Abstract
The study was originally designed to test the hypothesis that the compensatory increase in intestinal P450 (cytochrome P450) expression in the intestinal epithelium-specific P450 reductase (CPR) knockout (IE-Cpr-null) mice was attributable to decreased metabolism of putative P450 inducers present in the diet. Thus, we determined the impact of a dietary change from regular rodent chow to a synthetic diet devoid of phytochemicals on the expression of P450 enzymes in the small intestine (SI) and liver of wild-type (WT) and IE-Cpr-null mice. The dietary change diminished expression of CYP1A, 2B, 2C, and 3A in SI and CYP2B, 2C, and 3A in liver of both WT and IE-Cpr-null mice. However, the compensatory increase in SI P450 expression still occurred in IE-Cpr-null, compared with WT, mice, on the synthetic diet. The diet change–induced decrease in P450 expression was accompanied by decreases in microsomal midazolam-hydroxylase activity in vitro and first-pass clearance of midazolam in vivo in WT mice. Further studies showed that the dietary change, but not Cpr deletion, caused large decreases in bile acid (BA) levels in plasma, liver, SI, and intestinal content and that treatment of WT mice on the synthetic diet with GW4064, a farnesoid-X-receptor agonist, restored the levels of CYP3A expression in both liver and SI to those seen in mice fed with regular chow. Taken together, these results highlight the vital role of diet in maintaining adequate expression of major drug-metabolizing P450s and their associated drug-metabolizing activities in the digestive tract and suggest potential involvement of BA signaling in the regulatory mechanisms.
Introduction
Orally administered xenobiotics, including nutrients and therapeutic drugs, are potentially subject to first-pass metabolism before reaching systemic circulation. The liver and small intestine (SI) are major organs for this first-pass metabolism. Many biotransformation enzymes are expressed in the liver and SI, the most prominent of which are the cytochrome P450 (P450) monooxygenases. P450-mediated metabolism in both liver and SI could have a major impact on the bioavailability of a given drug, consequently affecting its therapeutic efficacy and/or the risks of chemical toxicity or adverse drug-drug interactions (Shen et al., 1997; Doherty and Charman, 2002; Kaminsky and Zhang, 2003).
P450-mediated metabolism also regulates the homeostasis of many endogenous compounds, such as cholesterol and bile acids (BAs). BAs are synthesized from cholesterol in the liver and transported to SI through bile. In the SI, the BAs facilitate the absorption of lipid and fat-soluble vitamins. Most of the BAs in the intestine are reabsorbed by intestinal enterocytes and then released into portal circulation. After taken up by hepatocytes, BAs are excreted into bile again to complete the enterohepatic circulation (Chiang, 1998). Excess BAs can be toxic; therefore, BA metabolism is highly regulated. BAs are also signaling molecules, which regulate gene expression through nuclear receptors, including farnesoid-X-receptor (FXR) and pregnane-X-receptor (PXR) (Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999; Xie et al., 2001). PXR, which regulates the expression of several P450s, can be activated by FXR (Jung et al., 2006); the latter plays a key role in the regulation of BA homeostasis (Goodwin et al., 2000; Kim et al., 2007).
The regular laboratory chow diet contains crude plant-derived ingredients and phytochemicals, some of which may induce P450 expression. In wild-type (WT) mice, replacing a standard laboratory chow with a semisynthetic diet led to reduced hepatic expression of CYP2C29, 2C55, 3A11, and 3A25 mRNAs, as well as decreased liver microsomal activity toward midazolam 1′-hydroxylation (van Waterschoot et al., 2009); however, such dietary effects on expression of mouse SI P450 have not been examined. In an intestinal epithelium (IE)–specific cytochrome P450 reductase (CPR) knockout mouse model (IE-Cpr-null), in which the activities of all microsomal P450s are suppressed in the IE cells because of deletion of the Cpr gene, we observed large increases in the expression of multiple P450s (Zhang et al., 2009). This compensatory increase in P450 expression in the SI of the IE-Cpr-null mice may be partly explained by a decreased local metabolism and elimination of dietary P450 inducers, although it may also be attributable to decreased local metabolism of endogenous P450 inducers.
In the present study, we determined whether dietary changes (from regular chow to a synthetic diet) can have a major effect on the expression of various P450 enzymes in the SI and in liver of WT mice and whether such expression changes can lead to significant changes in the in vivo disposition of an oral drug (midazolam). We also investigated whether the compensatory increase in P450 expression seen previously in the SI of the IE-Cpr-null mice was dependent on dietary exposure to the regular chow, by comparing P450 expression levels between IE-Cpr-null and WT mice that are receiving a synthetic diet. Toward understanding the mechanisms underlying the effects of dietary changes and/or SI Cpr deletion on SI P450 expression, we further determined whether the dietary change causes changes in BA homeostasis and whether diet-change induced alterations in SI P450 expression can be reversed by treatment of mice with GW4064, a FXR agonist.
Materials and Methods
Materials.
AIN-93G synthetic rodent diet was purchased from Dyets (Bethlehem, PA). Prolab RMH 3500 regular diet was from LabDiet (Hudson, NH). Ingredients of the two diets are described at the manufacturers’ websites. Midazolam (MDZ), diazepam, 1’-hydroxymidazolam (1’-OH-MDZ), 4-hydroxymidazolam (4-OH-MDZ), t-butyl-dimethylsilyl-trifluoroacetamide, cholic acid, taurocholic acid (T-CA), and GW4064 were purchased from Sigma-Aldrich (St. Louis, MO). Cholic-2,2,4,4-d4-acid (d4-CA) was obtained from C/D/N Isotopes (Pointe-Claire, Quebec, Canada); α- and β-muricholic acid (MCA) and tauro β-muricholic acid (T-β-MCA) sodium salt were obtained from Steraloids (Newport, RI). All solvents (acetonitrile, methanol, and water) were of high-performance liquid chromatography grade (Fisher Scientific, Houston, TX).
Animals and Treatments.
Adult male and female IE-Cpr-null (Zhang et al., 2009) mice (2–3 months of age and congenic on the C57BL/6 background) and age-matched WT littermates were used. Animals were given food and water ad libitum. For dietary studies, mice, raised on regular rodent diet (Prolab RMH 3500), were fed with synthetic diet (AIN-93G) for 3 consecutive weeks. MDZ (30 mg/kg, dissolved in 0.01% hydrochloric acid) was given to mice via oral gavage for pharmacokinetics analysis. GW4064 was administered (once) at 100 mg/kg (in polyethylene glycol 400:Tween 80; 4:1 [v/v]) via oral gavage, and control animals received the vehicle only; all animals were killed 18 hours later for tissue isolation and microsome preparation. All animal studies were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center.
Microsome Preparation and Immunoblot Analysis.
Microsomal samples were prepared as previously described (Zhang et al., 2009). Proteins were resolved on 10% NuPAGE Bis-Tris-gels (Invitrogen, Carlsbad, CA) and then transferred to nitrocellulose membranes. For immunodetection, polyclonal rabbit anti-rat CYP1A1 (Millipore, San Diego, CA), anti-CYP3A1 (Biomol Research Laboratories, Plymouth Meeting, PA), goat anti-rat CYP2B or CYP2C (BD Gentest, Woburn, MA), and rabbit anti-calnexin (loading control; Abcam Inc, Cambridge, MA) were used. Peroxidase-labeled goat anti-rabbit IgG or rabbit anti-goat IgG (Sigma-Aldrich) was used as secondary antibodies. The signal was detected with an enhanced chemiluminescence kit (GE Healthcare, Piscataway, NJ); immunoblot quantification was performed using a Bio-Rad GS-710 Calibrated Imaging Densitometer or a Bio-Rad ChemiDoc XRS+ System (Hercules, CA).
Assay for In Vitro Metabolism of MDZ.
The assay was performed essentially as described elsewhere (Granvil et al., 2003). Incubations were performed in a total volume of 200 μl containing 0.1 M phosphate buffer (pH, 7.4), either 0.05 mg/ml liver microsomes or 0.5 mg/ml SI microsomes, and 3 μM of MDZ. After a 5-minute preincubation at 37°C, the reactions were initiated by the addition of NADPH (1.0 mM) and then allowed to proceed for 5 minutes before they were terminated by the addition of 50 μl sodium hydroxide solution (100 mM) and 1 ml of an ether/hexane mixture (70/30, v/v). An internal standard (0.5 μg/ml diazepam, in 5 μl methanol) was added to the reaction mixture before extraction; the mixtures were shaken for 10 minutes and then centrifuged for 10 minutes at 4°C and 2000g. After being kept for 1 hour at −30°C, the supernatant was transferred to a new tube and evaporated under a stream of nitrogen at 40°C. Samples were reconstituted in 50 μl of 20% (v/v) t-butyl-dimethylsilyl-trifluoroacetamide in acetonitrile. Each sample was heated in a 200-μl injection vial for 2 hours at 70°C, and 2 μl was injected for gas chromatography-mass spectrometry (GC/MS) analysis.
GC/MS Analysis of MDZ and MDZ Metabolites.
GC/MS analysis was performed in accordance with previously reported methods (Thummel et al., 1994; Eeckhoudt et al., 1998). The GC/MS system consisted of an Agilent 7890A GC system and a 5975C inert XL EI/CI MSD with Triple-Axis Detector (Agilent Technologies, Santa Clara, CA), a 7693 autoinjector, and an Rxi-5ms fused capillary column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness; RESTEK, Bellefonte, PA). The helium carrier gas was at a head pressure of 10 psi, and injector temperature was 260°C. The column temperature was increased 1 minute after injection from the initial 160°C to 280°C at 5°C/minute and further to 300°C for 5 minutes. Then, the column was maintained at 300°C for 4 minutes before full-speed cooling to 85°C. Under these conditions, the retention time of diazepam, MDZ, t-butyl-dimethylsilyl-4-hydroxy-MDZ, and t-butyldimethylsilyl-1′-hydroxy-MDZ was 21.6, 22.5, 24.3, and 24.8 minutes, respectively. The mass spectrometer inlet probe was kept at 280°C, and the ions used for quantification were m/z 310 for MDZ, 398 for t-butyl-dimethylsilyl-1′-hydroxy-MDZ and t-butyldimethylsilyl-4-hydroxy-MDZ, and 283 for diazepam.
Pharmacokinetic Analysis.
Blood samples were collected from the tail vein at 15, 30, 60, 120, 240, and 360 minutes after oral MDZ administration. To a 25-μl whole blood sample, 50 μl of 100 mM sodium hydroxide and 5 μl of internal standard (0.5 μg/ml diazepam, in methanol) were added before extraction, as described in the assay for in vitro metabolism. The plasma concentrations of MDZ, 1′-OH-MDZ, and 4-OH-MDZ were determined by GC/MS, as described above. The concentrations are given as means ± S.D. (n = 4–6, each time point). Pharmacokinetic parameters were calculated using the PK Solver software. Statistical significance of differences between groups was examined using Student’s t test.
LC-MS/MS Analysis of BAs.
Quantitative analysis of BAs was performed as described previously (Inoue et al., 2004), with use of an ABI 4000 Q-Trap LC-MS/MS system (Applied Biosystem, Foster City, CA) fitted with a 3.5-µm Symmetry-C18 column (2.1 × 150 mm; Waters, Milford, MA). The samples were eluted at a flow rate of 0.25 ml/min, with a mobile phase consisting of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The column was equilibrated with 60% A to 40% B; the solvent gradient consisted of linear increases from 40% B through 100% B for 2–20 minutes and then stayed at 100% B for 5 minutes. The MS was operated in the positive ion mode with use of electrospray ionization. The parent/product ion pairs of m/z 373/355 for cholic acid and α-MCA, 391/359 for β-MCA, 516/462 for T-CA and T-β-MCA, and 377/359 for the internal standard d4-CA were measured in the Multiple Reaction Monitoring scan mode. The parameters for the chamber were as follows: curtain gas, 30 psig; heated nebulizer temperature, 350°C; ion spray voltage, 4000 V; nebulizer gas, 50 psig; turbo gas, 50 psig, declustering potential, 50 V; and entrance potential, 10 V.
Liver and SI samples were extracted as described previously (Alnouti et al., 2008). Approximately 50 mg of liver and small intestine were homogenized in 2 vol of 50% methanol. A 150-μl portion of the homogenate from each sample was combined with 5 ng d4-CA and mixed with 2 ml of ice-cold acetonitrile for 1 hour; the mixture was then centrifuged at 11,000g for 10 minutes. The supernatant was transferred to a new tube, whereas the precipitant was extracted with another 1 ml of ice-cold acetonitrile. The pooled supernatant fractions were dried, and the residues were reconstituted in 50% (v/v) acetonitrile for LC-MS analysis.
For intestinal contents, the extraction of BAs was performed as described elsewhere (Hagio et al., 2009). In brief, 100 mg (wet weight) intestinal contents, collected by flushing the intact intestinal tract, were homogenized in 2 vol of 50% (v/v) methanol. One milliliter of ethanol was added to the homogenate for extraction. The samples were subjected to sonication twice (10 seconds each) and then heated at 60°C for 30 minutes. After cooling to room temperature, the samples were heated again at 100°C for 3 minutes before centrifugation at 3000g for 10 minutes at 15°C. The supernatants were collected, whereas the precipitates were extracted with another 1 ml of ethanol. The pooled supernatants were dried, and the residues reconstituted in 50% acetonitrile for LC-MS analysis.
For plasma samples, 50 μl of plasma together with 5 ng of d4-CA were extracted with 0.5 ml acetonitrile, as described previously (Alnouti et al., 2008). The extracted BAs were reconstituted in 50% acetonitrile for LC-MS analysis. Authentic compounds were added to charcoal-stripped bovine serum (Hyclone, Logan, UT) for construction of calibration curves.
Results
Effects of Dietary Change on P450 Expression in WT and IE-Cpr-Null Mice.
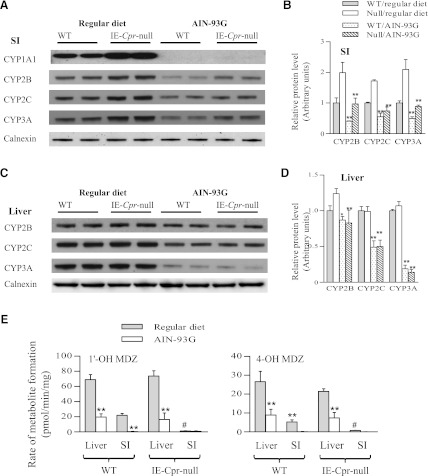
Dietary effects on SI P450 expression were examined by comparing microsomal CYP1A, 2B, 2C, and 3A protein levels between mice that were always receiving a regular laboratory chow diet and mice that were switched from the regular laboratory chow diet to a synthetic diet for 3 weeks. As shown in Fig. 1A, the dietary change led to large decreases in the intestinal levels of all four isoforms in both WT and IE-Cpr-null mice. Of note, the anti-P450 antibodies were all polyclonal and are expected to recognize multiple, if not all, members in the CYP2B, 2C, or 3A gene subfamily. As we have reported previously (Zhang et al., 2009), P450 expression levels in the null mice were higher than those in WT mice in the regular diet groups. Of note, with exception of CYP1A1, which under the conditions used, was not detected in mouse SI of either strain on the synthetic diet, the strain difference in SI P450 expression levels was also observed in mice receiving the synthetic diet, despite the overall decreases in P450 expression in both strains (Fig. 1, A and B).
Fig. 1.
Effects of dietary change on P450 expression and microsomal MDZ metabolism. Microsomes were prepared from adult, male WT and IE-Cpr-null mice that were either always receiving a regular laboratory chow diet (Prolab RMH 3500) or were switched from the regular diet to a synthetic diet (AIN-93G) for 3 weeks. (A–D) Immunoblot analysis of P450 expression. Each microsomal sample was pooled from 3 mice and analyzed in duplicate. Antibodies used are described in Materials and Methods. The results represent 2–3 independent experiments. (A) Expression of CYP1A, 2B, 2C, and 3A proteins in mouse SI. Each lane contained 20 (Synthetic/SI samples) or 10 (all others) μg total protein. Calnexin was also analyzed as a loading control. (B) Densitometry analysis of relative SI CYP2B, 2C, and 3A protein levels in WT and IE-Cpr-null mice. WT values from regular laboratory chow group were set as 1 (mean ± S.D. [n = 3]; **P < 0.01), compared with the corresponding regular diet group. (C) Expression of CYP2B, 2C, and 3A proteins in mouse liver. Each lane contained 10 μg total protein. (D) Densitometry analysis of relative hepatic CYP2B, 2C, and 3A protein levels in WT and IE-Cpr-null mice. WT values from regular laboratory chow group were set as 1 (mean ± S.D. [n = 3]; **P < 0.01; *P < 0.05), compared with the corresponding regular diet group. (E) In vitro metabolism of MDZ by liver or SI microsomes. Rates of formation of 1′-OH-MDZ and 4-OH-MDZ were determined. Reaction mixtures contained phosphate buffer (pH, 7.4), 3 μM MDZ, and 0.05 mg/ml liver microsomes or 0.5 mg/ml SI microsomes, in a total volume of 200 μl. The values reported are means ± S.D. (n = 6). **P < 0.01, compared with the corresponding regular diet group; #P < 0.01, compared with the WT SI in the regular diet group (Student's t test).
The dietary change also affected hepatic P450 expression (Fig. 1C). Levels of CYP2B, 2C, and 3A proteins were all decreased in mice (of either strain) fed with the synthetic diet, with the decreases in the CYP3A protein levels being most striking. In contrast to the situation in the SI, hepatic expression levels of CYP2B, 2C, and 3A proteins were not different between the IE-Cpr-null mice and WT mice receiving either regular chow or synthetic diet (Fig. 1, C and D).
The effects of the dietary change on the expression of CYP3A in lung and kidney were also examined. In contrast to the large decreases in CYP3A expression seen in SI and liver of mice fed the synthetic diet, only slight diet-related decreases in CYP3A levels were seen in the lung or kidney (data not shown). As was found in the liver, there was no strain difference in CYP3A expression levels in lung and kidney between WT and IE-Cpr-null mice receiving either regular chow or synthetic diet.
Impact of Dietary Change on MDZ Metabolism and In Vivo Clearance in WT and IE-Cpr-Null Mice.
The large effect of the dietary changes from a regular chow to a synthetic diet on both hepatic and intestinal CYP3A expression prompted us to also examine the impact of this dietary regulation on in vitro microsomal metabolism (Fig. 1E) and in vivo clearance (Table 1 and Supplemental Figs. 1 and 2) of MDZ, a CYP3A model substrate, in both WT and IE-Cpr-null mice. As shown in Fig. 1E, the dietary change led to significant decreases in rates of microsomal MDZ metabolism (formation of both 1′-OH-MDZ and 4-OH-MDZ) in both liver and SI of WT mice. In the null mice, the dietary change also led to large decreases in rates of hepatic microsomal MDZ metabolism, whereas the activity in SI microsomes were barely detectable with either diet, reflecting the tissue-specific absence of CPR in the SI. In pharmacokinetic studies (Supplemental Fig. 1 and Table 1), the dietary change led to a ∼2-fold increase in area under the curve (AUC) value for orally administered MDZ (at 30 mg/kg) in WT mice. However, a diet-related change in MDZ clearance in the IE-Cpr-null mice was much less noticeable (with only an ∼35% increase in AUC value), which is attributable largely to the fact that MDZ clearance was already decreased in the null mice by the loss of SI CPR in the regular diet groups (Table 1 and Supplemental Fig. 2). In experiments not shown, the diet change–induced decrease in MDZ clearance in WT mice was also associated with significant decreases in plasma levels of 1′-OH-MDZ (2.7-fold in AUC value).
TABLE 1.
Dietary effects on pharmacokinetic parameters for orally administered MDZ in WT and IE-Cpr-null mice
WT and IE-Cpr-null mice (2- to 4-month-old male) from the regular diet or synthetic diet groups were given a single oral dose of MDZ (30 mg/kg). Blood samples were collected from individual mice at 0.25 to 6 h after MDZ administration. Concentrations of MDZ in the plasma were determined using GC-MS, as described in Materials and Methods, and the plasma concentration-time curves are shown in Supplemental Figures 1 and 2. Values shown represent means ± S.D. (n = 4-6).
| Strain | Diet | Tmax |
Cmax |
t1/2 |
AUC0-6h |
|---|---|---|---|---|---|
| h | nmo/ml | h | nmol.h/ml | ||
| WT | Regular | 0.5 | 1.9 ± 0.1 | 4.6 ± 0.5 | 5.8 ± 0.5 |
| WT | Synthetic | 0.5 | 3.2 ± 0.3* | 3.2 ± 0.1 | 10.7 ± 0.4* |
| IE-Cpr-null | Regular | 0.5 | 3.0 ± 0.5** | 2.9 ± 0.2** | 8.4 ± 0.7** |
| IE-Cpr-null | Synthetic | 0.25 | 3.0 ± 0.3 | 3.1 ± 0.3 | 11.4 ± 0.6*** |
P < 0.01, compared with the WT/regular diet group; **P < 0.01, compared with the WT/regular diet group; ***P < 0.01, compared with the IE-Cpr-null/regular diet group.
Effects of Dietary Change on Systemic and Intestinal BA Homeostasis in WT and IE-Cpr-Null Mice.
The persistence of up-regulation of SI P450 expression in IE-Cpr-null mice, relative to WT mice, receiving the synthetic diet (Fig. 1) suggested that factors in addition to dietary P450 inducers may be involved in the regulation of SI P450 expression. Therefore, we next examined whether the homeostasis of BAs, which are abundant in the SI and known to activate pertinent nuclear receptors, such as FXR (Gnerre et al., 2004;Hylemon et al., 2009), is altered by the loss of SI CPR when mice are fed either regular or synthetic diet. The levels of the five major murine BAs (cholic acid, T-CA, α-MCA, β-MCA, and T-β-MCA) were measured in liver, SI, plasma, and intestinal contents from both WT and IE-Cpr-null mice. In both WT (Fig. 2) and IE-Cpr-null (Supplemental Fig. 3) mice, the abundance of all five BAs was significantly decreased by the change from regular chow to the synthetic diet in liver, SI, plasma, and intestinal contents. However, there was no significant difference between WT and IE-Cpr-null mice in the levels of any of the BAs determined when the mice were fed either regular or synthetic diet (data not shown).
Fig. 2.
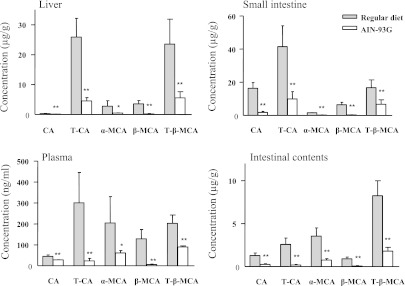
BA levels in plasma, liver, SI epithelium, and intestinal contents of adult male WT mice fed a regular or synthetic diet. Five different BAs were determined using LC-MS/MS as described in Materials and Methods. Results are shown as means ± S.D (n = 6). *P < 0.05; **P < 0.01, compared with the corresponding regular diet group. Corresponding data for IE-Cpr-null mice are shown in Supplemental Fig. 3. CA, cholic acid.
Effects of FXR Agonist GW4064 on SI CYP3A Expression in WT Mice Fed the Synthetic Diet.
It is currently unclear how the dietary change led to decreases in BA levels; however, the downstream link between decreases in BA levels and reductions in SI and liver CYP3A expression may be explained by a reduced activation of FXR and/or PXR by BAs, which are known ligands for these receptors. In that connection, treatment of mice receiving the synthetic diet with GW4064, a FXR agonist, restored the levels of CYP3A expression in both liver and SI to those seen in mice fed regular chow (Fig. 3). This result suggests that a decreased activation of FXR by BAs is likely to be involved in the dietary change–induced suppression of CYP3A expression in the liver and SI.
Fig. 3.
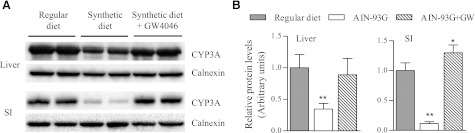
Effect of GW4064 treatment on liver and SI CYP3A expression in mice fed synthetic diet. Adult male WT mice fed the synthetic diet for 3 weeks were treated once with GW4064 by oral gavage or with the vehicle alone, and tissues were obtained 18 hours later for microsome preparation. Age-matched male WT mice fed with regular chow were also included for comparison. (A) Immunoblot analysis of CYP3A expression was performed as described in Fig. 1. Microsomes were prepared from pooled enterocytes of 2 mice in each group and analyzed in duplicates. Calnexin was analyzed as a loading control. Each lane contained 10 μg total protein. The results represent three independent experiments. (B) Results of densitometry analysis for relative CYP3A protein levels (mean ± S.D. [n = 3]; **P < 0.01; *P < 0.05, compared with the regular diet group.
Discussion
In this study, we determined the effects of dietary change on the expression of both intestinal and hepatic CYP2B, 2C, and 3A enzymes and the consequences for systemic drug clearance, with use of MDZ as a model drug. Furthermore, we found that the previously described up-regulation of CYP2B, 2C, and 3A expression in the SI of the IE-Cpr-null mice (Zhang et al., 2009) does not require exposure of the mice to dietary P450 inducers present in regular chow. The effects of a dietary change on hepatic (but not intestinal) P450 expression have also been examined in a previous study (van Waterschoot et al., 2009),in which expression of CYP2C29, 2C55, 3A11, and 3A25 mRNAs was found to be decreased when WT FVB mice were changed from a standard laboratory chow (AM-II; Hope Farms) to a semi-synthetic diet (20% casein, 4068.02; Hope Farms). Collectively, these studies indicate that daily diets can play a vital role in maintaining adequate expression of major drug-metabolizing enzymes in the digestive tract and point to the need to pay greater attention to potential adverse drug-drug interactions involving diet-induced alteration of drug biotransformation in patients.
Much remains to be determined about the mechanism underlying the changes in SI P450 expression that result from either the diet switch or the Cpr deletion. On the basis of results from the present study and literature, it appears that ingredients uniquely present in the regular rodent chow sustain normal P450 expression in the SI by maintaining circulating and intestinal tissue levels of BAs. BAs are well-known activators for both FXR and PXR (Xie et al., 2001; Gnerre et al., 2004, Staudinger et al., 2001 ; Hylemon et al., 2009). FXR can also up-regulate PXR expression (Jung et al., 2006). Thus, an increase in BA can activate FXR and PXR in the SI (and in liver), ultimately stimulating the expression of CYP3A and other P450s that are regulated by PXR. Conversely, with synthetic diet, BA levels are reduced, leading to less activation of FXR and PXR and decreased P450 expression. The effects of a synthetic diet on cholesterol metabolism and BA synthesis have been reported previously (McNamara et al., 1982).
Dietary P450 inducers (Mandlekar et al., 2006) may also directly activate nuclear receptors, such as PXR and CAR (constitutive androstane receptor), in the SI, leading to increased expression of their target P450 genes (e.g., Cyp2b, 2c, and 3a), as was found for the dietary regulation of AhR-mediated Cyp1a1 expression in the SI and extra-gut organs (Ito et al., 2007; Zhang et al., 2009). Nonetheless, the fact that activation of FXR by GW4046 led to restoration of CYP3A expression in mice fed the synthetic diet suggests that dietary P450 inducers (e.g., plant-derived PXR agonists) are not absolutely required for normal P450 expression when FXR is activated. Furthermore, because the dietary change caused decreased expression of CYP2B, 2C, and 3A in both WT and IE-Cpr-null mice, it seems reasonable to conclude that intestinal P450 activity is not responsible for or involved in the observed dietary regulation of these Cyp genes in SI and liver.
It remains to be determined how the diet change led to changes in BA homeostasis. Although the synthetic diet and the regular rodent chow used in this study are both nutritionally adequate, the two diets have major differences in the presence or absence of plant-derived raw material and subtle differences in chemical composition. For example, although the total protein or fiber content (percentage) in the two diets is similar, the types of proteins and fibers differ by diet. Previous studies have shown that changes in dietary constituents, including fiber contents (Kritchevsky, 1978) and protein sources (Liaset et al., 2011), can influence size of the BA pool. Here, we further demonstrated, for the first time, that the change from the regular chow to the synthetic diet causes large decreases in all five major murine BAs in plasma, liver, SI, and intestinal content.
Changes in P450 expression or activity in the SI may affect the metabolism and, consequently, local (SI) and/or systemic bioavailability of dietary P450 inducers. Studies on mice with intestine-specific deletion of the aryl hydrocarbon receptor nuclear translocator (Arnt) or the Cpr gene revealed the important role of intestinal P450–mediated metabolism in regulating bioavailability of dietary CYP1A1 inducer (Ito et al., 2007; Zhang et al., 2009) and provided the basis for the hypothesis that the loss of P450-mediated metabolism in the intestine leads to increased supply of dietary CYP1A1 inducers not only to the intestine, but also to extra-gut organs. The latter hypothesis was also supported by our more recent study of the IE-Cpr-null mice, which demonstrated a pivotal role of intestinal P450, most likely CYP1A1, in controlling the systemic exposure to orally administered benzo(a)pyrene, a prototypical CYP1A1 inducer (Fang and Zhang, 2010). In the current study, we further demonstrated that feeding mice a synthetic diet (devoid of plant-derived ingredient) not only abrogated the expression of CYP1A1 in the intestine of both WT and IE-Cpr-null mice (Fig. 1A), but also eliminated the induction of CYP1A1 in the lung, an extra-gut organ, by the loss of intestinal CPR (unpublished data).
SI P450-mediated metabolism also seems to control systemic bioavailability of dietary inducers for other P450 enzymes. Germline deletion of all mouse Cyp3a genes led to upregulation of Cyp2c55 and several other Cyp genes (but not Cyp3a, which could not be examined) in the liver, presumably because of reduced disposition of dietary PXR and/or CAR ligands; the latter hypothesis was supported by the mitigating effects of hepatic or SI transgenic expression of human CYP3A4 and by a reduction in the extent of the compensatory increase in P450 expression seen when mice were fed a semi-synthetic diet (van Waterschoot et al., 2009). However, no change in the expression of CYP2B, 2C, or 3A was observed in the livers of the IE-Cpr-null mice, compared with WT mice, receiving either regular or synthetic diet (Zhang et al., 2009; this study), suggesting that any role of SI P450 in metabolizing dietary PXR and/or CAR ligands may only become critical when hepatic CYP3A is compromised (or vice versa).
We still do not fully understand how the tissue-specific Cpr deletion in the SI leads to up-regulation of the intestinal expression of multiple P450 genes. Our recent study of the genomic and metabolomic changes in the SI of the IE-Cpr-null mice revealed remarkable changes in the sterol biosynthesis and metabolism pathways (D’Agostino et al., 2012). It will be worthwhile to determine whether the latter changes lead to activation of nuclear receptors, such as CAR, in the SI; the accumulation of endogenous CAR activators that are intermediate metabolites in the sterol biosynthesis pathway has been proposed previously to explain the up-regulation of P450 expression in the livers of the liver-Cpr-null mice (Weng et al., 2005).
In summary, we have shown that a dietary change from a regular rodent chow to a synthetic diet devoid of phytochemicals can cause large decreases in the expression of major drug-metabolizing P450 enzymes in the SI and in liver, with consequent decreases in the metabolic disposition of an oral drug. Our initial efforts toward identification of the underlying mechanisms uncovered the potential involvement of BA signaling. Furthermore, our study of the IE-Cpr-null mice provided additional insights to the previously proposed role of SI P450-mediated metabolism of dietary P450 inducers in physiologic regulation of P450 expression.
Supplementary Material
Acknowledgments
The authors thank Weizhu Yang for assistance with animal breeding, and Dr. Jaime D’Agostino for assistance with GC-MS analysis.
Abbreviations
- AUC
area under the curve
- BA
bile acid
- CPR
cytochrome P450 reductase
- d4-CA
cholic-2,2,4,4-d4-acid
- DMSO
dimethyl sulfoxide
- FXR
farnesoid-X-receptor
- GC/MS
gas chromatography–mass spectrometry
- IE
intestinal epithelium
- IE-Cpr-null
intestinal epithelium-specific Cpr-null
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MCA
muricholic acid
- MDZ
midazolam
- OH-MDZ
hydroxymidazolam
- PBS
phosphate-buffered saline
- P450
cytochrome P450
- PXR
pregnane X receptor
- SI
small intestine
- SPE
solid phase extraction
- T-CA
taurocholic acid
- WT
wild-type
Authorship Contributions
Participated in research design: P. Zhang, K. Jia, C. Fang, X. Ding, Q.-Y. Zhang.
Conducted experiments: P. Zhang, K. Jia, C. Fang, X. Zhou.
Performed data analysis: P. Zhang, K. Jia, C. Fang, Qing-Yu Zhang.
Wrote or contributed to the writing of the manuscript: P. Zhang, K. Jia, C. Fang, X. Ding, Q.-Y. Zhang.
Footnotes
This study was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant GM082978].
References
- Alnouti Y, Csanaky IL, Klaassen CD. (2008) Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 873:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JY. (1998) Regulation of bile acid synthesis. Front Biosci 3:d176–d193 [DOI] [PubMed] [Google Scholar]
- D’Agostino J, Ding X, Zhang P, Jia K, Fang C, Zhu Y, Spink DC, Zhang QY. (2012) Potential biological functions of cytochrome P450 reductase-dependent enzymes in small intestine: novel link to expression of major histocompatibility complex class II genes. J Biol Chem 287:17777–17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MM, Charman WN. (2002) The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism? Clin Pharmacokinet 41:235–253 [DOI] [PubMed] [Google Scholar]
- Eeckhoudt SL, Desager JP, Horsmans Y, De Winne AJ, Verbeeck RK. (1998) Sensitive assay for midazolam and its metabolite 1′-hydroxymidazolam in human plasma by capillary high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 710:165–171 [DOI] [PubMed] [Google Scholar]
- Fang C, Zhang QY. (2010) The role of small-intestinal P450 enzymes in protection against systemic exposure of orally administered benzo[a]pyrene. J Pharmacol Exp Ther 334:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnerre C, Blättler S, Kaufmann MR, Looser R, Meyer UA. (2004) Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics 14:635–645 [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, et al. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526 [DOI] [PubMed] [Google Scholar]
- Granvil CP, Yu AM, Elizondo G, Akiyama TE, Cheung C, Feigenbaum L, Krausz KW, Gonzalez FJ. (2003) Expression of the human CYP3A4 gene in the small intestine of transgenic mice: in vitro metabolism and pharmacokinetics of midazolam. Drug Metab Dispos 31:548–558 [DOI] [PubMed] [Google Scholar]
- Hagio M, Matsumoto M, Fukushima M, Hara H, Ishizuka S. (2009) Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J Lipid Res 50:173–180 [DOI] [PubMed] [Google Scholar]
- Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. (2009) Bile acids as regulatory molecules. J Lipid Res 50:1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Yu AM, Inoue J, Gonzalez FJ. (2004) Hepatocyte nuclear factor 4alpha is a central regulator of bile acid conjugation. J Biol Chem 279:2480–2489 [DOI] [PubMed] [Google Scholar]
- Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. (2007) Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest 117:1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Mangelsdorf DJ, Meyer UA. (2006) Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem 281:19081–19091 [DOI] [PubMed] [Google Scholar]
- Kaminsky LS, Zhang QY. (2003) The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos 31:1520–1525 [DOI] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. (2007) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48:2664–2672 [DOI] [PubMed] [Google Scholar]
- Kritchevsky D. (1978) Influence of dietary fiber on bile acid metabolism. Lipids 13:982–985 [DOI] [PubMed] [Google Scholar]
- Liaset B, Hao Q, Jørgensen H, et al. (2011) Nutritional regulation of bile acid metabolism is associated with improved pathological characteristics of the metabolic syndrome. J Biol Chem 286:28382–28395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365 [DOI] [PubMed] [Google Scholar]
- Mandlekar S, Hong J-L, Kong A-N. (2006) Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metab 7:661–675 [DOI] [PubMed] [Google Scholar]
- McNamara DJ, Proia A, Edwards KD. (1982) Cholesterol homeotasis in rats fed a purified diet. Biochim Biophys Acta 711:252–260 [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368 [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, et al. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Shen DD, Podoll TD, et al. (1994) Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exp Ther 271:549–556 [PubMed] [Google Scholar]
- Shen DD, Kunze KL, Thummel KE. (1997) Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev 27:99–127 [DOI] [PubMed] [Google Scholar]
- van Waterschoot RA, Rooswinkel RW, Wagenaar E, van der Kruijssen CM, van Herwaarden AE, Schinkel AH. (2009) Intestinal cytochrome P450 3A plays an important role in the regulation of detoxifying systems in the liver. FASEB J 23:224–231 [DOI] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553 [DOI] [PubMed] [Google Scholar]
- Weng Y, DiRusso CC, Reilly AA, Black PN, Ding X. (2005) Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. J Biol Chem 280:31686–31698 [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. (2001) An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA 98:3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QY, Fang C, Zhang J, Dunbar D, Kaminsky L, Ding X. (2009) An intestinal epithelium-specific cytochrome P450 (P450) reductase-knockout mouse model: direct evidence for a role of intestinal p450s in first-pass clearance of oral nifedipine. Drug Metab Dispos 37:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.