Abstract
Maternal cigarette smoking during pregnancy is associated with increased risk of perinatal morbidity and mortality. However, the mechanisms underlying adverse birth outcomes following prenatal exposure to cigarette smoke remain unknown due, in part, to the absence or unreliability of information regarding maternal cigarette smoke exposure during pregnancy. Our goal was to determine if placental cotinine could be a reliable biomarker of fetal cigarette smoke exposure during pregnancy. Cotinine levels were determined in placentas from 47 women who reported smoking during pregnancy and from 10 women who denied cigarette smoke exposure. Cotinine levels were significantly higher in placentas from women reporting cigarette smoking (median = 27.2 ng/g) versus women who reported no smoke exposure (2.3 ng/g, P < 0.001). Receiver operating characteristic curve analysis identified an optimal cut point of 7.5 ng/g (sensitivity = 78.7%, specificity = 100%) to classify placenta samples from mothers who smoked versus those from mothers who did not. Among 415 placentas for which maternal cigarette smoking status was unavailable, 167 had cotinine levels > 7.5 ng/g and would be considered positive for cigarette smoke exposure. Data from quantitative reverse-transcription polymerase chain reaction analyses demonstrated that in utero cigarette smoke exposure predicted by cotinine in placenta is associated with changes in the expression of xenobiotic-metabolizing enzymes in fetal tissues. CYP1A1 mRNA in fetal lung and liver tissue and CYP1B1 mRNA in fetal lung tissue were significantly induced when cotinine was detected in placenta. These findings indicate that cotinine in placenta is a reliable biomarker for fetal exposure and response to maternal cigarette smoking during pregnancy.
Introduction
Cigarette smoking by women during pregnancy is associated with increased risk of adverse birth outcomes, including low birth weight, prematurity, neonatal mortality, childhood respiratory illnesses, and abnormal nervous system development. Decreased birth weight in infants born to mothers who smoke was first reported in 1957 (Simpson, 1957) and has since become the most consistently demonstrated consequence of maternal cigarette smoking during pregnancy (Walsh, 1994). In spite of multiple adverse outcomes associated with maternal cigarette smoking, the mechanisms underlying these outcomes are not well understood.
Typically, determination of prenatal cigarette smoke exposure is assessed by self-report through the use of interviews or questionnaires. However, self-reports of cigarette smoking are unreliable, and rates of nondisclosure are high, especially among women of child-bearing age and pregnant women themselves (Walsh et al., 1996; Ford et al., 1997; Dietz et al., 2011). In addition, studies of self-reports of cigarette smoking during pregnancy generally do not include environmental tobacco smoke exposure (ETS, including sidestream and exhaled cigarette smoke), which remains widespread (El-Mohandes et al., 2011). Therefore, high rates of nondisclosure and under-reporting of cigarette smoking among pregnant women indicate the need for a sensitive and objective biomarker to accurately determine smoking status and exposure to ETS.
Cotinine (C10H12N2O), the major metabolite of nicotine (C10H14N2), has been determined to be the most sensitive and specific biomarker for distinguishing smokers from nonsmokers (Jarvis et al., 1987) and is routinely measured in blood, urine, saliva, and hair. Cotinine in meconium and cord blood at the time of delivery has been employed as a biomarker of recent prenatal exposure to maternal cigarette smoking (Pichini et al., 2000; Derauf et al., 2003; Montgomery et al., 2006; Chazeron et al., 2008; Berlin et al., 2010; Wright et al., 2011; Baheiraei et al., 2012). However, it is not always possible or feasible to collect these samples, particularly in studies utilizing samples from anonymous donors or those deposited in biobanks for which little or no information is available regarding prenatal cigarette smoke exposure. Consequently, alternative methods for estimating exposure are required to understand the effects of maternal smoking on the developing fetus. Previous studies have shown that cotinine is detectable in term and preterm placenta from women who smoke (Luck et al., 1985; Joya et al., 2010); however, the threshold level of cotinine in placenta to distinguish smokers from nonsmokers has not been determined.
Gene–environment interactions have been suggested to be important in modifying individual susceptibility to low birth weight and preterm delivery associated with maternal cigarette smoking. The expression of enzymes responsible for the biotransformation and detoxification of polycyclic aromatic hydrocarbons and arylamines, the toxic components of cigarette smoke, are induced in placenta and fetal tissues by maternal cigarette smoking (Rifkind et al., 1978; Whyatt et al., 1995; Huuskonen et al., 2008; Bruchova et al., 2010; O'Shaughnessy et al., 2011). Furthermore, mothers carrying polymorphisms in CYP1A1, GSTT1, GSTM1, and EPHX1 are reported to have increased risk of having an infant with low birth weight if they smoke during pregnancy (Wang et al., 2002; Nukui et al., 2004; Wu et al., 2007). The mechanisms underlying the increased risk are understood to be related to the biotransformation of constituents of cigarette smoke to toxic intermediates (via CYP1A1) and pathways that detoxify those intermediates (via GSTT1, GSTM1, and EPHX1).
The majority of human studies investigating the effects of cigarette smoking on prenatal human development have used readily available samples from full-term pregnancies (i.e., placenta and cord blood). However, susceptibility of the fetus to the toxic effects of components of cigarette smoke is likely to be stage- and tissue-specific. On the other hand, there is limited availability of potentially relevant fetal tissues, particularly samples with reliable information regarding maternal cigarette smoking during pregnancy. Therefore, as part of a larger study investigating the effects of maternal cigarette smoking on development of the prenatal lung as it relates to postnatal lung disease, the purpose of this work was to compare cotinine levels in human preterm placentas from mothers who reported cigarette smoking during pregnancy with those from mothers who denied smoking. Through analysis of these data, we sought to determine the optimal threshold to classify cigarette smoke exposure of fetal samples from the same pregnancy. In addition, we wanted to elucidate how cotinine levels in placenta reflect exposure of the fetus to components of cigarette smoke through gene expression of xenobiotic-metabolizing enzymes in fetal lung and liver tissue.
Materials and Methods
Human Tissue Samples.
Anonymized human placenta, lung, and liver samples [49 to 137 days estimated post conceptional age (PCA)] were obtained from the National Institute of Child Health and Human Development–supported Laboratory of Developmental Biology at the University of Washington (Seattle, WA). Fetal genders were as follows: 205 female, 241 male, and 26 unknown. For a subset of samples (n = 57; fetal genders: 23 female, 29 male, and 5 unknown), a history of cigarette smoking as reported by the mother was obtained at the time of tissue collection. All tissues were flash frozen and maintained at −80°C prior to preparation of whole cell extracts or RNA. The use of these tissues was declared non–human subject research by the University of Missouri-Kansas City Pediatric Health Sciences Review Board.
Murine Model of Maternal Cigarette Smoking.
Study animals were housed in pathogen-free conditions, with ad libitum food and water. Daily exposure of female C57Bl/6 mice (Charles River Laboratories, Waltham, MA) to the smoke of three 3R4F research cigarettes (University of Kentucky, Lexington, KY), five times per week, started at 10 weeks of age. Placenta and serum samples were harvested from pregnant females with and without cigarette smoke exposure at embryonic day e17.5 through e18.5. Lung tissue samples for gene expression studies were collected from offspring on postnatal day 5. The protocols for the animal studies were approved in advance by the Harvard Medical School Institutional Animal Care and Use Committee.
Cotinine Determination.
Crude placenta tissue extracts were prepared from 100–150 mg placenta tissue by homogenization in 200 μl homogenization buffer (50 mM Tris-HCl, 150 mM KCl, 2 mM EDTA, pH 7.5). Homogenates were centrifuged at 1000g for 15 minutes at 4°C. Cotinine was detected using 10 μl placenta extract or blood (diluted 1:2 in tissue homogenization buffer) with the Cotinine Direct ELISA kit (Calbiotech, Spring Valley, CA) according to the manufacturer’s directions. Standard curves were prepared from serial dilutions of cotinine ranging from 100 ng/ml to 1 ng/ml. Cotinine concentrations in placenta extracts and blood were calculated from a 4-parameter log analysis of the standard curves (SigmaPlot 6.0) as previously described (Plikaytis et al., 1991).
Gene Expression Assays.
Total RNA was isolated from tissues as previously described for human and mouse samples, respectively (Leeder et al., 2005; Manoli et al., 2012). Gene expression assays for murine Cyp1b1 and Cyp1a1 were performed on RNA extracted from mouse lungs using TaqMan Gene Expression assays shown in Table 1 (Invitrogen, Carlsbad, CA) as previously described (Haley et al., 2008). RNA from human lung and liver was reverse transcribed using the Omniscript Reverse Transcription kit (Qiagen, Germantown, MD) according to manufacturer’s recommendations. Gene expression assays were performed using commercially available TaqMan Gene Expression assays (Invitrogen) for CYP1B1, CYP2A6, EPHX1, and GAPDH (Table 1) and PerfeCTa qPCR SuperMix (Quanta Biosciences, Gaithersburg, MD). Gene expression assays to detect CYP1A1 were performed using gene-specific primers (Table 1) and Perfecta SYBR Green SuperMix (Quanta Biosciences). Standard curves were generated using serial dilutions from 10 to 107 copies/μl of cDNA plasmids. Gene expression levels from human tissues were expressed as copy numbers determined from linear regression of the standard curves. At least three replicate determinations of gene expression were averaged for each subject and normalized with GAPDH expression.
TABLE 1.
Assay information for quantitative RT-PCR
| Gene | Primers/Assay ID | Assay Length | Assay Location |
|---|---|---|---|
| CYP1A1 | For 5′GTCAAGGAGCACTACAAAACC | 270 bp | Exons 2—– |
| Rev 5′CCGTGACCTGCCAATCACTG | |||
| CYP1B1 | Hs00164383_m1 (Life Technologies) | 118 bp | Exons 2-3 |
| CYP2A6 | Hs00868409_s1 (Life Technologies) | 131 bp | Exon 9 |
| EPHX | Hs01116806_m1 (Life Technologies) | 76 bp | Exons 6–7 |
| CYP19A1 | Hs00240671_m1 (Life Technologies) | 122 bp | Exons 122 |
| GAPDH | Hs99999905_m1 (Life Technologies) | 122 bp | Exon 3 |
| Cyp1a1 | Mm00487218_m1 (Life Technologies) | 120 bp | Exons 2–3 |
| Cyp1b1 | Mm00487229_m1 (Life Technologies) | 76 bp | Exons 2–3 |
| 18S | Hs99999901_s1 (Life Technologies) | 187 bp | Exon 1 |
Data Analysis.
To account for variation in tissue sampling, cotinine levels determined by enzyme-linked immunosorbent assays are expressed as ng/g placental tissue. Cotinine levels in placentas from mothers who reported smoking during their pregnancy were compared with levels in women who denied smoking using a two-sided Student’s t test. Receiver operating characteristic (ROC) curve was employed to determine the optimal threshold to classify a placenta sample as positive for maternal cigarette smoking based on 57 samples with reported smoking status. Area under the curve (AUC), sensitivity (true positive rate), and specificity (true negative rate) were determined as measures of prediction accuracy. An optimal cut point was determined by the cotinine level corresponding to the maximum sensitivity when specificity equals 1. A bootstrap approach was employed to statistically assess the fitness of the optimal cut point determined with ROC curve analysis. To do so, we re-sampled 50, 100, and 200 subjects from the original data, and for each sample the optimal cut point was recalculated. This process was repeated 10,000 times to determine the mean and standard deviation of the cut point, AUC, and sensitivity. Gene expression data were log-transformed and compared between samples with or without detectable cotinine in placenta derived from the same pregnancy by using a two-sided Student’s t test. All analyses were performed with R Project software version 2.13.2 (Lucent Technologies, Murray Hill, NJ), SPSS software version 18.0 (IBM, Armonk, NY), SAS software version 9.2 (SAS Institute, Cary, NC), and JMP statistical discovery software version 10.0 (SAS Institute, Cary, NC).
Results
Cotinine Levels Are Increased in Placentas from Mothers Who Report Smoking Cigarettes during Pregnancy.
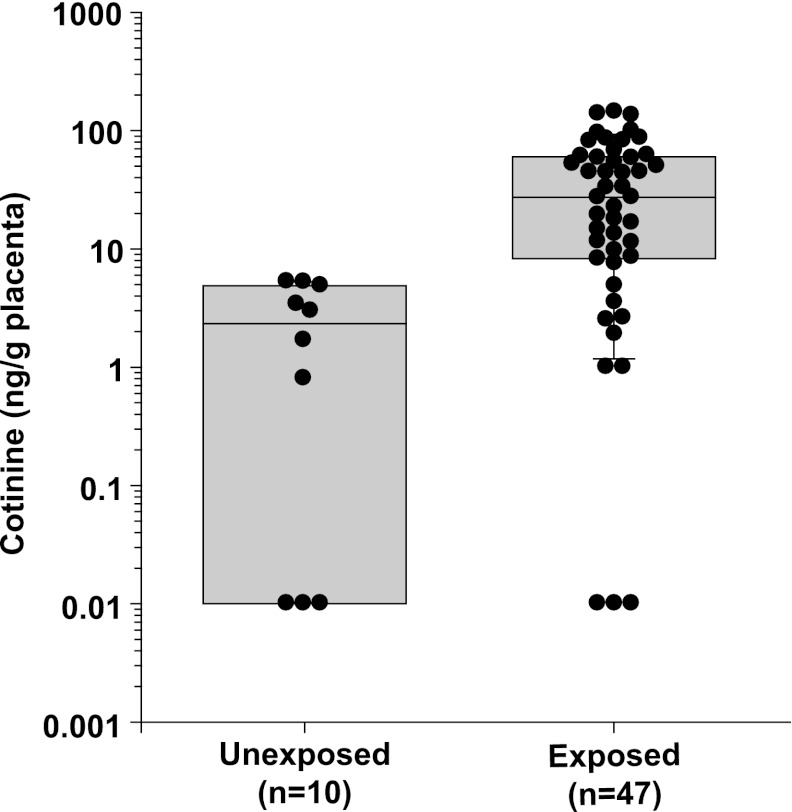
To characterize the range of placental cotinine exposure associated with maternal smoking, cotinine levels (ng/g total placental tissue) were determined in whole cell extracts prepared from human placenta samples obtained from 47 women who reported smoking at some point in their pregnancy (PCA 49 to 137 days). Cotinine values in placenta from self-reported smokers ranged from below the limits of detection (<1 ng/ml of extract, n = 3) to 143.5 ng/g of tissue with a median of 27.2 ng/g placenta (interquartile range 8.2–60.6 ng/g). One sample with no detectable cotinine was from a woman who reported “occasional” cigarette smoking, whereas the other two samples with no detectable cotinine did not have associated information provided regarding the frequency of cigarette smoking. The highest cotinine level was observed in the placenta from a woman who reported smoking a pack of cigarettes (20 cigarettes) per day. As negative controls, cotinine levels were determined in the placentas from 10 women who denied smoking during their pregnancy (PCA 67 to 122 days). Cotinine values in placenta in women who denied smoking ranged from not detectable (n = 4) to 5.27 ng/g of tissue, with a median of 2.3 ng cotinine/g of tissue (interquartile range 0.01–4.98 ng/g). Cotinine levels in the placentas from women who reported smoking during pregnancy were significantly higher than cotinine levels from those who denied cigarette smoking [Fig. 1A, mean (S.D.) = 2.42 (2.21) versus 40.06 (39.05), P < 0.001]. These data demonstrate significant differences in cotinine levels in human placenta of women who smoke cigarettes during pregnancy. Furthermore, seven samples from women who provided information regarding the number of cigarettes smoked per day [range 1–2 cigarettes per day to 1 pack (20 cigarettes) per day] suggest a positive relationship between cotinine levels in placenta and the level of cigarette exposure.
Fig. 1.
Cotinine levels (ng/g) in human placentas from mothers reporting no cigarette smoking versus mothers who reported smoking during their pregnancy. Cotinine concentrations in whole cell extracts were determined (as described in Materials and Methods) from placentas from women who denied smoking during their pregnancy (n = 10) and from those who reported cigarette smoking during pregnancy (n = 47).
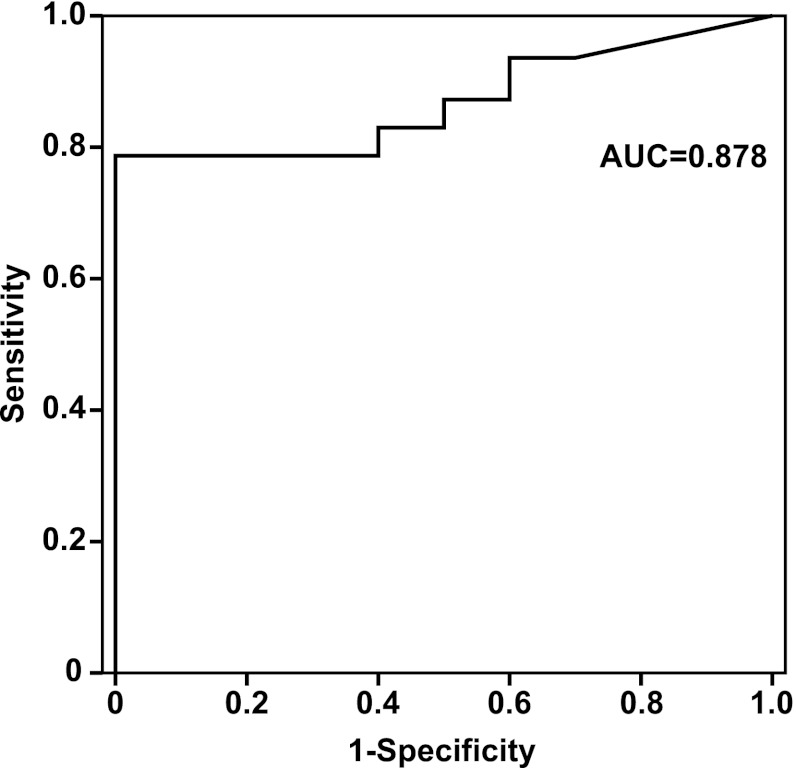
ROC curve analysis was performed to determine the optimal cut point required to classify a placental sample as one from a woman who smokes cigarettes. Figure 2 shows the sensitivity and 1 minus specificity (false positive rate) of placenta cotinine levels with an AUC of 0.878. The threshold of 7.5 ng/g placenta provided a sensitivity of 78.7% and a specificity of 100%. Lower thresholds between 5.2 and 7.5 ng cotinine/g placenta decreased specificity without an increase in sensitivity (Table 2). This analysis is based on a small number of samples with reported cigarette smoking by the mother during pregnancy; therefore, a bootstrapping approach was employed to statistically assess the reliability of the threshold of 7.5 ng/g. In three separate determinations with 10,000 iterations each, the mean threshold at which the specificity was 100% ranged from 7.5 to 7.6 ng/g, with a mean sensitivity ranging from 78.7% to 79.2% and an AUC of at least 0.877 for all three simulations (Table 3).
Fig. 2.
Receiver operating characteristics (ROC) curve for placenta cotinine concentration and maternal smoking status.
TABLE 2.
Sensitivity and specificity of cotinine in first trimester placenta
| Cotinine Concentration (ng/g) | Sensitivity | Specificity |
|---|---|---|
| 2.98 | 0.83 | 0.5 |
| 3.40 | 0.83 | 0.6 |
| 3.53 | 0.83 | 0.7 |
| 4.87 | 0.81 | 0.7 |
| 4.87 | 0.81 | 0.8 |
| 5.23 | 0.79 | 0.8 |
| 5.27 | 0.79 | 0.9 |
| 7.53 | 0.79 | 1 |
| 8.20 | 0.77 | 1 |
| 8.52 | 0.74 | 1 |
| 9.65 | 0.72 | 1 |
| 11.33 | 0.70 | 1 |
| 11.52 | 0.68 | 1 |
| 13.36 | 0.66 | 1 |
| 14.59 | 0.64 | 1 |
| 16.57 | 0.62 | 1 |
TABLE 3.
Mean bootstrap estimates for ROC thresholds and area under the curve
| Simulation | 50-Sample* | 100-Sample* | 200-Sample* |
|---|---|---|---|
| Mean threshold at specificity of 1 (S.D.) | 7.58 (1.45) | 7.58 (0.57) | 7.53 (0) |
| Mean AUC (S.D.) | 0.877 (0.05) | 0.878 (0.03) | 0.878 (0) |
| Mean sensitivity (S.D.) | 0.792 (0.06) | 0.788 (0.05) | 0.787 (0) |
AUC, area under the curve; ROC, receiver operating characteristics.
Data are calculated from 10,000 iterations of the bootstrap simulation.
Cotinine Concentrations in Placenta Suggest the Existence of Multiple Populations of Exposure.
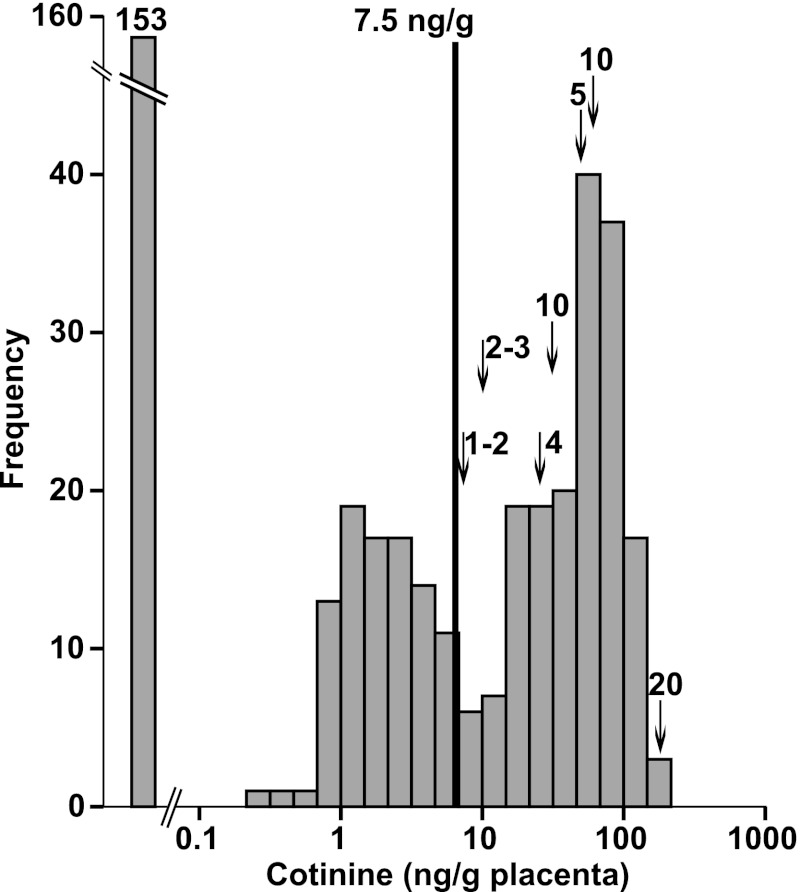
Cotinine levels were determined in an additional 415 placental samples (PCA 49–137 days) from pregnancies in which the history of maternal smoking was unknown. Cotinine was below the limits of detection of the assay for 153 samples and at detectable levels for 262 (63.1%) of the samples tested, ranging to over 180 ng/g placenta (n = 2). Based on the threshold of 7.5 ng/g determined by ROC curve analysis and confirmed by bootstrapping, 40.2% (n = 167) of the unknown samples would be classified as positive for maternal cigarette smoke exposure (either active smoking or ETS) on the basis of cotinine levels in the placenta. There was no relationship observed between estimated PCA and cotinine levels in human placenta, suggesting that placental cotinine levels are not the result of accumulation in the placenta throughout the first 20 weeks of pregnancy and most likely reflect recent exposure. The frequency histogram of log-transformed cotinine levels (ng/g placental tissue) in human placenta indicated the presence of at least two populations with detectable cotinine levels, in addition to a third group with cotinine levels below the limits of detection of the assay (Fig. 3). This distribution is similar to serum cotinine levels observed in over 16,000 participants in the National Health and Nutrition Examination Survey (NHANES) (Benowitz et al., 2009a). Furthermore, the threshold of 7.5 ng/g placenta as determined with the ROC curve analysis lies between the two population peaks.
Fig. 3.
Frequency distribution of placenta cotinine concentration (ng/g placenta) in 415 placentas from mothers for whom information regarding cigarette smoking was unavailable. The three peaks are consistent with unexposed, low/passively exposed, and actively intrauterine smoke exposed fetuses. The cut point of 7.5 ng/g determined by ROC analysis is shown. In addition, arrows indicate cotinine levels for seven mothers who reported the number of cigarettes consumed per day. Numbers above the arrows are the reported cigarettes per day.
Cotinine in Placenta Predicts Changes in Gene Expression in Fetal Tissues.
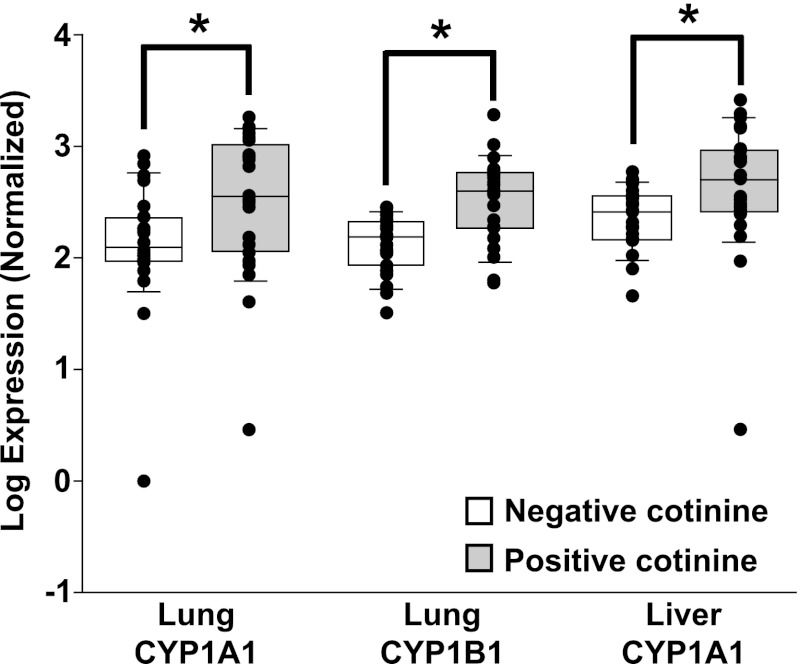
Maternal cigarette smoking has been shown to increase the expression of drug/xenobiotic-metabolizing enzymes in placenta and fetal liver. To determine if maternal smoking status, as determined by cotinine levels in placenta, is associated with changes in gene expression in fetal tissues, quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) was performed to detect the expression of CYP1A1, CYP1B1, CYP19A1, CYP2A6, and EPHX1 in fetal liver and/or lung tissue from 23 fetuses with no cigarette smoke exposure (defined as no detectable cotinine in the placenta) and from 23 with maternal cigarette smoke exposure [defined as cotinine >7.5 ng/g placenta (range 12.1–186.4 ng/g)]. Target gene expression was normalized using GAPDH, expression of which was not affected by PCA or the presence of cotinine in placenta in this set of fetal lung and liver tissues. After normalization with GAPDH, mRNA expression levels were significantly higher for both CYP1A1 (2.88-fold, P = 0.013) and CYP1B1 (2.57-fold, P = 0.0001) in lung samples for which placenta from the same pregnancy indicated maternal cigarette smoke exposure relative to lung samples from fetuses with no detectable cotinine in the placenta (Fig. 4). In addition, there was a statistically significant dose-response relationship observed between mRNA expression and cotinine concentration in placenta for both CYP1A1 and CYP1B1 in fetal lung (CYP1A1, r2=0.23, P = 0.0007; CYP1B1, r2=0.33, P < 0.0001). There were no statistically significant changes in mRNA expression in CYP2A6 or EPHX1 in lung tissue with respect to maternal cigarette smoke exposure. In fetal liver samples, CYP1A1 mRNA expression was increased (2-fold, P = 0.045) in samples with maternal cigarette smoking relative to those that had no detectable cotinine (Fig. 4). CYP19A1 and EPHX1 mRNA expression in fetal liver tissue was not associated with cotinine levels in the placenta. These data demonstrate that cotinine in placenta is associated with changes in gene expression in fetal liver tissue that are consistent with those seen in response to maternal cigarette smoke exposure (O'Shaughnessy et al., 2011). Furthermore, these data indicate that the developing fetal lung is exposed to potentially harmful components of cigarette smoke.
Fig. 4.
Expression of xenobioticmetabolizing enzymes in prenatal lung and liver in response to maternal cigarette smoking as predicted by placental cotinine concentrations. Quantitative RT-PCR was performed as described on RNA isolated from fetal liver or lung from pregnancies with (n = 23) or without (n = 23) maternal cigarette smoke exposure as determined by cotinine levels in the placenta. CYP1A1 mRNA expression was induced in fetal liver and lung tissue, and CYP1B1 expression was induced in fetal lung in response to maternal cigarette smoking. * P < 0.05.
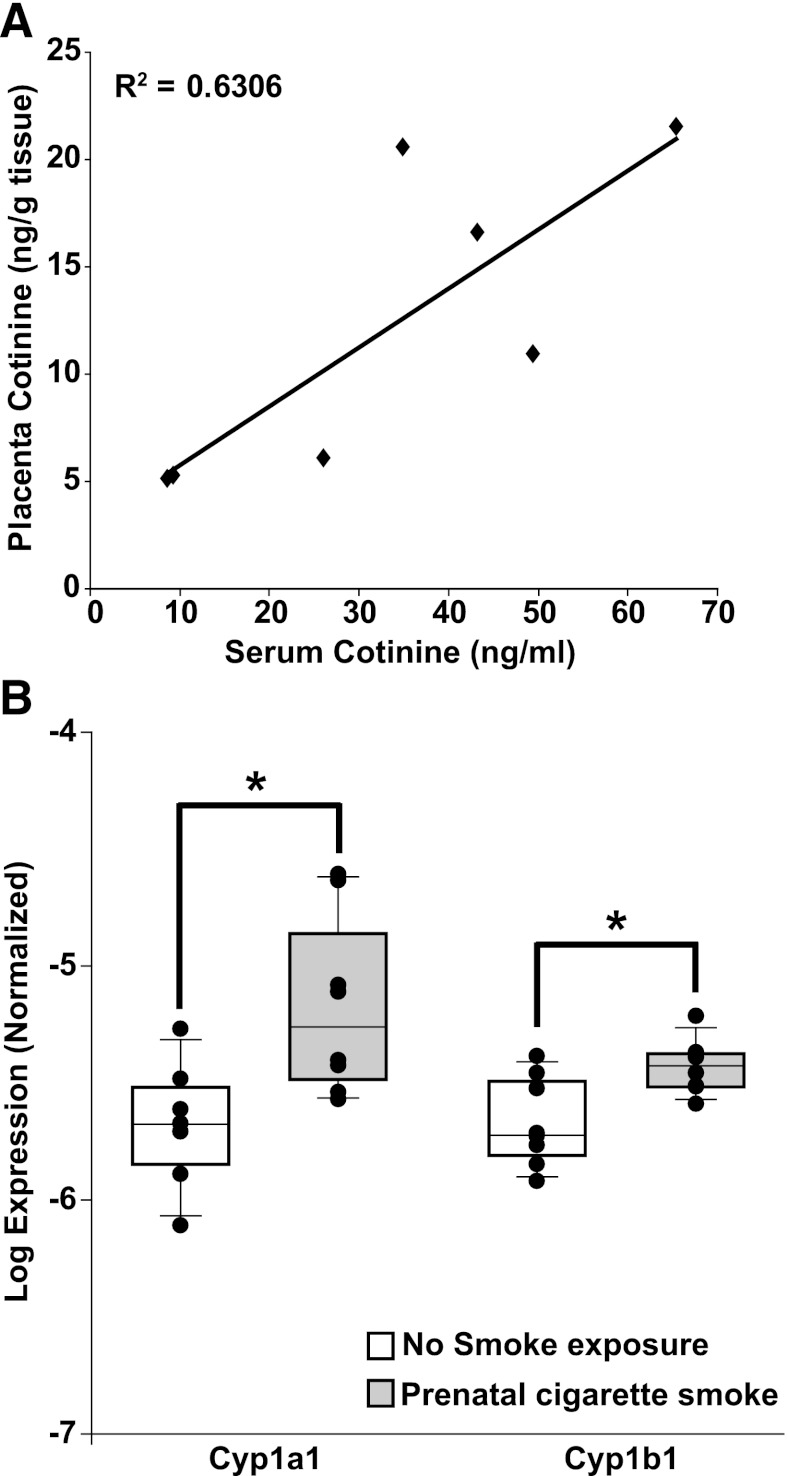
To further confirm that there exists a relationship between placental cotinine levels, maternal serum cotinine concentrations, and changes in the expression of drug-metabolizing enzymes in the fetal lung, a murine model of cigarette smoke exposure was employed. Cotinine levels were determined in placenta and serum samples collected on embryonic day 17.5 through 18.5 from unexposed pregnant adult female mice (n = 8) and from those exposed to mainstream cigarette smoke (n = 8). Serum cotinine levels in mice exposed to cigarette smoke were significantly higher than those in unexposed mice [mean (S.D.) = 30.15 (21.99) ng/ml versus 2.96 (0.34) ng/ml, P < 0.001]. Likewise, cotinine levels in placenta were higher in exposed mice versus unexposed (11.0 ng/g tissue versus below the limits of detection). In cigarette smoke–exposed mice, a statistically significant correlation between serum and placental cotinine levels was observed (Fig. 5A, r2=0.63, P = 0.03), with placenta cotinine levels 2.6-fold lower, on average, than cotinine levels in serum (range 1.7–4.5).
Fig. 5.
(A) Correlation between serum and placenta cotinine levels determined in a mouse model of maternal cigarette smoking. (B) Expression of Cyp1a1 and Cyp1b1 mRNA are induced in postnatal lung tissue after maternal cigarette smoke exposure during pregnancy. Quantitative RT-PCR was performed as described on RNA isolated from lungs of pups on postnatal day 5 that were from dams either unexposed (n = 8) or exposed (n = 8) to cigarette smoke during pregnancy (n = 8). * P < 0.05.
In the murine model of maternal cigarette smoke exposure, gene expression levels of Cyp1a1 and Cyp1b1 were determined in pups at postnatal day 5 from dams with (n = 8) or without (n = 8) exposure to cigarette smoke during gestation. In pups exposed to maternal cigarette smoke prenatally, mRNA expression for Cyp1a1 and Cyp1b1 was induced in the distal lungs. Cyp1a1 expression was induced 3.2-fold (P = 0.012), whereas Cyp1b1 was induced 1.7-fold (P = 0.009), confirming that maternal smoke exposure results in changes in gene expression in the lungs of the developing fetus (Fig. 5B).
Discussion
In this study, we report the detection of cotinine in human placentas from women who reported smoking during their pregnancy and correlate maternal cigarette smoke exposure predicted by placental cotinine levels with changes in gene expression in fetal liver and lung. The median cotinine level in placentas from women who smoked cigarettes was 27.2 ng/g placenta, whereas the median cotinine level in placentas from women who denied smoking cigarettes was significantly lower at 2.3 ng/g (P < 0.001). Cotinine is a widely used biomarker for determining cigarette smoke consumption and has been measured in serum, saliva, urine, hair, cord blood, and amniotic fluid. Optimal threshold levels have been estimated for the biologic materials most commonly used to measure cotinine, including serum, urine, and hair, but not other matrices. In serum, a threshold of 14 ng/ml proposed by Jarvis et al. has been widely used to distinguish active smokers from nonsmokers (Jarvis et al., 1987). Although ETS exposure can result in serum cotinine levels in excess of 10 ng/ml, a global cut point of 3 ng/ml (4.5 ng/ml in women) serum has been recently proposed to distinguish active smokers from nonsmokers on the basis of data from NHANES (Benowitz et al., 2009a).
Based on ROC curve analysis, results from 47 placental samples obtained from mothers who reported cigarette smoking suggest a threshold of 7.5 ng/g placenta to distinguish mothers who actively smoke cigarettes from those who do not smoke. Compared with self-reported cigarette smoking, this threshold appropriately classified all nonsmoking mothers and 68% of smoking mothers. As cotinine levels in cord blood are similar to maternal blood at term (Perkins et al., 1997), previous studies used a cotinine concentration in cord blood of 14 ng/ml to determine prenatal smoke exposure. This widely used threshold was determined in the 1980s with a reported 96% sensitivity and 100% specificity (Jarvis et al., 1987). More recently, a lower cut point of 4.5 ng/ml was proposed to distinguish smokers from nonsmokers in women ranging from 20 to 85 years of age (specificity 98.4%, sensitivity 96.4%), based on data from NHANES (Benowitz et al., 2009a). This lower cut point is in close agreement with the cut-off determined in placenta.
Data from seven samples in which the number of cigarettes consumed per day was reported suggest a linear relationship to cotinine levels in placenta. We could not reliably determine a dose-response relationship in human tissues given the small number of samples with information regarding the number of cigarettes smoked per day; however, cotinine levels determined in samples from pregnant mice demonstrate a positive correlation between cotinine levels in serum and placentas.
The distribution of cotinine levels in 415 placentas from women for whom the history of cigarette smoking was unknown suggests the presence of at least three populations: unexposed individuals and two populations within the samples with detectable levels of cotinine (Fig. 2). This distribution is very similar to serum cotinine levels in a small study of adult emergency room patients (Benowitz et al., 2009b) as well as in smokers and nonsmokers participating in the much larger NHANES study between 1999 and 2004 (Benowitz et al., 2009a). The two evident populations with detectable levels of cotinine in placenta are hypothesized to represent nonsmokers exposed to ETS and active smokers. One limitation of this study is that there were no samples with known ETS exposure only; therefore, the extent of overlap between women with extensive ETS exposure and low-level active smokers cannot be determined with certainty. However, one mother who reported smoking 1–2 cigarettes per day had a placenta cotinine concentration of 8.2 ng/g, suggesting that a threshold of 7.5 ng cotinine/g is capable of distinguishing low-level smoking mothers from nonsmoking mothers. In two separate studies, cotinine concentrations in cord blood at time of delivery have been proposed for distinguishing unexposed babies (<1 ng/ml) from babies whose mothers were exposed to ETS (1–14 ng/ml) and those whose mothers smoked cigarettes during their pregnancy (>14 ng/ml) (Bearer et al., 1997; Pichini et al., 2000). These findings were consistent with ROC analysis and the distribution of cotinine concentrations in placenta observed in this study.
Using a threshold of 7.5 ng/g in placenta, we found the number of placental samples that could be classified as from mothers who were actively smoking in this population would be 40.2% versus 22.9% who were exposed to ETS. This is approximately 2-fold higher than would be expected, based upon the estimated smoking prevalence among women of childbearing age in the United States (Dietz et al., 2011). In the United States, 36.7% of nonsmoking adults ≥20 years of age had evidence of recent secondhand smoke exposure (serum cotinine >0.05 ng/ml) (Centers for Disease Control and Prevention, 2010), suggesting that the higher-than-expected rate of placenta samples positive for cotinine may represent not only actively smoking mothers but also those with extensive exposure to ETS. Assuming similar cotinine levels in cord blood and whole placenta, a more conservative threshold of 14 ng/ml (Bearer et al., 1997; Pichini et al., 2000) would result in the classification of 37.3% (n = 155) of samples as placentas from mothers who were actively smoking and 21.4% (n = 89) as mothers exposed to ETS, which is still well above the reported prevalence of smoking among women of child-bearing age. An alternative explanation for the higher-than-expected number of samples classified as positive for cigarette smoking may be a higher rate of smoking among the source population (here, pregnant women who electively terminate a pregnancy). Population data from the Centers for Disease Control and Prevention indicate that approximately one-third of all electively terminated pregnancies in the United States in 2008 were sought by women 20–24 years of age (Jones and Kavanaugh, 2011; Pazol et al., 2011), a group that also has a high prevalence of smoking (26%–29% in Caucasian women <25 years of age). Specific information regarding age, ETS exposure, and economic status for the population from which the placental samples were obtained was not available; therefore, the larger-than-expected number of placenta samples that are positive for cotinine in this study may reflect the combination of environmental exposures as well as the demographics of the specific study population rather than cigarette smoking prevalence among all women of childbearing age in the United States. Given the small sample size of the control population, we cannot exclude the possibility that the ROC threshold based on fewer than 60 placentas cannot accurately predict smoking status. Since our mouse data show that placental cotinine levels consistently reflect cigarette smoke exposure, the likelihood of this possibility is low.
In humans, cotinine concentrations in cord blood, umbilical cord tissue, meconium, and amniotic fluid throughout gestation correlate strongly with maternal serum or urine levels (Luck et al., 1985; Bearer et al., 1997; Jauniaux et al., 1999; Pichini et al., 2000; Marin et al., 2011; Wright et al., 2011). As maternal blood was unavailable for comparison with human placentas in this study, a mouse model of maternal cigarette smoking was used to investigate the relationship between maternal blood and placental cotinine levels. Mouse and human placentas are hemochorial, characterized by fetal trophoblasts in direct contact with maternal blood; however, mouse placenta has three layers of trophoblasts (hemotrichorial), whereas human placenta has a single layer of trophoblasts (hemomonochorial). Though structurally different, studies suggest that the placentas from the two species are functionally similar with respect to the exchange of small molecules (reviewed in Dilworth and Sibley, 2012).
To further validate that cotinine levels in placenta are capable of distinguishing between fetuses exposed to maternal cigarette smoking and unexposed fetuses, quantitative RT-PCR was performed on fetal lung and liver tissue from pregnancies with no detectable cotinine in placenta (n = 23) and from pregnancies with evidence of maternal cigarette smoke exposure (placental cotinine concentrations >12 ng/g). In both human and mouse samples, we observed a statistically significant increase in the expression in mRNA of members of the CYP1 family of drug-metabolizing enzymes in fetal lung and liver tissues with respect to maternal cigarette smoking as predicted by placental cotinine levels. Furthermore, in human fetal lung samples, a dose-response relationship was observed between the concentration of cotinine detected in placenta and the level of expression of CYP1A1 and CYP1B1 mRNA. These data support the use of cotinine in placenta as a biomarker to predict fetal exposure to constituents of cigarette smoke as a result of maternal cigarette smoking.
In conclusion, results from this study demonstrate the utility of cotinine levels in human placenta as a biomarker of fetal exposure to cigarette smoke in utero. Additional studies are required to confirm the proposed thresholds for distinguishing nonsmokers with ETS exposure from active smokers and to define the dose-response relationship between the number of cigarettes consumed by the mother and the cotinine levels in placenta. Finally, cotinine levels in 415 placenta samples suggest that the prevalence of cigarette smoking may be higher than national averages for certain segments of the population. Therefore, placenta cotinine levels will be most useful as biomarker to assign cigarette smoking status in studies where information regarding maternal smoking behavior is absent.
Abbreviations
- AUC
area under the curve
- ETS
environmental tobacco smoke
- PCA
post-conceptional age
- ROC
receiver operating characteristics
- RT-PCR
reverse-transcription polymerase chain reaction
Authorship Contributions
Participated in research design: Vyhlidal, Haley, Leeder, Tantisira.
Conducted experiments: Vyhlidal, Riffel, Haley.
Performed data analysis: Vyhlidal, Haley, Dai.
Wrote or contributed to the writing of the manuscript: Vyhlidal, Haley, Sharma, Tantisira, Weiss, Leeder.
Footnotes
This work was supported by National Institutes of Health [Grant R01-HL097144] and by the Lovelace Respiratory Research Institute/Brigham and Women’s Hospital consortium for lung research. The project entitled “Laboratory of Developmental Biology” was supported by National Institutes of Health Award Number 5R24HD0008836 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
References
- Baheiraei A, Banihosseini SZ, Heshmat R, Mota A, Mohsenifar A. (2012) Association of self-reported passive smoking in pregnant women with cotinine level of maternal urine and umbilical cord blood at delivery. Paediatr Perinat Epidemiol 26:70–76 [DOI] [PubMed] [Google Scholar]
- Bearer C, Emerson RK, O’Riordan MA, Roitman E, Shackleton C. (1997) Maternal tobacco smoke exposure and persistent pulmonary hypertension of the newborn. Environ Health Perspect 105:202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. (2009a) Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 169:236–248 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P., 3rd (2009b) Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol 170:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Heilbronner C, Georgieu S, Meier C, Spreux-Varoquaux O. (2010) Newborns’ cord blood plasma cotinine concentrations are similar to that of their delivering smoking mothers. Drug Alcohol Depend 107:250–252 [DOI] [PubMed] [Google Scholar]
- Bruchova H, Vasikova A, Merkerova M, Milcova A, Topinka J, Balascak I, Pastorkova A, Sram RJ, Brdicka R. (2010) Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta 31:186–191 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2010) Vital signs: nonsmokers’ exposure to secondhand smoke --- United States, 1999-2008. MMWR Morb Mortal Wkly Rep 59:1141–1146 [PubMed] [Google Scholar]
- Chazeron I, Daval S, Ughetto S, Richard D, Nicolay A, Lemery D, Llorca PM, Coudoré F. (2008) GC-MS determined cotinine in an epidemiological study on smoking status at delivery. Pulm Pharmacol Ther 21:485–488 [DOI] [PubMed] [Google Scholar]
- Derauf C, Katz AR, Easa D. (2003) Agreement between maternal self-reported ethanol intake and tobacco use during pregnancy and meconium assays for fatty acid ethyl esters and cotinine. Am J Epidemiol 158:705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, Bernert JT. (2011) Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol 173:355–359 [DOI] [PubMed] [Google Scholar]
- Dilworth MR, Sibley CP. (2012) Review: Transport across the placenta of mice and women. Placenta DOI: 10.1016/j.placenta.2012.10.011 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- El-Mohandes AA, El-Khorazaty MN, Kiely M, Gantz MG. (2011) Smoking cessation and relapse among pregnant African-American smokers in Washington, DC. Matern Child Health J 15 (Suppl 1):S96–S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford RP, Tappin DM, Schluter PJ, Wild CJ. (1997) Smoking during pregnancy: how reliable are maternal self reports in New Zealand? J Epidemiol Community Health 51:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley KJ, Sunday ME, Porrata Y, Kelley C, Twomey A, Shahsafaei A, Galper B, Sonna LA, Lilly CM. (2008) Ontogeny of the eotaxins in human lung. Am J Physiol Lung Cell Mol Physiol 294:L214–L224 [DOI] [PubMed] [Google Scholar]
- Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysä J, Hakkola J, Pasanen M. (2008) Microarray analysis of the global alterations in the gene expression in the placentas from cigarette-smoking mothers. Clin Pharmacol Ther 83:542–550 [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. (1987) Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77:1435–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C. (1999) Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet Gynecol 93:25–29 [DOI] [PubMed] [Google Scholar]
- Jones RK, Kavanaugh ML. (2011) Changes in abortion rates between 2000 and 2008 and lifetime incidence of abortion. Obstet Gynecol 117:1358–1366 [DOI] [PubMed] [Google Scholar]
- Joya X, Pujadas M, Falcón M, Civit E, Garcia-Algar O, Vall O, Pichini S, Luna A, de la Torre R. (2010) Gas chromatography-mass spectrometry assay for the simultaneous quantification of drugs of abuse in human placenta at 12th week of gestation. Forensic Sci Int 196:38–42 [DOI] [PubMed] [Google Scholar]
- Leeder JS, Gaedigk R, Marcucci KA, Gaedigk A, Vyhlidal CA, Schindel BP, Pearce RE. (2005) Variability of CYP3A7 expression in human fetal liver. J Pharmacol Exp Ther 314:626–635 [DOI] [PubMed] [Google Scholar]
- Luck W, Nau H, Hansen R, Steldinger R. (1985) Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 8:384–395 [DOI] [PubMed] [Google Scholar]
- Manoli SE, Smith LA, Vyhlidal CA, An CH, Porrata Y, Cardoso WV, Baron RM, Haley KJ. (2012) Maternal smoking and the retinoid pathway in the developing lung. Respir Res 13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin SJ, Christensen RD, Baer VL, Clark CJ, McMillin GA. (2011) Nicotine and metabolites in paired umbilical cord tissue and meconium specimens. Ther Drug Monit 33:80–85 [DOI] [PubMed] [Google Scholar]
- Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. (2006) Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol 26:11–14 [DOI] [PubMed] [Google Scholar]
- Nukui T, Day RD, Sims CS, Ness RB, Romkes M. (2004) Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics 14:569–576 [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Monteiro A, Bhattacharya S, Fowler PA. (2011) Maternal smoking and fetal sex significantly affect metabolic enzyme expression in the human fetal liver. J Clin Endocrinol Metab 96:2851–2860 [DOI] [PubMed] [Google Scholar]
- Pazol K, Zane S, Parker WY, Hall LR, Gamble SB, Hamdan S, Berg C, Cook DA, Centers for Disease Control and Prevention (CDC) (2011) Abortion surveillance - United States, 2007. MMWR Surveill Summ 60:1–42 [PubMed] [Google Scholar]
- Perkins SL, Belcher JM, Livesey JF. (1997) A Canadian tertiary care centre study of maternal and umbilical cord cotinine levels as markers of smoking during pregnancy: relationship to neonatal effects. Can J Public Health 88:232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichini S, Basagaña XB, Pacifici R, Garcia O, Puig C, Vall O, Harris J, Zuccaro P, Segura J, Sunyer J. (2000) Cord serum cotinine as a biomarker of fetal exposure to cigarette smoke at the end of pregnancy. Environ Health Perspect 108:1079–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis BD, Turner SH, Gheesling LL, Carlone GM. (1991) Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J Clin Microbiol 29:1439–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind AB, Tseng L, Hirsch MB, Lauersen NH. (1978) Aryl hydrocarbon hydroxylase activity and microsomal cytochrome content of human fetal tissues. Cancer Res 38:1572–1577 [PubMed] [Google Scholar]
- Simpson WJ. (1957) A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol 73:807–815 [PubMed] [Google Scholar]
- Walsh RA. (1994) Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Hum Biol 66:1059–1092 [PubMed] [Google Scholar]
- Walsh RA, Redman S, Adamson L. (1996) The accuracy of self-report of smoking status in pregnant women. Addict Behav 21:675–679 [DOI] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. (2002) Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA 287:195–202 [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Garte SJ, Cosma G, Bell DA, Jedrychowski W, Wahrendorf J, Randall MC, Cooper TB, Ottman R, Tang D, et al. (1995) CYP1A1 messenger RNA levels in placental tissue as a biomarker of environmental exposure. Cancer Epidemiol Biomarkers Prev 4:147–153 [PubMed] [Google Scholar]
- Wright TE, Milam KA, Rougee L, Tanaka MD, Collier AC. (2011) Agreement of umbilical cord drug and cotinine levels with maternal self-report of drug use and smoking during pregnancy. J Perinatol 31:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FY, Wu HD, Yang HL, Kuo HW, Ying JC, Lin CJ, Yang CC, Lin LY, Chiu TH, Lai JS. (2007) Associations among genetic susceptibility, DNA damage, and pregnancy outcomes of expectant mothers exposed to environmental tobacco smoke. Sci Total Environ 386:124–133 [DOI] [PubMed] [Google Scholar]