Abstract
Over the past 19 years, we have developed a novel myxoma virus-derived anti-inflammatory serine protease inhibitor, termed a serpin, as a new class of immunomodulatory therapeutic. This review will describe the initial identification of viral serpins with anti-inflammatory potential, beginning with preclinical analysis of viral pathogenesis and proceeding to cell and molecular target analyses, and successful clinical trial. The central aim of this review is to describe the development of two serpins, Serp-1 and Serp-2, as a new class of immune modulating drug, from inception to implementation.
We begin with an overview of the approaches used for successful mining of the virus for potential serpin immunomodulators in viruses. We then provide a methodological overview of one inflammatory animal model used to test for serpin anti-inflammatory activity followed by methods used to identify cells in the inflammatory response system targeted by these serpins and molecular responses to serpin treatment. Finally, we provide an overview of our findings from a recent, successful clinical trial of the secreted myxomaviral serpin, Serp-1, in patients with unstable inflammatory coronary arterial disease.
1. Introduction
1.1. Background
1.1.1. Serpins
What is the time when all thought
Becomes a flame
All flame
a flight, a lark, a game
Serpentine, gibberose,
paths to thought
slowly met, or sudden sight
So long sought
Silent thought
An inspiration
Serpent wings
Gem of creation
Dragon dreams
Anonymous, 2010
Serpins are an ancient class of protease regulators present from the time of the dinosaurs to the present day, found in prokaryotes, viruses, worms, birds, and mammals (Silverman et al., 2010). Serpins regulate a large spectrum of protease functions with remarkable efficiency. Serpins modulate mammalian clot forming (thrombosis) and clot dissolving (thrombolysis) pathways, complement, immune responses, connective tissue breakdown, apoptosis, hormone transport, neuronal function, and blood pressure. Serpins are reported to represent from 2% to 10% of plasma proteins.
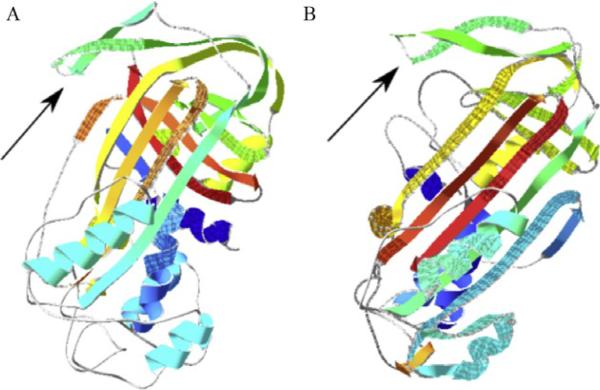
Serpins are suicide inhibitors, acting as bait for a target serine protease. During an attempt to cleave the P1–P1′ site in the reactive site loop (RSL), target proteases become covalently bonded to the RSL and are dragged across the face of the serpin to the opposite pole of the serpin, rendering both inactive (Arnold et al., 2006; Kiefer et al., 2009; Peitsch et al., 1995; Whisstock et al., 2010; Fig. 15.1).
Figure 15.1.
Hypothetical models of Serp-1 (left, A) and Serp-2 (right, B) based on known crystallized homologous protein structures, PAI-1 (PBD ID: 3CVM) for Serp-1 and CrmA (PBD ID: 1C8O) for Serp-2, respectively. The arrows point to the reactive site loops (RSL).
When a serpin pathway is dysfunctional, there is often significant pathologic consequence, providing testimony to the importance of serpin regulation of proteases. Serpins such as plasminogen activator inhibitor-1 (PAI-1) and antithrombin III (AT-III) regulate thrombosis and thrombolysis and when over- or underactive can cause severe clotting or bleeding problems (Gramling and Church, 2010). Serpinopathies, or serpin-based pathologies, occur with mutations of selected serpins; mutation of alpha-1 antitrypsin causes emphysema, cirrhosis, and pancreatitis, whereas mutation of neuroserpin causes early dementia and increased tendency to seizures. Serpinopathies, as elegantly described by Lomas, Whistock, and others, occur when the normal function of a serpin is disrupted and interactions between serpins form aggregates, produced by insertion of the RSL into the beta sheet of an adjacent serpin (Ekeowa et al., 2010; Gooptu and Lomas, 2008, 2009; Whisstock et al., 2010). Conversely, in a normal state, serpins maintain a functional balance in multiple protease pathways. Treatments for various diseases have now been designed to enhance serpin functions. For example, treatment with the glycosaminoglycan (GAG) heparin activates the serpin AT-III and provides a highly effective therapy for treatment of clotting disorders.
Mammalian serpins have been developed for the clinic, primarily as replacements for those who lack or have low levels of the endogenous proteins. Many of these are simply isolated from blood products, but one is a recombinant molecule isolated from transgenic goats. These clinical serpins include Aralast (alpha-1 antitrypsin), Zemaira (alpha-1 antitrypsin), and Thrombate (AT-III) isolated from blood products as well as Atryn (recombinant antithrombin), made from milk of transgenic goats (Berry et al., 2009; Bouchecareilh et al., 2010; Kowal-Vern et al., 2001; Mordwinkin and Louie, 2007). Thus tapping into the viral reservoir of potential serpin therapeutics is a natural extension of ongoing work with mammalian serpin replacement therapies.
1.1.2. Viral serpins
Viruses have commandeered the pathways regulated by mammalian serpins through engineering serpins of their own design. Current theory suggests that either these serpins are derived from mammals and modified for the benefit of the virus or conversely that mammalian serpins originate from viruses (Finlay and McFadden, 2006; Lucas et al., 2009). Either way, viral serpins provide highly effective inhibitory reagents that block host attacks against viral proliferation, spread, and invasion in an infected host.
Serp-1 is a secreted 55 kDa myxomaviral serpin that binds proteases in the thrombolytic and thrombotic pathways. Serp-1 inhibits tissue- and urokinase-type plasminogen activators (tPA and uPA, respectively), plasmin in the thrombolytic cascade, and factor Xa (fXa) in the thrombotic cascade (Dai et al., 2003; Lomas et al., 1993; Nash et al., 1998). When expressed and active in a myxomaviral infection, Serp-1 leads to a lethal infection in 90% of European rabbits, whereas mutation of Serp-1 leads to a benign infection that is cleared within weeks (Macen et al., 1993; Upton et al., 1990). Serp-2 is an intracellular myxomaviral serpin that targets granzyme B, a serine protease, and caspases 1 and 8, cysteine proteases (Messud-Petit et al., 1998a,b; Nathaniel et al., 2004; Turner et al., 1999; Turner and Moyer, 2001). The cross-class serpins Serp-2 and CrmA, the latter from cowpox virus (Komiyama et al., 1994), modify apoptosis and inflammatory responses and Serp-2, as for Serp-1, alters viral pathogenesis. In prior cellular and animal model work, both Serp-1 and Serp-2 have been identified as possessing anti-inflammatory activity:
With this review, we define the process whereby these two potential viral serpin immunotherapeutics were identified, isolated, and tested for activity in animal models of vascular disease. We begin with the discovery process through which viral serpins with immunomodulatory activity are identified, followed by approaches to the initial testing of viral serpins for immune modulating actions in animal models of disease. We will then describe the animal surgical models and cell-based assays in detail.
2. From Viral Pathogenesis to Identification of Immunomodulatory Potential
2.1. Serp-1 and viral pathogenesis—Identification of a secreted anti-inflammatory viral serpin
The discovery that viruses and other pathogens encode gene products that resemble elements of the host immune system dates back over 25 years and has generated the discipline of “anti-immunology,” which emphasizes the coevolution of the vertebrate immune system with the counteractive strategies evolved by pathogens to subvert its functions (Finlay and McFadden, 2006). In the case of viruses, many of these anti-immune proteins are secreted from virus-infected cells and these have been categorized as either virokines (secreted molecules that resemble host cytokines, growth factors, or extracellular immune regulators) or viroceptors (cell surface or secreted viral molecules that resemble cellular receptors; McFadden, 1995, 2003; McFadden and Murphy, 2000). Poxviruses encode a broader variety of virokines and viroceptors than any other virus family and are the only known viruses to encode bioactive serpins. Of these, only one viral serpin is secreted from virus-infected cells, namely Serp-1 of myxoma virus, which is modified by both host and viral glycosyltransferases and expressed as a secreted 55-60 kDa glycoprotein (Nash et al., 2000).
Myxoma virus is a rabbit-specific poxvirus that causes a severe disease known as myxomatosis in European rabbits but is completely nonpathogenic for all other known vertebrate hosts (Kerr and McFadden, 2002; McFadden, 2005). In studies to investigate the basis for the extreme lethality of myxoma virus in rabbits, a variety of viral virulence genes have been identified by targeted gene knockout analysis showing that the knockout virus exhibits attenuated pathogenesis in infected rabbits (Johnston and McFadden, 2004; Stanford et al., 2007). In the case of the myxoma virus Serp-1 gene, which is also called M008.1 (Cameron et al., 1999), targeted deletion of the gene caused severe attenuation of virus-induced disease symptoms in infected rabbits and was characterized by an exacerbated early inflammatory response to the infection (Macen et al., 1993; Upton et al., 1990). Specifically, infection with the Serp-1 knockout virus construct resulted in a more rapid inflammatory cell infiltration into primary lesions, a robust resolution of disease symptoms, and pronounced attenuation of the disease in immunocompetent hosts (Macen et al., 1993; Upton et al., 1990). The observation of increased myeloid cell infiltration into Serp-1 knockout viral lesions led to the model that Serp-1 functions as an anti-inflammatory reagent which antagonizes the migration and activation of innate immune cells into sites of damaged or infected tissues (Lucas et al., 1996). Biochemical studies indicated that the secreted Serp-1 protein was, as predicted by its serpin motif sequences, a bona fide inhibitor of host serine proteinases, including uPA and tPA, respectively, as well as plasmin and fXa (Lomas et al., 1993; Nash et al., 1998). Further, biologically active Serp-1 protein could be expressed independently of the other viral gene products and shown to still possess anti-inflammatory properties as a stand-alone reagent following intravenous infusion (Lucas et al., 1996). Further, Serp-1 shares homology with well-known mammalian serpins, PAI-1 and neuroserpin which inhibit some shared proteases (tPA and uPA) and have demonstrated both anti- and proinflammatory functions in man and animal models (Gooptu and Lomas, 2008, 2009; Munuswamy-Ramanujam et al., 2010). This has led to the realization that Serp-1, like certain other viral anti-immune proteins, could be adapted as novel anti-inflammatory drugs to treat diseases of hyperactive inflammation or exacerbated immune reactions (Lucas and McFadden, 2004).
2.2. Serp-2—Discovery of viral cross-class anti-inflammatory and antiapoptotic serpins
A second Myxoma virus-derived serpin, Serp-2, has very different biological targets, inhibiting the cysteine proteases, caspases 1 and 8, and the serine protease, granzyme B (Messud-Petit et al., 1998a,b; Nathaniel et al., 2004; Petit et al., 1996; Turner et al., 1999; Turner and Moyer, 2001). Serp-2 from myxoma virus and CrmA from cowpox virus (Komiyama et al., 1994; MacNeill et al., 2006; Turner and Moyer, 2001; Turner et al., 1999) are related but distinct cross-class serpins that similarly inhibit granzyme B and caspases 1 and 8 in vitro. CrmA has greater inhibitory activity in vitro, whereas Serp-2 has more inhibitory activity in viral infections in vivo (Messud-Petit et al., 1998a,b; Nathaniel et al., 2004; Turner and Moyer, 2001; Turner et al., 1999). Granzyme B and caspase 8 activate the apoptotic cascade, while caspase 1 is associated with the inflammasome (Messud-Petit et al., 1998a, b). Granzyme B is considered an extracellular mediator of apoptosis, thus inhibition of granzyme B modulates apoptosis by blocking activation of downstream executioner caspases. Inhibiting caspase 1 blocks the activation of inflammatory responses, specifically preventing activation of interleukin 1 beta (IL-1β) and IL-18. The inflammasome is also able to initiate pyroptosis (inflammation driven macrophage cell death; Franchi et al., 2009). Serp-2 shares homology with two mammalian serpins, murine serine protease inhibitor 6 (SPI-6) and human protease inhibitor 9 (PI-9), that target granzyme B and protect cells from cytotoxic T lymphocyte (CTL)-induced apoptosis (Medema et al., 2001).
2.3. Rationale for choosing viral serpins for analysis as potential immunotherapeutics
The rationale for identifying viral proteins as potential immune modifying therapeutics is thus based upon the following factors:
Demonstrated role in viral pathogenesis as verified by infectious disease models and viral gene mutation
Related serpin functions in a broad range of species
Binding to and/or inhibition of proteases associated with innate immune responses
Correlation with mammalian serpins with similar protease targets and/or function
Proteins that are secreted or proteins with potential for extracellular function
Inhibitory activity for human inflammatory cells, in vitro
Proven efficacy in animal models of vascular inflammation.
The following sections will describe one of the several mouse models of vascular inflammatory disease used to study anti-inflammatory actions of viral serpins, studies used to examine the cell and molecular targets and the mechanisms of serpin activity, and a recent successful clinical trial of a viral serpin in man.
3. Testing Biological Potential
3.1. Mouse model of arterial angioplasty injury-induced atherosclerosis and aneurysm
The innate immune, or inflammatory, response occurs during interactions between cells in the lining of the arterial wall with cells in the circulating blood and vascular tissues. Specifically, interactions between the endothelial cells, T cells, macrophages, and in some cases, neutrophils (Dollery, et al., 2003; Lucas et al., 2006; Munuswamy-Ramanujam et al., 2006; Serhan et al., 2008a,b) drive the initial inflammatory reaction. These cells make up the inflammatory response and are often activated by circulating factors, for example, cytokines, chemoattractants or chemokines in the blood, serine proteases in the clotting and thrombolytic pathways, and serine and cysteine proteases in the apoptotic/inflammasome pathways.
Activation of an inflammatory response in any organ system (including the vasculature) requires transport of innate immune system cells through the circulating blood to the site in need, and from there, the cells must ingress into the arterial, or more precisely capillary, wall to reach the organ which has ongoing infection or trauma. Thus the arterial vasculature is central to all inflammatory responses. Prior research has attempted the use of vascular explant outgrowths in vitro in culture, but this cannot reproduce in entirety the vascular inflammatory responses. There is no replacement or alternative approach for the use of animals in these studies, wherein there is a physiologic and pathogenic interactive response between the vascular wall and circulating inflammatory cells in the blood stream.
A mechanical stretch injury model in the mouse aorta has been reported in previous publications from other researchers (Petrov et al., 2005). We have further developed this mouse angioplasty injury model in mice that express high cholesterol levels in the blood, apolipoprotein E knockout (ApoE-/-). ApoE-/- mice develop increased atherosclerotic arterial occlusions and also aneurysmal dilatations with long-term follow-up (Daugherty and Cassis, 2004). With angioplasty injury, we have produced accelerated atheroma as well as rapid aneurysmal dilatation. Using this mouse angioplasty model, we can obtain insight into the structure and cellular mechanisms leading to accelerated atherosclerosis, restenosis, and aneurysm growth.
Observing the characteristics of knockout mice provides a method to investigate how similar genes may cause or contribute to disease in humans. In our study, angioplasty injury, in a series of knockout (KO) mice with differing genetic deficiencies (KO mouse models), was assessed for effects on inflammation, plaque growth, and aneurysmal dilatation. Use of the KO mouse models allow us to precisely identify key molecules involved in plaque growth and aneurysmal dilation. This then enables understanding of the pathways through which these proteins alter atherosclerotic plaque growth after mechanical angioplasty injury and during aneurysm development, in vivo.
3.1.1. Mouse angioplasty injury model
3.1.1.1. Preoperative preparation
Mouse type: Male—ApoE-/-, Apolipoprotein E deficient (B6.129P2-Apoetm1Unc/J); CCR2-/-, CC chemokine receptor deficient (B6.129S4-Ccr2tm1Ifc/J); PAI-1-/-, plasminogen activator inhibitor-1 deficient (B6.129S2-Serpine1tm1Mlg/J); Parp-1-/-, poly(adenosine 5′-diphosphate-ribose) polymerase-1 (129S-Parp1tm1Zqw/J), and wild-type (WT) control mice (C57BL/6J).
Mouse age: 12–14 weeks
Mouse diet: “Western diet” (WD): 16% fat, 1.25% cholesterol, and 0.5% Na-Cholate (Harlan Teklad, Madison, USA) starting 14 days before operation.
3.1.1.2. Operation
Anesthesia and analgesia for mice perioperative: ketamine/xylazine mixture: 1.1 ml Xylazine (100 mg/ml stock) is added to a 10-ml vial of Ketamine (100 mg/ml stock; Webster Veterinary Supply Inc., Chicago, USA) and this cocktail mixture has a concentration of 95 mg/ml Ketamine and 5 mg/ml Xylazine, and is then diluted 1 part premix plus 3 parts sterile saline. For a 25 g mouse, we give 0.1 ml premix intraperitoneal (i.p.) injection; 0.1 ml/25 g = 0.004 ml/g.
Isofluorane gas: Isofluorane gas anesthetic given by mask with initial anesthetic delivered at 2% with oxygen and then titrated to efficacy at 1–3%.
Analgesia: At the beginning of the surgical procedure, each animal receives buprenorphine at the dosage of 0.05–0.1 mg/kg subcutaneously (SC).
Skin preparation: Wash and shave the surgical area with a three-stage betadine soap/alcohol/betadine topical wash.
Make a long median abdominal laparotomy incision ending above the pubic symphysis and displace the abdominal contents to expose the right iliac artery.
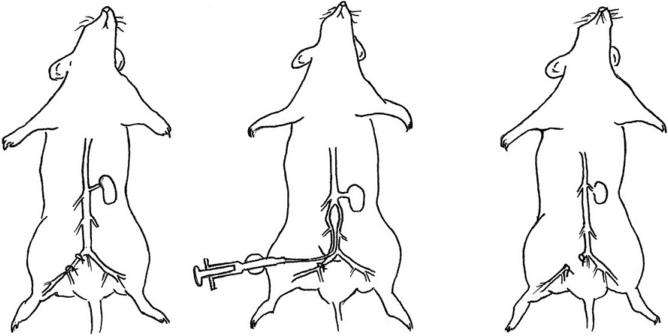
Dissect free the right iliac artery from the right iliac vein and ligate proximally and distally using 8-0 suture line (Fig. 15.2).
Place a retraction suture on the front wall of the right iliac artery and below then make a tiny cut using microsurgical scissors.
Insert a microcatheter balloon (a 0.62-mm caliber polyurethane tube with a latex balloon; MED PLUS perfecseal, Inc., Oshkosh, WI) into the right common iliac artery (Figs. 15.2 and 15.3), loosen the proximal ligature, and advance the catheter retrograde into the aorta as far as the aortic arch (for thoracic vascular injury) or just below the renal branches (for abdominal vascular injury only).
Inflate the angioplasty balloon (2.1 mm diameter for thoracic, 1.6 mm for abdominal injury) with saline (9 μl for thoracic, 7 μl for abdominal injury), delivered via a microsyringe, and the balloon is then pulled back along the vessel. Repeat balloon passage twice and then withdraw the catheter. The iliac artery is ligated.
Close the inner muscle and connective tissue with sterile 3-0 absorbable Maxon suture and the dermal layers of the abdominal wall are closed with either interrupted or continuous sterile 4-0 nonabsorbable nylon suture.
Figure 15.2.
Illustration of aortic balloon angioplasty injury in mouse model of accelerated atherosclerosis and arterial aneurysmal dilatation. The right iliac artery is dissected and ligated proximally and distally (left panel). A microcatheter is inserted and advanced into the aorta and the balloon is inflated and then pulled back along the vessel (middle panel). Balloon passage is repeated twice and then the catheter is withdrawn and the iliac artery ligated (right panel).
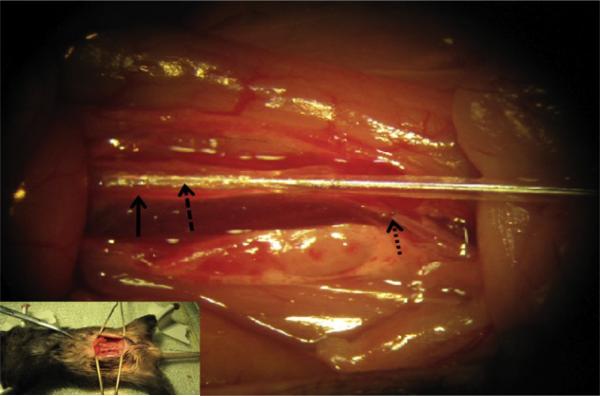
Figure 15.3.
Picture of balloon angioplasty in mouse. Lower left inserted picture shows abdominal incision and surgical exposure of the aorta for angioplasty balloon insertion and injury. Mag 10×.  , represents the right iliac artery into which the balloon is inserted;
, represents the right iliac artery into which the balloon is inserted;  , represents the abdominal aorta;
, represents the abdominal aorta;  , represents the balloon inserted into the right iliac artery and advanced into the abdominal aorta where it is inflated with saline and dragged back and forth in the aorta to stretch the aorta and to induce damage.
, represents the balloon inserted into the right iliac artery and advanced into the abdominal aorta where it is inflated with saline and dragged back and forth in the aorta to stretch the aorta and to induce damage.
3.1.1.3. Postoperative care
Temperature control: After surgery, animals are kept warm with a certified temperature controller, ATC 1000. Monitor animals every 5–20 min followed by every half hour intervals until stable.
Analgesia: Buprenophine at the dosage of 0.05–0.1 mg/kg is given every 12 h for a minimum of 48 h postoperatively or until veterinarian examination deems that analgesic administration is no longer necessary.
Nonabsorbable suture removal: Remove sutures at 7–10 days postsurgery.
Follow-up: Monitor animals daily for 4 weeks following the surgery. Any animals that show evidence of complications as a result of the procedure, such as nerve damage, hemorrhage (usually seen up to 5 days), infection, or difficulty with normal locomotion are euthanized. In some cases, if a mouse is lame temporarily on one side due to poor blood flow, the animal will be monitored for 24–48 h. At 4 weeks postoperatively, the animals are euthanized by pentobarbital sodium 150 mg/kg i.p. injection.
In follow-up analysis, the aorta and various other tissues such as heart, spleen, liver, bone marrow, and blood are harvested and isolated for histology and inflammatory/immune cell analysis. Tissue specimens, specifically aorta, are fixed in neutral buffered formalin, embedded cut, and stained using hematoxylin and eosin (H & E) or Masson's trichrome staining. Each aorta is cut into 2-3 equal length sections at measured intervals to ensure reproducible nonbiased sample analysis. Plaque area, aortic diameter, and cell invasion are measured by morphometric and immunohistochemical analysis of plaque area and cell number per microscopic high power field, as previously described.
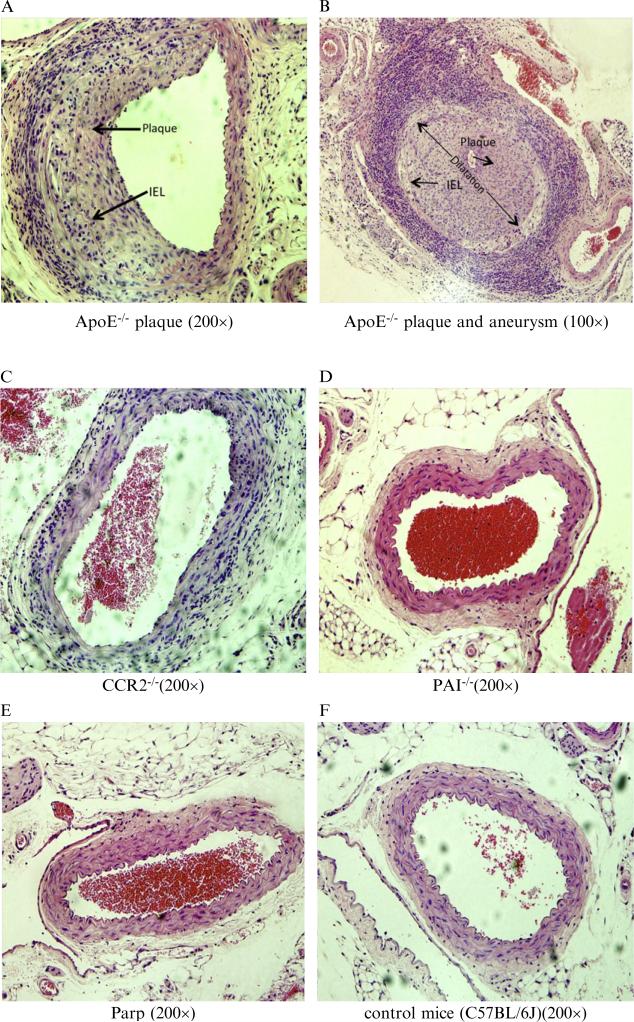
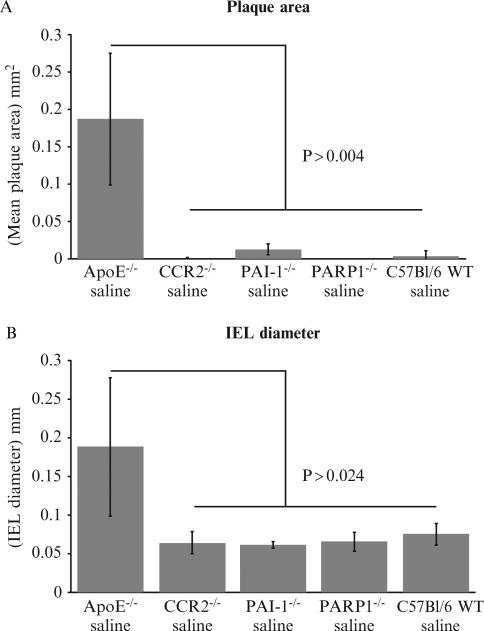
3.1.1.4. Angioplasty injury in ApoE-/-, but not WT (C57Bl/6) or other KO models, accelerates atheromatous plaque growth and aneurysmal dilatation
Compared to the other knockout mice, the plaque area and the internal elastic lamina (IEL) diameter were dramatically increased in the ApoE-/- mice 4 weeks postballoon angioplasty (Figs. 15.4 and 15.5). No increase in plaque area or aneurysmal dilatation was observed in CCR2-/- (N = 5), PAI-1-/- (N = 12), Parp1-/- (N = 6), and C57BL/6 (N = 19) mice at 4 weeks follow-up after angioplasty injury (Fig. 15.5). All told, this indicates that PAI-1, CCR2, and Parp1 do not alter the development of atherosclerotic plaque or aneurysm formation in this balloon angioplasty model. ApoE deficiency plays an important role in the development of atherosclerotic plaque or aneurysm formation after balloon angioplasty in this mouse model.
Figure 15.4.
Cross-sections of Hematoxylin and eosin (H & E) stained aorta isolated from mice 4 weeks after balloon angioplasty injury. Large areas of intimal plaque growth (arrows, A. Mag 200×) and aneurysmal dilatation (B. Mag 100×) were detected in ApoE-/- mice, but not in CCR2-/- (C. Mag 200×), PAI-1-/- (D. Mag 200×), Parp1-/- (E. Mag 200×), nor WT C57Bl/6 (F. Mag 200×) mice. A lower magnification picture is used in panel B to illustrate the marked increase in internal elastic lamina (IEL) diameter as marked by a double sided arrow demonstrating aneurysmal dilatation.
Figure 15.5.
(A) Bar graphs illustrate differing plaque size with each mouse model. ApoE-/-mice with balloon injury have significantly larger plaque area (P < 0.004) than the other KO (knock out) mouse models or WT mice. (B) Bar graphs demonstrate increased mean internal elastic lamina (IEL) diameter in ApoE-/- mice when compared to WT and KO mouse models after angioplasty injury, evaluated at 4 weeks postangioplasty.
3.2. Analysis of cellular mechanisms of anti-inflammatory activity
Once an effect is confirmed with serpin treatment in the animal models, one of the first questions that arises is “what particular stage of the inflammatory response is the serpin targeting?” Flow cytometry provides insight into cell responses through an analysis of changes in representative populations, both in response to ongoing vascular disease and serpin modulation of the disease state. We assess cells isolated from the bone marrow, circulating blood, spleen, and lymph nodes for changes in monocytes, dendritic cells (DCs), T cells, B cells, and stem cells. In prior work, we have focused on pro- and anti-inflammatory monocytes (MC1 and MC2, respectively) and pro- and anti-inflammatory T lymphocytes (Th1, Th17, and Th2, Treg, respectively, in a very broad sense). In current work, we expand our analyses to incorporate a broader range of potential cell targets. With eight-color flow cytometry, we can analyze a set of cell types using unique antibodies that allow for a clear separation of cell populations. In the following sections, we will describe cell isolation and labeling for flow cytometry as well as subsequent analysis and differentiation of cell types (Shapiro, 1995; Wang et al., 2007).
3.2.1. Flow cytometric immunophenotyping of splenocytes
As a part of lymphatic system, the spleen hosts a large quantity of monocytes, T cells, B cells, and other leukocytes. Immunophenotyping of these cells sheds light on how the immune system responds to selected diseases as well as to serpin treatment. Prior work has demonstrated that the viral serpin, Serp-1, and the mammalian serpin, neuroserpin, have effects on a variety of cell types in inflammatory vascular transplant disease models (Hausen et al., 2001; Miller et al., 2000; Munuswamy-Ramanujam et al., 2010; Viswanathan et al., 2009). To extend our understanding of the disease pathogenesis and serpin-mediated regulation of innate and acquired immune responses, we have expanded our analyses of cell responses to incorporate more cell types. In our current studies in mouse models of vascular diseases, we examine monocyte, B cell, T cell subgroups, natural killer, dendritic, and progenitor cells using flow cytometry. The cell types and the markers we have chosen for the cells are summarized in Table 15.1. Using this approach, we are able to determine the timing and direction of change in selected cell types in the immune response to disease.
Table 15.1.
Immune system cell types and corresponding fluorochrome-labeled antibodies
| Cell types | Marker |
|---|---|
| Cytotoxic T cell | Anti-CD3-PerCP-Cy5.5; Anti-CD8-APC-eFluor780 |
| T helper cell | Anti-CD3-PerCP-Cy5.5; Anti-CD4-PE-Cy7 |
| Th1 cell | Anti-IFNγ-FITC |
| Th2 cell | Anti-IL4-PE |
| Th17 cell | Anti-IL17a-AF647 |
| Treg cell | Anti-FoxP3-eFluor450 |
| B cell | Anti-CD19-Cy7 |
| Hematopoietic stem cell | Anti-CD34-PerCP-Cy5.5 |
| NK cell | Anti-NK1.1-eFluor450 |
| Monocyte | Anti-CD11c-APC-eFluor780 |
| Dendritic cell (Mature) | Anti-CD83-PE |
| Dendritic cell (Immature) | Anti-CD206-FITC |
| Memory T cell | Anti-CCR6-APC |
In our experiments, antibodies conjugated with different fluorochromes are accommodated in an antibody mixture to allow simultaneous labeling of multiple cell types in the sample. Due to the spectral overlap between different fluorochromes, it is necessary to adjust the amount of specific fluorescence emission by subtracting the “spillover” (overlap) component from the selected emission wavelength, a process called compensation. To perform these assays, a series of samples single-stained with each fluorochrome are utilized as controls (for more detailed discussion of compensation methodology, please refer to Shapiro, 1995). To rule out nonspecific staining, we also include isotype control staining using antibodies that have no specificity for the markers of interest in order to assess for nonspecific, irrelevant staining.
3.2.1.1. Preparation of single splenocyte suspension
Place mouse spleen on a 70-μm nylon mesh in a Petri dish with 5 ml of RPMI media with 10% FBS and 1% penicillin and streptomycin; mash the spleen with the rubber end of a plastic syringe plunger to release the cells.
Discard nylon mesh and the cell clumps; transfer the cell suspension in the Petri dish to a centrifuge tube.
Centrifuge cells at 400×g for 5 min and discard the supernatant.
To lyse erythrocytes, 5 ml of 0.84% ammonium chloride is added to resuspend the cells and then incubated at room temperature for 10 min.
Centrifuge at 400×g for 5 min, discard the supernatant.
Wash the cell pellet twice by resuspending cells in phosphate-buffered saline (PBS) and centrifuging at 400×g for 5 min; discard the supernatant.
Suspend cells in PBS at a concentration of 5×106 cells/ml.
3.2.1.2. Labeling of cells with fluorescent-conjugated antibodies
Transfer cells to 96-well plate total volume of 100 μl/well.
Centrifuge at 400×g for 5 min, discard the supernatant.
Antibodies to surface or intracellular antigens are diluted to 2 ng/μl in antibody dilution buffer (PBS + 2% fetal bovine serum (FBS)). Add 25 μl of surface antibody mix to each well; incubate for 30 min in the dark at room temperature.
Add 150 μl of PBS to each well, centrifuge at 400×g for 5 min; discard the supernatant.
If no intracellular antigen staining is planned, resuspend labeled cells in each well with 150 μl of PBS and transfer them to individual plastic tube, proceed to flow cytometry analysis, or add equal volume of 4% paraformaldehyde and store for later analysis.
If staining of intracellular antigens is desired, resuspend cell pellet from step 4 in 500 μl of fixation/permeabilization buffer (eBioscience, San Diego, CA) and transfer the cell suspension to a polystyrene tube, incubate in dark for 45 min at 4 °C.
Add 500 μl of 1× permeabilization buffer (eBioscience), centrifuge at 400×g for 5 min at 4 °C, and discard the supernatant. Repeat one more time.
Add 25 μl of intracellular antibody mix, shake gently, and incubate in dark for 30 min at 4 °C.
Add 500 μl of PBS to each tube, vortex gently, centrifuge at 400×g for 5 min at 4 °C, and discard the supernatant.
Resuspend labeled cells in 150 μl of PBS, proceed to flow cytometry analysis, or add same volume of 4% paraformaldehyde and store for later analysis.
3.2.1.3. Flow cytometry analysis
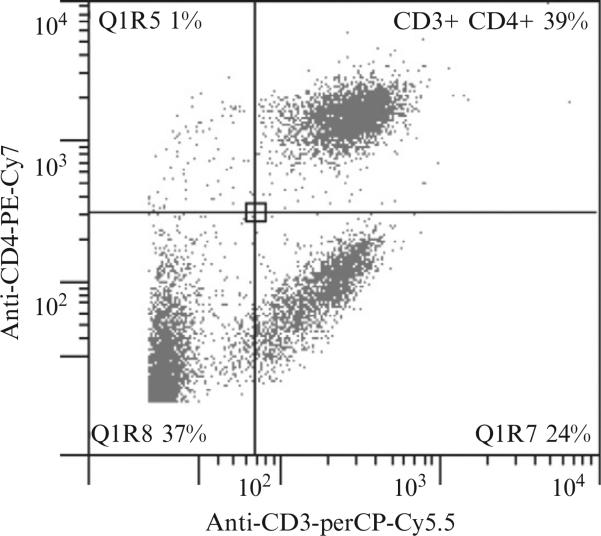
We performed flow cytometry using a CyAn ADP Analyzer (Dako, Ft Collins, CO). The data were analyzed with Gatelogic (eBioscience). The percentage of a specific cell population is illustrated with a two-dimensional histogram. Figure 15.6 is a sample histogram that provides the percentage of T helper cell population (CD3+, CD4+) in a splenocyte sample.
Figure 15.6.
Fluorescence flow cytometric assay of mouse spleen cell isolates with dot plot displaying CD3-PerCP-Cy5.5 on the x-axis and CD4-PE-Cy7 on the y-axis.
3.2.2. Detection of serpin inhibitory activity for human cells, in vitro: Analysis of adhesive activity of human leukocytes
While the mouse models provide an initial analysis of serpin immune modulating activity in a disease state and also an assessment of cells targeted in the mouse model, as has been noted frequently, mice and men differ significantly (Kennedy et al., 2007; Munuswamy-Ramanujam et al., 2010). We thus analyze serpin effects on human cells, in vitro, using cells isolated from patients with aneurysms and other vascular diseases, as well as assessing effects of serpins on individual human endothelial cell, monocyte, and T cell lines. As a preliminary screen for effects on human cell activation, we use cell adhesion assays with and without activators and serpin treatment. In our prior studies, both peripheral blood mononuclear cells (PBMCs) and cultured cell lines were used for this purpose. (Viswanathan et al., 2006, 2009; Zalai et al., 2001).
3.2.2.1. Isolation of PBMCs
Isolate PBMCs by density gradient centrifugation on Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ).
10 ml of heparinized blood is centrifuged at 400×g for 8 min.
Remove the serum layer on the top and resuspend the cells with an equal volume (~5 ml) of PBS +2% FBS.
Aliquot Ficoll-Paque PLUS into a 50-ml tube. The volume of Ficoll-Paque PLUS is equal to the cell suspension.
Carefully overlay the Ficoll-Paque PLUS with cell suspension, minimizing the interruption of cell to Ficoll interface.
Centrifuge at 400×g for 30 min at room temperature and wait for the rotor to stop without applying the brake.
PBMCs form a band between the upper buffer layer and the lower Ficoll layer. Carefully retrieve the PBMCs and transfer to a new centrifuge tube with a pipette, avoiding contamination by the buffer layer or Ficoll layer.
Wash the PBMCs twice with 10 ml of PBS and centrifuge at 400×g for 5 min. Resuspend the cell with proper media for further use.
3.2.2.2. In vitro adhesion assay after serpin treatment
Coat black 96-well plate with 100 μl of 50 ng/μl of fibronectin (33010–018, Invitrogen, Carlsbad, CA) per well.
After incubation at 37 °C for 1 h, discard the content of each well.
Wash each well twice with cold PBS.
Resuspend PBMCs at a concentration of 5×06 cells/ml in RPMI media without serum.
Label PBMCs with calcein acetoxymethyl (C1430, Invitrogen, Carlsbad, CA); add 5 μl of 1 mg/ml calcein acetoxymethyl per milliliter of cells, incubate for 1 h at 37 °C in the dark.
Wash cells with RPMI media and centrifuge at 400×g for 5 min; resuspend cells in RPMI media at 5×106 cells/ml.
To activate PBMCs, add Phorbol 12-myristate 13-acetate (PMA) to a final concentration of 10 μg/ml. At the same time, serpin can be added to treat the PBMCs at desired dose.
Transfer 100 μl of PBMCs to each fibronectin-coated well for cell assay repeated in triplicate. Set up standard curve by serial dilution of the calcein-labeled cells.
Incubate at 37 °C for 1 h.
Remove unattached cells by gentle washing 4× with RPMI media.
Add 100 μl of PBS to each well and measure the fluorescence at 527 nm emission during 485nm excitation.
3.3. Detection analysis of altered gene expression in cells isolated after arterial injury with and without serpin treatment
3.3.1. PCR arrays
In order to identify a cohort of genes involved in Serp-2's purported antiapoptotic effects and differentiate Serp-2 effects from the effects of antithrombotic serpin Serp-1 and chemokine modulating protein M-T7, three human cell lines were studied from the American Type Culture Collection (ATCC); Human umbilical endothelial vein cells (HUVECs, ATCC CRL-1730, passages 2–5), THP-1 monocytes (ATCC TIB-202), and Jurkat T cells (E6.1 clone, ATCC TIB-152). Prior studies have demonstrated a difference in apoptosis between Serp-2 and CrmA effectiveness in apoptosis induced by camptothecin (CPT), a topoisomerase I inhibitor, but not for any other apoptosis inducing agent.
3.3.1.1. RNA isolation
Treat cells with saline, 10 μM CPT in DMSO (Sigma), or CPT and 500 ng Serp-1, Serp-2, or M-T7 per million cells for either 30 min or 4.5 h. CPT and protein treatments are concurrent and continue until cell lysis.
Lyse cells and purify RNA using the Qiagen RNeasy kit (Valencia, CA).
Measure RNA concentration using a NanoDrop spectrofluorometer (Nanodrop, Wilmington, DE), and synthesize 500 ng of RNA into cDNA by SABioscience's First Strand kit (Frederick, MD).
Mix cDNA with SABioscience's premixed SYBR green/ROX fluor and PCR mix, and apply to the premanufactured plate containing apoptosis gene-specific primers in the bottom of each well.
Run plates on an ABI 7300 Real-time PCR Machine (Applied Biosystems, Foster City, CA) according to the recommended protocol—Stage 1: 10 min at 95 °C; Stage 2: 15 s at 95 °C, 1 min at 60 °C, 40 cycles; Stage 3: 15 s at 95 °C, 1 min at 60 °C, 15 s at 95 °C. Collect data points at Stage 2, Step 2 for each cycle.
Analyze gene expression fold changes using the ABI software. Changes deemed significant if they score P = 0.005 or lower on ANOVA analysis with Fisher's protected least significant difference (PSLD) post hoc testing provided by Statview statistics program (SAS Institute, Cary, NC).
For later studies, primers were manufactured to replicate the premanufactured plates, thus increasing the cost effectiveness of the technique. To increase the speed of the PCR array plate assembly, we recommend making a “master” primer plate, which contains ~200 μl of each primer pair in the correct well location, then use a multichannel pipette to transfer 5 μl of each primer pair into a fresh plate that will be used for sample analysis.
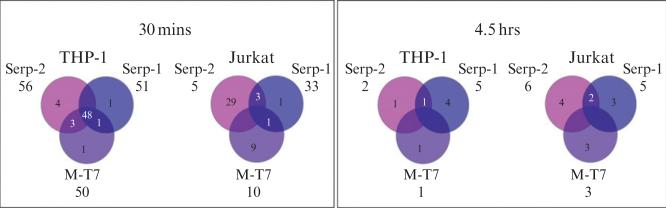
Early studies reveal that serpin and M-T7 treatments had the greatest effect in THP-1 monocytes at 30 min treatment (Fig. 15.7). Jurkat and HUVEC cell lines at both time points and THP-1 at 4.5 h demonstrated regulation of only a few concurrent genes (HUVEC not shown). This data underscores the significance of viral proteins in early regulation of monocyte responses to insult, particularly in regulation of host cell apoptosis.
Figure 15.7.
Dramatic differences between the number of genes regulated by all treatments in THP-1 monocytes at 30 min and Jurkat T cells, then again with the total number of genes regulated at 4.5 h. A set of apparently shared target genes is detectable in THP-1 cells at 30 min after treatment with each of the viral proteins.
4. Assessing Clinical Therapeutic Potential for a Viral Serpin in Clinical Trial: Trial of Serp-1 Treatment in Patients with Acute Coronary Syndrome and Stent Implant
4.1. Rationale
In order to assess the efficacy of a new therapeutic, in this case the viral serpin as a new class of immunotherapeutic, the ultimate analysis is a clinical trial. While clinical trial assessment of this new class of virus-derived serpins has been reported recently and is beyond the scope of this review, we would like to briefly present an overview of the steps used to complete the clinical trial of Serp-1 in acute coronary syndromes (ACS). The usual process from initial inception and testing of a new therapeutic and clinical trial (early stages) requires 15–20 years, on average. We provide here a brief outline of the steps required for clinical trial of a new class of immunotherapeutic protein and then provide some limited data on the findings in the Serp-1 Phase 2A clinical trial in patients with ACS and stent implant. This trial demonstrates safety, efficacy in reducing markers of myocardial damage, and trends toward modulation of inflammation.
4.2. Stages for proceeding into clinic
Taking a new class of drug into clinical testing in humans requires multiple preparatory steps in order to safely test a new protein immunotherapeutic. An outline of the requisite steps is provided below. While this outline provides an overview toward an approach to clinical testing, this is neither a detailed nor a comprehensive review of the approaches to be used to take this serpin protein into clinic.
Good lab practice (GLP) testin—Test potential immunomodulatory protein for toxicity prior to testing in man. Toxicity testing of the purified protein therapeutic is performed under highly controlled and reproducible conditions assessing for adverse effects, in a facility not connected to the initial research lab making the discovery.
Expression system for good manufacturing protocol (GMP) production—a production system for expression and purification of the final protein to be used for clinical testing is developed and established. In the case of the serpins tested, we use a cell line approved for protein expression by the Food and Drug Administration (FDA). The serpin gene is inserted into a CHO cell line and expressed from this cell line as a secreted protein. Alternative expression systems can include either Escherichia coli or Baculoviral expression vectors. Proteins are tested for purity, contaminants, and activity. A serpin activity assay is established to measure serpin inhibitory activity both during the isolation and purification steps and in blood samples for testing during clinical testing.
GMP product development—expression and purification of protein is then performed at a site experienced in expressing and purifying clinically active and safe drug.
Final GMP product is again tested for safety and toxicity, as well as half-life, in animal safety trials. Toxicity testing involves both broad spectrum analysis of potential adverse effects on test animal health, organ damage (liver, lung, spleen, etc.), and also selective testing for toxicity related to the disease and/or organ system designated for clinical testing. In some cases, this can require primate testing at an experienced lab.
FDA approval for Phase 1 safety trial—FDA approval is required for initial testing in man. This requires review by the FDA of all efficacy and also toxicity data as well as the protein therapeutic and its manufacture. A Phase 1 safety trial is designed in consultation with a clinical trials group and the FDA. We performed our initial safety trial using a group that specializes in Phase 1 trials, GFI (Go For It) in Indiana. The initial trial is monitored by the FDA, and progress to a true clinical trial is assessed and potentially approved by the FDA if the Phase 1 testing demonstrated safety.
FDA approval of a Phase 2A trial in patients with the targeted disease—Safety, with and without efficacy, of the serpin therapeutic is assessed in patients with clinical disease. In the case of Serp-1, a randomized, dose escalating, double blind trial was designed and performed and managed through a clinical regulatory organization as well as physicians designated as the site leaders for each hospital involved. A data safety monitoring board (DSMB) is established to monitor for any potential adverse effects of the drug tested.
Efficacy testing—This Serp-1 Phase 2A trial design allowed for both measurement of the circulating serpin half-life (t1/2), monitoring for any changes in serum markers of organ dysfunction (liver and renal and cardiac as well as hematological markers), and also collection of serum markers for effects of serpin treatment on markers of inflammation.
4.3. Clinical trial findings
Serp-1 was tested as a potential anti-inflammatory therapeutic for reduction of inflammation in patients with unstable coronary syndromes, so-called acute coronary syndromes, which include unstable angina and non-ST elevation MI. The details of this trial have been recently published. Forty-eight patients with coronary lesions appropriate for stent implant were enrolled, after obtaining informed consent, and randomized in a 3:1 pattern for either Serp-1 or saline control treatments; Serp-1 at two doses, with dose escalation. Coronary stenosis (narrowing) appropriate for stent implant as well as efficacy of stent implant was assessed by contrast angiography and intravascular ultrasound (IVUS). With this Phase 2A, clinical trial Serp-1 treatment was given for three consecutive days starting immediately after stent implant and then as an intravenous bolus every 24 h.
Of interest, this trial demonstrated excellent safety for serpin treatment when used in this clinical population with unstable coronary plaque. Major adverse cardiovascular events (MACE) were low and no significant adverse effects were demonstrated (MACE—control 2 of 12 patients with placebo, 5 of 19 in low-dose 5 μg/kg×3 days Serp-1 treatment group, and 0 of 17 patients with high-dose 15 μg/kg×3 days Serp-1 (P = 0.058)). In analysis of markers, a dose- and time-dependent reduction in markers of myocardial damage, specifically in troponin I levels with Serp-1 at 8, 16, 24, and 54 h (P < 0.05) and in CK-MB levels at 8, 16, and 24 h postdose (P < 0.05), was detected. (Tardif et al., 2010).
On further analysis of inflammatory biomarkers, both trends as well as some significant changes in markers of inflammation at selected times postinfusion and PCI were also detected (Tables 15.2 and 15.3). The markers tested included PAI-1, C reactive protein (CRP), myeloperoxidase (MPO), monocyte chemoattractant protein (MCP-1), D dimmer (DD), and brain natriuretic peptide (BNP). These changes in circulating markers of inflammation were most pronounced when data was separated into patients treated with or without statins at the time of stent implant (Table 15.2) or into groups of patients receiving either bare metal stents (BMS) or drug eluting stents (DES) (Table 15.3). While these analyses did not achieve dose and time-dependent significance, as detected for markers of myocardial damage (Troponin I or CK-MB), particularly when both statins and stent types were incorporated into the analysis, this lack of significance is thought to be in part limited by the small number of patients in the trial (N = 48) for all treatment groups, making detection of significance more challenging.
Table 15.2.
Inflammatory and Thrombotic markers in patients separated into those treated with statins and those without stating treatment prior to stent implant
| Treatment/ biomarker |
0 h |
8 h |
16 h |
24 h |
48 h |
54 h |
336 h |
612 h |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | No statin | Statin | All | No statin | Statin | All | No statin | Statin | All | No statin | Statin | All | No statin | Statin | All | No statin | statin | All | No statin | statin | All | No statin | Statin | |
| Pl/MCP-1 | 28.18 + 2.46 | 25.41 + 4.18 | 30.16 + 3.04 | 36.29 + 3.53 | 29.48 + 5.21 | 41.97 + 3.68 | 37.54 + 6.08 | 29.63 + 9.09 | 42.06 + 8.00 | 33.24 + 3.12 | 26.94 + 7.14 | 35.94 + 3.08 | 32.06 + 5.57 | 31.21 + 9.65 | 32.91 + 7.15 | 34.56 + 3.31 | 39.83 + 6.28 | 31.55 + 3.65 | 39.49 + 5.64 | 46.54 + 15.38 | 35.46 + 2.88 | 40.86 + 7.35 | 52.19 + 18.53 | 34.38 + 4.91 |
| s-1 5 μg/ MCP-1 | 28.03 + 4.40 | 27.85 + 6.48 | 28.17 + 6.29 | 30.28 + 3.38 | 33.63 + 6.31 | 27.85 + 3.71* | 29.23 + 2.73 | 30.53 + 4.46 | 28.28 + 3.58* | 28.45 + 2.53 | 33.20 + 4.90 | 25.00 + 2.22* | 23.92 + 2.71 | 25.76 + 5.94 | 22.59 + 2.12 | 26.29 + 2.26 | 25.46 + 5.10 | 26.89 + 1.61 | 31.14 + 3.22 | 33.76 + 7.28 | 29.23 + 2.07 | 30.32 + 3.16 | 29.80 + 6.07* | 30.74 + 3.32 |
| s-1 15 μg/ MCP-1 | 28.82 + 4.29 | 26.75 + 6.57 | 26.87 + 5.89 | 31.05 + 3.75 | 35.35 +7.28 | 28.71 + 4.31** | 34.92 + 4.40 | 31.85 + 6.13 | 36.77 + 6.18 | 26.68 + 3.08 | 24.94 + 4.21 | 27.72 + 4.37 | 24.28 + 3.50 | 21.68 + 4.25 | 25.58 + 4.90 | 25.69 + 3.80 | 20.72 + 3.92** | 28.44 + 5.44 | 27.08 + 3.31* | 28.22 + 3.55** | 26.45 + 4.91 | 33.15 + 4.04 | 33.59 + 6.86 | 32.98 + 5.17 |
| Pl/MPO | 85.96 + 23.99 | 71.46 + 29.26 | 96.32 + 38.86 | 19.51 + 3.77 | 15.22 + 1.86 | 23.08 + 6.65 | 28.76 + 9.43 | 19.07 + 3.75 | 34.30 + 14.66 | 24.61 + 2.95 | 34.66 + 1.65 | 20.30 + 2.81 | 17.60 + 3.40 | 22.70 + 5.84 | 12.50 + 1.54 | 24.59 + 3.83 | 30.92 + 6.92 | 20.97 + 4.33 | 23.18 + 4.18 | 25.65 + 3.94 | 21.77 + 6.36 | 22.54 + 3.62 | 31.88 + 5.42 | 17.21 + 3.58 |
| s-1 5 μg/ MPO | 68.63 + 15.1 | 61.68 + 26.91 | 74.20 + 17.79 | 25.16 + 4.21 | 29.05 + 8.08 | 22.33 + 4.46 | 31.73 + 5.19 | 31.27 + 9.36 | 32.07 + 6.26 | 47.74 + 13.12 | 67.04 + 27.4! | 33.70 + 10.21 | 26.25 + 5.24 | 30.47 + 11.72 | 23.19 + 3.64 | 24.28 + 3.55 | 25.81 + 4.95 | 23.16 + 5.14 | 30.96 + 9.37 | 18.93 + 2.28 | 39.72 + 15.87 | 20.63 + 3.50 | 18.01 + 2.06 | 22.74 + 6.15 |
| s-1 15 μg/ MPO | 90.29 + 21.25 | 90.78 + 32.02 | 90.00 + 29.37 | 40.34 + 22.69 | 88.25 + 62.79* | 14.21 + 2.18 | 29.59 + 6.43 | 36.66 + 9.48 | 25.35 + 8.67 | 38.31 + 12.20 | 46.78 + 29.0 | 33.23 + 10.39 | 22.24 + 3.33 | 19.71 + 3.05 | 23.51 + 4.83 | 25.36 + 6.90 | 17.29 + 1.79 | 29.84 + 10.60 | 16.70 + 1.84 | 19.66 + 2.68 | 15.05 + 2.36 | 36.43 + 9.70 | 37.84 + 20.83 | 35.87 + 11.50 |
| Pl/DD | 485.3 + 267.4 | 179.6 + 48.8 | 703.7 + 452.3 | 664.4 + 320.0 | 263.4 + 86.1 | 989.6 + 566.9 | 857.5 + 239.5 | 735.3 + 415.6 | 927.4 + 314.1 | 939.2 + 288 | 611.9 + 256 | 1080 + 397 | 785.4 + 158 | 825.4 + 286 | 745.4 + 181 | 1125 + 251 | 797.1 + 336 | 1312 + 340.6 | 965.0 + 351.8 | 315.1 + 73.9 | 1336 + 509.2 | 645.4 + 201.5 | 213.8 + 47.8 | 892.0 + 278.9 |
| s-1 5 μg/ DD | 223.6 + 43.3 | 177.4 + 37.8 | 260.5 + 71.7* | 373.. 5 + 74.9 | 456.2 + 139.4 | 313.3 + 81.8* | 532.4 + 100.5 | 629.1 + 206.9 | 462.0 + 91.7** | 538.7 + 114.1 | 705.1 + 261 | 422.1 + 62.5* | 698.0 + 136 | 554.5 + 173 | 802.3 + 199 | 524.1 + 69.6* | 540.8 + 140 | 511.9 + 71.3* | 405.4 + 61.8* | 441.3 + 89.2 | 379.3 + 87.5* | 281.3 + 53.8** | 246.9 + 45.2 | 308.9 + 91.6 |
| s-1 15 μg/ DD | 202.7 + 51.2 | 202.4 + 53.5 | 202.9 + 77.7** | 433.7 + 91.8 | 577.4 + 186.6 | 355.4 + 97.9* | 406.7 + 103* | 450.9 + 189.8 | 380.1 + 126.3** | 601.0 + 184.3 | 957.7 + 446 | 387.0 + 103* | 583.1 + 109 | 749.5 + 233 | 500.0 + 116 | 438.5 + 72.8* | 505.5 + 72.8* | 401.2 + 85.1* | 283.7 + 55.5* | 410.5 + 115 | 213.3 + 49.0* | 216.6 + 36.8* | 288.0 + 96.6 | 184.9 + 31.2* |
Variable reductions in circulating markers of inflammation are detectable at follow up
P ≤ 0.05 comparison Serp-1 treatment to Placebo by ANOVA with Fisher's PLSD;
P ≤ 0.10 comparison Serp-1 treatment to Placebo by ANOVA with Fisher's PLSD.
All values expressed as mean + SE, Pl — Placebo; S-1 — Serp-1; DD - D dimer, MCP-1 - monocyte chemoattractant protein - 1, MPO — myeloperoxidase.
Table 15.3.
Inflammatory and Thrombotic markers in patients separated into those treated with Bare Metal Stent (BMS) and those with Drug Eluting Stent (DES) implants
| Treatment/ biomarker |
0 h |
8 h |
16 h |
24 h |
48 h |
54 h |
336 h |
612 h |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | BMS | DES | All | BMS | DES | All | BMS | DES | All | BMS | DES | All | BMS | DES | All | BMS | DES | All | BMS | DES | All | BMS | DES | |
| Pl/MCP-1 | 28.18 + 2.46 | 26.68 + 3.72 | 30.27 + 3.04 | 36.29 + 3.53 | 35.06 + 5.39 | 38.45 + 5.92 | 37.54 + 6.08 | 30.22 + 5.78 | 46.33 + 10.87 | 33.24 + 3.12 | 30.39 + 3.33 | 35.24 + 8.74 | 32.06 + 5.57 | 33.34 + 8.55 | 29.92 + 6.49 | 34.56 + 3.31 | 36.75 + 4.39 | 31.945.32 | 39.49 + 5.64 | 39.23 + 8.93 | 39.93 + 3.49 | 40.86 + 7.35 | 39.19 + 10.87 | 43.77 + 8.79 |
| s-1 5 μg/MCP-1 | 28.03 + 4.40 | 28.61 + 6.44 | 26.88 + 3.79 | 30.28 + 3.38 | 27.72 + 4.06 | 35.82 + 5.92 | 29.23 + 2.73 | 31.58 + 3.58 | 24.13 + 3.24* | 28.45 + 2.53 | 30.69 + 3.22 | 23.60 + 3.52* | 23.92 + 2.71 | 26.01 + 3.74 | 19.41 + 2.29 | 26.29 + 2.26 | 26.58 + 3.26 | 25.64 + 1.79 | 31.14 + 3.22 | 34.37 + 4.45 | 24.12 + 0.89 | 30.32 + 3.16 | 29.12 + 4.04 | 32.71 + 5.33 |
| s-1 15 μg/ MCP-1 | 28.82 + 4.29 | 23.97 + 4.68 | 39.18 + 8.48 | 31.05 + 3.75 | 31.25 + 4.71 | 30.39 + 5.27 | 34.92 + 4.40 | 33.93 + 5.31 | 37.91 + 8.61 | 26.68 + 3.08 | 25.73 + 3.87 | 29.50 + 4.67 | 24.28 + 3.50 | 23.58 + 4.23 | 27.07 + 5.43 | 25.69 + 3.80 | 25.68 + 4.45 | 25.71 + 2.81 | 27.08 + 3.31* | 28.35 + 4.17 | 22.42 + 1.03 | 33.15 + 4.04** | 33.33 + 5.08 | 32.51 + 4.71 |
| Pl/CRP | 3.49 + 0.76 | 2.86 + 0.67N | 4.36 + 1.59 | 3.85 + 0.76 | 2.81 + 0.57 | 5.68 + 1.54 | 3.92 + 0.95 | 3.43 + 1.36 | 4.51 + 1.44 | 3.65 + 0.82 | 2.82 + 0.50 | 5.33 + 2.42 | 3.51 + 1.02 | 2.11 + 0.53 | 5.84 + 2.11 | 3.95 + 1.09 | 2.64 + 1.16 | 5.52 + 1.83 | 2.29 + 0.62 | 1.99 + 0.75 | 2.80 + 1.18 | 1.11 + 0.19 | 0.82 + 0.05 | 1.63 + 0.42 |
| s-1 5 μg/CRP | 3.15 + 0.80 | 3.45 + 0.96 | 2.53 + 1.52 | 3.11 + 0.69 | 2.86 + 0.73 | 3.64 + 1.59* | 3.18 + 0.68 | 3.04 + 0.77 | 3.48 + 1.49 | 3.78 + 0.75 | 4.23 + 0.99 | 2.81 + 1.04 | 4.52 + 0.79 | 4.45 + 0.94** | 4.68 + 1.55* | 4.41 + 0.83 | 4.39 + 0.95 | 4.45 + 1.77 | 2.07 + 0.53 | 2.18 + 0.73 | 1.84 + 0.69 | 0.97 + 0.15 | 1.04 + 0.02 | 0.83 + 0.23 |
| s-1 15 μg/CRP | 2.76 + 0.70 | 3.21 + 0.81 | 0.79 + 0.36 | 2.25 + 0.63 | 2.65 + 0.79 | 0.92 + 0.29 | 3.75 + 1.05 | 4.18 + 1.25 | 2.47 + 2.05 | 2.65 + 0.74 | 3.04 + 0.94 | 1.50 + 0.92** | 2.53 + 0.49 | 2.76 + 0.60 | 1.60 + 0.33** | 2.90 + 0.62 | 3.09 + 0.71 | 1.80 + 0.01 | 1.78 + 0.43 | 1.88 + 0.75 | 1.39 + 0.53 | 1.08 + 0.21 | 1.17 + 0.25 | 0.77 + 0.37 |
| Pl/DD | 485.3 + 267.4 | 241.4 + 37.7 | 826.8 + 647.3 | 664.4 + 320.0 | 447.5 + 142 | 1044 + 890.3 | 857.5 + 239.5 | 871.0 + 259 | 841.4 + 463.8 | 939.2 + 288.0 | 693.9 + 138 | 1446 + 944 | 785.4 + 157.5 | 756.8 + 243 | 833.1 + 183 | 1125 + 251 | 713.7 + 228 | 1618 + 394.6 | 965.0 + 351.8 | 824.6 + 490 | 1211 + 512.9 | 645.4 + 201.5 | 348.7 + 124 | 1165 + 420.7 |
| s-1 5 μg/DD | 223.6 + 43.3 | 217.2 + 49.9 | 236.3 + 89.97 | 373..5 + 74.9 | 328.1 + 84.4 | 471.8 + 155.2 | 532.4 + 100.5 | 531.8 + 125 | 533.6 + 183.7 | 538.7 + 114.1** | 474.4 + 117 | 656.5 + 252.3 | 698.0 + 136.0 | 725.0 + 179 | 639.4 + 207 | 524.1 + 69.6 | 557.4 + 91* | 451.8 + 103.4* | 405.4 + 61.8* | 444.5 + 76.9 | 320.7 + 103.4** | 281.3 + 53.8** | 297.9 + 74.1 | 248.2 + 70.9** |
| s-1 15 μg/DD | 202.7 + 51.2 | 203.3 + 611.1 | 200.3 + 88.33 | 433.7 + 91.8 | 489.0 + 115 | 254.2 + 62.08 | 406.7 + 103* | 461.5 + 134.2 | 242.2 + 34.01 | 601.0 + 184.3 | 720.7 + 238 | 241.7 + 31.9* | 583.1 + 109.3 | 557.8 + 118 | 684.4 + 322.5 | 438.5 + 72.8* | 451.9 + 83.6* | 357.6 + 111* | 283.7 + 55.5* | 318.5 + 66.8* | 156.3 + 39.2** | 216.6 + 36.8** | 241.4 + 44.4 | 134.1 + 34.1** |
Variable reductions in circulatine markers of inflammation are detectable at follow up.
P ≤ 0.05 comparison Serp-1 treatment to Placebo by ANOVA with Fisher's PLSD;
P = 0.10 comparison Serp-1 treatment to Placebo by ANOVA with Fisher's PLSD.
All values expressed as mean + SE, Pl - Placebo; S-1 - Serp-1; DD - D dimmer, CRP - C reactive protein, MCP-1 - monocyte chemoattractant protein -1.
In separating patient treatment groups into treatment with statins and serpin dose at the time of stent implant, there are apparent reductions in MCP-1 and DD beginning at 8 h follow-up in the statin-treated patients (comparison to a combined placebo group). MPO had minimal demonstrated change in expression in the non statin-treated patients, and this change is only detectable at 8 h. By later follow-up times (54 and 336 h), DD was significantly reduced in both the total groups and in patients treated with statins (Table 15.2).
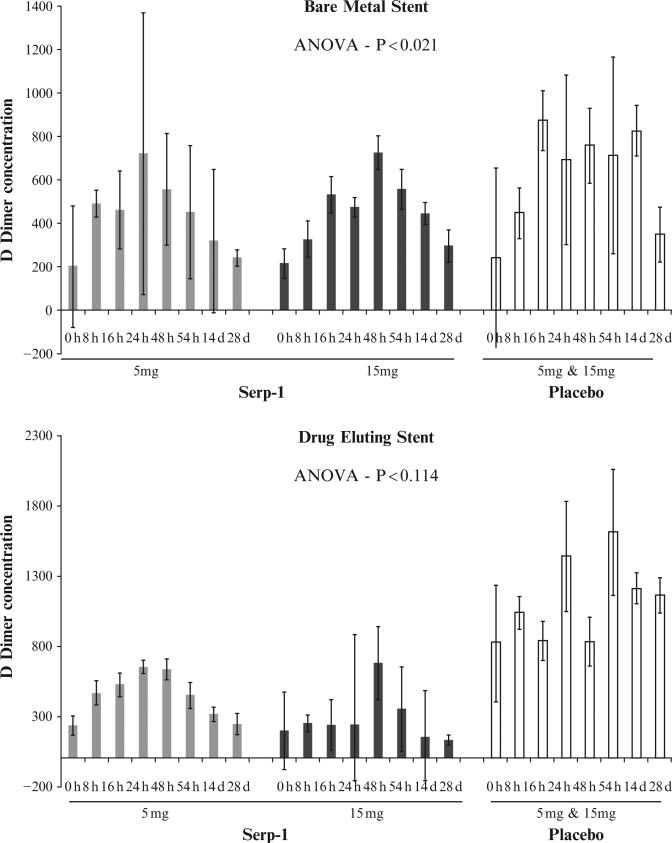
Similar trends were detected for patients when separated into those receiving different stent types, BMS or DES. In these analyses, MCP-1 and DD again showed more significant changes, with CRP showing only one limited change at the 8 h follow-up time point in patients with DES. Of interest, again MCP-1 and more significantly DD were significantly reduced, DD showing greater reductions in the whole group of patients and in patients with BMS 54–336 h follow-up (Table 15.3). The changes in DD are illustrated as a time course in bar graph format in Fig. 15.8, separated by placebo, serpin treatment and dosage, and time to follow-up. DD has been correlated with inflammatory, unstable coronary syndromes (ACS), as well as with pulmonary emboli (Empana et al., 2008; Hamaad et al., 2009) and is a by-product of fibrin degradation (fibrinolysis), the pathways targeted in part by the serpin treatment.
Figure 15.8.
Serum D Dimer levels in blood samples from ACS patients treated with Serp-1 at 5 or 15 μg/kg or placebo after either Bare Metal Stent (BMS, A) or Drug Eluting Stent (DES, B) coronary implants after treatment with 5 or 15 mg of Serp-1 or Placebo.
REFERENCES
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Berry LR, Thong B, Chan AK. Comparison of recombinant and plasma-derived antithrombin biodistribution in a rabbit model. Thromb. Haemost. 2009;102:302–308. doi: 10.1160/TH09-01-0062. [DOI] [PubMed] [Google Scholar]
- Bouchecareilh M, Conkright JJ, Balch WE. Proteostasis strategies for restoring {alpha}1-antitrypsin deficiency. Proc. Am. Thorac. Soc. 2010;7:415–422. doi: 10.1513/pats.201001-016AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron* C, Hota-Mitchell* S, Chen L, Barrett J, Cao JX, Macaulay C, Willer D, Evans D, McFadden G. The complete DNA sequence of myxoma virus. Virology. 1999;264:298–318. doi: 10.1006/viro.1999.0001. (*denotes co-authorship) [DOI] [PubMed] [Google Scholar]
- Dai E, Guan H, Liu L, Little S, McFadden G, Vaziri S, Cao H, Ivanova IA, Bocksch L, Lucas AR. Serp-1, a viral anti-inflammatory serpin,regulates cellular serine proteinase and serpin responses to vascular injury. J. Biol. Chem. 2003;278:18563–18572. doi: 10.1074/jbc.M209683200. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- Dollery CM, Owen CA, Sukhova GK, Krettek A, Shapiro SD, Libby P. Neutrophil elastase in human atherosclerotic plaques: Production by macrophages. Circulation. 2003;107:2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- Ekeowa UI, Freeke J, Miranda E, Gooptu B, Bush MF, Pérez J, Teckman J, Robinson CV, Lomas DA. Defining the mechanism of polymerization in the serpinopathies. Proc. Natl. Acad. Sci. USA. 2010;107:17146–17151. doi: 10.1073/pnas.1004785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empana JP, Canoui-Poitrine F, Luc G, Juhan-Vague I, Morange P, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, et al. Contribution of novel biomarkers to incident stable angina and acute coronary syndrome: The PRIME study. Eur. Heart. J. 2008;29:1966–1974. doi: 10.1093/eurheartj/ehn331. [DOI] [PubMed] [Google Scholar]
- Finlay B, McFadden G. Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooptu B, Lomas DA. Polymers and inflammation: Disease mechanisms of the serpinopathies. J. Exp. Med. 2008;205:1529–1534. doi: 10.1084/jem.20072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooptu B, Lomas DA. Conformational pathology of the serpins: Themes, variations, and therapeutic strategies. Annu. Rev. Biochem. 2009;78:147–176. doi: 10.1146/annurev.biochem.78.082107.133320. [DOI] [PubMed] [Google Scholar]
- Gramling MW, Church FC. Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment. Thromb Res. 2010;125:377–381. doi: 10.1016/j.thromres.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaad A, Sosin MD, Blann AD, Lip GY, MacFadyen RJ. Markers of thrombosis and hemostasis in acute coronary syndromes: Relationship to increased heart rate and reduced heart-rate variability. Clin. Cardiol. 2009;32:204–209. doi: 10.1002/clc.20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen B, Boeke K, Berry GJ, Morris RE. Viral serine proteinase inhibitor (SERP-1) effectively decreases the incidence of graft vasculopathy in hetero-topic heart allografts. Transplantation. 2001;72:364–368. doi: 10.1097/00007890-200108150-00003. [DOI] [PubMed] [Google Scholar]
- Johnston JB, McFadden G. Technical knockout: Understanding poxvirus pathogenesis by selectively deleting viral immunomodulatory genes. Cell. Microbiol. 2004;6:695–705. doi: 10.1111/j.1462-5822.2004.00423.x. [DOI] [PubMed] [Google Scholar]
- Kennedy SA, van Diepen AC, van den Hurk CM, Coates LC, Lee TW, Ostrovsky LL, Miranda E, Perez J, Davies MJ, Lomas DA, Dunbar PR, Birch NP. Expression of the serine protease inhibitor neuroserpin in cells of the human myeloid lineage. Thromb. Haemost. 2007;97:394–399. [PubMed] [Google Scholar]
- Kerr P, McFadden G. Immune responses to myxoma virus. Viral Immunol. 2002;15:229–246. doi: 10.1089/08828240260066198. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Ray CA, Pickup DJ, Howard AD, Thornberry NA, Peterson EP. Inhibition of interleukin-1-beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J. Biol. Chem. 1994;269:19331–19337. [PubMed] [Google Scholar]
- Kowal-Vern A, Walenga JM, McGill V, Gamelli RL. The impact of antithrombin (H) concentrate infusions on pulmonary function in the acute phase of thermal injury. Burns. 2001;27:52–60. doi: 10.1016/s0305-4179(00)00057-7. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Evans DL, Upton C, McFadden G, Carrell RW. Inhibition of plasmin, urokinase, tissue plasminogen activator and C1S by a myxoma virus serine proteinase inhibitor. J. Biol. Chem. 1993;268:516–521. [PubMed] [Google Scholar]
- Lucas A, McFadden G. Secreted immunomodulatory proteins as novel biotherapeutics. J. Immunology. 2004;173:4765–4774. doi: 10.4049/jimmunol.173.8.4765. [DOI] [PubMed] [Google Scholar]
- Lucas A, Liu L, Macen J, Nash P, Dai E, Stewart M, Graham K, Etches W, Boshkov L, Nation P, Humen D, Hobman MZ, et al. A virus-encoded serine protease inhibitor, SERP-1, inhibits atherosclerotic plaque development following balloon angioplasty. Circulation. 1996;94:2890–2900. doi: 10.1161/01.cir.94.11.2890. [DOI] [PubMed] [Google Scholar]
- Lucas A, Korol R, Pepine C. Inflammation in Atherosclerosis. Circulation. 2006;113:728–732. doi: 10.1161/CIRCULATIONAHA.105.601492. [DOI] [PubMed] [Google Scholar]
- Lucas A, Liu L, Dai E, Bot I, Viswanathan K, Munuswamy-Ramanujam G, Davids JA, Bartee MY, Richardson J, Christov A, Wang H, Macaulay C, et al. The Serpin Saga; Development of a New Class of Virus Derived Anti-inflammatory Protein Immunotherapeutics. In: Fallon Padraic., editor. Pathogen-Derived Immunomodulatory Molecules. Vol. 666. Landes Bioscience; Austin, TX: 2009. pp. 132–156. [DOI] [PubMed] [Google Scholar]
- Macen J, Upton C, Nation N, McFadden G. SERP-1, a serine proteinase inhibitor encoded by myxoma virus, is a secreted glycoprotein that interferes with inflammation. Virology. 1993;195:348–363. doi: 10.1006/viro.1993.1385. [DOI] [PubMed] [Google Scholar]
- MacNeill AL, Turner PC, Moyer RW. Mutation of the Myxoma virus SERP2P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology. 2006;356:12–22. doi: 10.1016/j.virol.2006.07.049. [DOI] [PubMed] [Google Scholar]
- McFadden G, editor. Viroceptors, Virokines, and Related Mechanisms of Immune Modulation by DNA Viruses. R.G. Landes Co; Austin, TX: 1995. [Google Scholar]
- McFadden G. Viroceptors: Virus-encoded receptors for cytokines and hemokines. In: Kotb M, Calandra T, editors. Cytokines and Chemokines in Infectious Diseases Handbook. Humana Press; 2003. pp. 285–299. [Google Scholar]
- McFadden G. Poxvirus tropism. Nat. Rev. Microbiology. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden G, Murphy PM. Host-related immunomodulators encoded by poxviruses and herpesviruses. Curr. Opin. Microbiol. 2000;3:371–378. doi: 10.1016/s1369-5274(00)00107-7. [DOI] [PubMed] [Google Scholar]
- Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: Differential modulation by T helper type 1 and type 2 cells. J. Exp. Med. 2001;194:657–667. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messud-Petit F, Geifi J, Delverdier M, Amardeilh MF, Py R, Sutter G. Serp2, an inhibitor of the interleukin-1beta-converting enzyme, is critical in the pathobiology of myxomavirus. J. Virol. 1998a;72:7830–7839. doi: 10.1128/jvi.72.10.7830-7839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messud-Petit F, Gelfi J, Delverdier M, Amardeilh MF, Py R, Sutter G. Serp2, an inhibitor of the interleukin-1-converting enzyme, is critical in the pathobiology of myxoma virus. J. Virol. 1998b;72:7830–7839. doi: 10.1128/jvi.72.10.7830-7839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LW, Dai E, Nash P, Liu L, Icton C, Klironomos D, Fan L, Nation PN, Zhong R, McFadden G, Lucas A. Inhibition of transplant vasculopathy in a rat aortic allograft model after infusion of anti-inflammatory viral serpin. Circulation. 2000;101:1598–1605. doi: 10.1161/01.cir.101.13.1598. [DOI] [PubMed] [Google Scholar]
- Mordwinkin NM, Louie SG. Aralast: An alpha 1-protease inhibitor for the treatment of alpha-antitrypsin deficiency. Expert Opin. Pharmacother. 2007;8:2609–2614. doi: 10.1517/14656566.8.15.2609. [DOI] [PubMed] [Google Scholar]
- Munuswamy-Ramanujam G, Khan KA, Lucas AR. Viral anti-inflammatory reagents: The potential for treatment of arthritic and vasculitic disorders. Endocr. Metab. Immune Disord. Drug Targets. 2006;6:331–343. doi: 10.2174/187153006779025720. [DOI] [PubMed] [Google Scholar]
- Munuswamy-Ramanujam G, Dai E, Liu LY, Shnabel M, Sun YM, Bartee M, Lomas D, Lucas A. Neuroserpin, a thrombolytic serine protease inhibitor (serpin), blocks transplant vasculopathy with associated modification of T-helper cell subsets. Thromb. Haemost. 2010;103:545–555. doi: 10.1160/TH09-07-0441. [DOI] [PubMed] [Google Scholar]
- Nash P, Whitty A, Handwerker J, Macen J, McFadden G. Inhibitory specificity of the anti-inflammatory myxoma virus serpin, SERP-1. J. Biol. Chem. 1998;273:20982–20991. doi: 10.1074/jbc.273.33.20982. [DOI] [PubMed] [Google Scholar]
- Nash P, Barry M, Seet BT, Veugelers K, Hota S, Heger J, Hodgkinson C, Graham K, Jackson RJ, McFadden G. Post-translational modification of the myxoma virus anti-inflammatory serpin, SERP-1 by a virally encoded sialyltransferase. Biochem. J. 2000;347:375–382. doi: 10.1042/0264-6021:3470375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel R, MacNeill AL, Wang YX, Turner PC, Moyer RW. Cowpox virus CrmA, Myxoma virus SERP2 and baculovirus P35 are not functionally interchangeable caspase inhibitors in poxvirus infections. J. Gen. Virol. 2004;85:1267–1278. doi: 10.1099/vir.0.79905-0. [DOI] [PubMed] [Google Scholar]
- Peitsch MC. Protein modeling by E-mail Bio/Technology. 1995;13:658–660. [Google Scholar]
- Petit F, Bertagnoli S, Gelfi J, Fassy F, Boucraut-Baralon C, Milon A. Characterization of a myxoma virus encoded serpin-like protein with activity against interleukin-1 β-converting enzyme. J. Virol. 1996;70:5860–5866. doi: 10.1128/jvi.70.9.5860-5866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov L, Laurila H, Hayry P, Vamvakopoulos JE. A mouse model of aortic angioplasty for genomic studies of neointimal hyperplasia. J. Vasc. Res. 2005;42:292–300. doi: 10.1159/000085905. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti- inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008a;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008b;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro HM. Practical Flow Cytometry. 3rd edn. Wiley-Liss; New York: 1995. p. 164. [Google Scholar]
- Silverman GA, Whisstock JC, Bollomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichart JM, Bird PI. Serpins flex their muscle I. Putting the clamps on proteolysis in diverse biological systems. J. Biol. Chem. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford M, Werden S, McFadden G. Myxoma virus in the European rabbit: Interactions between the virus and its susceptible host. Vet. Res. 2007;38:299–318. doi: 10.1051/vetres:2006054. [DOI] [PubMed] [Google Scholar]
- Tardif JC, L'Allier P, Grégoire J, Ibrahim R, McFadden G, Kostuk W, Knudtson M, Labinaz M, Waksman R, Pepine CJ, Macaulay C, Guertin MC, et al. A phase 2, double-blind, placebo-controlled trial of a viral Serpin (Serine Protease Inhibitor), VT-111, in patients with acute coronary syndrome and stent implant. Circ. Cardiovasc. Intervent. 2010;3:543–548. doi: 10.1161/CIRCINTERVENTIONS.110.953885. [DOI] [PubMed] [Google Scholar]
- Turner PC, Moyer RW. Serpins enable poxviruses to evade immune defenses. Am. Soc. Microbiol. News. 2001;67:201–209. [Google Scholar]
- Turner PC, Sancho MC, Thoennes SR, Caputo A, Bleackley RC, Moyer RW. Myxoma virus Serp2 is a weak inhibitor of granzyme B and interleukin- 1-converting enzyme in vitro and unlike CrmA cannot block apoptosis in cowpox virus-infected cells. J. Virol. 1999;73:6394–6404. doi: 10.1128/jvi.73.8.6394-6404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C, Macen JL, Wishart DS, McFadden G. Myxoma virus and malignant rabbit fibroma virus encode a serpin-like protein important for virus virulence. Virology. 1990;179:618–631. doi: 10.1016/0042-6822(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Liu L, Vaziri S, Richardson J, Togonu-Bickersteth B, Vatsya P, Christov A, Lucas AR. Myxoma viral serpin, Serp-1, a unique interceptor of coagulation and innate immune pathways. Thromb. Haemost. 2006;95:499–510. doi: 10.1160/TH05-07-0492. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Richardson J, Bickersteth B, Dai E, Liu L, Vatsya P, Sun Y, Yu J, Ramunujam G, Baker H, Lucas AR. Myxoma viral serpin, Serp-1. Inhibits human monocyte activation through regulation of Actin binding protein Filamin. B. J. Leukoc. Biol. 2009;85:418–426. doi: 10.1189/jlb.0808506. [DOI] [PubMed] [Google Scholar]
- Wang F, Roberts SM, Butfiloski EJ, Morel L, Sobel ES. Acceleration of autoimmunity by organochlorine pesticides: A comparison of splenic B-cell effects of chlordecone and estradiol in (NZBxNZW)F1 mice. Toxicol. Sci. 2007;99:141–152. doi: 10.1093/toxsci/kfm137. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Silverman GA, Bird PI, Bottomley SP, Kaiserman D, Luke CJ, Pak SC, Jean-Marc Reichhart JM, Huntington JA. Serpins flex their muscle II. Structural insights into target peptidase recognition, polymerization, and transport functions. J. Biol. Chem. 2010;285:24307–24312. doi: 10.1074/jbc.R110.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalai CV, Kolodziejczyk MD, Pilarski L, Christov A, Nation PN, Lundstrom-Hobman M, Tymchak W, Dzavik V, Humen DP, Kostuk WJ, Jablonsky G, Pflugfelder PW, et al. Increased circulating monocyte activation in patients with unstable coronary syndromes. J. Am. Coll. Cardiol. 2001;38:1340–1347. doi: 10.1016/s0735-1097(01)01570-4. [DOI] [PubMed] [Google Scholar]