Abstract
Leukotrienes are classical mediators of inflammatory response. New aspects of leukotriene function have recently been described. We examine here the previously unreported role that leukotrienes play in the regulation of cytokines in a murine model of histoplasmosis. We demonstrate that administration of MK 886, a leukotriene synthesis inhibitor, caused Histoplasma capsulatum-infected mice to die by the day 15 of infection, whereas the correlating death rate in untreated infected mice was 0%. Treating infected animals with MK 886 inhibited leukotriene synthesis but increased leukocyte recruitment to the lungs. Subsequent to this phenomenon, levels of tumor necrosis factor alpha, interleukin-1 (IL-1), IL-6, and KC chemoattractant cytokines and fungi in the lung parenchyma increased, as did inflammatory response. In contrast, IL-2, IL-5, IL-12, and gamma interferon cytokine levels actually decreased. Thus, murine response to pulmonary histoplasmosis may be leukotriene modulated. This finding may enable us to alter the course of the immune response and inflammation caused by histoplasmosis. The data from the present study suggest an important new strategy for immunologic or drug intervention in human patients.
In recent decades, the incidence of histoplasmosis has increased dramatically, mainly as the result of immune status alterations associated with organ or bone marrow transplantation, AIDS, or cancer chemotherapy (33). Although most infections involving Histoplasma capsulatum are asymptomatic, this organism can cause a broad range of clinically apparent ailments, including progressive disseminated infections and, in immunocompromised patients, death (8). Histoplasmosis is a pulmonary disease characterized by chronic granulomatous and suppurative inflammatory reactions. The causative organism, H. capsulatum, is a dimorphic fungus with a yeast-like morphology in tissue (8, 33). In the body, resident alveolar macrophages engulf the yeast cells, which subvert the hostile environment and multiply. Presumably, dividing yeast destroy the alveolar macrophages and are phagocytosed by neighboring alveolar macrophages and inflammatory macrophages recruited to the site of infection. Repetition of this cycle leads to the spread of infection to the lymph organs and other tissues. In an immunocompetent host, the infection is eventually eliminated (26).
Maturation of cell-mediated immunity leads to cytokine production, directly or indirectly activating macrophages that inhibit yeast cell proliferation (17, 18). In a murine model of histoplasmosis, gamma interferon (IFN-γ), interleukin-12 (IL-12), tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor have been implicated in the immune response to H. capsulatum yeasts (1-3, 11, 30, 34, 36, 37). However, the potent lipid inflammation mediators known as leukotrienes have received far less attention for their role as antimicrobial host defense mediators. These leukotrienes are derived via the 5-lipoxygenase (5-LO) pathway in the arachidonic acid metabolism (20, 23). It has been shown that the levels of leukotriene B4 (LTB4) are elevated in the bronchoalveolar lavage fluid (BALF) of patients with bacterial pneumonia and that similarly high levels of LTB4 and leukotriene C4 (LTC4) are found in lung homogenates from bacterial pneumonia models (7, 16, 27). These findings provide evidence of 5-LO pathway activation during the course of lower respiratory tract infections. Moreover, we have previously demonstrated that leukotrienes are the main chemoattractants involved in the neutrophil, eosinophil, and mononuclear cell migration induced by H. capsulatum or cell wall fraction thereof (22). The compound MK 886 is a potent inhibitor of leukotriene biosynthesis, both in vivo and in intact cells in vitro (15, 29). It has no effect on other routes of arachidonic acid metabolism, including the cyclooxygenase and 12-LO pathways, and appears to have no effect on availability of the substrate arachidonic acid. It does, however, inhibit translocation of 5-LO (14).
In the present study, we investigated the effects of leukotriene synthesis inhibition during the immune response in a murine model of pulmonary histoplasmosis. The results reveal that leukotrienes play an intrinsic and essential role in eliminating pulmonary histoplasmosis infection and controlling related inflammatory responses.
MATERIALS AND METHODS
Animals.
Male 6-week-old C57BL/6 mice were obtained from the animal facilities of the Ribeirão Preto campus of the Universidade de São Paulo. All experiments were approved and conducted in accordance with guidelines of the Animal Care Committee of the University. Infected animals were kept in biohazard facilities and housed in cages within a laminar flow safety enclosure under standard conditions.
Preparation of H. capsulatum.
The H. capsulatum strain was isolated from a patient at the Hospital Universitário, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo. The live mycelial phase was obtained by culturing fungi at 25°C on Sabouraud dextrose agar plates. H. capsulatum yeasts were grown at 37°C in Ham F-12 medium (Gibco-BRL, Grand Island, N.Y.) for 36 h. Yeast cells were washed three times and suspended in balanced salt solution. Yeast cells were used when fluorescein diacetate and ethidium bromide staining revealed their viability to be >95% (10).
Infection of mice with H. capsulatum yeast cells and treatment with MK 886.
Mice were anesthetized with tribromoethanol 2.5% and restrained on a small board. An anterior midline incision was made for trachea exposition. A 30-gauge needle attached to a tuberculin syringe was inserted into the trachea, and intratracheal (i.t.) dispersion was used to introduce either 100 μl of phosphate-buffered saline (PBS) or a sublethal dose (2 × 105 yeast cells in a volume of 100 μl) of H. capsulatum into the lungs. Animals were divided into five groups. Group 1 animals were subjected to i.t. infection with H. capsulatum and water (0.5 ml given perorally [p.o.] by gavage) was given 1 h before infection and then every 24 h for 30 days. Group 2 animals were treated with MK 886 p.o. (5 mg/kg/0.5 ml) 1 h prior to infection i.t. with H. capsulatum and again every 24 h until day 15 (maximum survival for animals in this group). The last treatment was 1 h before sacrifice. Group 3 animals received i.t. injections of PBS and were given water p.o. for 30 days. Group 4 animals underwent i.t. inoculation with dead H. capsulatum and were treated daily with MK 886 p.o. Group 5 animals received i.t. injections of PBS and were treated daily with MK 886 p.o. MK 886 was a generous gift from Merck Frosst Canada, Inc.
BALF.
On days 1, 2, 7, 14, and 30 after infection, animals were euthanized with sodium pentobarbitone. The chest cavity of each animal was carefully opened, and the trachea was exposed and catheterized. The catheter was tied in place, and sterile PBS was infused in three 1-ml aliquots. Lavage fluid was recovered and placed on ice. Total cell counts were immediately performed in a Neubauer Chamber. Differential counts were obtained by using Rosenfeld-stained cytospin preparations (12).
Organ culture for H. capsulatum.
Recovery of H. capsulatum was performed as described previously (2). Fungal burden was assessed as the mean CFU per whole organ ± the standard error of the mean (SEM; n = 12).
Measurement of leukotrienes and cytokines.
For cytokine and leukotriene measurement, lungs were removed on days 1, 2, 7, and 14 postinfection. Tissue was homogenized (Mixer Homogenizer; Labortechnik, Staufen, Germany) in 2 ml of RPMI 1640, centrifuged at 1,500 × g, filtered, sterilized, and stored at −70°C until assayed. Quantification of LTB4 and LTC4 in the samples was performed by specific enzyme immunoassay (Cayman Chemical, Ann Arbor, Mich.) according to the method of Pradelles et al. (28). Supernatant dilutions were incubated with conjugated eicosanoid-acetylcholinesterase and with specific antiserum in 96-well plates precoated with anti-rabbit immunoglobulin G antibodies. After overnight incubation at 4°C, plates were washed and enzyme substrate (Ellman's reagent) was added for 60 to 120 min at 25°C. The optical density of samples was determined at 412 nm in a microplate reader, and concentrations of eicosanoids were calculated based on a standard curve. Commercially available enzyme-linked immunosorbent assay (ELISA) antibodies were used to measure TNF-α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and IFN-γ (Pharmingen, San Diego, Calif.), as well as IL-1 and KC (R&D Systems, Minneapolis, Minn.). Sensitivities were >10 pg/ml.
Quantitation of NO.
On days 1, 2, 7, and 14 postinfection, BALF cells from mice infected or uninfected with H. capsulatum and treated with MK 886 (n = 6) were obtained. After being washed twice, the cells were seeded at 105 per well in 96-well plates in Dulbecco modified Eagle medium (Gibco-BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum. Cells were stimulated with 1 μg of lipopolysaccharide (LPS; Sigma, St. Louis, Mo.) and 50 ng of rIFN-γ (Pharmingen)/ml. Supernatants were collected at 24 h after seeding, and nitrite was measured by Griess reaction as described previously (18). The data are presented as micromoles of NO2 (mean ± the SEM).
Histology.
Lungs were removed on days 7 and 14 postinfection and tissues were fixed in 10% formalin and embedded in paraffin blocks. For fungal elements, 5-μm sections were stained with hematoxylin and eosin (HE) or Grocott’s methanemine silver (GMS). Analysis of the sections was performed in a “blinded” fashion.
Statistical analyses.
Each experiment was repeated three times. Statistical variations were analyzed by using the Student t test, and a P value of <0.05 was considered significant.
RESULTS
Effect of the 5-LO inhibitor, MK 886, on leukocyte recruitment in the BALF.
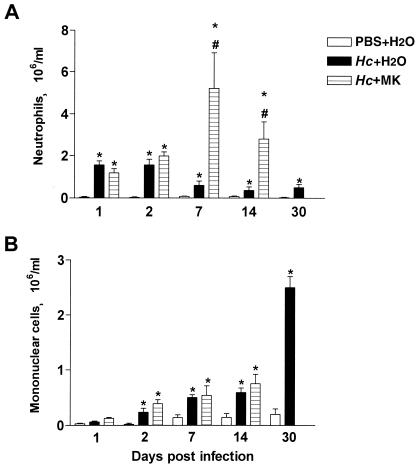
The i.t. inoculation of a sublethal dose of H. capsulatum induced significant neutrophil recruitment to the lungs after the first day of infection. The migration of leukocytes in this group peaked between the first and second days. No such migration occurred in the control group (Fig. 1A). The number of inflammatory cells in the BALF progressively decreased during the course of infection, although the number of neutrophils at 30 days after infection was still greater than in the control group. Comparing H. capsulatum-infected and treated animals, with infected-only animals, MK 886 treatment did not alter neutrophil numbers in the bronchoalveolar space on the first 2 days after infection (Fig. 1A). Nevertheless, on day 7, H. capsulatum-infected animals treated with MK 886 exhibited neutrophil counts in the bronchoalveolar space 500% greater than those of infected untreated animals. Even on day 14, neutrophil counts in the MK 886-treated animals were 300% higher than in the untreated animals. After the second day of infection, mononuclear cell counts into the bronchoalveolar space as a response to H. capsulatum were significant in comparison to the control group (Fig. 1B). Mononuclear cell numbers progressively increased until day 30 postinfection, which was the end of the observation period. By day 7, infected animals treated with MK 886 presented an influx of mononuclear cells that was similar to that observed in untreated animals. However, on day 14, mononuclear cell counts in the bronchoalveolar space of infected animals treated with MK 886 was significantly greater than those of infected untreated animals. After 30 days of infection and MK 886 treatment, it was not possible to measure the number of inflammatory cells into the BALF because all of the animals had died.
FIG. 1.
Effect of endogenous leukotriene synthesis inhibition on alveolar leukocyte numbers after H. capsulatum infection. BALF cells were obtained from mice after (i) i.t. injection of PBS and daily p.o. administration of water (PBS+H2O), (ii) i.t. infection with H. capsulatum yeast cells and daily p.o. administration of water (Hc+H2O), and (iii) i.t. infection with H. capsulatum yeast cells and daily p.o. administration of MK 886 (MK) as indicated. (A) Neutrophils; (B) mononuclear cells. Cells were enumerated and identified after Rosenfeld staining. The data are presented as mean ± the SEM from three experiments (n = 9). ✽, PBS+H2O versus either Hc+H2O or Hc+MK; #, Hc+H2O versus Hc+MK; P < 0.05. Animals that were infected and treated died before day 30.
Effect of endogenous leukotriene synthesis inhibition on H. capsulatum growth and mouse survival.
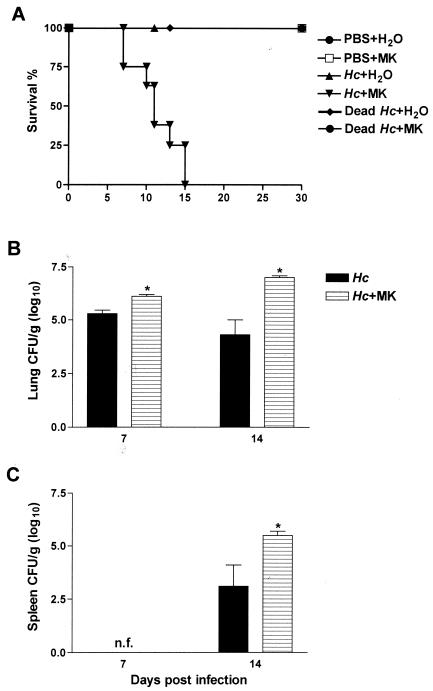
H. capsulatum-infected animals treated with MK 886 did not survive past day 15 of infection, whereas animals infected but not treated with MK 886 survived until day 30, the end of the observation period (Fig. 2A). In order to evaluate whether the increase in cell recruitment and mortality in the treated animals was due to MK 886 toxicity, i.t. injections of either dead H. capsulatum or PBS were given to a group of animals who were then submitted to p.o. MK 886 treatment for 30 days. Animals injected with dead H. capsulatum and treated with MK 886 had BALF leukocyte counts lower than those observed in animals injected with only dead H. capsulatum. In contrast, animals inoculated with PBS and treated with MK 886 showed counts similar to those of the group receiving PBS and H2O (data not shown). Moreover, all animals in these groups survived until day 30 (Fig. 2A).
FIG. 2.
Effect of endogenous leukotriene synthesis inhibition on mouse survival rate and on fungal recovery. (A) Survival of mice after i.t. PBS injection and daily p.o. administration of water or MK 886, after i.t. infection with H. capsulatum yeast cells and daily p.o. administration of water or MK 886, after i.t. inoculation with dead Hc yeast cells and daily p.o. administration of water or MK 886 as indicated. Mice were monitored for 30 days (n = 6 to 8). (B) Fungal burden in lungs. (C) Fungal burden in the spleen. Tissue samples were harvested at 7 and 14 days of H. capsulatum infection with daily administration of water or MK 886. The data are expressed as mean ± the SEM from three experiments (n = 12). ✽, Hc+H2O versus Hc+MK; P < 0.05. n.f., No yeast cells found; MK, MK 886; Hc, H. capsulatum.
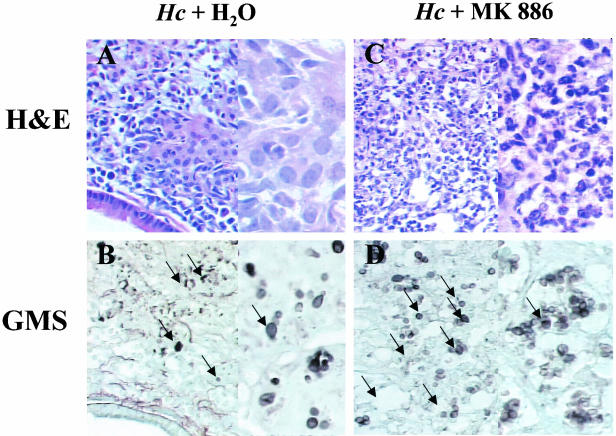
The number of H. capsulatum CFU in the lungs decreased during the course of infection (Fig. 2B). Recovery of yeast CFU in spleens was not noted until day 14 (Fig. 2C). However, recovery of yeast CFU from lungs of infected animals treated with MK 886 increased 1.2 and 2.7 log10 at 7 and 14 days, respectively, after infection in comparison to infected, untreated animals (Fig. 2B). The CFU in the spleens of MK 886-treated animals were 2.9 log10 greater than in infected, untreated mice (Fig. 2C). Histopathologic changes in lungs after treatment with MK 886 were determined on day 14 of infection. Lung tissue of H. capsulatum-infected animals treated with MK 886 presented greater leukocyte infiltration, with a predominance of neutrophils (Fig. 3C) and higher numbers of yeast cells (Fig. 3D) throughout the parenchyma than did untreated infected mice (Fig. 3A and B). Moreover, infected mice treated MK 886 exhibited a diffuse granulomatous pneumonia with destruction of the lung parenchyma (Fig. 3C). There was moderate to severe peribronchiolar and perivascular lymphoid cuffing. In MK 886-treated mice the extent of the inflammatory response was substantially greater than in untreated animals, and complete alveolar destruction was seen. Despite the persistence of peribronchiolar and perivascular lymphoid cuffing in MK 886-treated mice, nonfocal granulomatous infiltration of the alveoli was observed.
FIG. 3.
Increased leukocyte recruitment and fungal burden in the lung parenchyma resulting from endogenous leukotriene inhibition. Representative lung sections from mice infected with H. capsulatum (Hc) and receiving p.o. water or MK 886 daily. (A and C) Hematoxylin-and-eosin staining for leukocytes; (B and D) GMS staining for yeast cells (black arrow). Magnifications: ×360 (A and B) and ×900 (C and D).
Because we observed greater numbers of yeast cells in the lungs of MK 886-treated animals, we analyzed the direct effect of this compound on survival and multiplication of yeast in vitro. In an additional experiment, H. capsulatum was incubated with MK 886 (1, 10, and 50 μM). On days 2 and 7, yeast viability and CFU were determined. No difference was observed in the growth or viability of H. capsulatum in the presence of MK 886 compared to the fungus cultured in the absence of the drug (data not shown).
Leukotriene production in lungs of H. capsulatum-infected mice untreated or treated with MK 886.
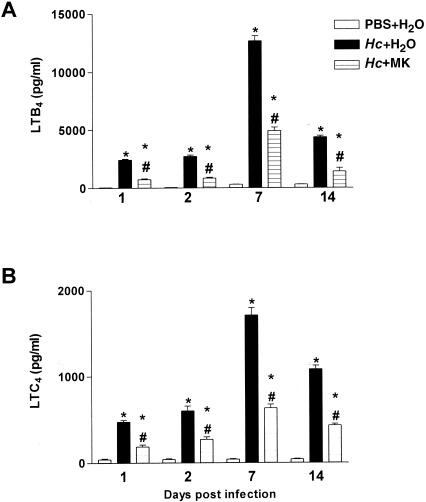
Our results demonstrated that LTB4 and LTC4 cell production in H. capsulatum-infected animals begins on the first day of infection and that levels of both peaked on day 7, not decreasing until day 14. MK 886 treatment of infected animals inhibited LTB4 and LTC4 synthesis compared to infected untreated mice (Fig. 4A and B). Throughout the observation period, PBS-inoculated animals presented no detectable levels of these mediators (Fig. 4A and B).
FIG. 4.
Effect of MK 886 on LTB4 and LTC4 synthesis in lung tissue. Enzyme immunoassay quantification of leukotriene concentrations in lungs from mice subjected to either i.t. PBS injection and daily p.o. doses of water or i.t. infection with H. capsulatum (Hc) yeast cells and daily p.o. doses of water or MK 886 (MK). (A) LTB4. (B) LTC4. Lungs removed on days 1, 2, 7, and 14 and homogenized in RPMI are shown. The data are presented as means ± the SEM from three experiments (n = 9). ✽ PBS+H2O versus Hc+H2O; #, Hc+H2O versus Hc+MK; P < 0.05.
Cytokine production by lungs of H. capsulatum-infected mice untreated or treated with MK 886.
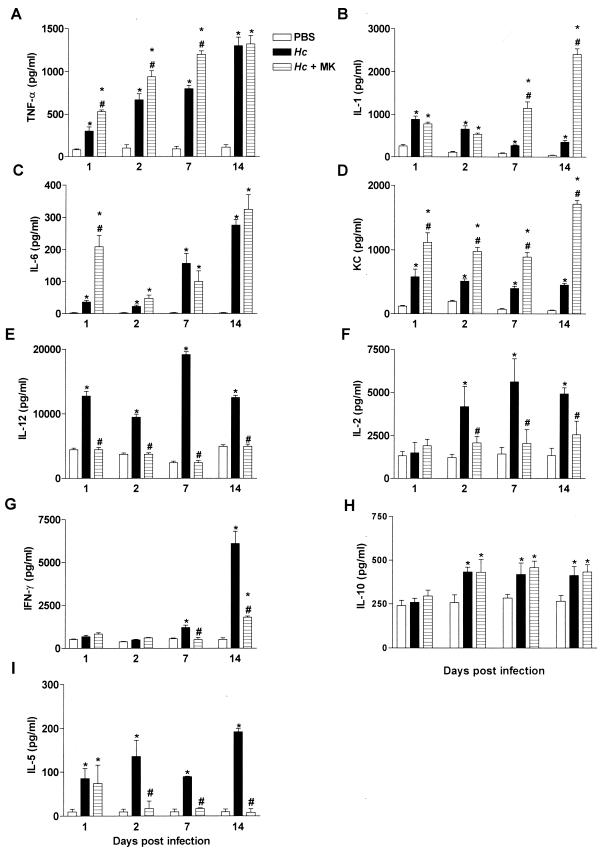
We determined whether MK 886 treatment altered cytokines that are involved in inflammatory and immune responses to H. capsulatum. In H. capsulatum-infected animals, TNF-α and IL-6 levels in the lungs increased progressively from the first day of infection, reaching their peak on day 14. Treatment of infected animals with MK 886 induced a constant increase in TNF-α levels, but IL-6 increased only on the first day of infection (Fig. 5A and C). The levels of IL-1 and KC in lungs of H. capsulatum-infected animals were greater than in control mice but decreased progressively until day 14. From the first day of infection, the levels of KC were greater in infected animals treated with MK 886 than in infected-only animals, whereas IL-1 increased only after day 7 (Fig. 5B and D). At 14 days postinfection, levels of both cytokines were 2- to 13-fold higher than in untreated animals. Levels of IL-12 and IL-5 in the lungs of H. capsulatum-infected animals increased from the first day of infection, but IL-2 was only detected after day 2 postinfection, and IFN-γ was only detected after day 7 postinfection. Nevertheless, the levels of all cytokines remained above those observed in control animals until day 14. Treatment of infected animals with MK 886 inhibited synthesis of IL-2, IL-5, IFN-γ, and IL-12 (Fig. 5E, F, G, and I). Between days 2 and 14 postinfection, the production of IL-10 in H. capsulatum-infected animals was higher than in controls. Treatment with MK 886 did not modify IL-10 release (Fig. 5I). Levels of IL-4 in lungs were not detectable on any postinfection day (data not show). Although the production of IL-4 during H. capsulatum lung infection has been previously described (3), we were unable to detect this Th2 cytokine in our study. This could be due to the low sensitivity of our ELISA assay (data not shown). Between PBS-inoculated mice treated with MK 886 for 30 days and untreated PBS-inoculated mice, no differences were detected in lung levels of IFN-γ, IL-12, TNF-α, or IL-1 (data not show).
FIG. 5.
Cytokine levels in lung tissue of mice subjected to either i.t. PBS injection and daily p.o. doses of water or i.t. infection with H. capsulatum (Hc) yeast and daily p.o. doses of water or MK 886 (MK). Lungs removed on days 1, 2, 7, and 14 and homogenized in RPMI are shown. Cytokine levels were determined by ELISA. The data are expressed as means ± the SEM from three experiments (n = 9). ✽, PBS+H2O versus Hc+H2O or Hc+MK; #, Hc+H2O versus Hc+MK 886; P < 0.05.
Production of NO by BALF cells.
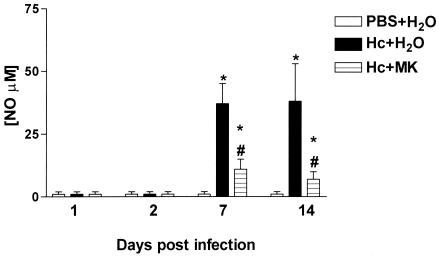
On days 1, 2, 7, and 14 after infection, BALF cells were obtained from H. capsulatum-infected animals who were either untreated or were treated with MK 886. In comparison to similar cells from control animals, the cells taken from infected mice and stimulated in vitro with LPS and IFN-γ produced increased levels of NO2 only after 7 to 14 days of infection. In contrast, similar cells obtained from infected MK 886-treated animals and stimulated with LPS and IFN-γ showed an approximate 65% reduction in NO2 production compared to infected untreated animals (Fig. 6).
FIG. 6.
NO2 production by BALF cells. Cells harvested from mice subjected to either i.t. PBS injection and daily p.o. doses of water or i.t. infection with H. capsulatum (Hc) yeast and daily p.o. doses of water or MK 886 (MK). NO2 production was quantified by Griess reaction in the supernatants of cells from BALF recovered on days 1, 2, 7, and 14. In vitro cells either unstimulated (spontaneous release) or stimulated with IFN-γ (50 μg/ml) or LPS (1 μg/ml). The data are expressed as ΔNO2 (unstimulated NO2 − stimulated NO2). The data are expressed as means ± the SEM from two experiments (n = 6). ✽, PBS+H2O versus Hc+H2O or Hc+MK; #, Hc+H2O versus Hc+MK; P < 0.05.
DISCUSSION
The present study demonstrates the important role that leukotrienes play during pulmonary histoplasmosis. Reduced leukotriene synthesis caused by administration of MK 886 resulted in 100% 14-day mortality in H. capsulatum-infected mice. Treatment with MK 886 was also associated with increased lung CFU at 7 and 14 days postinfection. On day 14, the fungal burden in the spleens of leukotriene-depleted mice was elevated in comparison with infected controls. Interestingly, histopathologic examination revealed that the components of the inflammatory response in BALF and in lung parenchyma differed between H. capsulatum-infected, MK 886-treated and untreated mice. In BALF, both groups exhibited a high and similar number of neutrophils infiltration 1 and 2 days postinfection, although leukotriene-depleted mice manifested more extensive inflammation on days 7 and 14. In addition, mononuclear cell counts in infected untreated mice rose progressively from the first day postinfection until day 30 (the last day of observation). However, by 14 days postinfection, MK 886 treatment of H. capsulatum-infected mice had not reduced the number, or even the potency, of mononuclear cells. We have previously demonstrated that leukotrienes are the main chemoattractants involved in the recruitment of neutrophils, eosinophils, and mononuclear cells into the peritoneal cavity after intraperitoneal inoculation with H. capsulatum. Although the present study shows that H. capsulatum infection also induces greater leukotriene release in the lungs than does PBS injection, these mediators do not appear to be implicated in inflammatory cell recruitment into the lung. Although functional leukotriene deficiency could be expected after MK 886 treatment of H. capsulatum-infected mice, animals infected with H. capsulatum and treated with MK 886 presented lower levels of LTB4 and LTC4 and higher numbers of inflammatory cells. In fact, the decreased leukotriene levels during infection appear to be correlated to both increased CFU and higher mortality, indicating a lack of fungal clearance in the MK 886-treated group. The results are consistent with the pivotal role of 5-LO and its products in the protective host response seen in this model of infection, as previously demonstrated by others in bacterial infection (5, 9, 27). Neutrophil phagocytosis of Klebsiella pneumoniae is augmented by LTB4 (21), which also participates in the antimicrobial host defense (5, 27). In light of these findings, we might hypothesize that the absence of leukotrienes during infection decreases the antimicrobial action of neutrophils and mononuclear cells, allowing persistence and proliferation of the fungus in the lung and its dissemination to other organs, such as the spleen. We ruled out the possibility that MK 886 is acting directly upon the fungus, accelerating its growth in the lungs. In vitro 6-day cultures of H. capsulatum augmented with MK 886 (1, 10, and 50 μM) presented the same level of growth and viability, as did identical cultures in F-12 medium alone. Thus, we advance two hypotheses to explain the persistence of cell recruitment in the lung after leukotriene inhibition. First, we propose that MK 886 treatment inhibits antimicrobial host defense in H. capsulatum-infected mice. This results in fungal proliferation that induces constant cell recruitment, independent of leukotriene release. Second, also in H. capsulatum-infected mice, we hypothesize that MK 886 induces an increase in compensatory mechanisms, similar to the chemoattractant signals, such as cytokines and chemokines, generated in 5-LO KO mice (5).
Inflammatory cytokines such as IL-1, IL-6, TNF-α, and KC are known to be potent neutrophil chemotactic agents in mice (6, 12, 31). Moreover, TNF-α and IL-1 have been implicated in murine histoplasmosis (4, 32). Therefore, we compared the levels of these cytokines in lung homogenates from H. capsulatum-infected mice that were treated with MK 886 with those from H. capsulatum-infected untreated mice. The major findings were that H. capsulatum infection induced release of TNF-α, IL-6, IL-1, and KC into the lungs from the first day of infection until day 14 and that inhibition of endogenous leukotriene biosynthesis upregulated production of all inflammatory cytokines, although there were differences in timing. Thus, the intact capacity of inflammatory cell recruitment in MK 886-treated mice correlated with the increase in cytokines/chemokines release and could explain the markedly elevated leukocyte counts in BALF and lung parenchyma after i.t. administration of H. capsulatum. In the lungs of H. capsulatum-infected mice treated with MK 886, a functional defect appears to occur in inflammatory cells. Since the host is enable to eliminate the fungus, this defect may occur even when high numbers of inflammatory cells are present. This leads to a worsening of the infection. We cannot discard the contribution of other mechanisms, such as lipoxin inhibition, induced by MK 886 treatment. Lipoxins are potent in vitro and in vivo counterregulatory signals for endogenous proinflammatory mediators, including leukotrienes, platelet-activating factor and cytokines (TNF-α and IL-6), resulting in the inhibition of leukocyte-dependent inflammation (13). In light of this, we could hypothesize that MK 886 treatment of H. capsulatum-infected mice inhibits synthesis of the specific lipoxins that block the anti-inflammatory action of these mediators, resulting in PMN-mediated inflammation and increased numbers of inflammatory cytokines.
Cytokine release by T cells (IFN-γ and IL-2) and myeloid lineage cells (IL-12 and TNF-α) is crucial to the immune response and to controlling pulmonary infection caused by H. capsulatum and other intracellular pathogens (1, 3, 36). Other authors have shown that LTB4 is involved in T-cell activation and upregulation of IL-2, IL-4, and IFN-γ (24). We also examined the effect of MK 886 treatment on H. capsulatum-infected mice, with a focus on lung production of IL-2, IL-12, IFN-γ, IL-10, IL-5, and NO during infection. The results demonstrate that decreased leukotriene biosynthesis was associated with impairment of IL-2, IL-12, and IFN-γ generation. In fact, lung levels of IL-2, IL-12, and IFN-γ were markedly lower in infected and MK 886-treated mice than in infected untreated mice. The results from previous studies show that IFN-γ knockout mice and anti-IL-12 MAb-treated mice succumb to H. capsulatum infection, suggesting that IFN-γ and IL-12 are necessary for the clearance of this type of pulmonary infections (2, 36).
Accumulated data demonstrate that NO is a potent killing mechanism for several intracellular pathogens, including H. capsulatum (17, 18, 25). Leukotrienes (19) and IFN-γ (17, 18) both induce NO production. Since they may also be involved in inducing NO production during H. capsulatum infection, we compared NO production in lungs of MK 886-treated mice to that of infected-only mice and detected impaired NO production in leukotriene-deficient mice. Nevertheless, we cannot rule out the possibility that differences between the cell populations in the lungs of infected untreated mice and in those of infected mice treated with MK 886 could be related to decreased NO production. In our study, the immunosuppressive effects of this leukotriene inhibitor appear to be also related with suppression of IL-2, IL-12, IFN-γ, and NO production. This could explain the increase proliferation and dissemination of fungus in MK 886-treated mice.
Analysis of cytokine production in the lungs of H. capsulatum-infected mice revealed pronounced differences in production of IL-5 and IL-10 between H. capsulatum-infected MK 886-treated mice and infected untreated mice. As has been previously demonstrated (3), levels of IL-10 were higher in infected mice than in uninfected controls. However, IL-10 levels in leukotriene-depleted mice were comparable to those observed in infected untreated animals. Although no differences were detected between IL-10 levels in lung homogenates from H. capsulatum-infected untreated and those in lung homogenates from infected mice treated with MK 886, there was a difference in the ratio of IFN-γ to IL-10. At 14 days postinfection, the ratio of IFN-γ to IL-10 in the infected untreated group was approximately 15, whereas it was approximately 4 in the infected group treated with MK 886. We suggest that there was an imbalance between Th1 and Th2 cytokines in H. capsulatum-infected mice treated with MK 886, similar to the imbalance that occurs in TNF-α-depleted mice during histoplasmosis (3). Nevertheless, significant differences were noted between IL-5 levels in infected MK 886-treated mice and infected untreated mice. Others have demonstrated that LTB4 regulates IL-5 generation by human T lymphocytes (35). This could explain the decrease of IL-5 in MK 886-treated animals. Moreover, we detected IL-5 release from the first day and, in contrast to data from our previous studies of the peritoneal cavity, no eosinophils were seen during H. capsulatum lung infection. Therefore, we cannot assign any specific relevance to the IL-5 findings in this experimental model.
In summary, we have demonstrated for the first time that H. capsulatum infection induces production of LTB4, LTC4, and KC in mice. We have also observed that treatment of H. capsulatum-infected mice with the leukotriene biosynthesis inhibitor MK 886 induces upregulation of the inflammatory chemoattractant cytokines IL-1, IL-6, KC, and TNF-α, as well as downregulation of immune response cytokines such as IL-2, IL-5, IL-12, and IFN-γ. This cytokine production imbalance causes continuous and persistent cell recruitment to the lungs and suppresses an appropriate immune response by decreasing NO production. Moreover, leukotriene inhibition decreases antimicrobial host defense and results in fungal proliferation, which contributes to continued cell recruitment. Despite the greater number of cells in the lungs, this phenomenon does not produce a granulomatous reaction. Subsequent to MK 886 treatment, the conflagration of these events culminates in the death of the infected animal. These findings indicate that, in the murine model, leukotriene production is necessary for efficient pulmonary antifungal host defense and for immunoregulation.
Acknowledgments
This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-95/9691-6 and -98/16082-4), the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Fundação de Amparo ao Ensino, Pesquisa e Assistência do Hospital das Clínicas Da FMRP-USP of Brazil.
We are grateful to Sônia Jancar and Richardt Gama Landgraft for excellent technical assistance in leukotriene detection, Claudia M. L. Maffei for graciously providing the H. capsulatum strain, and Merck Frosst Canada, Inc., for furnishing the MK 886.
Editor: T. R. Kozel
REFERENCES
- 1.Allendoerfer, R., and G. S. Deepe, Jr. 1997. Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect. Immun. 65:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allendoerfer, R., G. P. Biovin, and G. S. Deepe, Jr. 1997. Modulation of immune responses in murine pulmonary histoplasmosis. J. Infect. Dis. 175:905-914. [DOI] [PubMed] [Google Scholar]
- 3.Allendoerfer, R., and G. S. Deepe, Jr. 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160:6072-6082. [PubMed] [Google Scholar]
- 4.Allendoerfer, R., and G. S. Deepe, Jr. 2000. Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J. Immunol. 165:2657-2664. [DOI] [PubMed] [Google Scholar]
- 5.Bailie, M. B., T. J. Standiford, L. L. Laichalk, M. J. Coffey, R. Strieter, and M. Peters-Golden. 1996. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumoniae in association with decreased alveolar macrophage phagocytic and bactericidal activities. J. Immunol. 157:5221-5224. [PubMed] [Google Scholar]
- 6.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893-901. [DOI] [PubMed] [Google Scholar]
- 7.Buret, A., M. Dunkley, R. L. Clancy, and A. W. Cripps. 1993. Effector mechanisms of intestinally induced immunity to Pseudomonas aeruginosa in the rat lung: role of neutrophils and leukotriene B4. Infect. Immun. 61:671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano, M. V., and R. A. Hajjeh. 2001. The epidemiology of histoplasmosis: a review. Semin. Respir. Infect. 16:109-118. [DOI] [PubMed] [Google Scholar]
- 9.Chen, N., A. Restivo, and C. S. Reiss. 2001. Leukotrienes play protective roles early during experimental VSV encephalitis. J. Neuroimmunol. 120:94-102. [DOI] [PubMed] [Google Scholar]
- 10.Corrêa, B., A. Purchio, C. R. Paula, W. Gambale, and M. A. Shikanai-Yasuda. 1990. Fluorescent method (fluorescein diacetate and ethidium bromide) to study the viability of Cryptococcus neoformans in liquor. Rev. Inst. Med. Trop. Sao Paulo 32:46-50. [PubMed] [Google Scholar]
- 11.Deepe, G. S., Jr., R. Gibbons, and E. Woodward. 1999. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J. Immunol. 163:4985-4993. [PubMed] [Google Scholar]
- 12.Faccioli, L. H., G. E. Souza, F. Q. Cunha, S. Poole, and S. H. Ferreira. 1990. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration “in vivo” by indirect mechanisms. Agents Actions 30:344-349. [DOI] [PubMed] [Google Scholar]
- 13.Fierro, I. M., and C. N. Serhan. 2001. Mechanisms in anti-inflammation and resolution: the role of lipoxins and aspirin-triggered lipoxins. Braz. J. Med. Biol. Res. 34:555-566. [DOI] [PubMed] [Google Scholar]
- 14.Ford-Hutchinson, A. W. 1991. FLAP: a novel drug target for inhibiting the synthesis of leukotrienes. Trends Pharmacol. Sci. 12:68-70. [DOI] [PubMed] [Google Scholar]
- 15.Gillard, J., A. W. Ford-Hutchinson, C. Chan, S. Charleson, D. Denis, A. Foster, R. Fortin, S. Leger, C. S. McFarlane, H. Morton, H. Piechuta, D. Riendeau, C. A. Rouzer, J. Rokach, and R. Young. 1989. L-663,536 (MK 886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2-dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can. J. Physiol. Pharmacol. 67:456-464. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins, H., T. Stull, S. G. Von Essen, R. A. Robbins, and S. I. Rennard. 1989. Neutrophil chemotactic factors in bacterial pneumonia. Chest 95:1021-1027. [DOI] [PubMed] [Google Scholar]
- 17.Lane, T. E., B. A. Wu-Hsieh, and D. H. Howard. 1993. Gamma interferon cooperates with lipopolysaccharide to activate mouse splenic macrophages to an antihistoplasma state. Infect. Immun. 61:1468-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane, T. E., B. A. Wu-Hsieh, and D. H. Howard. 1994. Anti-histoplasma effect of activated mouse splenic macrophages involves production of reactive nitrogen intermediates. Infect. Immun. 62:1940-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larfars, G., F. Lantoine, M. A. Devynck, J. Palmblad, and H. Gyllenhammar. 1999. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood 93:1399-1405. [PubMed] [Google Scholar]
- 20.Lewis, R. A., K. F. Austen, and R. J. Soberman. 1990. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 323:645-655. [DOI] [PubMed] [Google Scholar]
- 21.Mancuso P, P. Nana-Sinkam, and M. Peters-Golden. 2001. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect. Immun. 69:2011-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros, A. I., C. L. Silva, A. Malheiro, C. M. Maffei, and L. H. Faccioli. 1999. Leukotrienes are involved in leukocyte recruitment induced by live Histoplasma capsulatum or by the β-glucan present in their cell wall. Br. J. Pharmacol. 128:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, D. K., J. W. Gillard, P. J. Vickers, S. Sadowski, C. Leveille, J. A. Mancini, P. Charleson, R. A. Dixon, A. W. Ford-Hutchinson, R. Fortin, J. Y., Gauthier, J. Rodkey, R. Rosen, C. Rouzer, I. S. Sigal, C. D. Strader, and J. F. Evans. 1990. Identification and isolation of a membrane protein necessary for leukotriene production. Nature 343:278-281. [DOI] [PubMed] [Google Scholar]
- 24.Morita, H., K. Takeda, H. Yagita, and K. Okumura. 1999. Immunosuppressive effect of leukotriene B4 receptor antagonist in vitro. Biochem. Biophys. Res. Commun. 264:321-326. [DOI] [PubMed] [Google Scholar]
- 25.Nathan C., and Q. W. Xie. 1994. Nitric oxide synthases: roles, tolls, and controls. Cell 78:915-918. [DOI] [PubMed] [Google Scholar]
- 26.Newman, S. L. 1999. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol. 7:67-71. [DOI] [PubMed] [Google Scholar]
- 27.Peters-Golden, M., and M. Coffey. 1998. Role of leukotrienes in antimicrobial defense of the lung. J. Lab. Clin. Med. 132:251-257. [DOI] [PubMed] [Google Scholar]
- 28.Pradelles, P., C. Antoine, J. P. Lellouche, and J. Maclouf. 1990. Enzyme immunoassays for leukotrienes C4 and E4 using acetylcholinesterase. Methods Enzymol. 187:82-89. [DOI] [PubMed] [Google Scholar]
- 29.Rouzer, C. A., A. W. Ford-Hutchinson, H. E. Morton, and J. W. Gillard. 1990. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 265:1436-1442. [PubMed] [Google Scholar]
- 30.Smith, J. G., D. M. Magee, D. M. Williams, and J. R. Graybill. 1990. Tumor necrosis factor-α plays a role in host defense against Histoplasma capsulatum. J. Infect. Dis. 162:1349-1353. [DOI] [PubMed] [Google Scholar]
- 31.van Enckevort, F. H., C. G. Sweep, P. N. Span, M. G. Netea, A. R. Hermus, and B. J. Kullberg. 2001. Reduced adrenal response and increased mortality after systemic Klebsiella pneumoniae infection in interleukin-6-deficient mice. Eur. Cytokine Netw. 12:581-586. [PubMed] [Google Scholar]
- 32.Watson, S. R., S. K. Schmitt, D. E. Hendricks, and W. E. Bullock. 1985. Immunoregulation in disseminated murine histoplasmosis: disturbances in the production of interleukins 1 and 2. J. Immunol. 135:3487-3493. [PubMed] [Google Scholar]
- 33.Woods, J. P. 2002. Histoplasma capsulatum molecular genetics, pathogenesis, and responsiveness to its environment. Fungal Genet. Biol. 35:81-97. [DOI] [PubMed] [Google Scholar]
- 34.Wu-Hsieh, B. A., G. S. Lee, M. Franco, and F. M. Hofman. 1992. Early activation of splenic macrophages by tumor necrosis factor alpha is important in determining the outcome of experimental histoplasmosis in mice. Infect. Immun. 60:4230-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaoka, K. A., and J. P. Kolb. 1993. Leukotriene B4 induces interleukin 5 generation from human T lymphocytes. Eur. J. Immunol. 23:2392-2398. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, P., M. C. Sieve, J. Bennett, K. J. Kwon-Chung, R. P. Tewari, R. T. Gazzinelli, A. Sher, and R. A. Seder. 1995. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J. Immunol. 155:785-795. [PubMed] [Google Scholar]
- 37.Zhou, P., G. Miller, and R. A. Seder. 1998. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J. Immunol. 160:1359-1368. [PubMed] [Google Scholar]