Abstract
Loss of function of the tumor suppressor LKB1 occurs in 30% to 50% of lung adenocarcinomas. Because LKB1 activates AMP-activated protein kinase (AMPK), which can negatively regulate mTOR, AMPK activation might be desirable for cancer therapy. However, no known compounds activate AMPK independently of LKB1 in vivo, and the usefulness of activating AMPK in LKB1-mutant cancers is unknown. Here, we show that lipid-based Akt inhibitors, phosphatidylinositol ether lipid analogues (PIA), activate AMPK independently of LKB1. PIAs activated AMPK in LKB1-mutant non–small cell lung cancer (NSCLC) cell lines with similar concentration dependence as that required to inhibit Akt. However, AMPK activation was independent of Akt inhibition. AMPK activation was a major mechanism of mTOR inhibition. To assess whether another kinase capable of activating AMPK, CaMKKβ, contributed to PIA-induced AMPK activation, we used an inhibitor of CaMKK, STO-609. STO-609 inhibited PIA-induced AMPK activation in LKB1-mutant NSCLC cells, and delayed AMPK activation in wild-type LKB1 NSCLC cells. In addition, AMPK activation was not observed in NSCLC cells with mutant CaMKKβ, suggesting that CaMKKβ contributes to PIA-induced AMPK activation in cells. AMPK activation promoted PIA-induced cytotoxicity because PIAs were less cytotoxic in AMPKα−/− murine embryonic fibroblasts or LKB1-mutant NSCLC cells transfected with mutant AMPK. This mechanism was also relevant in vivo. Treatment of LKB1-mutant NSCLC xenografts with PIA decreased tumor volume by ∼50% and activated AMPK. These studies show that PIAs recapitulate the activity of two tumor suppressors (PTEN and LKB1) that converge on mTOR. Moreover, they suggest that PIAs might have utility in the treatment of LKB1-mutant lung adenocarcinomas.
Introduction
LKB1, or serine/threonine kinase 11 (STK11), is a tumor suppressor gene located on the short arm of chromosome 19 (1). Germ line mutations of LKB1 occur in Peutz-Jeghers syndrome, which is characterized by intestinal hamartomas and an increased predisposition to many types of cancer. However, LKB1 mutations are uncommon in most sporadically occurring cancers. A notable exception is non–small cell lung cancer (NSCLC), in which somatic LKB1 inactivation is estimated to occur in 30% to 50% of sporadically occurring lung adenocarcinomas (1–4). More recently, LKB1 mutations have also been described in ∼20% of squamous cell carcinomas and ∼10% of large cell carcinomas of the lung (4). Because 212,000 cases of lung cancer are projected to be diagnosed in the U.S. in 2007 and 87% of lung cancer cases are NSCLC, it is therefore possible that >80,000 NSCLC patients will have tumors that bear LKB1 mutations (5).
LKB1 activates AMP-activated protein kinase (AMPK) when the intracellular AMP/ATP ratio is increased (6–9). AMPK is a heterotrimeric protein composed of α-catalytic, and β- and γ-regulatory subunits. Conditions that deplete intracellular energy activate AMPK by direct binding of AMP to four tandem repeats of cystathionine-β synthase sequences located in the γ (regulatory) subunit (10). Although AMP binding does not seem to directly promote LKB1 phosphorylation of T172 in the activation loop, it blocks dephosphorylation and inactivation by phosphatase PP2C (11). Phosphorylation at this site is required for the activation of AMPK.
The LKB1/AMPK pathway is linked to tumor growth and proliferation through regulation of the mTOR pathway. AMPK inhibits mTOR by directly phosphorylating the tumor suppressor TSC2 at S1227 and S1345, which causes its activation. TSC2 is a component of a complex that negatively regulates mTOR (12–14). TSC2 is also regulated by the serine/threonine kinase Akt, which phosphorylates TSC2 at T1462, leading to its inactivation (15). Importantly, regulation of TSC2 by AMPK is independent of Akt activation, which is the best-described stimulus for mTOR activation. mTOR is a bona fide target in cancer treatment and prevention because it regulates cellular growth and protein synthesis through downstream targets such as 4E-BP1, S6K, and S6 (reviewed in ref. 16). Based on its regulation of mTOR, activation of the LKB1/AMPK pathway could be a mechanism to prevent the formation and progression of cancer, and AMPK activators may have potential as anticancer agents. Despite the potentially strong rationale to target AMPK in LKB1-mutant cancers, this approach has not been tested because no compounds have been identified that can activate AMPK independently of LKB1 in vivo.
Phosphatidylinositol ether lipid analogues (PIAs) were designed to target the pleckstrin homology domain of the serine/threonine kinase, Akt. Screening of these compounds in cancer cell lines showed that 5 of 25 analogues inhibited Akt and preferentially induced apoptosis in cancer cell lines with high levels of endogenous Akt activity (17). When these compounds were screened in the NCI-60 cell line panel, cytotoxicity correlated with levels of phosphorylated Akt but not total Akt, but other molecular markers were also identified that had higher correlations with activity (18). In addition, these studies showed that PIAs induced more apoptosis than other pathway inhibitors despite similar levels of Akt inhibition, which suggested that other targets of PIAs might contribute to the cytotoxicity of these compounds. To assess effects on other kinases, we screened the 5 active PIAs against 29 purified kinases and showed that although no kinase was uniformly inhibited by the five active PIAs, two kinases were uniformly activated, p38α and AMPK (19). Subsequently, we characterized the mechanism of activation of p38 by PIAs and showed that p38 activation contributed to PIA-induced cytotoxicity (19). However, because a portion of cytotoxicity was still independent of p38 and Akt, and AMPK is an emerging target in cancer, we therefore investigated the role of AMPK in the cellular response to PIAs.
We report that PIAs induce the phosphorylation and activation of AMPK in lung cancer cells in vitro and in vivo by a mechanism that is independent of LKB1. Activation of AMPK was also independent of other previously described activities of PIAs (inhibition of Akt and activation of p38α), but was dependent on an upstream kinase of AMPK, CaMKKβ. Because AMPK activation contributes to PIA cytotoxicity, our data suggest that PIAs could potentially fulfill a unique therapeutic niche in treating NSCLC patients whose tumors bear LKB1 mutations.
Materials and Methods
Materials
The synthesis of PIAs has previously been described (20). P-AMPK (T172) monoclonal antibody, pan-AMPKα, P-ACC (S79), P-p38 (T180/Y182), P-hsp27 (S82), P-Akt (S473), P-GSK3α/β (S21/9), and P-S6K (S389) were purchased from Cell Signaling Technologies. LKB1 antibody was purchased from Upstate. STO-609, SB203580, and LY294002, were purchased from Calbiochem. Protease inhibitor cocktail was obtained from Sigma Chemical Co., and protein assay materials were from Pierce. Wild-type (wt) LKB1 cDNA and AMPK dominant-negative K(45)R plasmids were generously provided by Dr. Tomi Makela (University of Helsinki, Helsinki, Finland) and Dr. Craig Thompson (Abramson Family Cancer Research Institute, University of Pennsylvania, Philadelphia, PA), respectively, and have been previously described (1, 21).
Cell culture
The NSCLC cell lines A549 and H460 both contain a point mutation in codon 37 (Gln to stop) of the LKB1 gene, as previously described (3). The H157 NSCLC cell line contains a 1.5 kb deletion spanning exons 2 and 3 (kinase domain) of the LKB1 gene (22). The H1155 NSCLC cell line contains wt LKB1 (3). All NSCLC cell lines were established at NCI/Navy Medical Oncology. The prostate cancer cell line PC3 was obtained from the American Type Culture Collection. The isogenic wt and p38α−/− murine embryonic fibroblasts (MEFs) were a kind gift from Dr. Michael Karin (University of California, San Diego, CA). The immortalized isogenic wt and LKB1−/− MEFs were established by Dr. Tomi Makela (University of Helsinki, Helsinki, Finland). The lung cancer cell line H1770 contains a missense mutation (P127L) in the CaMMKβ gene, as previously described (23). The immortalized isogenic AMPKα wt and −/− MEFs were created by Dr. Keith Laderoute and are described in ref. (24). All cell lines were maintained in 175 cm2 flasks in RPMI and supplemented with 5% (v/v) fetal bovine serum (FBS), 100 units/mL of penicillin, and 100 mg/mL of streptomycin. Cells were incubated at 37°C in a 5.0% CO2 atmosphere.
Transient transfections
Cells (1 × 106) were nucleofected with 4 μg of LKB1-wt cDNA construct, AMPK DN construct, or vector using nucleofector technology (Amaxa). Following nucleofection, 4 × 105 cells/well were plated in six-well plates in RPMI + 5% FBS. Cells were treated 24 h posttransfection.
Glucose depletion study
Cells were plated at a density of 4 × 105 cells per well in six-well plates in RPMI containing 5% FBS and incubated for 24 h. The medium was then changed to RPMI containing 5% FBS with or without 25 mmol/L of glucose. Cells were incubated under these conditions for 2 h and were harvested for immunoblot analysis as described below.
Pharmacologic treatments
Cells were plated at a density of 4 × 105 cells per well in six-well plates in RPMI containing 5% FBS and incubated for 24 h. The medium was then changed to RPMI with 0.1% FBS just prior to pharmacologic treatment. Cells were treated with 10 μmol/L of PIAs dissolved in DMSO for the indicated time periods. For experiments involving pretreatment of cells with kinase inhibitors, the medium was changed to 0.1% RPMI and cells were treated with LY294002 or SB203580 dissolved in DMSO or STO-609 dissolved in 50% DMSO and 100 mmol/L of NaOH, prior to treatment with PIA5. In all experiments, vehicle was added to control samples and had no effect on kinase phosphorylation or activity. After incubation with PIAs, the cells were harvested for immunoblot analysis or for analysis of total cell death or apoptosis, as described below.
Immunoblotting
Cell extracts were prepared by adding 2× Laemmli sample buffer supplemented with protease inhibitor cocktail as described previously (25). Lysates were sonicated for 15 s with a Vibra Cell sonicator. The protein yield was quantified using the bicinchoninic acid protein assay kit. Equivalent protein was loaded and the lysates were separated by SDS-PAGE and then transferred to 0.45-μm nitrocellulose membranes. Equivalent loading was confirmed by staining membranes with fast green as previously described (26). The membranes were blocked for 1 h in blocking buffer (1× TBS, 5% milk, 0.1% Tween 20) and placed in primary antibody (1× TBS, 5% bovine serum albumin, 0.1% Tween 20, 1:1,000 antibody) overnight at 4°C. Primary antibody was detected using horseradish peroxidase–linked goat anti-mouse or goat anti-rabbit IgG antibodies (Cell Signaling) and visualized with the enhanced chemiluminescent detection system (Pierce Super Signal). Immunoblot experiments were performed at least thrice. Densitometry was performed using NIH Image software.
PIA treatment of H157 and PC3 xenografts
H157 LKB1-mutant NSCLC cells and PC3 prostate cancer cells were obtained from the American Type Culture Collection and cultured in RPMI + 5% FBS. Male 6-week-old athymic nude mice (athymic NCr-nu/nu) were inoculated s.c. with 1 × 107 H157 cells (efficacy study) or PC3 cells (biomarker study) in each rear flank. When tumors reached 200 mm2 (day 15 post-tumor implant), the mice were randomized into vehicle (n = 12 for H157 xenograft study or n = 3 for PC3 xenograft study) and PIA-treated (n = 12 or 3) groups. PIAs were dissolved in a vehicle of 10% DMSO in saline/Tween 80. The mice received i.p. injections of vehicle, 90 mg/kg of PIA5 qd ×5 (efficacy study), or 40 mg/kg of PIA23 every 8 h ×3 (biomarker study). Four hours after the last dose, three mice from each group were sacrificed, and tumors were fixed in 10% formalin for immunohistochemical analysis of AMPK activation. For immunoblotting, tumor lysates were prepared in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. Immunoblots were performed as described above. For the efficacy study, tumor volume was measured on days 1, 3, 5, and 9 of the study. Data points represent the median tumor volume ± SE.
Immunohistochemistry
Immunohistochemistry was performed on tumors harvested from three of the vehicle-treated mice from each xenograft study and three of the mice treated with PIA5 or PIA23. Tumors were paraffin-imbedded, sectioned, and placed on slides (Histoserv, Inc.). Antigen retrieval was performed in target retrieval solution (DakoCytomation California, Inc.). P-AMPK antibody (Cell Signaling) was used at a dilution of 1:50. To verify the specificity of staining, one tissue section was incubated in a solution containing P-AMPK antibody in the presence of a 1:10 dilution of blocking peptide obtained from Cell Signaling. Detection was performed by standard avidin-biotin complex methods. Immunohistochemistry was quantified by assigning a score of absent (0), minimal (1), moderate (2), or high (3) staining to each cell in three 40× magnification fields. The staining index was then calculated by multiplying the staining intensity by its distribution. An overall score was assigned to each slide, and the scores were averaged for vehicle versus PIA23-treated groups.
Cell death assays
Cells were treated with PIAs for 12 h as described above. Floating cells were collected, and adherent cells were harvested by trypsinization and then centrifuged at 1,000 × g for 5 min. Cells were incubated with 1 μg/mL of propidium iodide. Cell uptake of propidium iodide was quantified using a Becton Dickinson FACsort and by manual gating using CellQuest software. Cell death experiments were performed in triplicate and were repeated at least thrice.
Apoptosis assays
Cells were treated with PIAs for 12 h and collected as described above. Cells were fixed in ice-cold 70% methanol added dropwise, and then incubated at −20°C for 30 min. Cells were centrifuged and incubated with propidium iodide (25 μg/mL) supplemented with RNase A (30 μg/mL) for 30 min at room temperature. Quantification of sub-2N DNA was determined by flow cytometry. Apoptosis experiments were performed in triplicate and were repeated at least thrice.
Results
PIAs activate AMPK in LKB1-mutant NSCLC cells
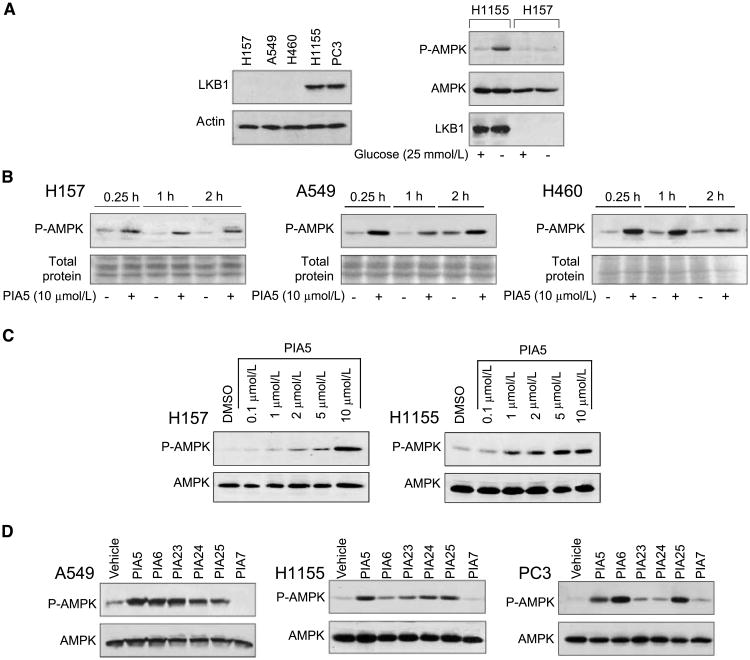
Because PIAs increased the activity of purified AMPK by ∼40% in the absence of LKB1 in vitro (19), we investigated if they could activate AMPK in a panel of cancer cell lines that varied in LKB1 mutational status. Four NSCLC cell lines (H157, H1155, H460, and A549) and one prostate cancer cell line (PC3) were tested. Immunoblotting analysis showed that LKB1 protein expression was absent in the H157, A549, and H460 cells and present in H1155 and PC3 cells, consistent with their mutational status (Fig. 1A, left). To determine if AMPK could be activated in response to a normal stimulus (glucose depletion), H1155 and H157 cells were incubated in the absence of glucose (Fig. 1A, right). Glucose depletion induced AMPK phosphorylation in cells with wild-type but not mutant LKB1. To investigate if PIAs activated AMPK in the absence of LKB1, we treated the three LKB1-mutant NSCLC cell lines (H157, A549, and H460) with PIA5. PIA5 caused rapid phosphorylation of AMPK at T172 that was sustained to 2 h in these cell lines (Fig. 1B). Despite increased AMPK phosphorylation in the absence of LKB1, these effects were not likely due to increases in AMP levels, because PIAs did not change the intracellular AMP/ATP ratio in H157 cells (data not shown). Activation of AMPK was also dose-dependent. PIA5 activated AMPK in H157 cells at doses of >1 μmol/L, and maximal activation occurred at 10 μmol/L (Fig. 1C, left). Dose dependence was also assessed in H1155 cells that have wild-type LKB1 (Fig. 1C, right). As was observed in LKB1-mutant H157 cells, 1 μmol/L of PIA5 was sufficient to induce AMPK phosphorylation, but maximal phosphorylation was achieved at a lower dose (5 μmol/L). Collectively, these experiments show that PIA5 rapidly activates AMPK in NSCLC cell lines independently of LKB1, and that AMPK activation occurs with similar time- and dose-dependence as inhibition of Akt and activation of p38α.
Figure 1.
PIAs activate AMPK in LKB1-mutant NSCLC cells. A, left, correlation of LKB1 mutational status with protein expression. Immunoblotting for levels of LKB1 protein expression was performed in a panel of NSCLC cell lines and PC3 cells. Right, activation of AMPK in response to glucose deprivation in NSCLC cells that vary in LKB1 status. H1155 and H157 cells were incubated in the presence or absence of 25 mmol/L of glucose for 2 h. Immunoblotting was performed using antibodies specific for the phosphorylated or native states of AMPK, as well as native LKB1. B, LKB1-mutant NSCLC cell lines H157, A549, and H460 were treated with PIA5 for the indicated times. C, H157 and H1155 cells were treated with varying concentrations of PIA5 for 2 h. D, LKB1-mutant A549 cells, LKB1-wt H1155 cells or PC3 cells were treated with PIA5, PIA6, PIA7, PIA23, PIA24, or PIA25 for 2 h. Immunoblotting in C and D was performed using antibodies specific to phosphorylated or native AMPK.
To assess whether AMPK activation was common among the five PIAs that inhibit Akt, we treated LKB1-mutant and wild-type NSCLC cell lines with the five active PIAs (Fig. 1C, left and middle). In both LKB1-mutant and -wt cells, the active PIAs increased AMPK phosphorylation in comparison to an inactive PIA (PIA7; Fig. 1D). The magnitude of induction of AMPK phosphorylation was specific for individual PIAs and was cell line–dependent. PIA-induced activation of AMPK was not specific to NSCLC cells because the active PIAs, but not PIA7, increased AMPK phosphorylation in the prostate cancer cell line, PC3 (Fig. 1D, bottom). Because the active PIAs and inactive PIA differ by the presence or absence of an inositol ring, respectively, these studies suggest that the ability of PIAs to activate AMPK depends on the inositol ring and not the lipid side chain that is shared by all PIAs.
Phosphorylation of AMPK by PIAs occurs independently of Akt inhibition and p38α activation
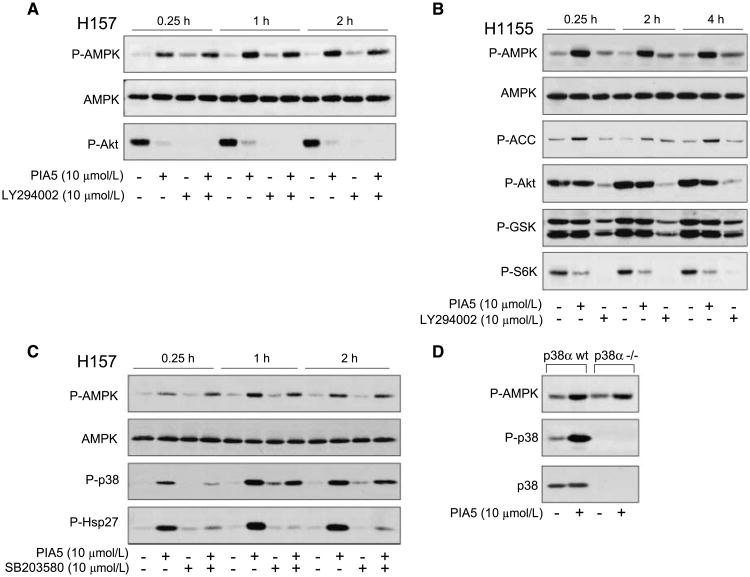
Because PIAs independently inhibit Akt and activate p38α (19), we sought to determine whether AMPK activation was independent of these activities. To test independence from Akt inhibition, we pretreated NSCLC cell lines with LY294002 to inhibit the phosphoinositide-3-kinase/Akt pathway prior to PIA administration. In H157 cells, PIA5 increased phosphorylation of AMPK and inhibited Akt phosphorylation within 15 min, which was sustained throughout the 2-h time course (Fig. 2A). LY294002 alone rapidly inhibited Akt and only slightly increased phosphorylation of AMPK over baseline levels. Pretreatment of H157 cells with LY294002 did not affect PIA-induced AMPK phosphorylation. Pretreatment with LY294002 for longer periods (18 h) before addition of PIA5 also did not affect PIA-induced AMPK activation (data not shown). These data show that even when Akt is completely inhibited, PIAs could activate AMPK.
Figure 2.
Phosphorylation of AMPK by PIA5 occurs independently of other activities of PIAs. A, H157 cells were pretreated with vehicle or LY294002, then incubated with PIA5 or LY294002 for the indicated times. Immunoblotting was performed using antibodies specific for phosphorylated and native AMPK or phosphorylated Akt. B, H1155 cells were treated with PIA5 or LY294002 for the indicated times. Phosphorylation of AMPK, total AMPK, as well as phosphorylation of Akt, downstream substrates of AMPK (ACC) and Akt (GSK3α/β), and a downstream substrate of mTOR (S6K) are shown. C, H157 cells were pretreated with vehicle or SB203580, and then treated with vehicle or PIA5 for the indicated times. Phosphorylation of AMPK, p38, and hsp27, as well as total levels of AMPK were assessed. D, isogenic wild-type or p38α−/− MEFs were treated with PIA5 for 2 h prior to immunoblotting for phosphorylated AMPK, as well as phosphorylated and total p38α.
To confirm the independence of Akt inhibition and AMPK activation, we treated H1155 cells, which do not decrease Akt activation with PIA administration, with PIA5. PIA5 alone only minimally inhibited phosphorylation of Akt and its substrate GSK3β (Fig. 2B), even when treatment was extended to 18 h (data not shown). In contrast, LY294002 markedly inhibited Akt phosphorylation. Although PIA5 minimally inhibited Akt, it markedly activated AMPK and inhibited the mTOR pathway, as shown by decreased phosphorylation of one of the downstream components of the mTOR pathway, S6K. This observation is consistent with AMPK's established role as a negative regulator of the mTOR pathway (12, 14). These studies show that PIAs cause rapid and sustained phosphorylation of AMPK in cells independently of their ability to inhibit Akt. Moreover, in some cell types such as H1155 cells, activation of AMPK might be a major mechanism for mTOR inhibition.
To show that phosphorylation of AMPK by PIAs also occurs independently of p38α activation, two approaches were employed. First, we inhibited p38 with a small molecule inhibitor, SB203580, prior to PIA administration. In H157 cells, PIA5 alone rapidly induced the phosphorylation of AMPK, p38α, and one of its substrates, hsp27 (Fig. 2C). These effects were maintained throughout the 2-h time course. Pretreatment with SB203580 inhibited PIA5-induced phosphorylation of p38 and hsp27, but did not affect the ability of PIA5 to induce the phosphorylation of AMPK. Similar results were observed when we treated p38α−/− MEFs or wt MEFs with PIA5 and assessed AMPK and p38α phosphorylation (Fig. 2D). In both cell lines, induction of AMPK phosphorylation was observed. These results show that activation of AMPK and p38α are two distinct activities of PIAs.
CaMKKβ, but not LKB1, is necessary for PIA-induced AMPK activation in cells
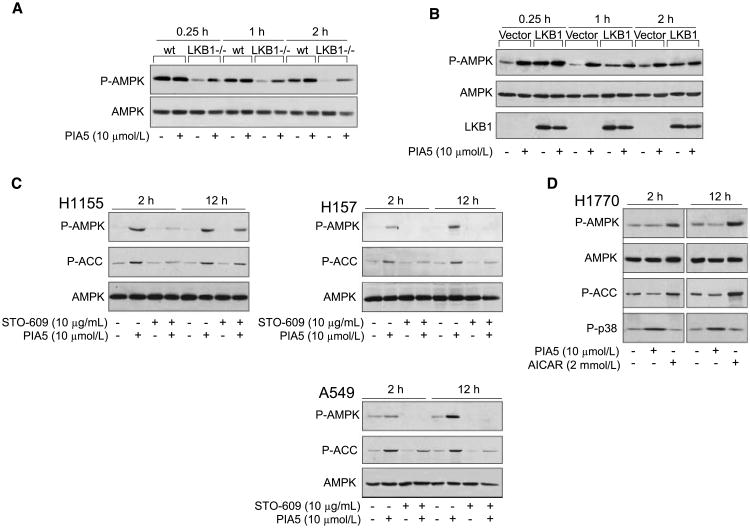
Because we observed that LKB1 was not required for activation of AMPK by PIAs, we wanted to assess whether the presence of LKB1 could contribute to AMPK activation by PIAs. We treated immortalized LKB1−/− or wt MEFs with PIA5. Basal levels of AMPK phosphorylation were higher in wt MEFs. Treatment of LKB1−/− MEFS with PIA5 resulted in rapid AMPK phosphorylation similar to the NSCLC cell lines (Fig. 3A). In the wt MEFs, treatment with PIA5 caused a smaller relative increase in the phosphorylation of AMPK, and did not occur until a later time point (2 h), suggesting that the contribution of LKB1 to AMPK activation by PIAs is minimal. We then reconstituted LKB1 into LKB1−/− cells and assessed AMPK phosphorylation with PIA treatment (Fig. 3B). LKB1−/− MEFs transfected with vector alone exhibited the same rapid induction of AMPK phosphorylation as that observed in untransfected LKB1−/− MEFs (Fig. 3A). In contrast, LKB1−/− MEFs reconstituted with LKB1 showed a diminished ability to induce AMPK phosphorylation that was similar to the wt MEFs. Taken together, these studies suggest that LKB1 does not significantly contribute to PIA-induced activation of AMPK.
Figure 3.
CaMKKβ but not LKB1, is necessary for PIA-induced AMPK activation in cells. A, immortalized isogenic wild-type or LKB1−/− MEFs were treated with PIA5 for the indicated times, and phosphorylation of AMPK was assessed by immunoblotting. B, reconstitution of wt LKB1 into LKB1−/− MEFs. LKB1−/− MEFs were nucleofected with vector or wt LKB1 cDNA construct as described in Materials and Methods. Transfected cells were treated with vehicle or PIA5 for he indicated times. Expression of LKB1 and phosphorylation and expression of AMPK were assessed by immunoblotting. C, H1155 (left), H157 (top right), or A549 (bottom right) cells were pretreated with vehicle or STO-609, followed by treatment with vehicle or PIA5 for the indicated times. D, the CaMKKβ mutant lung cancer cell line H1770 was treated with PIA5 for the indicated times. Immunoblotting in C and D was performed using antibodies that recognize the phosphorylated forms of AMPK, ACC, and p38, as well as native AMPK.
Similar to LKB1, CaMKKβ could also directly activate AMPK by phosphorylation of T172 (27–29). To determine if CaMKKβ contributed to PIA-induced AMPK phosphorylation at this residue, we pretreated LKB1-wt (H1155) and LKB1-mutant (H157 and A549) NSCLC cells with an inhibitor of CAMKK, STO-609 (30). STO-609 markedly inhibited PIA-induced phosphorylation of AMPK and ACC at 2 h in H1155 cells (Fig. 3C, left), but phosphorylation partially recovered at 12 h. Repeated dosing of STO-609 did not affect recovery at 12 h (data not shown), which suggests that this recovery is not due to the metabolism of STO-609 and could be related to the utilization of LKB1.
We repeated these studies in two LKB1-mutant cell lines, H157 and A549. As we observed in H1155 cells with wt LKB1, pretreatment with STO-609 effectively inhibited PIA-induced phosphorylation of AMPK and ACC at 2 h in both LKB1-mutant cell lines (Fig. 3C, top and bottom right). However, in contrast to what we observed in the LKB1-wt cells, STO-609 inhibition of PIA-induced AMPK activation was sustained through 12 h in both cell lines. To confirm a role for CaMKKβ in the activation of AMPK by PIAs, we treated a NSCLC cell line that bears a mutation in CaMKKβ (H1770) with PIA5 (23). Although PIA5 activated p38 in H1770 cells, activation of AMPK or ACC was not observed at either time point (Fig. 3D). Treatment of H1770 cells with AICAR, which activates AMPK by an LKB1-dependent mechanism, confirmed that AMPK could be activated in these cells. Collectively, these studies suggest that PIA-induced activation of AMPK in cells is dependent on CaMKKβ when LKB1 is mutated.
PIAs inhibit the growth of LKB1-mutant NSCLC tumors and induce AMPK activation in vivo
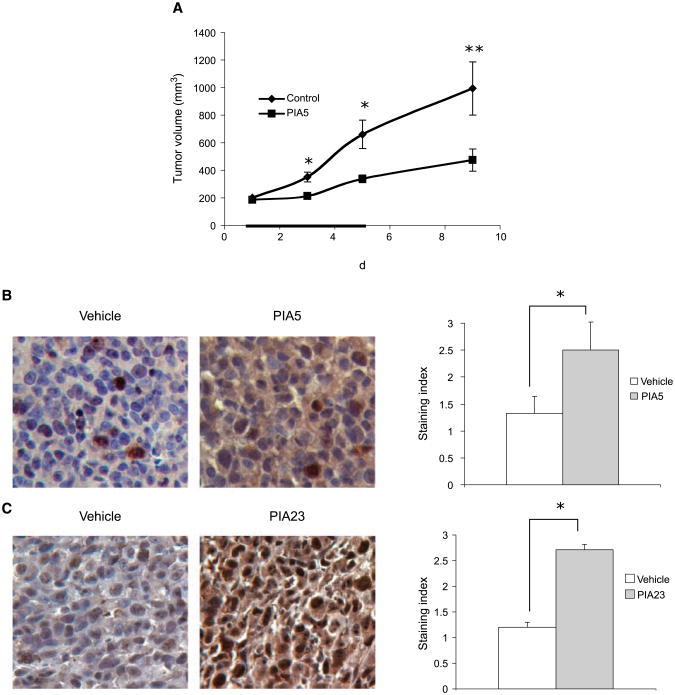
To determine if PIAs can inhibit tumor growth and activate AMPK independently of LKB1 in vivo, nude mice bearing LKB1-mutant H157 human NSCLC xenografts were given i.p. injections of vehicle or PIA5 once a day for 5 days after tumors were allowed to form. PIA caused a 55% decrease in tumor volume, an effect that was sustained over the course of the study (Fig. 4A). PIA-induced phosphorylation of AMPK occurred independently of LKB1 in vivo because immunohistochemical analysis of tumors from mice treated with PIA showed a 2-fold increase in staining index for phosphorylation of AMPK (Fig. 4B). These results show that PIA5 inhibits tumor growth and activates AMPK in LKB1-mutant NSCLC cells in vivo.
Figure 4.
PIAs inhibit tumor growth and induce the phosphorylation of AMPK independently of LKB1 in vivo. A, nude mice bearing H157 xenografts received 90 mg/kg of PIA5 qd ×5 i.p. (days 1–5, black line). Assessment of tumor volume in xenografts. *, P < 0.005; **, P < 0.05. B, representative immunohistochemistry of tumors for P-AMPK (left) and quantification of immunohistochemistry (right). *, P < 0.001. C, representative staining for P-AMPK of vehicle and PIA23-treated tumors from nude mice bearing PC3 xenografts (left), and quantification of immunohistochemistry (right). *, P < 0.001.
To confirm that activation of AMPK in vivo was not cell line–specific or limited to a specific PIA, we performed a short-term xenograft study with a different cell line (PC3) and a different PIA (PIA23), and assessed the phosphorylation of AMPK after 1 day. Activation of AMPK in tumors was assessed by immunoblotting or immunohistochemical analysis. Immunoblotting for phosphorylated AMPK in these tumors showed a statistically significant >2-fold increase in AMPK phosphorylation in mice that received PIA23 versus mice that received vehicle (data not shown). Immunohistochemical analysis showed that PIA23 caused a 2.3-fold increase in the staining index for phosphorylation of AMPK in tumors (Fig. 4C). These studies indicate that induction of AMPK phosphorylation in vivo can be detected by immunoblotting or immunohistochemistry, and that induction is not cell line–specific or PIA-specific. Moreover, they suggest that AMPK phosphorylation might be useful as a biomarker for PIA administration in vivo.
Loss of AMPK attenuates the cytotoxicity of PIAs
To investigate the contribution of AMPK to PIA-induced cytotoxicity, we assessed apoptotic and nonapoptotic cell death induced by PIA5 in immortalized AMPKα−/− MEFs or isogenic AMPKα wt MEFs. PIA5 caused 60% of wt MEFs to undergo cell death (Fig. 5A). Loss of AMPK increased resistance to PIA5 because PIA5 only caused the death of 31% of the AMPKα−/− MEFs. Similar results were observed when apoptosis was assessed. Thirty-two percent of PIA-treated AMPKα wt MEFs were apoptotic, but PIA treatment caused only 17% of AMPKα−/− cells to undergo apoptosis (Fig. 5B, left). These results show that loss of AMPK attenuates apoptotic and nonapoptotic cell death caused by PIAs.
Figure 5.
AMPK activation contributes to the cytotoxicity of PIAs. A, assessment of total cell death (left) and apoptosis (right) in AMPKα wt or −/− MEFs treated with PIA5 for 12 h. Columns, mean of cells used in triplicate; bars, SD. *, P < 0.001 for drug treatment; **, P < 0.001 for cell type. B, AMPKα wt or −/− MEFs were treated with PIA5 for the indicated times. Immunoblotting was performed using antibodies that recognize the phosphorylated forms of AMPK, ACC, Akt, S6K, as well as total Akt. C, assessment of total cell death for H157 cells transfected with vector or a dominant-negative AMPK construct and treated with vehicle or PIA5 for 12 h. Columns, mean of cells used in triplicate; bars, SD. *, P < 0.001 for drug treatment; **, P < 0.001 for cell type.
To evaluate changes in pathway activation that accompany cell death, we assessed the phosphorylation of AMPK, ACC, Akt, and S6K in each type of MEF. In wt MEFs, PIA5 increased the phosphorylation of AMPK as well as one of its substrates, ACC, and inhibited the phosphorylation of Akt and S6K (Fig. 5B). However, in AMPKα−/− MEFS, PIA5 did not decrease S6K phosphorylation despite decreased Akt phosphorylation (Fig. 5A). This again suggests that AMPK activation is a major mechanism of mTOR pathway inhibition by PIAs.
To confirm that AMPK activation contributes to PIA-induced cell death in NSCLC cells, we transiently transfected H157 cells with a construct that encodes a dominant-negative mutant form of AMPK (K45R) or a vector control, and measured total cell death induced by PIA5. Consistent with data obtained in AMPKα−/− MEFs, transfection of H157 cells with the dominant-negative AMPK construct resulted in 50% less cell death in response to treatment with PIA5 than cells transfected with vector (Fig. 5C). These results confirm that AMPK activation by PIAs contributes to their cytotoxicity.
Discussion
Our studies show that the lipid-based Akt inhibitors, PIAs, independently activate AMPK through multiple mechanisms. PIAs directly activate purified AMPK in the absence of upstream kinases. In cells, PIAs activate AMPK by inducing the phosphorylation of AMPK on T172, an effect that seemed to be dependent on CaMKKβ but was independent of LKB1. Pretreatment of NSCLC cells with the CaMKK inhibitor STO-609 completely abolished PIA-induced activation of AMPK in LKB1-mutant cells, and delayed activation in cells with wt LKB1. Moreover, PIAs failed to activate AMPK in CaMKKβ mutant cells. These results show that activation of AMPK preferentially occurs through CaMKKβ.
Although PIAs activated AMPK with similar kinetics and dose dependence as Akt inhibition and p38α activation, AMPK activation is a distinct activity of PIAs because inhibition of phosphoinositide-3-kinase or p38 did not affect AMPK activation by PIAs. Likewise, Akt inhibition and p38 activation by PIAs are not likely to be dependent on AMPK because PIAs modulated these targets in AMPKα−/− MEFs. The ability of PIAs to independently affect the activities of AMPK, Akt, and p38α shows the broad biological activities of these compounds, and highlights their diverse potential as anticancer agents.
As PIAs independently inhibit Akt and activate AMPK, they are distinct among existing cancer chemotherapeutics because they recapitulate the activity of two tumor suppressors, LKB1 and PTEN, to negatively regulate the mTOR pathway (Fig. 6). For example, even in cell lines such as H1155 in which PIAs only marginally inhibited Akt, they still inhibited the mTOR pathway through activation of AMPK. Interestingly, PIAs failed to inhibit the mTOR pathway in AMPKα−/− MEFS, suggesting that AMPK activation is a major mechanism by which PIAs inhibit mTOR. Because mTOR pathway activation promotes tumorigenesis and tumor growth, the ability of PIAs to inhibit this pathway through two mechanisms could contribute to their potential efficacy as anticancer agents.
Figure 6.
PIAs recapitulate the activity of two tumor suppressors that converge on mTOR. PIAs independently inhibit Akt and activate AMPK to negatively regulate the mTOR pathway in cells. Cellular activation of AMPK by PIAs occurs by a CaMKKβ-dependent mechanism. Conversely, other activators of AMPK (AICAR and metformin) activate AMPK by an LKB1-dependent mechanism. Gray boxes, tumor suppressors.
The ability of PIAs to activate AMPK in an LKB1-independent manner in vivo is unique among existing activators of AMPK and could have clinical implications. For example, activators of AMPK such as AICAR and metformin activate AMPK by an LKB1-dependent mechanism (7, 8). Although 2-deoxyglucose can activate AMPK in the absence of LKB1 (28), this compound significantly alters the intracellular AMP/ATP ratio, and the level of AMPK activation induced by 2-deoxyglucose is directly related to the presence of LKB1 (22, 31). Moreover, 2-deoxyglucose is unlikely to activate AMPK in vivo because millimolar concentrations of 2-deoxyglucose are required to activate AMPK in cultured cells, which is not likely to be achievable in vivo. Another AMPK activator, the thienopyridone A769662, can activate purified AMPK, but the role of upstream kinases in mediating cellular activation of AMPK by A769662 is unclear and A769662 has not been shown to activate AMPK in vivo (32, 33). Therefore, PIAs are the first compounds, to our knowledge, that activate AMPK independently of LKB1 in vivo, which may be useful in the treatment of LKB1-mutant tumors. This may be especially relevant for NSCLC, in which LKB1 mutations are common. The fact that inhibition of tumor growth by PIAs in vivo correlated with the induction of AMPK phosphorylation in tumor tissue suggests that AMPK phosphorylation could serve as a useful biomarker of PIA administration. More broadly, our study lends support to the development of cancer chemotherapeutics that target AMPK, and represents the first characterization of compounds that induce cytotoxicity in NSCLC cells by LKB1-independent activation of AMPK.
Acknowledgments
Grand support: Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and federal funds from the National Cancer Institute, NIH, under NCI contract NO1-CO-12400.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 2.Ghaffar H, Sahin F, Sanchez-Cepedes M, et al. LKB1 protein expression in the evolution of glandular neoplasia of the lung. Clin Cancer Res. 2003;9:2998–3003. [PubMed] [Google Scholar]
- 3.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- 4.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;7155:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 6.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 11.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–48. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura N, Tokunaga C, Dalal S, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 15.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 16.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–61. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Castillo SS, Brognard J, Petukhov PA, et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64:2782–92. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 18.Gills JJ, Holbeck S, Hollingshead M, Hewitt SM, Kozikowski AP, Dennis PA. Spectrum of activity and molecular correlates of response to phosphatidylinositol ether lipid analogues, novel lipid-based inhibitors of Akt. Mol Cancer Ther. 2006;5:713–22. doi: 10.1158/1535-7163.MCT-05-0484. [DOI] [PubMed] [Google Scholar]
- 19.Gills JJ, Castillo SS, Zhang C, et al. Phosphatidylinositol ether lipid analogues that inhibit AKT also independently activate the stress kinase, P38α, through MKK3/6-independent and -dependent mechanisms. J Biol Chem. 2007;37:27020–9. doi: 10.1074/jbc.M701108200. [DOI] [PubMed] [Google Scholar]
- 20.Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PIanalogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125:1144–5. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- 21.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Zhong D, Guo L, de Aguirre I, et al. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer. 2006;53:285–94. doi: 10.1016/j.lungcan.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Greenman C, Stephens P, Smith R, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laderoute KR, Amin K, Calaoagan JM, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canman CE, Wolff AC, Chen CY, Fornace AJ, Jr, Kastan MB. The p53-dependent G1 cell cycle checkpoint pathway and ataxia-telangiectasia. Cancer Res. 1994;54:5054–8. [PubMed] [Google Scholar]
- 26.Kastan MB, Zhan Q, el-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 27.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 29.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J Biol Chem. 2002;277:15813–8. doi: 10.1074/jbc.M201075200. [DOI] [PubMed] [Google Scholar]
- 31.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–5. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 32.Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Goransson O, McBride A, Hawley SA, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;45:32549–60. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]