 | 7.7 ± 1.2 | 3.1 ± 0.4 | 4.0 ± 0.7 | 8.6 ± 1.5 | 14.3 ± 1.2* | 8.2 ± 0.8 | 11.9 ± 0.7* |
P < 0.05 vs. ratios at 25°C in mice.
At 25°C, the ratio of parent compound (MDMA) to metabolites was significantly higher in mice than in rats (Table 4). This apparent species difference largely dissipated, however, when lower doses of MDMA, namely 20 and 40 mg/kg, were tested in mice (Table 4).
TABLE 4.
Ratio of MDMA to metabolites in rats and mice at 25°C
Calculations are based on peak plasma concentrations (Cmax) and areas under the curve (AUC) obtained in rats treated with a single oral dose of 20 mg/kg of MDMA and mice treated with single oral doses of 60, 40, or 20 mg/kg of MDMA. Both rats and mice were treated at an ambient temperature of 25°C. The significance of differences between means was determined using one-way analyses of variance, and significance was assumed when P < 0.05. Values represent the mean ± S.E.M. (n = 5–9).
| Rat (20 mg/kg) | Mouse (60 mg/kg) | Mouse (40 mg/kg) | Mouse (20 mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Cmax Ratio | AUC Ratio | Cmax Ratio | AUC Ratio | Cmax Ratio | AUC Ratio | Cmax Ratio | AUC Ratio | |
| MDMA/MDA | 2.2 ± 0.5 | 1.2 ± 0.1 | 5.9 ± 0.6*§ | 4.6 ± 0.3*§# | 3.9 ± 0.7 | 3.3 ± 0.5* | 3.8 ± 0.4 | 3.3 ± 0.3* |
| MDMA/HMMA | 4.8 ± 1.0 | 2.2 ± 0.2 | 7.1 ± 1.1§ | 7.4 ± 0.7*§# | 3.7 ± 0.6 | 4.0 ± 0.3 | 2.8 ± 0.2 | 3.0 ± 0.2 |
| MDMA/HHMA | 6.0 ± 0.8 | 3.1 ± 0.4 | 8.6 ± 1.5§# | 8.2 ± 0.8*§# | 2.4 ± 0.4 | 2.5 ± 0.4 | 2.5 ± 0.2 | 2.7 ± 0.1 |
P < 0.05 vs. rat (20 mg/kg); §P < 0.05 vs. mouse (20 mg/kg); #P < 0.05 vs. mouse (40 mg/kg).
Neurochemistry.
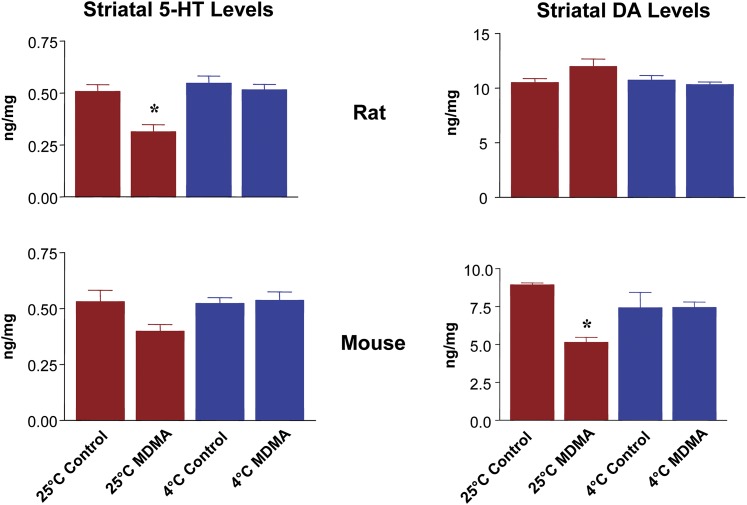
Striatal 5-HT and DA levels in rats and mice 1 week after treatment with single oral doses of MDMA (20 and 60 mg/kg, respectively) at two different ambient temperatures (25°C and 4°C) are shown in Fig. 4. After treatment at 25°C, striatal 5-HT levels were decreased by 40% in rats, whereas there were no changes in striatal DA concentrations (Fig. 4). In mice, DA levels were depleted by 40%, with no changes in 5-HT concentrations. MDMA administration in the cooler environment (4°C) afforded complete neuroprotection against the depletion of either neurotransmitter in both species (Fig. 4).
Fig. 4.
Effect of hypothermia on striatal 5-HT and DA levels in rats (top) and mice (bottom) treated with single oral doses of MDMA (20- and 60-mg/kg, respectively) 1 week previously. Values represent the mean ± S.E.M. (n = 8 per group). Data were analyzed by one-way analyses of variance followed by Tukey’s tests for multiple comparisons. *P < 0.05 vs. saline-treated controls treated at the same temperature.
Core Temperature.
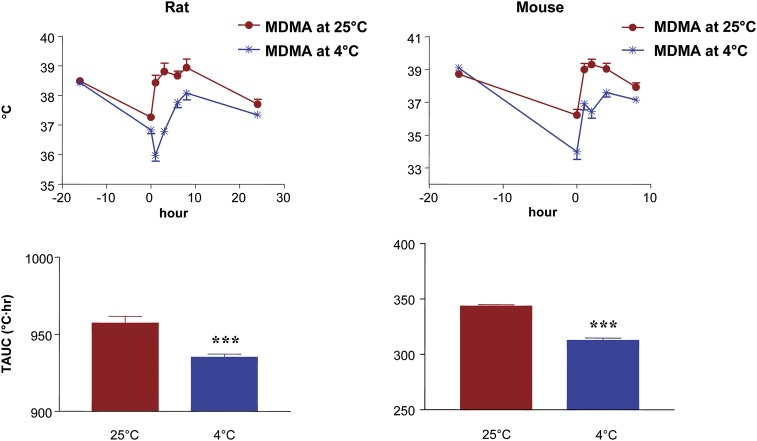
Body temperatures and the corresponding TAUCs in rats and mice treated with single oral doses of MDMA (20 and 60 mg/kg, respectively) at two different ambient temperatures (25°C and 4°C) are shown in Fig. 5. In rats, MDMA administration at the cooler temperature caused an initial drop of body temperature followed by an increase, a phenomenon that has been previously noted by a number of investigators (Malberg and Seiden, 1998; Green et al., 2003). In both species, the body temperature rose above baseline levels when MDMA was administered at 25°C, but remained below the baseline temperature when given at 4°C (Fig. 5). TAUCs of rats and mice treated in colder ambient temperatures were significantly lower than those in animals treated at 25°C (Fig. 5).
Fig. 5.
Effect of single oral doses of MDMA on core body temperature in rats (left) and mice (right) treated at two different ambient temperatures (25°C and 4°C). Rats received 20 mg/kg and mice received 60 mg/kg. Shown are temperature-time curves (top) along with the corresponding TAUCs (bottom). Values represent the mean ± S.E.M. (n = 9 per group). ***P < 0.05 vs. alternative ambient temperature.
Duration of Drug Exposure.
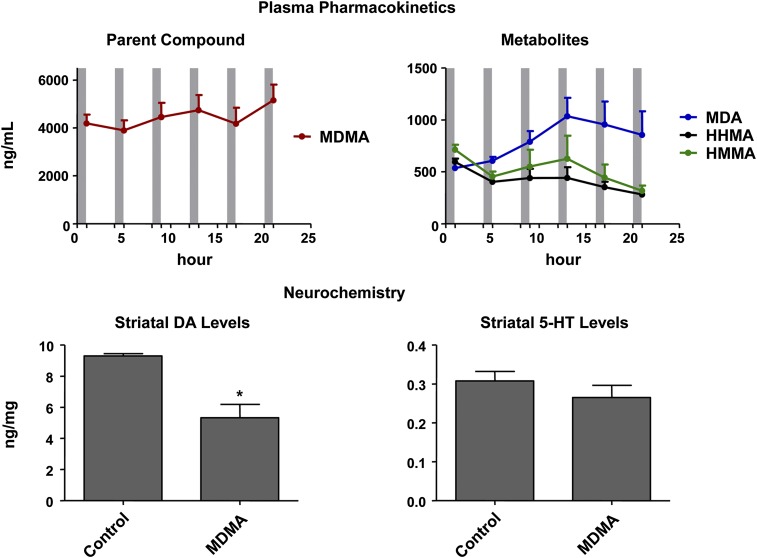
Despite prolonged exposure of mice to MDMA and its metabolites (Fig. 6, top), they developed selective DA toxicity. That is, a multiple-dose regimen of MDMA (six 15-mg/kg doses of MDMA given at 4-hour intervals) produced selective DA deficits in mice, without significant 5-HT deficits (Fig. 6, bottom). Note, peak plasma levels of MDMA and its metabolites after administration of six 15-mg/kg doses of MDMA (Fig. 6) were on the order of those seen after a single administration of 60 mg/kg (Fig. 3).
Fig. 6.
(Top) Plasma time-concentration profiles of MDMA and its metabolites in mice given six subcutaneous doses of MDMA (15 mg/kg) at 4-hour intervals. The beginning of each gray bar indicates the time of drug treatment; the end of each gray bar reflects the time at which blood was collected for determination of drug levels. Concentrations of HMMA and HHMA represent total amounts of free HMMA and HHMA obtained after enzymatic conjugate cleavage. (Bottom) Striatal 5-HT and DA levels in the same animals 1 week later, in comparison with control mice. Values represent the mean ± S.E.M. (n = 9). Data were analyzed by unpaired two-tailed Student’s t test. *P < 0.05 vs. vehicle-treated controls (n = 5).
Discussion
It is well established that the neurotoxic effects of MDMA are species-specific and strongly influenced by temperature. In particular, whereas MDMA-treated rats and nonhuman primates typically develop selective 5-HT neurotoxicity (Steele et al., 1994; Green et al., 2003), mice generally incur selective DA neurotoxicity (O'Callaghan and Miller, 1994). In both rats and mice, hypothermia attenuates MDMA neurotoxicity, whereas hyperthermia exacerbates MDMA neurotoxicity (Miller and O'Callaghan, 1994, 1995; Malberg and Seiden, 1998; O'Shea et al., 2006; Goni-Allo et al., 2008). It has been hypothesized that differences in MDMA metabolism underlie MDMA’s different neurotoxic profile in rats and mice (Lim et al., 1992; de la Torre and Farre, 2004; Green et al., 2012). Similarly, it has been suggested that the neuroprotective effects of hypothermia on MDMA neurotoxicity are secondary to temperature influences on MDMA metabolism (Goni-Allo et al., 2008). The present research aimed to directly test the hypothesized role of MDMA metabolism in temperature-related and species-specific effects of MDMA.
Contrary to what was expected, based upon previously published findings (Goni-Allo et al., 2008), hypothermia did not significantly alter MDMA metabolism in rats. In particular, the pharmacokinetics of MDMA in rats treated at 25°C and 4°C were similar (Fig. 2). That is, time-concentration profiles of MDMA and its major metabolites (MDA, HHMA, and HMMA) and the MDMA/metabolite ratios were not significantly different in rats treated at the two ambient temperatures, even though animals treated at 4°C developed significant hypothermia (Fig. 5). These results differ from those indicating that rats treated at 15°C had decreases in MDMA metabolism (Goni-Allo et al., 2008). Of note, however, in that study, rats treated at the lower ambient temperature (15°C) did not develop hypothermia. As such, changes in MDMA metabolism observed in the Goni-Allo study cannot be attributed to reductions in body temperature. Although the present study differed from that of Goni-Allo et al. in several respects (e.g., rat strains, MDMA regimens, routes of administration, study designs, and ambient temperatures), it seems unlikely that any of these differences accounts for the different findings.
The failure of hypothermia to inhibit MDMA metabolism in the rat could conceivably be related to the fact that the dose of MDMA presently tested (20 mg/kg) already fully inhibited MDMA metabolism in this experimental animal, leaving no room for hypothermia to further inhibit MDMA metabolism. In particular, previous work has shown that O-demethylenation of MDMA, which is catalyzed by species-specific homologs of the human CYP2D6 (cytochrome P450) enzyme, is subject to saturation and/or inhibition by MDMA (Chu et al., 1996; Baumann et al., 2009; Scheidweiler et al., 2011), and that such inhibition and/or saturation occurs at relatively low plasma MDMA concentrations (approximately 125–200 ng/ml) (Mueller et al., 2008, 2011b; Baumann et al., 2009). As such, the plasma MDMA concentrations of 800–900 ng/ml generated by the 20 mg/kg dose of MDMA used in this study would be more than sufficient to inhibit and/or saturate O-demethylenation, leaving no room for hypothermia to further inhibit MDMA metabolism. In light of this consideration, the question arises as to whether MDMA dose differences between this study and that of Goni-Allo et al. (2008) might account for the different findings of the two studies. In short, this is unlikely, because the MDMA dose used by Goni-Allo and colleagues (2008) generated plasma MDMA concentrations that were even higher than those presently observed (approximately 1500 ng/ml versus 900 ng/ml).
Hypothermia did not alter MDMA metabolism in the rat (Fig. 2) but afforded complete neuroprotection (Fig. 4). These results indicate that the neuroprotective effect of hypothermia in rats is not related to an effect on MDMA metabolism. Thus, at least in rats, some factor other than inhibition of MDMA metabolism must underlie the neuroprotective effect of lower body and ambient temperatures. As mentioned above (see Introduction), hypothermia may be modulating the interaction of MDMA with DAT and SERT in a manner that decreases cellular processes hypothesized to underlie neurotoxicity (e.g., ionic dysregulation) (Callahan et al., 2001). Alternatively, hypothermia may decrease formation of reactive oxygen species that have been postulated in MDMA neurotoxicity (Yamamoto et al., 2010). It is also conceivable that hypothermia dampens hormonal responses that have been implicated in MDMA neurotoxicity (Johnson and Yamamoto, 2010). It remains to be determined which, if any, of these mechanisms plays a role in the neuroprotective effect of hypothermia in MDMA-treated rats.
In contrast to the lack of its effect on MDMA metabolism in rats, hypothermia inhibited MDMA metabolism in mice, albeit modestly. This is reflected by the higher MDMA/metabolite ratios at 25°C compared with 4°C (Table 3). Notably, however, the higher MDMA/metabolite ratio seen in mice at 4°C was due to a small, nonsignificant increase in MDMA concentration and small, nonsignificant decreases in metabolite concentrations (Table 2). That is, plasma time-concentration profiles of MDMA and its metabolites were not significantly different in mice maintained at the two ambient temperatures (Fig. 3). Nevertheless, given the apparent modest inhibitory effect of hypothermia on MDMA metabolism in mice, the question arises as to whether the neuroprotective effect of hypothermia in mice is due to decreased MDMA metabolism. We believe this is unlikely for several reasons. First, although MDMA/metabolite ratios were higher in hypothermic mice, the absolute levels of MDMA metabolites in the mouse were still quite high [e.g., HHMA Cmax, 654 ng/ml (25°C) versus 604 ng/ml (4°C); HHMA AUC, 2418 ng⋅h/ml (25°C) versus 2321 ng⋅h/ml (4°C)]. Indeed, they were much higher than those seen in rats. Second, if metabolites are responsible for MDMA neurotoxicity, the small, nonsignificant decrease in metabolite levels would be expected to attenuate, rather than completely block, neurotoxicity. Taken together, these considerations suggest that the neuroprotective effect of hypothermia in mice is not fully accounted for by alterations in MDMA metabolism.
The present study also sought to determine whether the different profiles of MDMA neurotoxicity in rats and mice (i.e., 5-HT neurons affected in rats, DA neurons in mice) were related to species differences in MDMA metabolism or disposition. MDMA metabolism in rats and mice differed in three aspects.
Concentrations of MDMA, HHMA, and HMMA were substantially higher in mice than in rats.
Clearance of MDMA and metabolites was significantly faster in the mouse compared with the rat.
The ratio of MDMA to metabolites was significantly higher in the mouse than in the rat.
It is difficult to envision how any of these pharmacokinetic differences would account for the different profile of MDMA toxicity in the two species. If demethylenated metabolites of MDMA (HHMA and HMMA) were responsible for 5-HT neurotoxicity, mice would be expected to develop greater 5-HT neurotoxicity than rats because they are exposed to much higher concentrations of these metabolites than rats (Figs. 2 and 3). However, this was not observed. Differences in clearance of MDMA and metabolites are also unlikely to be responsible for the observed species differences in neurotoxicity profiles because, when the presence of MDMA and metabolites in mice is prolonged (by administering repeated doses; [Fig. 6, top]), DA neurons are still selectively affected (Fig. 6, bottom panel). With regard to the higher ratio of MDMA to metabolites seen in mice, this apparent metabolic difference largely dissipates when mice are treated with lower doses of MDMA (Table 4). The only exception is the ratio of MDMA to MDA, which was still higher in the mouse at the lower dose. However, MDA, like MDMA, has been found to produce selective DA neurotoxicity in mice (O'Callaghan and Miller, 1994), and therefore, decreased formation of MDA in mice cannot explain differences in neurotoxic profile between the two species. Collectively, these considerations suggest that differences in MDMA metabolism in rats and mice are unlikely to account for the different profiles of MDMA neurotoxicity seen in the two species.
In summary, contrary to what has been recently suggested (Green et al., 2012), alterations or differences in MDMA metabolism do not account for either the neuroprotective effect of hypothermia or the different profile of MDMA neurotoxicity observed in mice and rats. Additional studies will, therefore, be needed to understand the basis for both of these phenomena. Given that pharmacokinetic explanations do not appear to account for species-specific neurotoxic profiles of MDMA, pharmacodynamic factors warrant attention. For example, differences in monoamine transporter structure and/or function in rats and mice may underlie the different profiles of MDMA neurotoxicity seen in the two species.
Abbreviations
- AUC
area under the plasma concentration–time curve
- Cmax
peak plasma concentration
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- HHA
3,4-dihydroxyamphetamine
- HHMA
3,4-dihydroxymethamphetamine
- 5-HIAA
5-hydroxyindoleacetic acid
- HMMA
4-hydroxy-3-methoxymethamphetamine
- 5-HT
5-hydroxytryptamine
- MDA
3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- SERT
serotonin transporter
- TAUC
area under the temperature-time curve
- Tmax
time of peak plasma concentration
Authorship Contributions
Participated in research design: Mueller, McCann, Ricaurte.
Conducted experiments: Mueller, Maldonado-Adrian, Yuan.
Performed data analysis: Mueller, Maldonado-Adrian, Yuan, McCann, Ricaurte.
Wrote or contributed to the writing of the manuscript: Mueller, McCann, Ricaurte.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA05707 and DA01796401].
References
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. (2009) Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos 37:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr (1995) Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/-)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther 275:325–333 [PubMed] [Google Scholar]
- Callahan BT, Cord BJ, Yuan J, McCann UD, Ricaurte GA. (2001) Inhibitors of Na(+)/H(+) and Na(+)/Ca(2+) exchange potentiate methamphetamine-induced dopamine neurotoxicity: possible role of ionic dysregulation in methamphetamine neurotoxicity. J Neurochem 77:1348–1362 [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remião F, Bastos ML, Meisel A, Carvalho F. (2009) Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol 39:210–271 [DOI] [PubMed] [Google Scholar]
- Chu T, Kumagai Y, DiStefano EW, Cho AK. (1996) Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51:789–796 [DOI] [PubMed] [Google Scholar]
- Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. (1987) Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 241:338–345 [PubMed] [Google Scholar]
- de la Torre R, Farré M. (2004) Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci 25:505–508 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J. (2004) Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26:137–144 [DOI] [PubMed] [Google Scholar]
- Goni-Allo B, O Mathúna B, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N. (2008) The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology (Berl) 197:263–278 [DOI] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC. (2012) Lost in translation: preclinical studies on 3,4-methylenedioxymethamphetamine provide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans. Br J Pharmacol 166:1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 55:463–508 [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Kumagai Y, Unger SE, Cho AK. (1990) Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther 254:521–527 [PubMed] [Google Scholar]
- Johnson BN, Yamamoto BK. (2010) Chronic stress enhances the corticosterone response and neurotoxicity to +3,4-methylenedioxymethamphetamine (MDMA): the role of ambient temperature. J Pharmacol Exp Ther 335:180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Zeng S, Chei DM, Foltz RL. (1992) Comparative investigation of disposition of 3,4-(methylenedioxy)methamphetamine (MDMA) in the rat and the mouse by a capillary gas chromatography-mass spectrometry assay based on perfluorotributylamine-enhanced ammonia positive ion chemical ionization. J Pharm Biomed Anal 10:657–665 [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. (1998) Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 18:5086–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA. (2004) Amphetamine neurotoxicity: accomplishments and remaining challenges. Neurosci Biobehav Rev 27:821–826 [DOI] [PubMed] [Google Scholar]
- Mechan A, Yuan J, Hatzidimitriou G, Irvine RJ, McCann UD, Ricaurte GA. (2006) Pharmacokinetic profile of single and repeated oral doses of MDMA in squirrel monkeys: relationship to lasting effects on brain serotonin neurons. Neuropsychopharmacology 31:339–350 [DOI] [PubMed] [Google Scholar]
- Meyer MR, Peters FT, Maurer HH. (2008) The role of human hepatic cytochrome P450 isozymes in the metabolism of racemic 3,4-methylenedioxy-methamphetamine and its enantiomers. Drug Metab Dispos 36:2345–2354 [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. (1994) Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:752–760 [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. (1995) The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol 11:177–192 [DOI] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, Lau SS. (2004) The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit 26:132–136 [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. (1989) The use of interspecies scaling in toxicokinetics, in Toxicokinetics in New Drug Development (Yacobi A, Kelly J, Batra V. eds) pp 42–96, Pergamon Press, New York [Google Scholar]
- Mueller M, Goodwin AK, Ator NA, McCann UD, Ricaurte GA. (2011b) Metabolism and disposition of 3,4-methylenedioxymethamphetamine (“ecstasy”) in baboons after oral administration: comparison with humans reveals marked differences. J Pharmacol Exp Ther 338:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Kolbrich-Spargo EA, Peters FT, Huestis MA, Ricaurte GA, Maurer HH. (2009b) Hydrolysis of 3,4-methylenedioxymethamphetamine (MDMA) metabolite conjugates in human, squirrel monkey, and rat plasma. Anal Bioanal Chem 393:1607–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Maurer HH, McCann UD, Ricaurte GA. (2008) Nonlinear pharmacokinetics of (+/-)3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327:38–44 [DOI] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Ricaurte GA, Maurer HH. (2007) Validated liquid chromatographic-electrospray ionization mass spectrometric assay for simultaneous determination of 3,4-methylenedioxymethamphetamine and its metabolites 3,4-methylenedioxyamphetamine, 3,4-dihydroxymethamphetamine, and 4-hydroxy-3-methoxymethamphetamine in squirrel monkey plasma. J Chromatogr B Analyt Technol Biomed Life Sci 855:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Yuan J, Felim A, Neudörffer A, Peters FT, Maurer HH, McCann UD, Largeron M, Ricaurte GA. (2009a) Further studies on the role of metabolites in (+/-)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. Drug Metab Dispos 37:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Yuan J, Maldonado Adrian C, McCann UD, Ricaurte GA. (2011a) Inhibition of 3,4-methylenedioxymethamphetamine metabolism leads to marked decrease in 3,4-dihydroxymethamphetamine formation but no change in serotonin neurotoxicity: implications for mechanisms of neurotoxicity. Synapse 65:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:741–751 [PubMed] [Google Scholar]
- O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. (1988) Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci 8:2788–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea E, Orio L, Escobedo I, Sanchez V, Camarero J, Green AR, Colado MI. (2006) MDMA-induced neurotoxicity: long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br J Pharmacol 148:778–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti X, O’Mathúna B, Pizarro N, Cuyàs E, Khymenets O, Almeida B, Pellegrini M, Pichini S, Lau SS, Monks TJ, et al. (2009) Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion. Drug Metab Dispos 37:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Schmued L. (2010) Neurotoxicity of ecstasy (MDMA): an overview. Curr Pharm Biotechnol 11:460–469 [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Ladenheim B, Barnes AJ, Cadet JL, Huestis MA. (2011) (±)-3,4-methylenedioxymethamphetamine and metabolite disposition in plasma and striatum of wild-type and multidrug resistance protein 1a knock-out mice. J Anal Toxicol 35:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele TD, McCann UD, Ricaurte GA. (1994) 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”): pharmacology and toxicology in animals and humans. Addiction 89:539–551 [DOI] [PubMed] [Google Scholar]
- Xie T, McCann UD, Kim S, Yuan J, Ricaurte GA. (2000) Effect of temperature on dopamine transporter function and intracellular accumulation of methamphetamine: implications for methamphetamine-induced dopaminergic neurotoxicity. J Neurosci 20:7838–7845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. (2010) Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci 1187:101–121 [DOI] [PMC free article] [PubMed] [Google Scholar]