Abstract
Little information is available in the literature regarding the expression and activity of transporters in fetal human liver or cultured cells. A synthetic progesterone structural analog, 17α-hydroxyprogesterone caproate (17-OHPC), is used in the prevention of spontaneous abortion in women with a history of recurrent miscarriage (habitual abortion). 17-OHPC has been reported to traverse the placental barrier and gain access to fetal circulation. In this study, the role of transporters in the disposition of 17-OHPC in fetal and adult human hepatocytes was examined. Progesterone metabolites have been reported to induce trans-inhibition of bile acid transporter, ABCB11. Thus, we investigated the effect of 17-OHPC or its metabolites on [3H]taurocholic acid transport in sandwich-cultured human fetal and adult hepatocytes. 17-OHPC was taken up rapidly into the cells and transported out partially by an active efflux process that was significantly inhibited by cold temperature, cyclosporine, verapamil, and rifampin. The active efflux mechanism was observed in both adult and fetal hepatocyte cultures. 17-OHPC produced a concentration-dependent inhibition of taurocholate efflux into canaliculi in sandwich-cultured adult and fetal human hepatocytes. However, given the high concentrations required to cause inhibition of these transport processes, no adverse effects would be anticipated from therapeutic levels of 17-OHPC. We also evaluated the expression of various hepatic transporters (ABCB1, ABCB4, SLCO1B1, SLCO1B3, SLCO2B1, ABCB11, SLC10A1, ABCC2, ABCC3, ABCC4, and ABCG2) in fetal and adult hepatocytes. With the exception of ABCB4, all transporters examined were expressed, albeit at lower mRNA levels in fetal hepatocytes compared with adults.
Introduction
Drug therapy during pregnancy exposes both the mother and fetus to the potential adverse effects associated with therapy. Despite the presence of a placental barrier to drug transfer, certain drugs, depending on their physicochemical properties, are able to gain access to the fetal circulation. The fetus, like the adult, has detoxifying mechanisms in the form of phase 1, 2, and 3 (efflux transporter) pathways to facilitate xenobiotic inactivation and elimination. Concerted efforts over the last several years have added considerably to our knowledge regarding the expression of the phase 1 enzymes and to a lesser extent, phase 2 enzymes, in the fetal liver (Ackermann and Richter, 1977; Aranda et al., 1979; Rollins et al., 1979; Rane and Tomson, 1980; Wiebkin et al., 1985; Komori et al., 1990; Chiba et al., 1997; Ladona et al., 2000; Hines, 2007, 2008). Much less is known regarding the expression and activity of various transporters that directly mediate xenobiotic elimination. Biliary excretion of therapeutic drugs plays an important role in detoxification and elimination of drugs. Inhibition of biliary transport has led to adverse hepatic effects. The hepatic transporter, bile salt export pump (BSEP, ABCB11), is responsible for the transport of bile acids such as taurocholic acid (TCA). Inhibition of BSEP activity can result in the accumulation of these toxic unconjugated bile acids, leading to pathologic conditions like intrahepatic cholestasis of pregnancy (ICP) (Fattinger et al., 2001; Funk et al., 2001; Kroumpouzos, 2002). In addition to ABCB11, other efflux and uptake hepatic transporters like ABCB1 (MDR1), ABCC2 (MRP2), the SLCO (OATP) family of organic anion transporters, and ABCG2 (BCRP) are also expressed in adults and have been implicated in drug hepatotoxicity due to transporter-based drug-drug interactions. Our first aim was to measure the functional activity of hepatic transporters in primary cultures of fetal hepatocytes using sodium taurocholate as a substrate of sodium-dependent uptake transporter, SLC10A1 (NTCP), and the bile salt efflux transporter, ABCB11 (BSEP). Our second aim was to determine whether transport processes were involved in the disposition of 17α-hydroxyprogesterone caproate (17-OHPC), a new progesterone analog administered to pregnant women to prevent preterm delivery (Meis et al., 2003; Sharma et al., 2008). Progesterone metabolites (PMs) have been reported to play a role in the etiology of ICP. A rise in the serum concentrations of PMs has been associated with impaired biliary excretion. PMs are reported to induce trans-inhibition of ABCB11 and the subsequent toxicity induced by the accumulation of bile acids; as such, they may play a role in the pathogenesis of ICP (Kroumpouzos, 2002; Vallejo et al., 2006). Furthermore, progesterone is reported to be an inhibitor of ABCB1, a transporter expressed on the canalicular membrane of hepatocytes (Barnes et al., 1996). We recently characterized the transplacental transfer of 17-OHPC and the presence of significant levels of this drug in the fetal circulation (unpublished data). Thus, our final aim was to evaluate the interaction between 17-OHPC and taurocholate to determine whether there was the potential for hepatotoxicity due to transporter-mediated drug-drug interactions involving 17-OHPC in the fetus. Given the paucity of knowledge regarding fetal transporter expression, we also evaluated the expression of several of these proteins in fetal hepatocytes.
Materials and Methods
Chemicals
Hepatocyte maintenance media (HMM) culture medium, Hank’s balanced salt solution (HBSS), dexamethasone, and insulin were obtained from Lonza (Walkersville, MD). Penicillin G/streptomycin and amphotericin B (Fungizone) were obtained from GIBCO Laboratories (Grand Island, NY). [3H]Taurocholic acid (2 Ci/mmol) was obtained from PerkinElmer Life Sciences (Boston, MA). Cyclosporine A (CyA), verapamil (VER), and rifampin (RIF) were purchased from Sigma (St. Louis, MO). 17-OHPC (molecular weight 428.6) was a gift from Diosynth Inc (Chicago, IL). The radioactive isotope of 17-OHPC, 17α-hydroxy-[1,2,6,7-3H]-progesterone-[1-14C]-caproate, was custom synthesized by RTI International (Research Triangle Park, NC). Matrigel matrix and type I (rat-tail) collagen were purchased from BD Biosciences (Bedford, MA). All reagents used were of the highest chemical purity available.
Tissue Procurement
Livers were obtained from human fetal tissues (Table 1) with a gestational age between 18 and 23 weeks. The tissues were obtained from Magee Women’s Hospital (Pittsburgh, PA) after obtaining informed consent by a protocol approved by the Human Research Review Committee of the University of Pittsburgh. Liver tissue was placed in chilled Eagle’s minimum essential medium (EMEM) containing 0.05% antibiotics and antimycotics, penicillin (10,000 U/ml), amphotericin B (25 µg/ml), and streptomycin (10,000 µg/ml) until cell isolation was begun (usually within 0.5–2 hours).
TABLE 1.
Donor demographics
| Tissue ID | Gestational Age | Sex | Viability (%) |
|---|---|---|---|
| Fetal Donors | |||
| Fetal 1 | 18 wk | M | 97 |
| Fetal 2 | 20 wk | M | 97 |
| Fetal 3 | 23 wk | Unknown | 75 |
| Fetal 4 | 20 wk | Unknown | 97 |
| Fetal 5 | Unknown | Unknown | 86 |
| Fetal 6 | Unknown | Unknown | 83 |
| Fetal 7 | 22 wk | Unknown | 82 |
| Fetal 8 | 22 wk | M | Unknown |
| Adult Donors | |||
| Adult 1 | 56 yr | F | 91 |
| Adult 2 | 37 yr | M | 81 |
| Adult 3 | 60 yr | F | 92 |
| Adult 4 | 24 yr | F | 82 |
| Adult 5 | 27 yr | F | 80 |
| Adult 6 | 44 yr | M | 89 |
| Adult 7 | 73 yr | M | 84 |
| Adult 8 | 67 yr | M | 66 |
| Adult 9 | 56 yr | F | 68 |
| Adult 10 | 37 yr | F | 80 |
| Adult 11 | 42 yr | F | 75 |
| Adult 12 | 46 yr | F | 85 |
Adult human liver tissue (Table 1) was procured under an institutional review board–approved protocol and with support from the Liver Tissue and Cell Distribution System.
Fetal Hepatocyte Isolation and Culture
Hepatocytes were isolated from fetal livers at 21–23 weeks of age. Transport activity with fetal hepatocytes was conducted as originally described by Ellis et al. (2008). The fetal tissue was kept in EMEM and transported on ice. The hepatic tissue was gently scraped with a sterile cell scraper in a 100-mm cell culture dish. HBSS (30 ml) containing 0.1 M EGTA was added to the tissue and the contents were pipetted up and down several times with a 25-ml pipette to obtain a homogenous mixture. The tissue was transferred to a sterile 50-ml conical tube and gently rotated by hand to suspend red blood cells. The tubes were centrifuged at 60g for 5 minutes and the supernatant was poured off and discarded. Cell pellets were resuspended in 30 ml HBSS and again centrifuged using the same conditions. The supernatant was poured off, and the pellet volume was recorded. The cell pellet was then resuspended in 25 ml of EMEM (at 37°C) containing 1 mg/ml collagenase XI, 0.2 mg/ml DNase I, and penicillin/streptomycin. The suspension was rotated at 37°C for approximately 25 minutes. Tissue was dispersed by pipetting up and down with a 10-ml pipette. The suspension was centrifuged at 50–100g for 5 minutes. The supernatant was decanted and the cell pellet was suspended in 50 ml EMEM (at 4°C). The resulting suspension was centrifuged again using the previously described conditions and the supernatant was discarded. The cell pellet was resuspended in 40 ml of cell culture media (Dulbecco's modified Eagle’s medium [DMEM (+)]) containing insulin (0.1 µM), dexamethasone (0.1 µM), and amphotericin B (25 µg/ml), and cell viability was estimated using the trypan blue exclusion method. The cells were diluted to the desired concentration with DMEM (+), and bovine calf serum was added to a final concentration of 10%. The cells were plated at a cell density of 0.3–0.5 × 106 cells per well in 12-well plates previously coated with 0.2 mg/ml type I collagen. Media were changed to serum-free DMEM (+) after 3–5 hours. The media were changed once every day for the next 3–4 days until the cells achieved the desired confluency. The media were subsequently aspirated and the cells overlaid with Matrigel (final concentration of 0.233 mg/ml) diluted in HMM (+) containing insulin (0.1 µM), dexamethasone (0.1 µM), and amphotericin B (25 µg/ml).
Adult Hepatocyte Isolation and Culture
Adult hepatocytes were isolated by a three-step collagenase perfusion technique as described previously (Strom et al., 1996), plated at a cell density of 0.5 × 106 cells per well in 12-well plates previously coated with 0.2 mg/ml type I collagen. The isolated hepatocytes (>80% viability) were maintained in HMM supplemented with 10−7 M dexamethasone, 10−7 M insulin, 100 U/ml penicillin G, 100 μg/ml streptomycin (HMM +), and 10% bovine calf serum and kept at 37°C in a humidified incubator with 95% air/5% CO2. The hepatocytes were allowed to attach to the plate for 4–6 hours, at which time the media were replaced with serum-free HMM. After 24–48 hours in culture, the medium was removed and the cells overlaid with Matrigel (final concentration of 0.233 mg/ml) diluted in HMM supplemented as described above.
Uptake and Release Studies
The following two protocols for evaluating the transport of 17-OHPC in hepatocyte cultures were used.
Protocol 1.
The transport assay for 17-OHPC and TCA was conducted in sandwich-cultured hepatocytes as described by Kostrubsky et al. (2003) with the exception that rather than collagen, the cells were overlaid with Matrigel at a concentration of 0.233 mg/ml. The hepatocytes were incubated for 20 minutes in standard HBSS buffer containing the test compounds, 1 µM 17-OHPC and 1 µM TCA, in the presence or absence of inhibitors (I), 40 µM cyclosporine (Kukongviriyapan and Stacey, 1988; Kostrubsky et al., 2003), 100 µM rifampin (Vavricka et al., 2002), and 100 µM verapamil (Germann et al., 1997) (loading phase). Uptake was stopped by removing the buffer and washing cells with ice-cold media. The efflux of the test compound was initiated by adding Ca2+/Mg2+-free HBSS (±inhibitor) to the cells. Aliquots of media were harvested after 20 minutes and counted in a liquid scintillation counter to estimate the amount in media. Subsequently, the remaining media were aspirated and cell lysis buffer was added to the cells. The cell lysates were collected and counted in a liquid scintillation counter to estimate the amount in cell lysate. Results were normalized to total cellular protein measured by the Bradford method (Bio-Rad, Richmond, CA).
A procedure similar to protocol 1 was followed for evaluating the effect of 17-OHPC on bile acid transport (estimated as the total TCA efflux into the canalicular space). Briefly, 1 μM [3H]taurocholic acid, with or without increasing concentrations of test compound (17-OHPC, CyA) in standard HBSS buffer was added to hepatocytes at 37°C. The uptake was stopped by removing the buffer and washing cells with ice-cold media. The taurocholate efflux was initiated by adding standard HBSS (±17-OHPC, ±CyA) or Ca2+/Mg2+-free HBSS (±17-OHPC, ±CyA) to the cells. Aliquots of media and cell lysates were collected and counted in a liquid scintillation counter. Results were normalized to total cellular protein measured by the Bradford method (Bio-Rad). In the presence of Ca2+/Mg2+, the bile canaliculi remain intact, whereas in the absence of Ca2+/Mg2+, the bile canaliculi tight junctions are disrupted. Therefore, the difference in the efflux between the standard HBSS and Ca2+/Mg2+-free HBSS buffer in the absence of test compound was defined as 100% TCA efflux (control) into the canalicular space. In the presence of the test compound, this difference became smaller and was expressed as %TCA (canalicular) efflux relative to control.
Protocol 2.
The transport assay was also conducted in warm (37°C) and cold (4°C) media to evaluate active transport. Briefly, the hepatocytes were incubated for 20 minutes in warm and cold standard HBSS buffer containing the test compound, 1 µM 17-OHPC, and 1 µM TCA (loading phase). The transport was stopped by removing the buffer and washing cells with ice-cold media. The efflux of the test compound was initiated by adding (warm and cold) Ca2+/Mg2+-free HBSS to the cells. Aliquots of media and cell lysates were harvested and counted in a liquid scintillation counter to estimate the amount in media and cell lysate. Results were corrected for total protein content.
For both protocols, the efflux of 17-OHPC or TCA was calculated using eq. 1:
| (1) |
where Total Uptake = Amount in Media + Amount in Cell Lysate.
The inhibition of efflux and uptake of 17-OHPC or TCA was calculated using eqs. 2 and 3:
| (2) |
 |
(3) |
Hepatobiliary Transport in Adult and Fetal Human Hepatocytes
Hepatobiliary transport of 17-OHPC, in comparison with TCA, was also assessed in human hepatocytes (Bi et al., 2006; Kalgutkar et al., 2007). Briefly, fresh human hepatocytes in culture were rinsed twice with 1 ml of either standard HBSS or Ca2+/Mg2+-free HBSS and then equilibrated in the same buffers for 10 minutes at 37°C. [3H]Taurocholic acid (1 µM) or [3H]17-OHPC (1 µM) in standard HBSS was then added to both sets of cultures. Taurocholate transport was used as a positive control for active hepatic uptake and efflux and also to quantify the activity of bile transporters in fetal hepatocytes. The protein concentration of cell lysates was determined using the Bradford assay (Bio-Rad). Equation 4 was used to calculate the biliary excretion index (BEI), and is as follows (Liu et al., 1999):
| (4) |
Quantifying the accumulation of test compound in the presence and absence of Ca2+/Mg2+ allows one to determine the amount of test compound in the bile canaliculi. Individual BEI values for adult and fetal hepatocyte cultures were calculated and the results reported as the mean %BEI (±S.D.).
Reverse Transcription–Polymerase Chain Reaction and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from the plated cells using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA was quantified spectrophotometrically and subjected to agarose gel electrophoresis to assess the integrity of RNA. The RNA was subsequently treated with RNase-free DNase (Promega, Madison, WI), mixed with random hexamers (Promega), heated to 70°C for 5 minutes, and then cooled to 4°C. A reaction mixture containing 200 U Moloney murine leukemia virus-reverse transcriptase, 1 mM deoxynucleoside-5′-triphosphates, and 25 U RNasin (Promega) was added to the previous mixture and incubated at 37°C for 60 minutes. The resulting cDNA was diluted 10-fold and stored at −20°C.
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) was conducted on an ABI Prism 7700 (Applied Biosystems, Foster City, CA). DNase-I treated total RNA of each sample was transcribed and mixed with specific primer sets and PCR master mix (Applied Biosystems). TaqMan analysis was used for all of the genes analyzed with primers and conditions designated by Assays on Demand, Gene Expression Products (Applied Biosystems). Data were analyzed with the ABI Prism 7700 SDS software (version 1.0; Applied Biosystems). Expression of specific genes was normalized to an internal control (cyclophilin) mRNA expression.
Data Analysis
Data are expressed as the mean ± S.D.. The Student’s t test was used to assess the significance of the results (GraphPad Software, Inc., La Jolla, CA).
Results
Effect of Temperature on 17-OHPC and TCA Transport in Human Hepatocytes.
To investigate the mechanisms of hepatic transport of 17-OHPC (passive diffusion or active uptake process), the effect of temperature (4°C versus 37°C) on 17-OHPC transport in hepatocytes was evaluated.
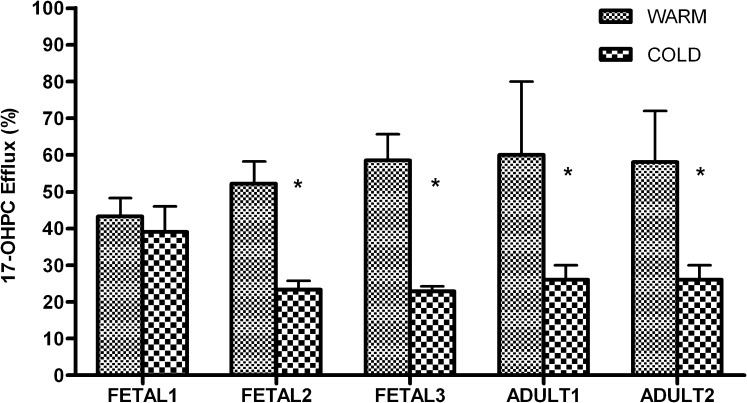
In fetal hepatocytes, inhibition of 17-OHPC efflux was observed in cold media (4°C) compared with warm media (37°C). The percent inhibition due to cold temperature varied from 10% to 61% (Fig. 1). The total uptake (media + cell lysate) of 17-OHPC in fetal hepatocytes was comparable between 4°C and 37°C. Similar results were observed in adult hepatocytes (Fig. 1). The percent inhibition of 17-OHPC efflux at 4°C varied from 44% to 61%; however, the total uptake was comparable.
Fig. 1.
Effect of temperature on 17-OHPC transport from primary human adult and fetal hepatocytes. The transport was evaluated at warm (37°C) and cold (4°C) temperatures. The results are expressed as 17-OHPC efflux relative to total 17-OHPC uptake (amount in media + cell lysate). Each bar represents the mean of triplicate treatments (±S.D.). Inhibition (statistically significant for *P < 0.05) of 17-OHPC efflux was observed in cold media.
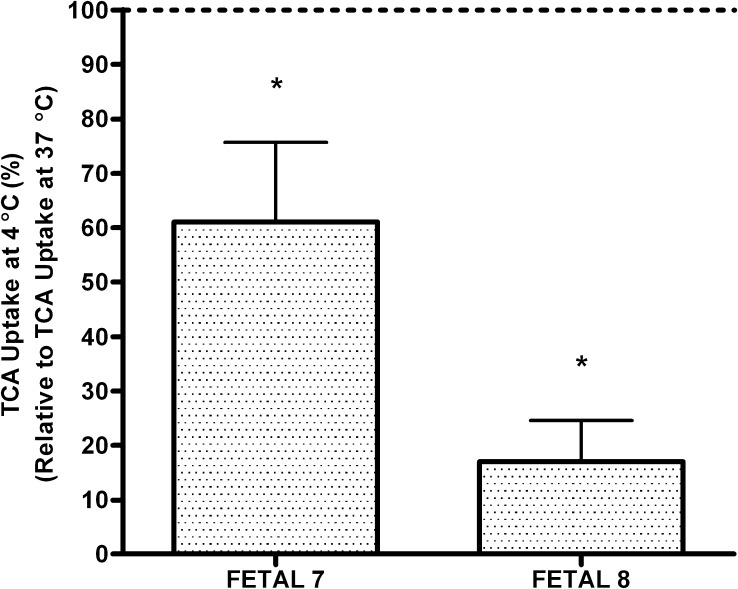
The effect of temperature on TCA transport was also evaluated in fetal hepatocytes. The efflux of TCA at 4°C was less than at 37°C. The percent inhibition of TCA efflux at 4°C was >90%. Furthermore, the total uptake of TCA was also inhibited at 4°C and varied from 26% to 93% (Fig. 2).
Fig. 2.
Effect of cold media (4°C) on TCA uptake from primary human fetal hepatocytes. The results are expressed as total TCA uptake at 4°C normalized to the total uptake at 37°C. Each bar represents treatments in triplicate (±S.D.). Efflux of TCA was significantly (*P < 0.05) inhibited in cold media (4°C) compared with warm media (37°C).
Effect of Cyclosporine on 17-OHPC and TCA Transport in Human Hepatocytes.
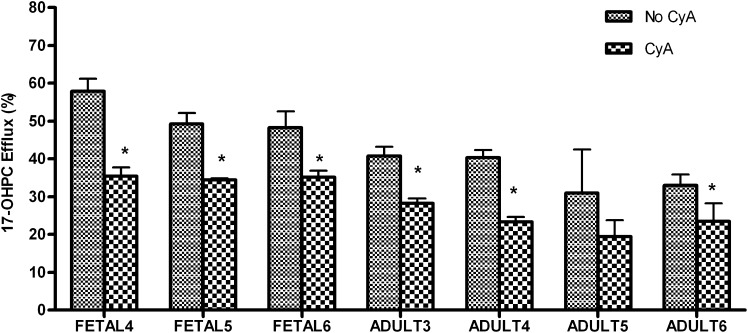
To confirm the involvement of a transport-mediated process in the disposition of 17-OHPC in primary human hepatocytes, cyclosporine, a well known inhibitor of ATP-mediated transport processes, was coincubated with fetal and adult human hepatocytes. The amount of 17-OHPC efflux (Fig. 3) was lower in the presence of cyclosporine for both fetal and adult human hepatocytes. In fetal hepatocytes, the percent inhibition of 17-OHPC efflux varied from 30% to 41%. In adult hepatocytes, 31%–52% inhibition was observed. However, the total uptake (media + cell lysate) of 17-OHPC by adult and fetal human hepatocytes was comparable in the presence and absence of cyclosporine (Table 2).
Fig. 3.
Efflux of 17-OHPC in the presence (CyA) and absence (No CyA) of cyclosporine A in primary human adult and fetal hepatocytes. The results are expressed as 17-OHPC efflux relative to total 17-OHPC uptake (amount in media + cell lysate). Each bar represents the mean of triplicate treatments (±S.D.). Inhibition (statistically significant for *P < 0.05) of 17-OHPC efflux was observed in the presence of CyA.
TABLE 2.
Total uptake of 17α-hydroxyprogesterone caproate in fetal and adult human hepatocytes
The total uptake was estimated by scintillation counting and calculated on the basis of total protein content. The results are expressed as mean (±S.D.).
| Hepatocytes | Condition/Inhibitor | Total Uptake |
|---|---|---|
| pmol/mg protein | ||
| Fetal | CyA | 304 ± 103 |
| Fetal | No CyA | 284 ± 70 |
| Adult | CyA | 494 ± 260 |
| Adult | No CyA | 476 ± 171 |
| Fetal | VER | 281 ± 47 |
| Fetal | No VER | 263 ± 93 |
| Adult | VER | 347 ± 86 |
| Adult | No VER | 341 ± 81 |
| Fetal | RIF | 241 ± 26 |
| Fetal | No RIF | 267 ± 86 |
| Adult | RIF | 565 ± 110 |
| Adult | No RIF | 611 ± 146 |
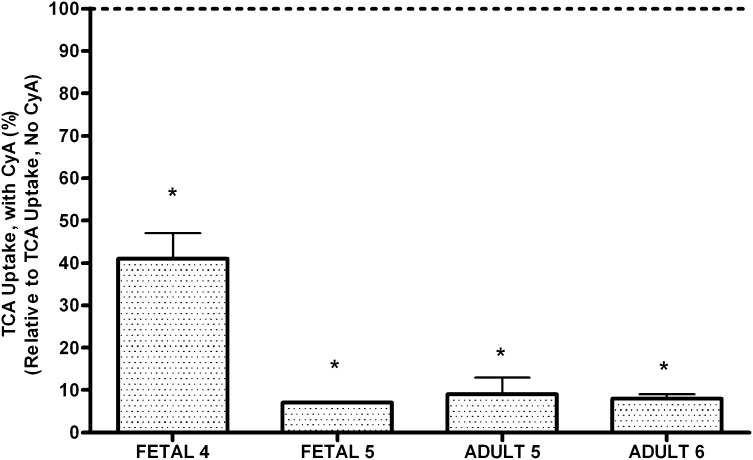
Similarly, the amount of TCA efflux was lower in the presence of cyclosporine in fetal and adult human hepatocytes. The percent inhibition of TCA efflux varied from 60% to 95% for fetal cells and from 60% to 98% in adult human hepatocytes. Furthermore, the total taurocholate uptake was also inhibited by CyA (Fig. 4) with percent inhibition in the range of 60%–90% for fetal hepatocytes and >90% for adult human hepatocytes.
Fig. 4.
Effect of cyclosporine (CyA) on taurocholate (TCA) uptake from primary human adult and fetal hepatocytes. The results are expressed as total TCA uptake in the presence of CyA normalized to the total uptake in the absence of CyA. Each bar represents the mean of triplicate treatments (±S.D.). Significant (*P < 0.05) inhibition of TCA efflux was observed in the presence of CyA.
Effect of Verapamil on 17-OHPC Transport in Human Hepatocytes.
ABCB1 (MDR1 or P-glycoprotein) is a member of the ATP-binding cassette transporter superfamily that is involved in the efflux of various chemical compounds. Verapamil has been reported to inhibit MDR1-mediated efflux activity (Zong and Pollack, 2003). To evaluate the involvement of an active, transporter-mediated efflux process in the disposition of 17-OHPC in primary human hepatocytes, verapamil was coincubated with fetal and adult human hepatocytes.
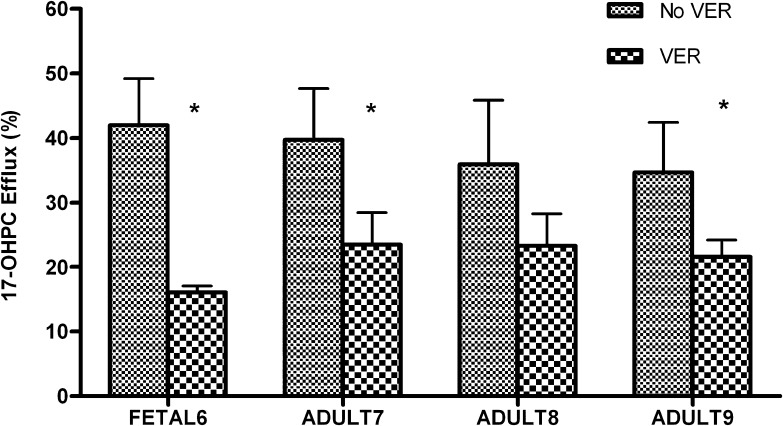
The amount of 17-OHPC efflux (Fig. 5) was lower in the presence of verapamil for both fetal and adult human hepatocytes. In fetal hepatocytes, the inhibition of 17-OHPC efflux was 58%–67% in the presence of verapamil. In adult hepatocytes, 25%–42% inhibition was observed. However, the total uptake (media + cell lysate) of 17-OHPC by adult and fetal human hepatocytes was comparable in the presence and absence of verapamil (Table 2).
Fig. 5.
Efflux of 17-OHPC in the presence (VER) and absence (No VER) of verapamil in primary human adult and fetal hepatocytes. The results are expressed as 17-OHPC efflux relative to total 17-OHPC uptake (amount in media + cell lysate). Each bar represents the mean of triplicate treatments (±S.D.). Inhibition (statistically significant for *P < 0.05) of 17-OHPC efflux was observed in the presence of VER.
Effect of Rifampin on 17-OHPC Transport in Human Hepatocytes.
The organic anion-transporting family of proteins (SLCO gene family or OATPs) plays a role in the uptake transport of bile salts and xenobiotics (Hagenbuch and Meier, 2004). Rifampin has been reported to inhibit human liver SLCOs and thus interfere with carrier-mediated organic anion uptake (Vavricka et al., 2002). To evaluate the involvement of an active, transporter-mediated uptake process in the disposition of 17-OHPC in primary human hepatocytes, rifampin was coincubated with fetal and adult human hepatocytes.
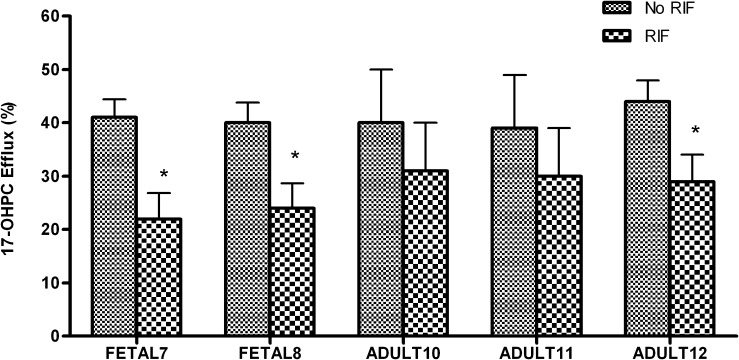
The amount of 17-OHPC efflux (Fig. 6) was lower in the presence of rifampin for both fetal and adult human hepatocytes. In fetal hepatocytes, inhibition of 17-OHPC efflux in the presence of rifampin varied from 46% to 55%. In adult hepatocytes, 21%–44% inhibition was observed. However, the total uptake (media + cell lysate) of 17-OHPC by adult and fetal human hepatocytes was comparable in the presence and absence of rifampin (Table 2).
Fig. 6.
Efflux of 17-OHPC in the presence (RIF) and absence (No RIF) of rifampin in primary human adult and fetal hepatocytes. The results are expressed as 17-OHPC efflux relative to total 17-OHPC uptake (amount in media + cell lysate). Each bar represents the mean of triplicate treatments (±S.D.). Inhibition (statistically significant for *P < 0.05) of 17-OHPC efflux was observed in the presence of RIF.
Effect of 17-OHPC on TCA Transport in Adult and Fetal Hepatocytes.
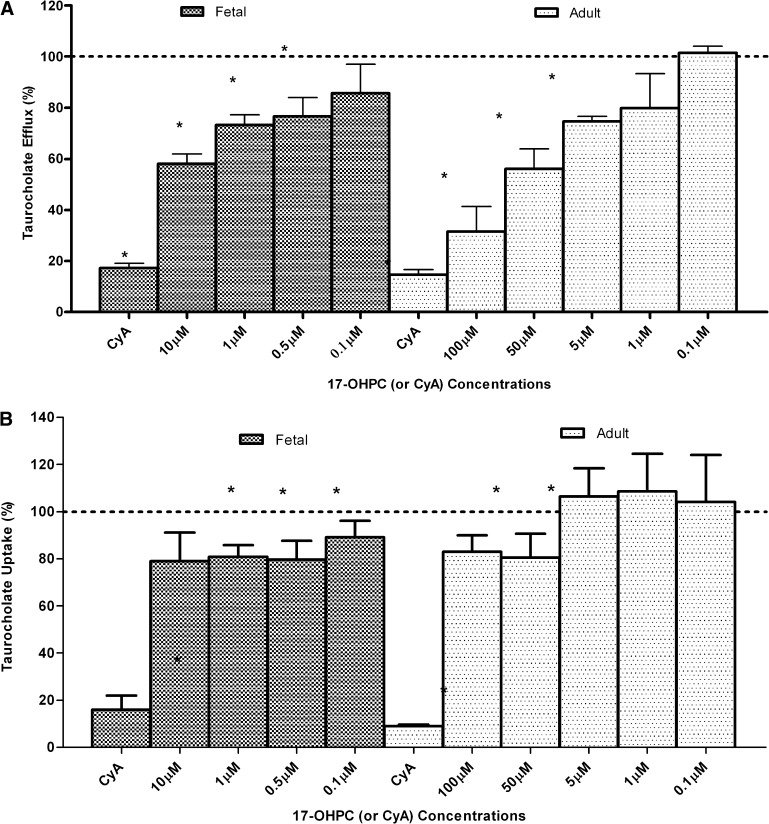
To evaluate the inhibitory potential of 17-OHPC on TCA efflux, we preincubated and coincubated different concentrations of 17-OHPC with TCA. In fetal hepatocytes, 17-OHPC reduced TCA efflux with significant (P < 0.05) inhibition observed at 0.5 µM, 1.0 µM, and 10.0 µM 17-OHPC concentrations (Fig. 7A). The total uptake of TCA was also reduced by 17-OHPC with significant (P < 0.05) inhibition at concentrations >0.5 µM (Fig. 7B). CyA (40 µM) was used as the positive control and depicted a significant (P < 0.05) inhibition of both efflux and total uptake of TCA.
Fig. 7.
Effect of 17-OHPC on TCA disposition in cultured fetal (n = 4) and adult (n = 6) human hepatocytes. (A) TCA efflux into the canaliculi in the absence of 17-OHPC (or CyA) was defined as control (100% TCA efflux). The results are expressed as %TCA efflux relative to control. Each bar represents the mean of triplicate treatments (±S.D.). CyA was employed as the positive control. Significant decrease (*P < 0.05) in taurocholate (canalicular) efflux was observed at ≥0.5 µM 17-OHPC in fetal hepatocytes, and at ≥5.0 µM 17-OHPC in adult hepatocytes. CyA significantly (P < 0.05) decreased taurocholate efflux in both fetal and adult cells. (B) Taurocholate uptake into the hepatocytes in the absence of 17-OHPC (or CyA) was defined as control (100% TCA uptake). The results are expressed as %TCA uptake relative to control. Each bar represents the mean of triplicate treatments (±S.D.). CyA was employed as the positive control. Significant decrease (*P < 0.05) in TCA uptake was observed at ≥0.5 µM 17-OHPC in fetal hepatocytes, and at ≥50 µM 17-OHPC in adult hepatocytes. CyA significantly (P < 0.05) decreased taurocholate uptake in both fetal and adult cells.
In adult hepatocytes, 17-OHPC also inhibited taurocholate efflux. A significant (P < 0.05) decrease in TCA efflux was observed at 5 µM, 50 µM, and 100 µM 17-OHPC concentrations (Fig. 7A). The total uptake of TCA was also inhibited by 17-OHPC with significant (P < 0.05) inhibition at concentrations >50 µM (Fig. 7B). Similarly to fetal hepatocytes, CyA (40 µM) was used as the positive control and showed a significant (P < 0.05) inhibition of both TCA efflux and uptake.
The mean %BEI (±S.D.) for 17-OHPC in adult (6.2 ± 2.0) and fetal (7.0 ± 2.0) hepatocyte cultures were similar. This was also demonstrated in the mean (±S.D.) accumulation values of 17-OHPC (pmol/mg protein) for adult (Ca2+/Mg2+: 680 ± 148; Ca2+/Mg2+ free: 628 ± 145) and fetal (Ca2+/Mg2+: 320 ± 168; Ca2+/Mg2+ free: 294 ± 163) hepatocytes.
The mean %BEIs (±S.D.) for taurocholate in adult (72.0 ± 10.0) and fetal hepatocyte (36.0 ± 8.0) cultures were significantly (P < 0.05) different. The mean (±S.D.) accumulation values of taurocholate (pmol/mg protein) for adult (Ca2+/Mg2+: 85 ± 12; Ca2+/Mg2+ free: 21 ± 11) and fetal (Ca2+/Mg2+: 34 ± 11; Ca2+/Mg2+ free: 23 ± 10) hepatocytes reflected the difference.
Expression of Transporters in Fetal and Adult Hepatocytes.
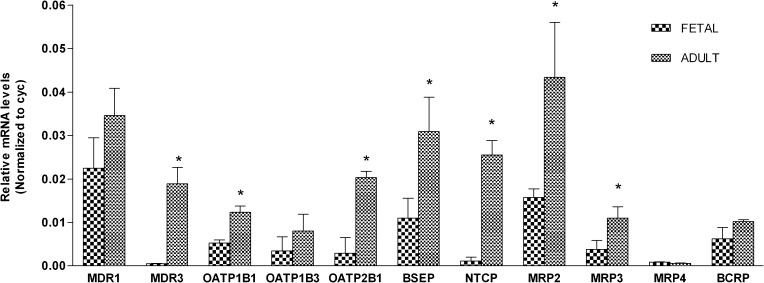
Quantitative real-time RT-PCR–based measurements of transporter mRNA levels in fetal hepatocyte cultures were determined and compared with results with adult hepatocytes (Fig. 8). The relative mRNA levels were normalized to the endogenous reference, cyclophilin (cyc). In adult hepatocytes, ABCC2 (MRP2) showed the highest expression level, followed by ABCB1 (MDR1) and ABCB11 (BSEP). In fetal hepatocytes, ABCB1 (MDR1) (approximately 64% of adults) showed the highest expression, followed by ABCB11 (BSEP) (approximately 40% of adults) and ABCC1 (MRP2) (approximately 38% of adults) transporters. ABCB4 (MDR3) was the only transporter that was not detected on a consistent basis in the fetal hepatocytes. Relative to adults, the transporters with the lowest expression in fetal liver were ABCB4 (MDR3) (approximately 2% of adults) and SLC10A1 (NTCP) (approximately 4% of adults).
Fig. 8.
Expression of transporters based on RT-PCR analysis in adult (n = 3) and fetal (n = 3) hepatocytes. Each bar represents the mean of triplicate observations (±S.D.). *P < 0.05. Expression of hepatic transporters in adults was observed to be significantly higher (P < 0.05) compared with the fetus except for ABCB1 (MDR1), SLCO1B3 (OATP1B3), ABCC4 (MRP4), and ABCG2 (BCRP).
Discussion
This study was designed to evaluate the hepatobiliary disposition of 17-OHPC and Taurocholate in primary cultures of fetal and adult human hepatocytes. Furthermore, the expression of drug transporters in fetal and adult human hepatocytes was evaluated. Dual-labeled 17-OHPC used in our study was observed to retain both (14C, 3H) labels after metabolism to mono-, di-, and tri-hydroxy derivatives, the major metabolites generated on incubations with adult and fetal hepatocyte cultures (Caritis et al., 2011 ; Sharma et al., 2008). Thus, the findings in our study refer to the disposition of 17-OHPC and its metabolites; in using the current study design, any difference between the parent compound and its metabolites cannot be differentiated.
To determine the presence of an active transport mechanism in the disposition of 17-OHPC, we evaluated the effect of temperature (4°C versus 37°C) in adult and fetal hepatocyte cultures. A decrease in 17-OHPC efflux was observed at a cold temperature (4°C) compared with 37°C. These results suggest the presence of a carrier-mediated efflux process in 17-OHPC transport along with the possible involvement of passive permeation. Significant variability in 17-OHPC transport was observed across fetal and adult hepatocytes. This may be attributed to interindividual variability in the expression and function of transporters or interbatch variability (due to variability in tissue storage and shipping conditions, hepatocyte isolation, and culturing conditions) in hepatocytes.
Hepatic TCA transport is well characterized, with its uptake being mediated by SLC10A1 (NTCP) (approximately 80%) and the SLCO (OATP) family of transporters (approximately 20%) and efflux into the canalicular space by ABCB11 (BSEP) (Kemp et al., 2005). On evaluating the effect of temperature on TCA transport, significant inhibition of both total uptake and efflux was observed in fetal hepatocytes at 4°C. Thus, the effect of temperature on TCA suggests the involvement of functional uptake and efflux processes.
Cyclosporine, a known inhibitor of multiple transporters such as ABCB1 (P-gp), SLCO1B1 (OATP1B1), ABCC2 (MRP2), and ABCG2 (BCRP) (US Food and Drug Administration, 2012), was used to evaluate the role of active transporters in the disposition of 17-OHPC. Cyclosporine produced a significant inhibition of 17-OHPC efflux, indicating that an active efflux process is involved in the disposition of 17-OHPC.
Similarly, significant taurocholate efflux inhibition was observed in the presence of cyclosporine in both adult and fetal hepatocytes. However, in contrast to 17-OHPC, the total accumulation of taurocholate was also significantly inhibited by cyclosporine in both adult and fetal hepatocytes. The results indicate that functional uptake and efflux processes are involved in the transport of taurocholate in fetal hepatocytes, whereas only an efflux process is primarily observed with 17-OHPC.
The organic anion-transporting polypeptide transporters (SLCO1B1, 2B1, and 1B3) are uptake proteins with broad substrate specificities including bile salts, prostaglandins, and steroid conjugates. It was recently reported that the sulfate conjugate of pregnenolone, precursor to endogenous hormones including dehydroepiandrosterone and progesterone, is a substrate of SLCO2B1 (OATP2B1) (Grube et al., 2006). To investigate the involvement of an uptake process mediated by the SLCO proteins, in addition to the predominant efflux process in the transport of 17-OHPC, we used rifampin, a known inhibitor of SLCO transporters (Vavricka et al., 2002). If the SLCO transporters played a role in the disposition of 17-OHPC or its metabolites, we expected to observe a decrease in the total uptake of 17-OHPC in the presence of rifampin. However, the total uptake of 17-OHPC was not affected by rifampin (Table 2) and instead a decrease in the percent efflux was observed. These observations were contrary to the expected results. Previous literature reports (Fardel et al., 1995; Zong et al., 2003; Reitman et al., 2011) indicate that rifampin has the ability to bind to ABCB1 (MDR1)-drug binding sites and thus cause an inhibition in the ABCB1 (MDR1) mediated efflux of substrates (e.g., digoxin, vinblastine). Furthermore, rifampin has been reported to inhibit ABCB11 (BSEP; Mita et al., 2006) and ABCC2 (MRP2; Cui et al., 2001) mediated efflux of substrates. Thus, the results suggest the possible involvement of one or a combination of the above-mentioned efflux transporters in the disposition of 17-OHPC. Subsequent studies confirmed the expression of these transporters (MDR1, BSEP, and MRP2) in fetal and adult hepatocytes, consistent with the observation. In addition to evaluating the functional activity of hepatic transporters in fetal hepatocytes, we performed quantitative RT-PCR analyses to compare the mRNA expression levels of various hepatic transporters in adult and fetal hepatocytes. Overall, the mean expression for all of the transporters was higher in adult than fetal cells, except ABCC4 (MRP4). Relative to adult hepatocytes, the expression level of ABCB11 (BSEP) in fetal hepatocytes (approximately half the expression observed in adults) correlated well with the observed canalicular taurocholate efflux activity (mean BEI in fetal cells approximately half of that observed in adult cells). The expression of SLC10A1 (NTCP) in fetal hepatocytes was only 5% of that in adult hepatocytes; however, the total uptake of taurocholate was approximately 50% relative to the adult cells. This might be attributed to the enhanced role of other uptake transporters, including SLCO1B1 and 1B3 (OATP1B1 and 1B3, observed at approximately 50% of that observed in adult hepatocytes) in fetal hepatocytes. The expression of ABCB1 (MDR1) in fetal hepatocytes varied between 40% and 150% of that in adults, whereas the efflux of 17-OHPC in fetal hepatocytes varied from 84% to 135% relative to adult hepatocytes. Thus, assuming that the mRNA expression and protein levels are correlated, these results indicate the involvement of ABCB1 (MDR1) in the hepatic transport of 17-OHPC.
The maximum plasma concentrations reported for 17-OHPC is 30 ng/ml (0.07 μM) in adults (Caritis et al., 2011) and 10 ng/ml (0.02 μM) in cord blood (unpublished data). These concentrations are much lower than the 1 µg/ml threshold reported for hepatotoxicity (Kostrubsky et al., 2003) due to bile salt transporter inhibition. However, the above-mentioned threshold is based on evaluations carried out in adult human hepatocytes and the model must be validated for fetal human hepatocytes. To the best of our knowledge, this is the first report that tests the applicability of the in vitro hepatotoxicity model in fetal human hepatocyte cultures.
To test the role of biliary excretion pathway in the disposition of 17-OHPC (molecular weight 428.7) or its metabolites, we estimated the BEI for 17-OHPC in both adult and fetal hepatocytes and compared it with taurocholate. A large BEI (as high as taurocholate) indicates extensive excretion of substrate into the canalicular space via transporters (Liu et al., 1999). Biliary excretion of 17-OHPC was observed to be similar in fetal and adult hepatocytes although it was 5–10 times less than the BEI for taurocholate. The BEI for taurocholate was significantly (P < 0.05) higher in adults than fetal hepatocytes, which can be possibly be explained by the higher expression level of ABCB11 (BSEP) in adult cells, assuming that there is a correlation between ABCB11 mRNA and protein expression. Thus, the results indicate that biliary excretion pathway might play a limited role in the disposition of 17-OHPC.
To evaluate the affinity of 17-OHPC for the bile salt transporters, we tested the ability of 17-OHPC to inhibit taurocholate transport in a concentration-dependent manner. Significant inhibition of taurocholate transport by 17-OHPC was observed at ≥0.5 µM in fetal hepatocytes indicating the potential for inhibiting bile transport. The uptake also decreased with increasing concentrations of 17-OHPC although the inhibition was of a much smaller magnitude compared with efflux. In adult hepatocytes, a similar trend was observed with both efflux and total uptake decreasing with increasing 17-OHPC concentrations. Further studies are needed to elucidate the mechanism of inhibition of TCA transport by 17-OHPC or its metabolites. The IC50 calculated for 17-OHPC–mediated inhibition of taurocholate efflux in fetal hepatocytes was approximately 20 µM or 4-fold lower than the IC50 (approximately 80 µM) observed in adult hepatocytes. This observation suggests the possibility that the fetal liver might be more sensitive to xenobiotics than the adult liver, largely due to its lower expression of bile salt and other sinusoidal or canalicular transporters that also play an important role in detoxification. Cyclosporine, used as positive control, showed significant inhibition of both the uptake and efflux of taurocholate in adult and fetal hepatocytes, thus confirming the functional activity of bile transporters. Although inhibition of taurocholate transport was observed in fetal and adult hepatocytes, the inhibition is not likely to be clinically significant given the very low 17-OHPC levels observed in human plasma.
In conclusion, this study demonstrates the functional expression of both uptake and efflux transporters for taurocholate and 17-OHPC in fetal human hepatocytes. The results also demonstrate the inhibitory potential of 17-OHPC toward taurocholate transport. It is likely that the presence of an active efflux process in fetal hepatocytes prevents the accumulation of 17-OHPC or its metabolites in fetal hepatocytes, thus minimizing the risk of any adverse event. The bile transport inhibition assay has proven useful to investigate hepatotoxicity with adult hepatocytes, and we show here that the transport assays can be applied to fetal hepatocytes to evaluate drugs that traverse the placental barrier and gain access to the fetal circulation after being administered to pregnant subjects.
Acknowledgments
This work was conducted as part of the Obstetric-Fetal Pharmacology Research Unit Network.
Abbreviations
- 17-OHPC
17α-hydroxyprogesterone caproate
- BEI
biliary excretion index
- CyA
cyclosporine A
- DMEM
Dulbecco's modified Eagle’s medium
- EMEM
Eagle’s minimum essential medium
- HBSS
Hanks’ balanced salt solution
- HMM
hepatocyte maintenance media
- ICP
intrahepatic cholestasis of pregnancy
- PM
progesterone metabolite
- RIF
rifampin
- RT-PCR
reverse transcription-polymerase chain reaction
- TCA
taurocholate acid
- VER
verapamil
Authorship Contributions
Conducted experiments: Sharma, Ellis, Gramignoli, Dorko, Tahan, Hansel.
Performed data analysis: Sharma.
Wrote or contributed to the writing of the manuscript: Sharma, Mattison, Caritis, Hines, Venkataramanan, Strom.
Footnotes
This work was supported in part by the Public Health Service [Grants HD-047905 and GM-081344], and the Liver Tissue and Cell Distribution System, funded by National Institutes of Health [Grant N01-DK-7-0004/HHSN26700700004C]
This article has supplemental material available at dmd.aspetjournals.org.
References
- Ackermann E, Richter K. (1977) Dizepam metabolism in human foetal and adult liver. Eur J Clin Pharmacol 11:43–49 [DOI] [PubMed] [Google Scholar]
- Aranda JV, Louridas AT, Vitullo BB, Thom P, Aldridge A, Haber R. (1979) Metabolism of theophylline to caffeine in human fetal liver. Science 206:1319–1321 [DOI] [PubMed] [Google Scholar]
- Barnes KM, Dickstein B, Cutler GB, Jr, Fojo T, Bates SE. (1996) Steroid treatment, accumulation, and antagonism of P-glycoprotein in multidrug-resistant cells. Biochemistry 35:4820–4827 [DOI] [PubMed] [Google Scholar]
- Bi YA, Kazolias D, Duignan DB. (2006) Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab Dispos 34:1658–1665 [DOI] [PubMed] [Google Scholar]
- Caritis SN, Sharma S, Venkataramanan R, Rouse DJ, Peaceman AM, Sciscione A, Spong CY, Varner MW, Malone FD, et al. (2011) Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation. Am J Obstet Gynecol 205:40.e1–40.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba M, Nshime JA, Lin JH. (1997) In vitro metabolism of indinavir in the human fetal liver microsomes. Drug Metab Dispos 25:1219–1222 [PubMed] [Google Scholar]
- Cui Y, König J, Keppler D. (2001) Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol 60:934–943 [DOI] [PubMed] [Google Scholar]
- Ellis ES, Bodin K, Griffiths WJ, Sellaro TL, Cai H, Strom SC. (2008) Bile acid transport and synthesis in human fetal hepatocytes: influence of culture conditions (Abstract no. 779). Hepatology 48 (Suppl. S1):609A–706A [Google Scholar]
- Fardel O, Lecureur V, Loyer P, Guillouzo A. (1995) Rifampicin enhances anti-cancer drug accumulation and activity in multidrug-resistant cells. Biochem Pharmacol 49:1255–1260 [DOI] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. (2001) The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 69:223–231 [DOI] [PubMed] [Google Scholar]
- Funk C, Ponelle C, Scheuermann G, Pantze M. (2001) Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol 59:627–635 [PubMed] [Google Scholar]
- Germann UA, Shlyakhter D, Mason VS, Zelle RE, Duffy JP, Galullo V, Armistead DM, Saunders JO, Boger J, Harding MW. (1997) Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs 8:125–140 [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK. (2006) Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70:1735–1741 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hines RN. (2007) Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol 21:169–175 [DOI] [PubMed] [Google Scholar]
- Hines RN. (2008) The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 118:250–267 [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, Feng B, Nguyen HT, Frederick KS, Campbell SD, Hatch HL, Bi YA, Kazolias DC, Davidson RE, Mireles RJ, et al. (2007) Role of transporters in the disposition of the selective phosphodiesterase-4 inhibitor (+)-2-[4-([2-(benzo[1,3]dioxol-5-yloxy)-pyridine-3-carbonyl]-amino-methyl)-3-fluoro-phenoxy]-propionic acid in rat and human. Drug Metab Dispos 35:2111–2118 [DOI] [PubMed] [Google Scholar]
- Kemp DC, Zamek-Gliszczynski MJ, Brouwer KL. (2005) Xenobiotics inhibit hepatic uptake and biliary excretion of taurocholate in rat hepatocytes. Toxicol Sci 83:207–214 [DOI] [PubMed] [Google Scholar]
- Komori M, Nishio K, Kitada M, Shiramatsu K, Muroya K, Soma M, Nagashima K, Kamataki T. (1990) Fetus-specific expression of a form of cytochrome P-450 in human livers. Biochemistry 29:4430–4433 [DOI] [PubMed] [Google Scholar]
- Kostrubsky VE, Strom SC, Hanson J, Urda E, Rose K, Burliegh J, Zocharski P, Cai H, Sinclair JF, Sahi J. (2003) Evaluation of hepatotoxic potential of drugs by inhibition of bile-acid transport in cultured primary human hepatocytes and intact rats. Toxicol Sci 76:220–228 [DOI] [PubMed] [Google Scholar]
- Kroumpouzos G. (2002) Intrahepatic cholestasis of pregnancy: what’s new. J Eur Acad Dermatol Venereol 16:316–318 [DOI] [PubMed] [Google Scholar]
- Kukongviriyapan V, Stacey NH. (1988) Inhibition of taurocholate transport by cyclosporin A in cultured rat hepatocytes. J Pharmacol Exp Ther 247:685–689 [PubMed] [Google Scholar]
- Ladona MG, Gonzalez ML, Rane A, Peter RM, de la Torre R. (2000) Cocaine metabolism in human fetal and adult liver microsomes is related to cytochrome P450 3A expression. Life Sci 68:431–443 [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KL. (1999) Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol 277:G12–G21 [DOI] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik MVarner MW, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network (2003) Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 348:2379–2385 [DOI] [PubMed] [Google Scholar]
- Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. (2006) Inhibition of Bile Acid Transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab Dispos 34:1575–1581 [DOI] [PubMed] [Google Scholar]
- Rane A, Tomson G. (1980) Prenatal and neonatal drug metabolism in man. Eur J Clin Pharmacol 18:9–15 [DOI] [PubMed] [Google Scholar]
- Reitman ML, Chu X, Cai X, Yabut J, Venkatasubramanian R, Zajic S, Stone JA, Ding Y, Witter R, Gibson C, et al. (2011) Rifampin’s acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther 89:234–242 [DOI] [PubMed] [Google Scholar]
- Rollins DE, von Bahr C, Glaumann H, Moldéus P, Rane A. (1979) Acetaminophen: potentially toxic metabolite formed by human fetal and adult liver microsomes and isolated fetal liver cells. Science 205:1414–1416 [DOI] [PubMed] [Google Scholar]
- Sharma S, Ou J, Strom S, Mattison D, Caritis S, Venkataramanan R. (2008) Identification of enzymes involved in the metabolism of 17alpha-hydroxyprogesterone caproate: an effective agent for prevention of preterm birth. Drug Metab Dispos 36:1896–1902 [DOI] [PubMed] [Google Scholar]
- Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG. (1996) Use of human hepatocytes to study P450 gene induction. Methods Enzymol 272:388–401 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2012) FDA (Food and Drug Administration) Guidance for Industry on Drug Interaction Studies —Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. Draft Guidance. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf (accessed 2012 Aug 25).
- Vallejo M, Briz O, Serrano MA, Monte MJ, Marin JJ. (2006) Potential role of trans-inhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol 44:1150–1157 [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. (2002) Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology 36:164–172 [DOI] [PubMed] [Google Scholar]
- Wiebkin P, Dees JH, Mathis JM, Prough RA. (1985) Drug metabolism by isolated fetal human hepatocytes in suspension and primary culture. Drug Metab Dispos 13:163–168 [PubMed] [Google Scholar]
- Zong J, Pollack GM. (2003) Modulation of P-glycoprotein transport activity in the mouse blood-brain barrier by rifampin. J Pharmacol Exp Ther 306:556–562 [DOI] [PubMed] [Google Scholar]