Abstract
Morphine conjugate vaccines have effectively reduced behavioral effects of heroin in rodents and primates. To better understand how these effects are mediated, heroin and metabolite distribution studies were performed in rats in the presence and absence of vaccination. In non-vaccinated rats 6-monoacetylmorphine (6-MAM) was the predominant opioid in plasma and brain as early as 1 minute after i.v. administration of heroin and for up to 14 minutes. Vaccination with morphine conjugated to keyhole limpet hemocyanin (M-KLH) elicited high titers and concentrations of antibodies with high affinity for heroin, 6-MAM, and morphine. Four minutes after heroin administration vaccinated rats showed substantial retention of all three opioids in plasma compared to controls and reduced 6-MAM and morphine, but not heroin, distribution to brain. Administration of 6-MAM rather than heroin in M-KLH vaccinated rats showed a similar drug distribution pattern. Vaccination reduced heroin-induced analgesia and blocked heroin-induced locomotor activity throughout 2 weeks of repeated testing. Higher serum opioid-specific antibody concentrations were associated with higher plasma opioid concentrations, lower brain 6-MAM and morphine concentrations, and lower heroin-induced locomotor activity. Serum antibody concentrations over 0.2 mg/ml were associated with substantial effects on these measures. These data support a critical role for 6-MAM in mediating the early effects of i.v. heroin and suggest that reducing 6-MAM concentration in brain is essential to the efficacy of morphine conjugate vaccines.
Introduction
An estimated 13 to 22 million people worldwide abuse opioids (UNODC, https://www.unodc.org/unodc/en/data-and-analysis/WDR-2010.html). Intravenous heroin use is associated with crime, social disruption, and transmission of blood-borne pathogens such as human immunodeficiency virus and hepatitis C (Tang et al., 2006). Pharmacological treatments available for opioid addicts include opioid agonists (methadone and buprenorphine) and opioid antagonists (naltrexone), which bind to opioid receptors to either mimic or block the effects of heroin. Although these medications are safe, effective, and decrease the risk of human immunodeficiency virus transmission (Fiellin et al., 2006), less than 20% of opioid addicts in the United States are treated with these medications and even fewer in many other countries (Tang and Hao, 2007; Mendelson et al., 2008; Krupitsky et al., 2010; Lobmaier et al., 2010). Compliance with antagonist use is low (O'Malley et al., 2000) and agonists have a high abuse potential, risk of diversion, and relatively short duration of efficacy, limiting their appeal and necessitating tight regulation. These issues suggest that longer acting treatments that do not elicit their own pharmacological effects could benefit addicts unwilling to use currently available treatments.
Opioid vaccines are being studied as an alternative or complementary treatment of heroin addiction because they are long acting, highly selective, and have few side effects. Although opioids are too small to be recognized by the immune system, they can be rendered immunogenic by conjugating them to foreign carrier proteins. Vaccination with heroin or morphine immunogens can elicit a robust immune response and reduce heroin self-administration and other opioid-related behavioral effects in animals (Bonese et al., 1974; Anton and Leff, 2006; Li et al., 2011; Stowe et al., 2011). These effects are presumably due to the binding of opioids by drug-specific antibodies in blood and reduction of opioid distribution to brain (Pravetoni et al., 2012b). Opioid vaccines have only been studied in animals, but vaccines for cocaine and nicotine have reached phase II-III clinical trials and have shown efficacy in subjects with high antibody responses (Martell et al., 2005; Haney et al., 2010; Hatsukami et al., 2011).
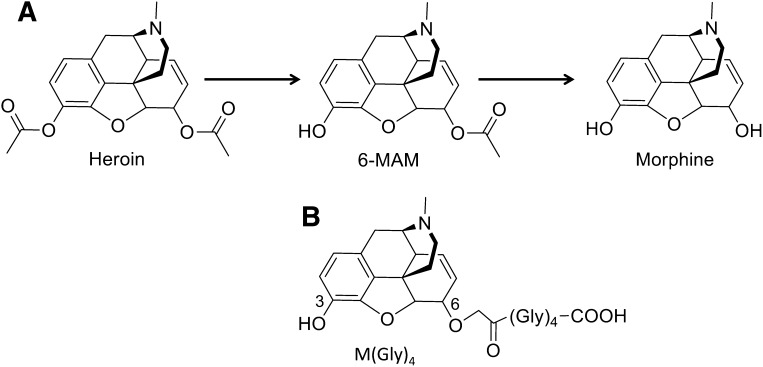
The potential use of morphine vaccines presents a number of challenges. Nicotine and cocaine vaccines have simpler targets than morphine conjugate vaccines because only the parent drug is active. In contrast, heroin is sequentially metabolized both peripherally and in the central nervous system (Fig. 1A) as: heroin → 6-monoacetylmorphine (6-MAM) → morphine → morphine-6-glucuronide (M-6-G) (Antonilli et al., 2005), which are all active in humans. Heroin enters brain but is presumed to be a pro-drug because it is rapidly metabolized, has considerably lower affinity for opioid receptors than 6-MAM or morphine (Inturrisi et al., 1983), and is generally found at low concentrations in brain (Andersen et al., 2009). 6-MAM is more likely to be the primary mediator of heroin’s early behavioral effects, because it is found at high levels in plasma and brain after heroin administration in mice (Way et al., 1960; Andersen et al., 2009) and administration of equimolar heroin or 6-MAM doses results in similar behavioral effects (Andersen et al., 2009). The contributions of morphine and M-6-G to the early effects of heroin are assumed to be smaller due to their lower levels and slower accumulation in brain (Andersen et al., 2009). With regard to the mechanism of action of an opioid vaccine, it is unclear whether the antibodies they generate must bind heroin, its downstream metabolites, or both to prevent opioid distribution from plasma to brain and reduce heroin’s behavioral effects.
Fig. 1.
(A) Heroin degradation pathway and (B) M-KLH hapten.
Heroin and metabolite distribution after i.v. heroin administration has not been well characterized in non-vaccinated rodents. Distribution studies have primarily focused on the s.c. route (Umans and Inturrisi, 1982; Pacifici et al., 2000; Andersen et al., 2009), which may subject heroin to increased peripheral degradation and slower distribution to brain compared to the i.v. route used by humans (Klous et al., 2005). Heroin distribution after i.v. administration has been studied, but an extremely high dose was used to compensate for less sensitive analytical methods and these studies were performed in mice (Way et al., 1960). The use of rats could allow a wider range of pharmacokinetic and behavioral studies to be performed.
In the current study heroin and metabolite distribution was studied after i.v. dosing in both non-vaccinated and vaccinated rats to determine the relative levels of heroin and metabolites in plasma and brain. Effects of vaccination on opioid-induced behaviors were also examined. Results support a predominant role for 6-MAM in mediating heroin effects and the importance of binding of 6-MAM by vaccine-generated antibodies in reducing those effects.
Materials and Methods
Reagents.
[Leu5]-enkephalin was purchased from Tocris Biosciences (Ellisville, MO). All other drugs were obtained through the NIH National Institute on Drug Abuse Drug Supply Program (Bethesda, MD) or Sigma-Aldrich (St. Louis, MO). Drug doses and concentrations are expressed as the weight of the base.
Synthesis of the M-KLH Vaccine.
Morphine hapten was synthesized as previously reported (Pravetoni et al., 2012b). Briefly, the C3 position of morphine sulfate was protected with di-tert-butyl carbonate and a tetraglycine (Gly)4 linker was attached at the C6 to create the hapten M(Gly)4 (Fig. 1B). M(Gly)4 was conjugated to bovine serum albumin (BSA) for use as a coating antigen for enzyme-linked immunosorbent assay (ELISA) and to keyhole limpet hemocyanin (KLH) for immunization of rats (Pravetoni et al., 2012a, b).
Vaccinations.
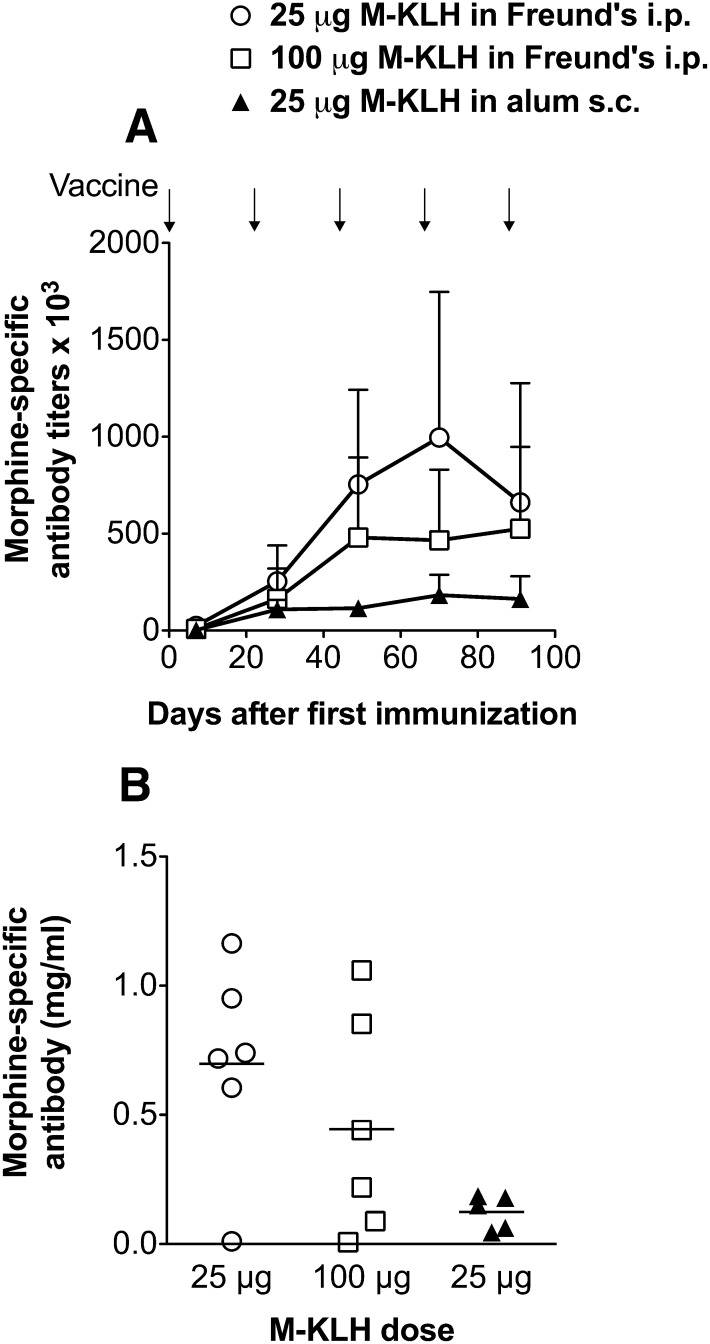
Animal protocols were approved by the Minneapolis Medical Research Foundation Animal Care and Use Committee. Male Holtzman rats (Harlan Laboratories, Madison, WI) weighing 350 g at arrival were single housed under a 12/12-hour standard light/dark cycle. All testing occurred during the light (inactive) phase. Rats were immunized with M-KLH or unconjugated KLH at an immunogen dose of 25–100 μg. Immunogen was injected in a volume of 0.4 ml using either Freund’s adjuvant i.p. or 2.5 mg/ml alum s.c. (Alhydrogel; Brenntag Biosector, Frederikssund, Denmark) (Pravetoni et al., 2011). Freund’s complete adjuvant (EMD Millipore, Billerica, MA) was used for the first immunization while Freund’s incomplete adjuvant (Sigma-Aldrich) was used for subsequent vaccine boosts. For initial experiments on heroin and 6-MAM distribution, rats were immunized on days 0, 21, 42, 64, and 86. Because titers were maximal after three vaccinations (Fig. 4A), subsequent behavioral experiments omitted the last two vaccine doses. Blood was obtained 1 week after the final vaccination for antibody characterization.
Fig. 4.
(A) Morphine-specific antibody titers and (B) concentrations after vaccination i.p. with either 25 or 100 μg M-KLH with Freund’s or s.c. with 25 μg M-KLH with alum (n = 5 per group). Rats were vaccinated once every 3 weeks (arrows indicate times of vaccinations). Antibody concentrations were measured after the fifth vaccination only.
Antibody Characterization.
ELISA plates were coated with 5 ng/well M-BSA in carbonate buffer at 9.6 pH and blocked with 1% gelatin. Goat anti-rat and anti-mouse antibodies conjugated to horseradish peroxidase were used as secondary antibodies. Antibody specificity was determined by competitive binding ELISA to calculate IC50 values. Morphine-specific antibody concentrations were measured as previously described (Pravetoni et al., 2012a, b) using mouse monoclonal anti-morphine IgG (Qed Biosciences, San Diego, CA).
Drug Level Analysis.
Plasma and brain heroin, 6-MAM, and morphine concentrations were measured by liquid chromatography/mass spectrometry within 3 days of extraction under conditions that minimize their degradation (Jones et al., 2013). The assay measures total (bound and unbound) drug. M-6-G was not measured because it is not appreciably formed in rats (Antonilli et al., 2005). Morphine-3-glucuronide was not measured because it lacks reinforcing effects (Vindenes et al., 2009), and appreciable levels were not expected in plasma and brain shortly after heroin or 6-MAM administration. Briefly, 8 ml of trunk blood was collected in a syringe containing 4 mg/ml of ice-cold NaF and heparin (100 IU/ml) and centrifuged immediately at 2400 RPM for 10 minutes at +4°C. Plasma was transferred to a 5-ml vial and diluted 1:1 with ice-cold 10 mM formate buffer (pH 3.0) prior to extraction. Brains were rinsed with 10 mM formate buffer (pH 3.0), and placed in preweighed vials; four parts (by weight) of 10 mM formate buffer pH 3.0 was added to each sample. Samples were homogenized for 30–40 seconds and stored at −20°C until extraction. Plasma and brain drug concentrations that were below the limit of quantitation (5 ng/ml for heroin and 6-MAM and 10 ng/ml for morphine) were estimated when above the detection limit and are indicated as such.
Protein Binding.
The protein binding of heroin in serum could not be measured accurately because of its rapid degradation. Instead, morphine 2 μg was added to 1 ml of drug-free serum from M-KLH vaccinated and KLH control rats. Equilibrium dialysis of the spiked serum was carried out in Sorenson’s buffer at pH 7.35 for 4 hours at 37°C using 1 ml Teflon cells. Protein binding determined in this manner represents binding to all serum proteins including opioid-specific IgG. The percent of unbound morphine was calculated as the buffer morphine concentration (unbound drug) divided by the total concentration of morphine on the serum side.
The percent unbound morphine calculated in this manner was then used to estimate the unbound plasma morphine concentrations in experimental animals that had received heroin. This was estimated from the product of the measured plasma morphine concentrations in the rats that had received heroin and the mean percent unbound morphine from spiked ex vivo samples.
Stoichiometric Relationships.
The total number of moles per kilogram of morphine-specific IgG in rats vaccinated with M-KLH was calculated as the product of the reported IgG volume of distribution (131 ml/kg) in rats and the plasma antibody concentration and converted to moles/kg using a molecular weight of 150 kDa for IgG (Bazin-Redureau et al., 1997; Pravetoni et al., 2012b). The corresponding number of drug-binding sites of IgG was twice that number because there are two binding sites per IgG.
Experimental Design Overview.
Heroin and metabolite distribution was studied in non-vaccinated rats after i.v. infusion of heroin to assess the early time course of opioid concentrations in plasma and brain. Opioid distribution after i.v. infusion of heroin or 6-MAM was then studied in vaccinated rats to determine the effect of vaccination on plasma and brain opioid levels. Finally, drug-induced anti-nociception and locomotor activity were studied in vaccinated rats to determine if vaccination could block behavioral effects of targeted opioids.
Distribution of Drug in Non-vaccinated Rats.
Rats (n = 12) were anesthetized with ketamine/xylazine i.m., and an indwelling catheter was placed in the right external jugular vein. Distribution of heroin and its metabolites in plasma and to brain was studied after a 1 minute i.v. infusion of 0.703 µmol/kg (0.26 mg/kg) heroin, dissolved in 2% (v/v) dimethylsulfoxide in physiologic saline immediately prior to infusion. Rats were decapitated for collection of trunk blood and brain 1, 4, and 14 minutes (n = 4 per time point) after heroin infusion. This heroin dose was chosen because it is within the range of reinforcing doses in heroin-dependent individuals (Comer et al., 1999), the resulting 6-MAM concentrations in plasma were similar to those found in humans receiving comparable heroin doses (Comer et al., 1999), and because it did not suppress respiration in anesthetized rats.
Effect of Vaccination on Drug Distribution.
The effect of immunization with M-KLH was compared to that of KLH control vaccine. Rats (n = 6 per group) were anesthetized 1 week after the fifth vaccination and 0.703 µmol/kg heroin or 6-MAM was infused i.v. as described above. Blood was withdrawn from catheters for antibody characterization prior to drug administration. Rats were decapitated for collection of trunk blood and brain 4 minutes after drug infusion.
Behavioral Studies.
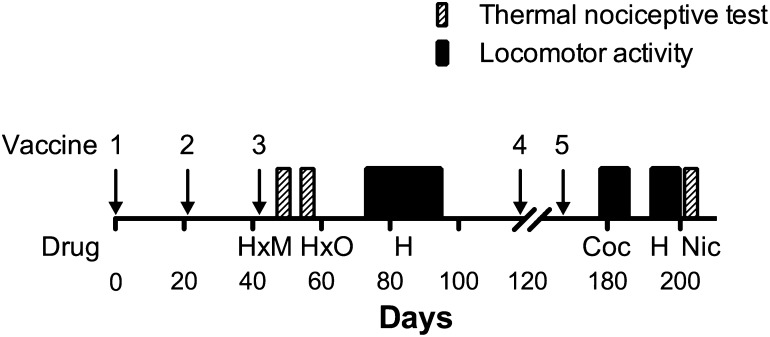
Rats were vaccinated with M-KLH (n = 14) or KLH (n = 8) 3 times (days 0, 21, and 42), and a series of behavioral studies was performed (see Fig. 2 for timeline). Two subsequent vaccine boosts were given on days 118 and 168 to maintain antibody levels. The thermal nociceptive tests were performed on days 49 and 50 (heroin and methadone), days 56 and 57 (heroin and oxycodone), and day 203 (nicotine). Locomotor activity was tested on days 75–93 (heroin), 180–184 (cocaine), and 194–198 (repeat heroin challenge). Blood collection for antibody characterization was performed on days 49, 99, and 189 when rats had not received opioids recently. Fentanyl anti-nociception was tested in a separate group of rats (n = 10).
Fig. 2.
Timeline for the thermal nociceptive and locomotor activity behavioral experiments. H, heroin; M, methadone; O, oxycodone; Coc, cocaine; Nic, nicotine. The "x" denotes a crossover design.
Effect of Vaccination on Drug-Induced Anti-nociception.
Two weeks after the third vaccination, rats were habituated for 2 hours to the testing environment, and nociception was measured on a hot plate (Columbus Instruments, Columbus, OH) set to a temperature of 54 ± 0.2°C. A nociceptive response of hindpaw lick or jumping was measured as the latency to respond. Baseline hot-plate responses were obtained 2 hours prior to drug dosing. A maximum cutoff of 60 seconds was used to avoid tissue damage. Percent maximum possible effect (%MPE) was calculated as (postdrug latency − predrug latency)/(maximum latency − predrug latency) × 100.
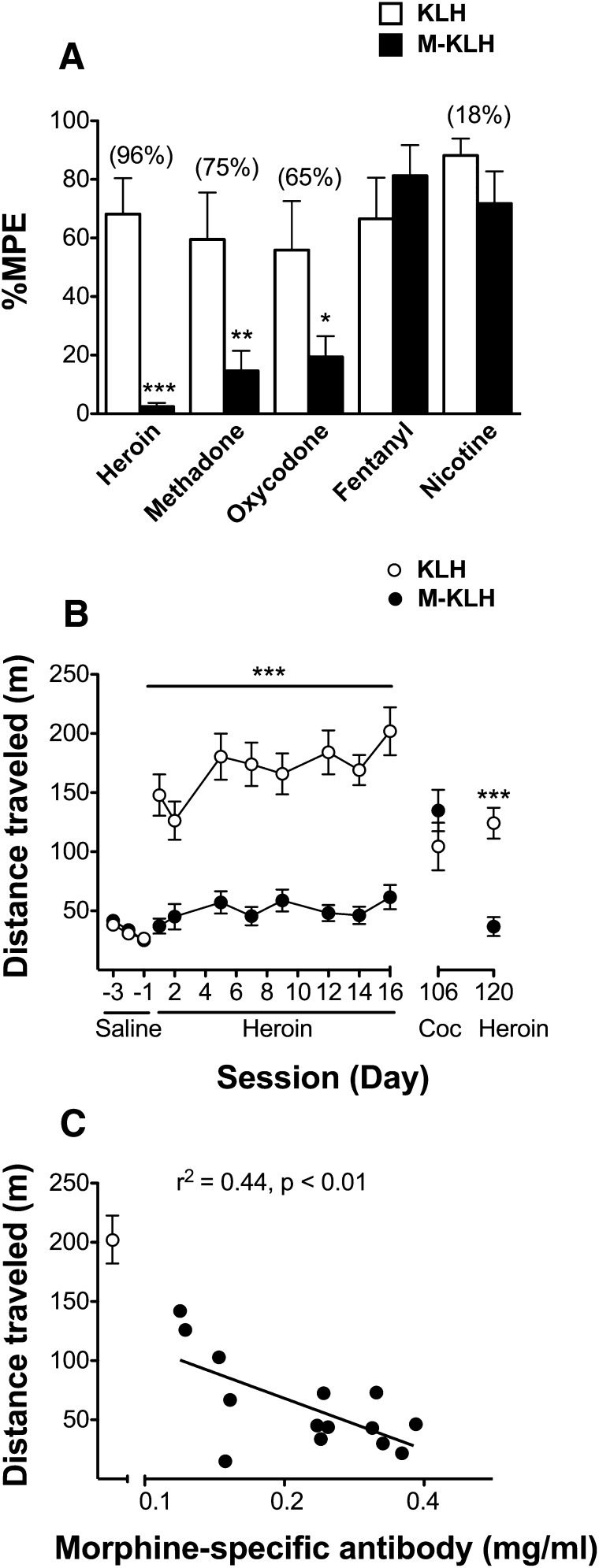
Hot plate response latency was measured 30 minutes after s.c. injection of heroin (1 mg/kg), methadone (2.25 mg/kg), oxycodone (2.25 mg/kg), or fentanyl (0.05 mg/kg). Rats were tested using a crossover design such that one-half of the animals in the M-KLH and KLH control groups received heroin on the first day and methadone, oxycodone, or fentanyl on the second day, and one-half received these drugs in the reverse order. These opioid doses were chosen because they elicited near-maximal effects in pilot studies. Rats were tested with nicotine to study vaccine specificity toward opioids. Habituation and pretest procedures used for nicotine anti-nociception were the same as for the opioid studies, except that rats were placed on the hot-plate (52 ± 0.2°C) 5 minutes after an injection of 0.35 mg/kg nicotine s.c. (McCallum et al., 1999). Because vaccine blocked heroin-induced locomotor activity the previous week, a crossover design was not used with nicotine anti-nociception.
Effect of Vaccination on Locomotor Activity.
Baseline locomotor activity was obtained on three consecutive days (days −3 through −1) by administering 1 ml/kg saline s.c. to M-KLH vaccinated and KLH control rats and placing them in open field activity chambers for 90 minutes immediately following injection (Roiko et al., 2008). The final saline day was used as baseline locomotor activity. On days 1, 2, 5, 7, 9, 12, 14, and 16 rats received 0.25 mg/kg heroin s.c. (Swerdlow et al., 1985) and were tested for activity for 90 minutes (Li et al., 2011). To confirm specificity of the assay for opioids, rats received 15 mg/kg cocaine i.p. This cocaine dose was used because it induces locomotor activity that can be blocked by immunization with a cocaine vaccine (Carrera et al., 2001). Two weeks later a final heroin challenge of 0.25 mg/kg heroin s.c. was administered to confirm that vaccination blockade of this response had been maintained. Baseline locomotor activity was re-established prior to cocaine and the final heroin challenge.
Statistical Analysis.
Effects of vaccination on drug distribution and thermal nociception between M-KLH and KLH control groups, as well as effect of drug administration order on % maximum possible effect using the crossover design, were analyzed using unpaired t-tests. Differences in antibody titers and concentrations between groups were compared using one-way analysis of variance. Relationships between antibody levels, opioid distribution, and locomotor activity were analyzed by linear regression. The effect of treatment on heroin-induced locomotor activity (measured as total horizontal distance traveled in meters) was analyzed by two-way analysis of variance with group as a between-subjects factor and day as a within-subject factor followed by Bonferroni post hoc tests. When group variances were unequal, t-tests were performed on log-transformed data.
Results
Heroin Time Course and Distribution in Non-vaccinated Rats.
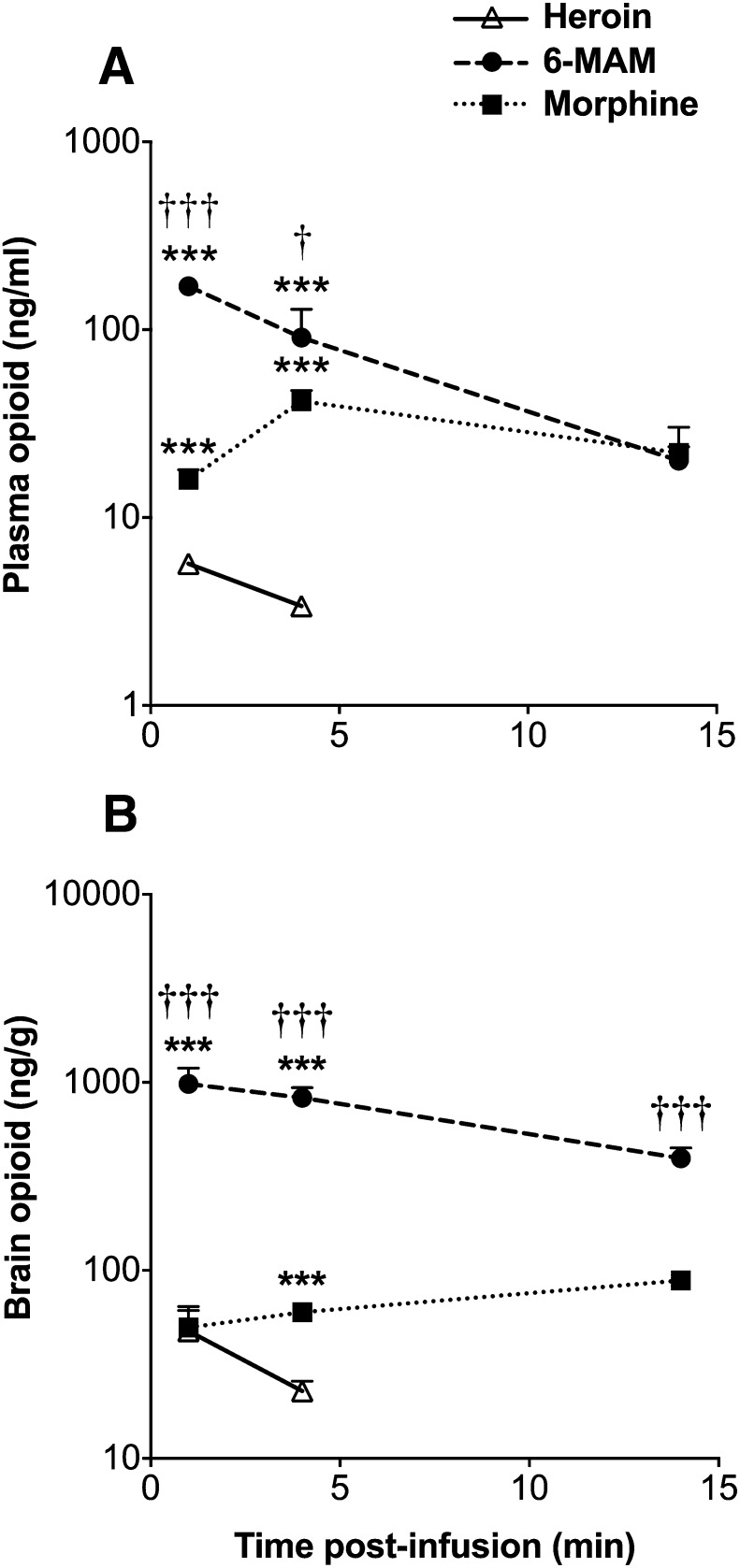
After i.v. infusion of heroin, 6-MAM was the predominant analyte at 1 and 4 minutes in plasma (Fig. 3A) and at all measured times in brain (Fig. 3B). Heroin concentration in plasma and brain was measurable at 1 minute but was below the limit of quantitation (5 ng/ml) at 4 minutes and below the limit of detection at 14 minutes. 6-MAM concentration in plasma and brain was highest at 1 minute. Morphine concentration in plasma was highest at 4 minutes. Morphine concentration in brain increased over time.
Fig. 3.
(A) Plasma and (B) brain concentrations of opioids (mean ± S.D.) after 1-minute infusion of heroin 0.26 mg/kg i.v (n = 4 per time point). Heroin concentrations were above the limit of quantitation (5 ng/ml) in plasma and brain at 1-minute post-infusion and estimated at 4 minutes. ***P < 0.001 compared to heroin concentrations; †P < 0.05 and †††P < 0.001 compared to morphine concentrations.
Serum Antibody Characterization.
Immunization with M-KLH either i.p. in Freund’s adjuvant or s.c. in alum elicited substantial antibody titers (Fig. 4A) and concentrations (Fig. 4B), although antibody levels were higher after vaccination i.p with Freund’s. Titers did not increase appreciably after the fourth or fifth dose. Immunization using 25 or 100 μg M-KLH in Freund’s elicited generally similar titers and antibody concentrations so the 25-μg dose was used for distribution and behavior studies. Averaging all experiments, not just those shown in Fig. 4, rats receiving 25 μg M-KLH i.p. in Freund’s elicited serum antibody titers of 460 ± 400 × 103 and antibody concentrations of 0.50 ± 0.38 mg/ml (mean ± S.D.). There was considerable individual variability in both antibody titers and concentrations (Fig. 4). Vaccination with M-KLH resulted in antibodies that were specific for heroin, 6-MAM, morphine, and morphine-6-glucuronide as measured by IC50 values of ≤0.1 μM (Table 1) compared to those of the off-target opioids (where binding was undesirable due to potential effects on their clinical use or the endogenous opioid system) methadone, buprenorphine, naloxone, naltrexone, oxycodone, and the endogenous opioid enkephalin, which had IC50 values two to three orders of magnitude higher.
TABLE 1.
Competitive binding ELISA IC50 values
| Drug | IC50 |
|---|---|
| μM | |
| Target | |
| Heroin | 0.03 |
| 6-MAM | 0.04 |
| Morphine | 0.05 |
| M-6-G | 0.10 |
| Off-target | |
| Methadone | 177.50 |
| Buprenorphine | 574.00 |
| Naloxone | 38.70 |
| Naltrexone | 41.90 |
| Oxycodone | 8.98 |
| Enkephalin | 16.07 |
ELISA, enzyme-linked immunosorbent assay.
Vaccine Effects on Drug Distribution After I.V. Administration of Heroin.
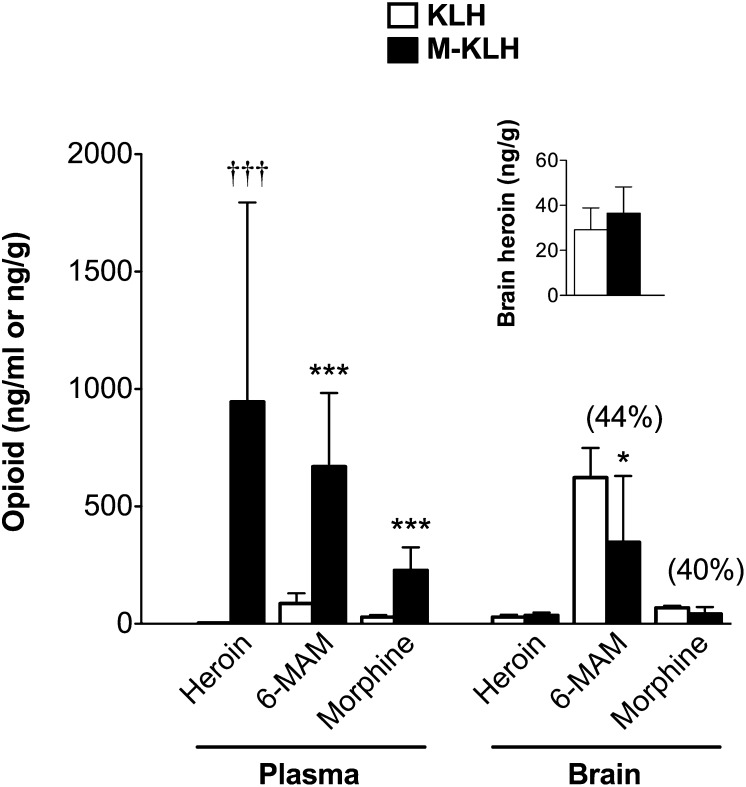
Plasma heroin concentrations in KLH controls were below the limit of quantitation but were still detectable at 4 minutes and were estimated at 3.7 ± 0.6 ng/ml (mean ± S.D.). By using this estimate, heroin retention in plasma was increased 280-fold in M-KLH vaccinated rats compared to KLH controls at 4 minutes (see Fig. 5). 6-MAM and morphine concentrations in plasma were increased eightfold in vaccinated rats compared to controls at 4 minutes.
Fig. 5.
Effect of vaccination on distribution of heroin and its metabolites (mean ± S.D.) 4 minutes after a 1-minute infusion of heroin 0.26 mg/kg i.v. in M-KLH and KLH rats (n = 6 per group). Rats were tested after the fifth vaccination i.p. with 25 μg M-KLH or KLH in Freund’s adjuvant. Inset shows heroin brain concentrations in M-KLH and KLH rats on a reduced scale. *P < 0.05 and ***P < 0.001 compared to KLH controls and †††P < 0.001 compared to estimated heroin concentrations in KLH controls. Numbers in parentheses are the percent decrease compared to controls.
Vaccination with M-KLH did not reduce heroin distribution to brain compared to controls (Fig. 5, inset), but brain heroin concentrations in both groups were low compared to 6-MAM and morphine concentrations. Vaccination reduced the distribution of 6-MAM to brain by 44% (P < 0.05). Morphine distribution to brain in vaccinated rats was decreased by 40% compared to controls, but the difference was not quite significant (P = 0.052). However, both 6-MAM and morphine distribution to brain were reduced by 70% (P < 0.01) if data from two rats with very low serum antibody concentrations (<0.08 mg/ml) were removed. Non-vaccinated (Fig. 3) and KLH control (Fig. 5) rats, although not directly compared, had similar plasma and brain opioid distribution at 4 minutes, showing that KLH vaccination did not alter drug distribution.
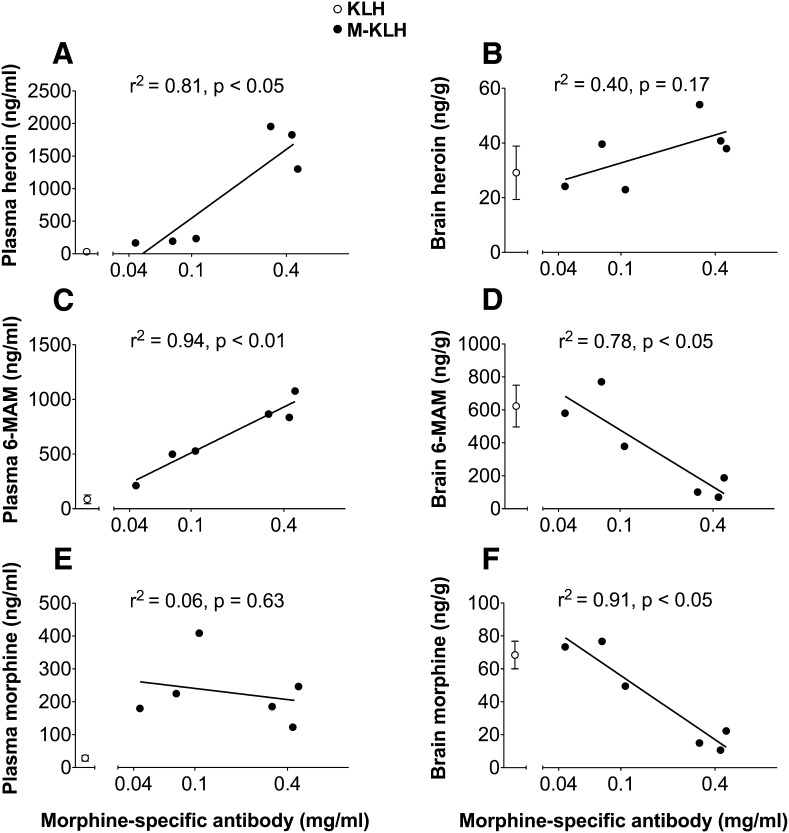
Serum antibody concentrations were positively correlated with plasma heroin and 6-MAM concentrations but not with plasma morphine concentrations (Fig. 6, A, C, and E). No significant correlation was found between heroin brain concentrations and serum antibody concentrations, but heroin concentrations in both groups were low (Fig. 6B). Higher antibody concentrations were associated with reduced brain 6-MAM and morphine concentrations (Fig. 6, D and F).
Fig. 6.
Relationship between morphine-specific antibody concentrations and opioid concentrations in plasma (A, C, and E) and brain (B, D, and F) 4 minutes after i.v. infusion of 0.26 mg/kg heroin in M-KLH vaccinated rats.
Vaccine Effects on Drug Distribution After I.V. Administration of 6-MAM.
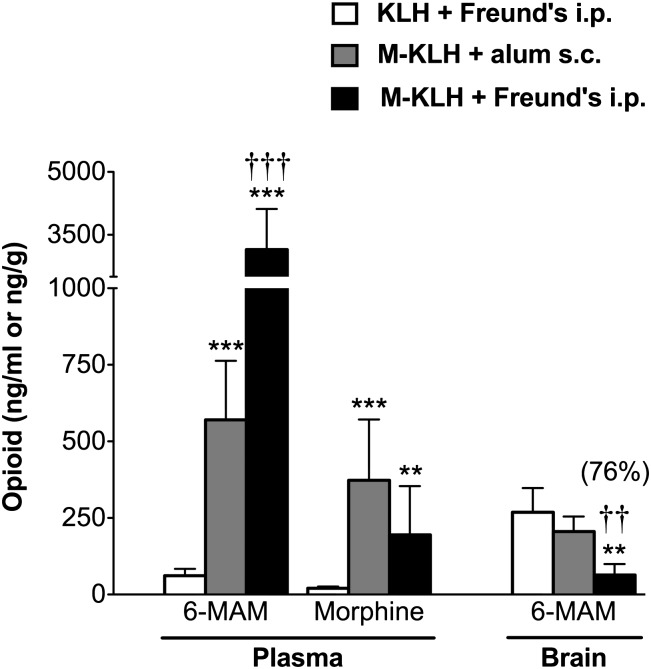
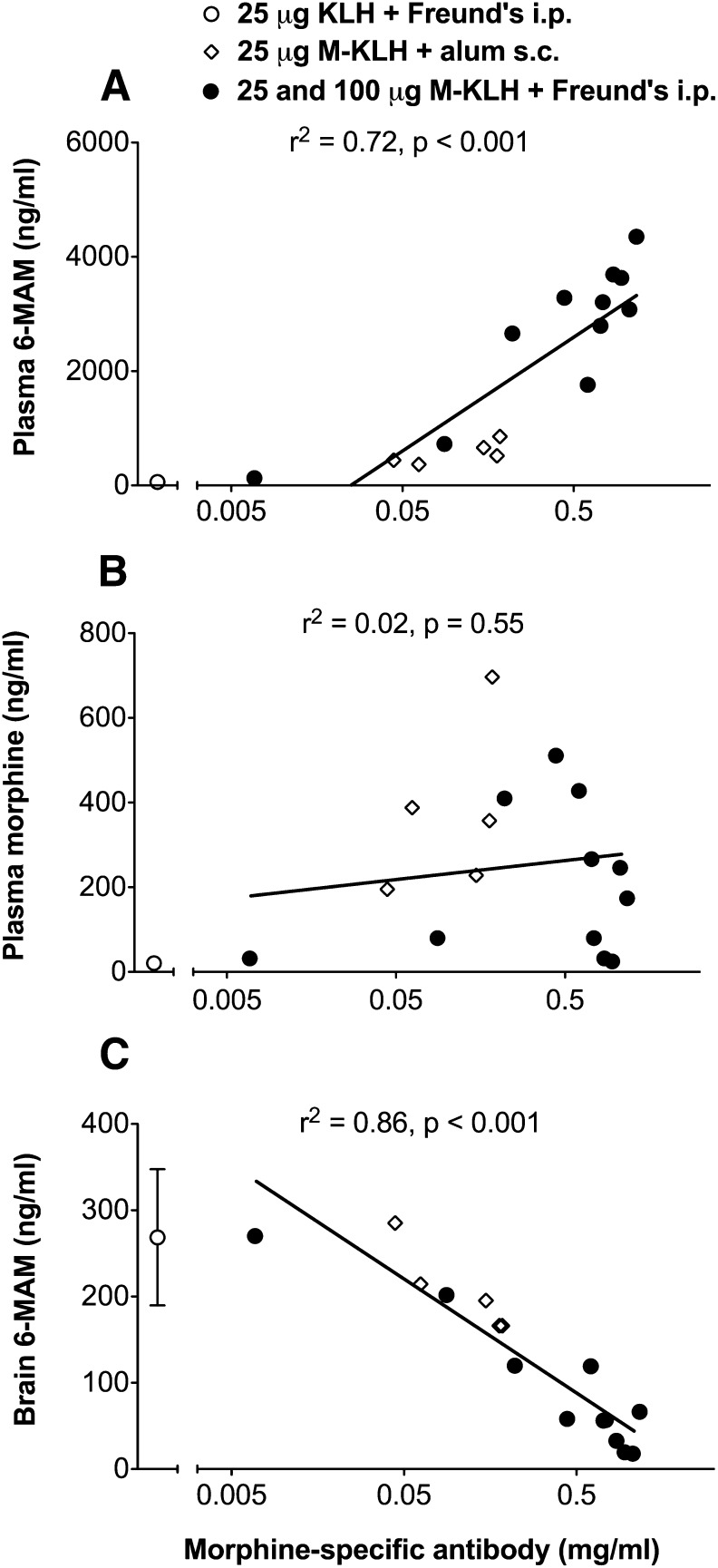
In rats vaccinated i.p. with M-KLH in Freund’s, 6-MAM retention in plasma was increased 51-fold compared to KLH controls (Fig. 7) and morphine concentrations were sixfold higher. Vaccination reduced 6-MAM concentration in brain by 76% compared to controls. Morphine concentrations in brain were too low to compare between groups. In rats vaccinated s.c. with M-KLH in alum effects were similar but smaller (Fig. 7). Serum antibody concentrations were directly correlated with plasma 6-MAM but not morphine concentrations (Figs. 8A and 7B). Serum antibody concentrations were inversely correlated with 6-MAM brain concentrations (Fig. 8C). Because effects on drug distribution were similar in rats vaccinated i.p. with either 25 and 100 μg M-KLH in Freund’s, the 100-μg group was not included in Fig. 7 for clarity but was added to Fig. 8 to provide more data for linear regression.
Fig. 7.
Plasma and brain concentrations of 6-MAM and morphine (mean ± S.D.) 4 minutes after a 1-minute infusion of 0.23 mg/kg 6-MAM in M-KLH and KLH rats (n = 5 per group). Rats were tested after the fifth vaccination i.p. with 25 μg M-KLH + Freund’s, s.c. with M-KLH + alum, and i.p. with 25 μg KLH + Freund’s (n = 5 per group). Brain concentrations of morphine were below detection in all groups. **P < 0.01 and ***P < 0.001 compared to KLH controls; ††P < 0.01 and †††P < 0.001 compared to M-KLH + alum s.c. Numbers in parentheses are the percent decrease compared to controls.
Fig. 8.
(A) Relationship between morphine-specific antibody concentrations and 6-MAM concentrations in plasma, (B) morphine concentrations in plasma, and (C) 6-MAM concentrations in brain 4 minutes after i.v. infusion of 0.23 mg/kg 6-MAM in M-KLH vaccinated rats. Morphine was not detected in brain 4 minutes after 6-MAM infusion.
Protein Binding.
Protein binding of morphine in spiked serum (of rats that had not received opioid in vivo) was substantially higher in the M-KLH serum (99.7%) than the KLH control serum (15.8%). Estimates of unbound morphine concentrations in plasma of experimental rats that had received heroin (see Fig. 5) were markedly lower in M-KLH vaccinated rats than KLH controls (0.7 ± 0.3 ng/ml versus 24.3 ± 7.3 ng/ml, mean ± S.D., respectively).
Thermal Nociception Test.
Vaccination with M-KLH significantly reduced response latency to heroin (P < 0.001), methadone (P < 0.01), and oxycodone (P < 0.05) compared to KLH controls (Fig. 9A), although the effect on heroin was greatest. There was no effect of drug administration order on %MPE using the crossover design. Vaccination was highly effective at reducing opioid anti-nociception at all measured antibody concentrations so no correlation was apparent. Vaccination did not alter nicotine or fentanyl-induced anti-nociception, showing that vaccine effects were specific to certain opioids (Fig. 9A).
Fig. 9.
(A) Vaccine effect on drug-induced anti-nociception in M-KLH (n = 14) and KLH (n = 8) rats after s.c. administration of heroin (1.0 mg/kg), methadone (2.25 mg/kg), oxycodone (2.25 mg/kg), and nicotine (0.35 mg/kg) and in M-KLH (n = 10) and KLH (n = 10) rats after s.c. administration of fentanyl (0.05 mg/kg). Data are the mean percent maximum possible effect [% maximum possible effect (MPE)] ± S.E.M. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to KLH controls. Numbers in parentheses are the percent decrease of %MPE in M-KLH compared to KLH controls. (B) Vaccine effect on heroin-induced locomotor activity in M-KLH (n = 14) and KLH (n = 8) rats. Total horizontal distance traveled (mean ± S.E.M.) was measured for 90 minutes after s.c. administration of 0.25 mg/kg heroin. ***P < 0.001 heroin-induced locomotor activity compared to KLH controls. (C) Relationship of log morphine-specific IgG concentrations and locomotor activity in M-KLH (n = 14) and KLH (n = 8) rats on day 16.
Heroin-Induced Locomotor Activity.
Rats vaccinated with M-KLH or KLH had comparable baseline locomotor activity (Fig. 9B). There was a significant effect of vaccination (P < 0.001), day (P < 0.001), and interaction (P < 0.001) on heroin-induced locomotor activity. M-KLH vaccinated rats had substantially lower heroin-induced locomotor activity than controls on all days (Bonferroni t = 4.6– 8.0, P < 0.001). Vaccination had no effect on cocaine-induced locomotor activity. On the final day of testing, higher antibody concentrations were associated with lower heroin-induced locomotor activity (Fig. 9C). Morphine-specific antibody titers were still elevated after completion of locomotor activity testing (180 ± 100 × 103, mean ± S.D.), but were 62% lower than after the final vaccination 2 months earlier.
Stoichiometry.
In all experiments the dose of heroin or 6-MAM administered was nearly equal to or above the antibody binding capacity (Table 2), except for one experiment in which M-KLH vaccinated rats had very high antibody concentrations.
TABLE 2.
Molar ratios of opioid-specific binding sites and opioid dose
| Opioid Administered | Opioid Dose | Vaccine Adjuvant and Route | Total Opioid-specific IgG Binding Sites | Molar Ratio of Drug Dose: IgG Binding Sites |
|---|---|---|---|---|
| μmol/kg | μmol/kg | |||
| Heroin i.v. | 0.703 | Freund’s, i.p. | 0.42 | 1.7 |
| 6-MAM i.v. | 0.703 | Freund’s, i.p. | 1.09 | 0.6 |
| Alum, s.c. | 0.22 | 3.2 | ||
| Heroin s.c. | 2.70a | Freund’s, i.p. | 0.95 | 2.8 |
| 0.68b | Freund’s, i.p. | 0.42 | 1.6* |
Heroin-induced anti-nociception study.
Heroin-induced locomotor activity study.
Molar ratios reflect single doses though repeated doses were used to test behavioral responses in these protocols.
Discussion
Distribution of heroin and its metabolites was studied in rats to understand how morphine conjugate vaccines alter the behavioral effects of heroin. The main findings of this study were that (1) in non-vaccinated rats 6-MAM was the predominant metabolite in brain shortly after i.v. heroin administration; (2) vaccination with M-KLH led to a reduction of brain 6-MAM but not heroin concentration after i.v. heroin administration; (3) vaccination reduced heroin’s behavioral effects; and (4) higher levels of opioid-specific antibodies were associated with greater effects on heroin pharmacokinetics and heroin-induced behavioral effects. These findings suggest that the binding of 6-MAM by vaccine-generated antibodies is critical for blockade of heroin’s behavioral effects and that relatively high serum levels of opioid-specific antibodies are needed to produce substantial blockade.
The finding that 6-MAM was the predominant opioid in plasma and brain in non-vaccinated rats for up to 14 minutes after i.v. heroin administration is consistent with several prior preclinical studies (Way et al., 1960; Andersen et al., 2009), despite differences in species and route of heroin administration. Because 6-MAM also has a higher affinity for the mu-opioid receptor than heroin (Inturrisi et al., 1983), these observations support a critical role of 6-MAM in mediating the early effects of heroin. These preclinical data contrast with several human studies that found heroin to be the predominant opioid in plasma shortly (2 to 10 minutes) after similar i.v. doses of heroin (Comer et al., 1999; Girardin et al., 2003). The metabolism of heroin to 6-MAM and morphine in plasma is attributable to esterases in blood as well as non-enzymatic degradation (Salmon et al., 1999; Selley et al., 2001; Rook et al., 2006). Rats have higher plasma esterase activity than humans (Minagawa et al., 1995; Bahar et al., 2012), which complicates extrapolation of opioid data across species but may explain why higher heroin and lower 6-MAM levels were found in humans.
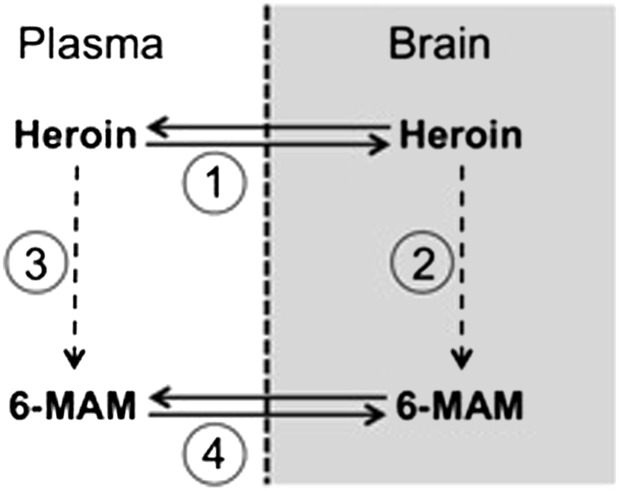
The current data suggest that morphine conjugate vaccines act mainly by reducing 6-MAM concentrations in brain. Accumulation of 6-MAM in brain after heroin administration may result from either heroin distribution to brain and subsequent hydrolysis to 6-MAM or conversion of heroin to 6-MAM in plasma and subsequent 6-MAM distribution to brain. Vaccine-generated antibodies could potentially target these pathways at several points (Fig. 10). (1) Vaccine-generated antibodies might bind heroin in plasma and prevent its distribution to brain. This is unlikely because brain heroin concentrations were not reduced in vaccinated rats compared to controls. (2) Vaccine-generated antibodies might reduce conversion of heroin to 6-MAM in brain. This is unlikely because drug-specific antibody is largely excluded from the central nervous system by the blood-brain barrier (Satoskar et al., 2003). (3) Vaccine-generated antibodies might reduce the conversion of heroin to 6-MAM in plasma, reducing its availability for distribution to brain. This did not appear to be the case because plasma 6-MAM concentrations in vaccinated rats were substantial, indicating that conversion of heroin to 6-MAM in plasma was not prevented by vaccination. (4) Vaccine-generated antibodies might bind 6-MAM in plasma and impair its distribution to brain. This mechanism is consistent with the very high serum 6-MAM concentrations measured in vaccinated rats, reflecting its extensive binding and retention in serum. In support of this mechanism being the predominant explanation for reduced 6-MAM levels in brain, vaccine-generated antibodies also reduced 6-MAM levels in brain when 6-MAM was administered in place of heroin.
Fig. 10.
Steps involved in the accumulation of 6-MAM in brain after heroin administration. Vaccination could potentially interfere by reducing (1) heroin distribution from plasma to brain, (2) hydrolysis of heroin to 6-MAM in brain, (3) hydrolysis of heroin to 6-MAM in plasma, or (4) distribution of 6-MAM from plasma to brain. The data generated in this study suggest that M-KLH acted primarily by binding 6-MAM in plasma and reducing its distribution to brain (Step 4).
Unbound heroin and 6-MAM concentrations could not be directly measured in plasma because rapid degradation complicated their measurement. However, estimates of unbound morphine concentrations in plasma based on in vitro protein binding data suggest that M-KLH rats had much lower unbound morphine concentrations than controls despite an overall increase in total plasma morphine concentrations. If this were also true for 6-MAM, it would account for the lower 6-MAM levels in brain in vaccinated rats.
Further support for the importance of 6-MAM binding is provided by a heroin vaccine composed of hapten conjugated to protein through the bridge nitrogen. This vaccine generated antibodies with a higher affinity for 6-MAM than heroin yet blunted heroin analgesia in rats (Stowe et al., 2011). Another immunogen, which had a lower affinity for 6-MAM than heroin, was not as effective (Stowe et al., 2011).
Higher plasma antibody concentrations in this study were associated with higher plasma and lower brain drug levels and reduced behavioral effects of heroin. Although a distinct threshold for effective antibody concentrations was not apparent, the combined distribution and behavioral studies suggest that antibody concentrations above 0.2 to 0.3 mg/ml produced substantial effects. No other such estimates are available for morphine conjugate vaccines, but this range is generally consistent with previous data for nicotine or cocaine vaccines in rodents (Kantak et al., 2000; Cornish et al., 2011).
The reduction of oxycodone- and methadone-induced anti-nociception found in vaccinated rats was unexpected, because the IC50 data showed relatively low antibody specificity for oxycodone and methadone compared to heroin, 6-MAM, or morphine (Table 1). This discrepancy may be due to the nature of the competitive ELISA method. The morphine conjugate used as a solid phase in the ELISA has many haptens available allowing antibody, which has two binding sites per molecule of IgG, to bind to two haptens at a time. In contrast the soluble competing opioid can bind only to one site. The ELISA may therefore reflect antibody avidity (binding to two sites on the ELISA coating conjugate), which is higher than its affinity (binding to one site on the competing antigen), and underestimate the ability of antibodies to bind some opioids in vivo. Nonetheless, vaccine did not block fentanyl or nicotine analgesia or reduce cocaine-induced locomotor activity, showing that the effects of vaccination are specific to certain opioids. Vaccination with M-KLH was previously shown to reduce brain oxycodone levels in rats by 33% after a 0.1 mg/kg i.v. dose (Pravetoni et al., 2012b). The ability to reduce oxycodone effects could be a desirable feature of a morphine conjugate vaccine, broadening its effects to another commonly abused opioid. Alternatively, if this is not desired, positioning the linker at the nitrogen bridge of the hapten has been shown to minimize cross-reactivity with oxycodone (Stowe et al., 2011). A similar approach could perhaps be explored to minimize methadone cross-reactivity if needed.
Vaccination with M-KLH was effective even after administration of large, clinically relevant heroin doses that matched or exceeded the estimated drug-binding capacity of the available antibody. Efficacy in the face of large heroin doses encourages further study of this and related morphine-conjugate vaccines. On the other hand, efficacy was associated with relatively high serum antibody levels, which, at least for analogous nicotine or cocaine vaccines, have been more difficult to achieve in humans than in rodents. Achieving high serum antibody levels will be important in translating this strategy to the treatment of humans.
Vaccination i.p. using Freund’s adjuvant produced a robust antibody response but it is not suitable for human use. The primary purpose of this study was to better understand vaccine effects on heroin and metabolite distribution so a potent adjuvant was desirable. A clinically acceptable adjuvant, such as alum, will be necessary for human use. Vaccination s.c. with alum did alter plasma drug levels compared to controls, but effects were not as large as those seen with Freund’s i.p. However the immunogen dose in the s.c. alum formulation was not optimized, and, by analogy with another morphine conjugate vaccine (Anton and Leff, 2006), higher immunogen doses may improve response.
A limitation of this study is that vaccine effects on heroin pharmacokinetics were only studied using single doses. Nevertheless, the heroin doses used were within the range abused by addicts, and vaccination remained effective in blocking heroin-induced locomotor activity over 2 weeks of repeated dosing. Other morphine conjugate vaccines of similar design have also blocked heroin self-administration (Bonese et al., 1974; Anton and Leff, 2006), which involves even more frequent heroin dosing. A similar morphine vaccine blocked heroin-induced motor activity, and these effects are predictive of efficacy in heroin self-administration in rats (Li et al., 2011). The current data contribute to understanding how these behavioral effects of morphine conjugate vaccines are mediated. Further studies under different heroin dosing conditions should be helpful in determining the extent to which vaccine effects can be sustained over time and the antibody levels needed to do so.
Acknowledgments
The authors thank Ashli Tucker and Theresa Harmon for technical support.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- KLH
keyhole limpet hemocyanin
- 6-MAM
6-monoacetylmorphine
- M-6-G
morphine-6-glucuronide
- M-KLH
morphine conjugated to keyhole limpet hemocyanin
- %MPE
percent maximum possible effect
Authorship Contributions
Participated in research design: Raleigh, Pravetoni, Harris, Pentel.
Conducted experiments: Raleigh.
Contributed new reagents or analytical tools: Pravetoni, Birnbaum.
Performed data analysis: Raleigh, Pravetoni, Harris, Pentel.
Wrote or contributed to the writing of the manuscript: Raleigh, Pravetoni, Harris, Birnbaum, Pentel.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA026300, DA030715, and T32-DA07097].
Part of this work was presented previously: Raleigh MD, Pravetoni M, Le Naour M, Jones JM, Portoghese PS, Birnbaum AK, Pentel PR (2012) Effects of a morphine conjugate vaccine on heroin pharmacokinetics in rats, 74th Annual Meeting of the College on Problems of Drug Dependence; 2012 June 9–14; Palm Springs, CA.
References
- Andersen JM, Ripel A, Boix F, Normann PT, Mørland J. (2009) Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J Pharmacol Exp Ther 331:153–161 [DOI] [PubMed] [Google Scholar]
- Anton B, Leff P. (2006) A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 24:3232–3240 [DOI] [PubMed] [Google Scholar]
- Antonilli L, Petecchia E, Caprioli D, Badiani A, Nencini P. (2005) Effect of repeated administrations of heroin, naltrexone, methadone, and alcohol on morphine glucuronidation in the rat. Psychopharmacology (Berl) 182:58–64 [DOI] [PubMed] [Google Scholar]
- Bahar FG, Ohura K, Ogihara T, Imai T. (2012) Species difference of esterase expression and hydrolase activity in plasma. J Pharm Sci 101:3979–3988 [DOI] [PubMed] [Google Scholar]
- Bazin-Redureau MI, Renard CB, Scherrmann JM. (1997) Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab’)2 and Fab after intravenous administration in the rat. J Pharm Pharmacol 49:277–281 [DOI] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. (1974) Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252:708–710 [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. (2001) A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci USA 98:1988–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. (1999) Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology (Berl) 143:327–338 [DOI] [PubMed] [Google Scholar]
- Cornish KE, Harris AC, LeSage MG, Keyler DE, Burroughs D, Earley C, Pentel PR. (2011) Combined active and passive immunization against nicotine: minimizing monoclonal antibody requirements using a target antibody concentration strategy. Int Immunopharmacol 11:1809–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Friedland GH, Gourevitch MN. (2006) Opioid dependence: rationale for and efficacy of existing and new treatments. Clin Iinfect Dis 43 (Suppl 4):S173–177 [DOI] [PubMed] [Google Scholar]
- Girardin F, Rentsch KM, Schwab MA, Maggiorini M, Pauli-Magnus C, Kullak-Ublick GA, Meier PJ, Fattinger K. (2003) Pharmacokinetics of high doses of intramuscular and oral heroin in narcotic addicts. Clin Pharmacol Ther 74:341–352 [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. (2010) Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry 67:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, et al. (2011) Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. (1983) Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 33 (Suppl 1):773–776 [DOI] [PubMed] [Google Scholar]
- Jones JM, Raleigh MD, Pentel PR, Harmon TM, Keyler DE, Remmel RP, Birnbaum AK. (2013) Stability of heroin, 6-monoacteylmorphine, and morphine in biological samples and validation of an LC-MS assay for delayed analyses of pharmacokinetic samples in rats. J Pharm Biomed Anal 74:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. (2000) Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 148:251–262 [DOI] [PubMed] [Google Scholar]
- Klous MG, Van den Brink W, Van Ree JM, Beijnen JH. (2005) Development of pharmaceutical heroin preparations for medical co-prescription to opioid dependent patients. Drug Alcohol Depend 80:283–295 [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Zvartau E, Woody G. (2010) Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr Psychiatry Rep 12:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, Zhai HF, Shi J, Lu L. (2011) A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem 119:1271–1281 [DOI] [PubMed] [Google Scholar]
- Lobmaier P, Gossop M, Waal H, Bramness J. (2010) The pharmacological treatment of opioid addiction—a clinical perspective. Eur J Clin Pharmacol 66:537–545 [DOI] [PubMed] [Google Scholar]
- Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. (2005) Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry 58:158–164 [DOI] [PubMed] [Google Scholar]
- McCallum SE, Caggiula AR, Epstein LH, Saylor S, Ploskina T, Sved AF. (1999) Mecamylamine blocks the development of tolerance to nicotine in rats: implications for the mechanisms of tolerance. Psychopharmacology (Berl) 141:332–338 [DOI] [PubMed] [Google Scholar]
- Mendelson J, Flower K, Pletcher MJ, Galloway GP. (2008) Addiction to prescription opioids: characteristics of the emerging epidemic and treatment with buprenorphine. Exp Clin Psychopharmacol 16:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T, Kohno Y, Suwa T, Tsuji A. (1995) Species differences in hydrolysis of isocarbacyclin methyl ester (TEI-9090) by blood esterases. Biochem Pharmacol 49:1361–1365 [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, O’Connor PG. (2000) Naltrexone-induced nausea in patients treated for alcohol dependence: clinical predictors and evidence for opioid-mediated effects. J Clin Psychopharmacol 20:69–76 [DOI] [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P. (2000) Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol 22:603–614 [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, Pettersson S, Pentel PR. (2011) Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem Pharmacol 81:1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. (2012a) An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 341:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, Pentel PR. (2012b) Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30:4617–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. (2008) Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther 325:985–993 [DOI] [PubMed] [Google Scholar]
- Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH. (2006) Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol 1:109–118 [DOI] [PubMed] [Google Scholar]
- Salmon AY, Goren Z, Avissar Y, Soreq H. (1999) Human erythrocyte but not brain acetylcholinesterase hydrolyses heroin to morphine. Clin Exp Pharmacol Physiol 26:596–600 [DOI] [PubMed] [Google Scholar]
- Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. (2003) Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol 3:957–970 [DOI] [PubMed] [Google Scholar]
- Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR. (2001) mu Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compare with morphine. Biochem Pharmacol 62:447–455 [DOI] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. (2011) A vaccine strategy that induces protective immunity against heroin. J Med Chem 54:5195–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Koob GF. (1985) Effects of naloxone on heroin-, amphetamine- and caffeine-stimulated locomotor activity in the rat. Pharmacol Biochem Behav 23:499–501 [DOI] [PubMed] [Google Scholar]
- Tang YL, Hao W. (2007) Improving drug addiction treatment in China. Addiction 102:1057–1063 [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao D, Zhao C, Cubells JF. (2006) Opiate addiction in China: current situation and treatments. Addiction 101:657–665 [DOI] [PubMed] [Google Scholar]
- Umans JG, Inturrisi CE. (1982) Heroin: analgesia, toxicity and disposition in the mouse. Eur J Pharmacol 85:317–323 [DOI] [PubMed] [Google Scholar]
- Vindenes V, Ripel A, Handal M, Boix F, Mørland J. (2009) Interactions between morphine and the morphine-glucuronides measured by conditioned place preference and locomotor activity. Pharmacol Biochem Behav 93:1–9 [DOI] [PubMed] [Google Scholar]
- Way EL, Kemp JW, Young JM, Grassetti DR. (1960) The pharmacologic effects of heroin in relationship to its rate of biotransformation. J Pharmacol Exp Ther 129:144–154 [PubMed] [Google Scholar]