Abstract
Most insects produce two or more storage hexamers whose constituents and developmental profiles are sufficiently different to suggest specialization in the ways that they support metamorphosis and reproduction. Hexamerin specializations are compared here in the Cecropia moth (Hyalophora cecropia), which produces eggs during the pupal-adult molt, and the Monarch butterfly (Danaus plexippus), which produces eggs under long-day conditions after adult eclosion. In both sexes of both species, reserves of arylphorin (ArH) were exhausted by the end of metamorphosis. In Cecropia, the same was true for the high-methionine hexamerins, V-MtH and M-MtH. But in short day Monarch females 20–30% of the pupal reserves of V-MtH and M-MtH survived metamorphosis, persisting until long-day conditions were imposed to stimulate egg formation. Differences in storage sites have been documented in other lepidopterans, with MtH reserves being found primarily in fat body protein granules and the ArH reserve being found primarily in the hemolymph. Similar differences could explain how a fraction of the MtH's, but not of ArH, escapes utilization during metamorphosis in a species with post-eclosion egg formation. No differences in utilization schedules were detected between V- and M-MtH, despite divergent compositions and antigenic reactivity.
Keywords: No Keywords Available
Introduction
Insects prepare for the synthetic demands of molting, metamorphosis and reproduction by accumulating hexamerins in their hemolymph and fat body. First to be described were two soluble storage hexamerins isolated from larvae of Calliphora erythrocephala (Munn and Greville, 1969). Two or more hexamerins were later reported in the hemolymph and fat body of many other insects, with some lepidopterans having as many as four. Hexamerins occurring within a single species can differ in amino acid composition, stage of synthesis, distribution between hemolymph and fat body, timing of clearance from the hemolymph, and antigenic reactivity (reviewed by Wyatt and Pan, 1978; Riddiford and Law, 1983; Kanost et al., 1990; Telfer and Kunkle, 1991).
The first storage proteins to be described in lepidopterans were two methionine-rich hexamerins from Hyalophora cecropia (Tojo et al., 1978). They differed in electrophoretic mobility in native PAGE and also in amino acid composition, methionine contents being 7.0% for the more slowly migrating and 4.9% for the faster. (We adopt here descriptive acronyms, V-MtH and M-MtH, for very and moderately methionine-rich hexamerins.) The two appear in the hemolymph late in the last larval instar, rise to maximum concentrations early in the larval-pupal molt, and are then largely endocytosed by the cells of the fat body, which store them in crystalline form until they are utilized during adult development. MtH's have been identified in a variety of moths, either as isolated proteins or from cDNA sequencing (Tojo et al., 1978; 1980; 1985; Ryan et al., 1985; Bean and Silhacek, 1988; Jones et al., 1990; Jones et al., 1993; Memmel et al., 1994). They are identified here in a butterfly by antigenic cross-reactions. They tend to be more abundant in female pupae than in males, and this has led to a prediction that they play special roles in egg formation.
A third lepidopteran hexamerin, first described in Manduca sexta (Kramer et al., 1980), has a high aromatic amino acid content, resembling in this regard one of the prototypical hexamerins of Calliphora. The importance of aromatic amino acids in sclerotization led to the suggestion that hexamerins in this class, now known as arylphorins (ArH) (Telfer et al., 1983), are adapted to support cuticle deposition. While many lepidopterans produce just these three, some also contain a riboflavin-binding hexamerin, RbH (Telfer and Massey, 1987; Miller and Silhacek, 1992; Magee et al., 1994). Like ArH, RbH is a major component of pupal hemolymph that disappears during adult development (Pan and Telfer, 1999).
Labeling experiments in saturniid moths failed to confirm the targeting of hexamerins for special developmental processes. In Actias luna M-MtH and ArH proved to be equivalent sources of labeled amino acids for the production of eggs and cuticle, as well as other adult tissues (Pan and Telfer, 1996), and RbH and ArH were found to be similarly equivalent in H. cecropia (Pan and Telfer, 1999). These tests were performed in species that produce eggs at the same time that they form the soma of moths. They thus left open the question of which of the hexamerins remain available to support egg formation when the latter is delayed until after adult eclosion.
To investigate this we compared the timing of hexamerin depletion in two lepidopterans that form eggs at different times relative to eclosion. The Cecropia silkmoth, Hyalophora cecropia, ecloses with a full complement of eggs that are ready to be fertilized and laid. The Monarch butterfly, Danaus plexippus, delays egg formation until it experiences a long-day photoperiod after eclosion (reviewed by Ackery and Vane-Wright, 1984). The strategy we used was to time in each species the disappearance of V-MtH, M-MtH and ArH during metamorphosis and egg formation. The accumulation of vitellogenin (Vg) was simultaneously measured, since synthesis of this abundant product by the adult fat body can be correlated with hexamerin depletion (Wheeler and Buck, 1996; Wheeler et al., 1999). RbH was not included because we could not detect it with rabbit antibodies in Monarch larvae or pupae (Pan and Telfer, 1999).
Materials and Methods
1. Experimental Insects
Diapausing pupae of field-reared Hyalophora cecropia were stored at 6°C for at least five months and then allowed to initiate adult development by transfer to 25°C. At this temperature development from apolyis to eclosion requires 23 days in females and 21 days in males.
Migrating butterflies of Danaus plexippus were captured during mid-October in eastern Tennessee. Most of the catches were sufficiently fresh to suggest recent emergence in nearby regions. They were kept in cages at outdoor temperature and day length, and fed once per day with a 30% honey solution. After two to three weeks under these conditions the ovaries contained only small, translucent follicles, indicating an absence of yolk formation. Their fat bodies, relative to those of freshly caught butterflies, were greatly enlarged and full of lipid droplets.
To obtain pupae and second generation adults, some captured Monarchs were kept at 30°C under long day conditions, and fed twice daily with the honey solution. They terminated reproductive diapause and began mating in 1 to 2 weeks. Potted milkweed, Asclepias curasavica, was provided for egg laying. Hatched caterpillars were left on the plants until they reached the 3rd instar, and were then transferred to field-collected cuttings of Asclepias syriaca. Additional eggs and caterpillars were generously provided by Mr. Paul deMarrais of Afton, Tennessee.
2. Antibodies
V-MtH, M-MtH, ArH and Vg were measured in both species with rabbit antibodies against the corresponding proteins of H. cecropia. The reactions with Monarch proteins, though weaker than those with Cecropia, were still strong enough to be useful. This relationship is shown in Figure 1 for antibodies to Cecropia V-MtH.
Figure 1.
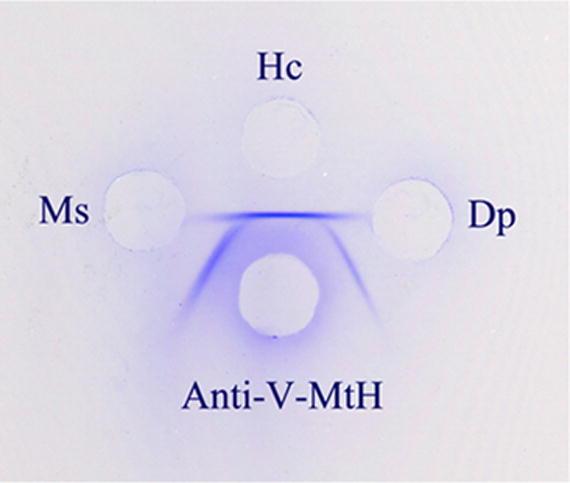
An Ouchterlony plate comparing the reactions between a rabbit antiserum against isolated Cecropia V-MtH and pupal extracts from Cecropia (Hc) and Monarchs (Dp). A pupal extract of the tobacco hornworm, Manduca sexta (Ms) is included in addition. Spur formation indicates that a fraction of the epitopes of Cecropia V-MtH are not present in either of the other two species. A practical consequence of this partial identity is that separate standard curves must be used for each species in measuring V-MtH changes with Oudin test.
ArH, Vg and what we identify below as M-MtH have been isolated from the hemolymph of Cecropia pupae by chromatographic methods, and the reactions of the antisera employed to measure them have already been described (Telfer et al., 1983; Telfer and Pan, 1988; Pan and Telfer, 1992). Each of the three antisera reacted with its homologous isolate to produce a single zone of heavy precipitation in immunodiffusion tests and showed no additonal reactions when combined with unfractionated hemolymph, whole-body extracts, or isolates other than the one used for immunization.
The antiserum against V-MtH has not been described before. We used native PAGE to isolate this hexamerin from pupal male hemolymph. Slab gels (0.3 × 12 × 17 cm) containing 4% acrylamide and lacking lane dividers were overlain with 40 µl of pupal male hemolymph diluted with twice its volume of native PAGE sample buffer. After electrophoresis a vertical strip from the center of the gel was stained with Coomassie Blue (Figure 2, lane 1). Horizontal strips corresponding to the positions of the two MtH's were cut from the unstained remainder of the gel and stored by freezing.
Figure 2.
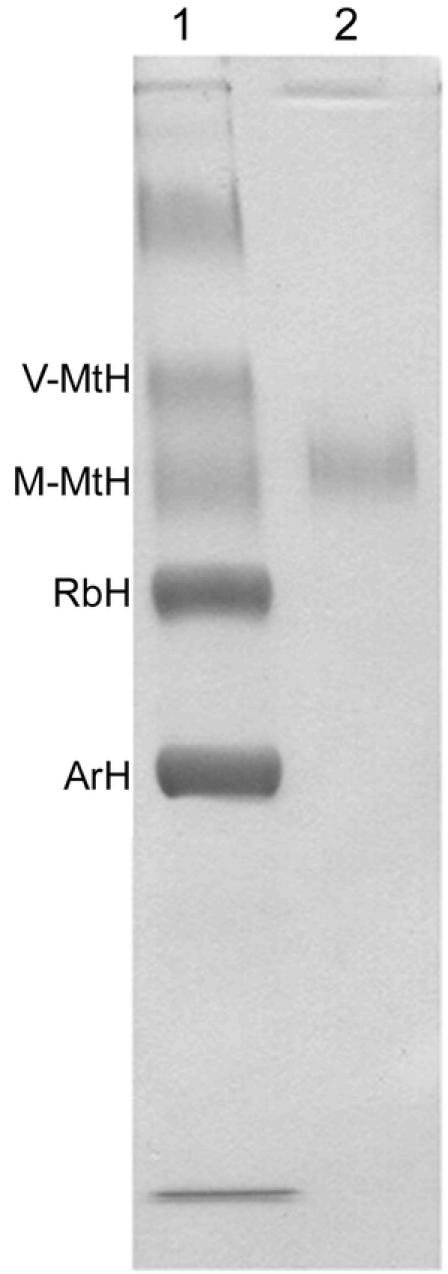
Coomassie Blue-stained native PAGE gel of male hemolymph from Cecropia pupae (lane 1) and M-MtH isolated by elution from the M-MtH band (lane 2). The M- and V-MtH bands were identified by microsequencing their N-terminal amino acids. Beginning with the V-MtH band and reading down, the hexamerin bands occur in the same order as proteins 1 through 4 in Fig. 17A of Tojo et al. (1978). The band near the top of lane 1 contained lipophorin (HDLp) (Telfer et al., 1991).
The two MtH's were identified by N-terminal amino acid microsequencing at a facility of the University of Pennsylvania School of Veterinary Medicine. For the faster of the two in native PAGE, the N-terminal sequence was RPDNDDVNFVVSM and for the slower band was SVVNDANYSF (40% of the fast component lacked the N-terminal RP but was otherwise identical to the other 60%.) These sequences matched N-terminal sequences deduced from cDNA clones of, respectively, M-MtH and V-MtH after signal polypeptide deletion (Massey, 1995; accession number AF032398 for M-MtH and AF032399 for V-MtH). The faster band corresponded to a 738 amino acid polypeptide with a methionine content of 4.76%, while the slower corresponded to a 737 amino acid polypeptide with a methionine content of 8.54%. Protein extracted from the slower band, thus identified as V-MtH, was used to immunize a rabbit (Harlow and Lane, 1988). In a variety of immunodiffusion tests with pupal hemolymph and extracts from H. cecropia, anti-V-MtH produced a single band of precipitation (Figure 1).
3. Tissue Preparation
Hemolymph was drained through a dorsal slit into a pre-chilled, graduated test tube containing 0.2 ml of a buffer solution—either, pH 7.2 phosphate-buffered saline (PBS) for Cecropia or 0.15 M Tris-citrate, pH 8.2, for the Monarch. (The Tris-citrate buffer used for the Monarch extraction was 6X that of the running buffer strength employed for Rocket Immunoelectrophoresis (Pan and Telfer, 1990). It was used in case the smaller amount of tissues collected from this species should prove to require a more sensitive technique for protein quantification, but this proved eventually not to be the case.) Both extraction media contained in addition a protease inhibitor cocktail, Control® (Boehringer Mannheim Biochemical), and 5mM phenylthiourea (PTU). The midgut and, when present, the bursa copulatrix were discarded and the rest of the soft tissues were scraped out of the abdominal cuticle and added to the collected blood. Additional buffer with the protease inhibitor and PTU was added to bring the suspended tissues to a volume that would yield antigen concentrations convenient to measure by the method described below. For Cecropia the final volume was 6 ml per pupa, and 3 or 1.5 ml per adult female and male, respectively. For Monarch pupae the final volume was 3 ml and for adults 0.75 ml. Comparisons between stages were made possible by normalizing to the pupal volume; e. g., concentrations for adult Cecropia females were divided by two and for males by four in order to compare them with the contents of pupal extracts.
The collected samples were stored frozen. For extraction, the tissue mixtures were thawed and transferred to a glass homogenizer. When chorionated eggs were present, they were crushed separately in a ceramic mortar and then combined with the rest of the tissues in the homogenizer.
4. Measurement of Antigen Concentrations
An immunodiffusion method was used to measure changes in extractable stores of the four proteins. Antigen concentrations were measured by layering the extracts over antisera that had been solidified in 3 mm (i. d.) glass tubes with 0.3% agarose, PBS and 0.05% sodium azide. The rate of penetration of an antigen-antibody precipitation front through the agarose is a function of the rate of diffusion of the antigen into the agarose layer and this in turn is a measure of antigen concentration in the upper reservoir. Procedures for setting up the tubes, for measuring rates of advance of precipitation fronts, and for constructing standard curves have already been described (Telfer et al., 1983; Telfer and Pan, 1988). The method was developed for studies on human serum proteins (Oudin, 1948), and has been used many times to measure antigen concentrations in insect hemolymph and egg extracts. It requires larger volumes and higher antigen concentrations than rocket immunoelectrophoresis (Pan and Telfer, 1990, 1992), but when these conditions are satisfied, as they proved to be for hexamerin measurements in Cecropia and Monarchs, it is convenient and reliable.
The measurements are of relative concentrations and are expressed as percentages of the concentration in a standard solution. For the hexamerins, extracts of abdomens from female pupae of each species were used as the standards; for VG, extracts of females containing chorionated eggs were used. The latter included newly eclosed adults in Cecropia and day 7 adults in long day Monarchs.
5. SDS-PAGE Electrophoresis
Insoluble residues that would escape detection by immunodiffusion were monitored by SDS-PAGE. For this purpose 0.1 ml of each of the homogenized samples from a chosen stage were vortexed and pooled. The pooled sample was centrifuged in a microfuge (Beckman®) for 4 min. The lipid cap was removed, and the supernatant decanted. Pellet and supernatant were then separately dissolved in SDS-PAGE sample buffer so that equal volumes contained equal fractions of, respectively, the total pellet and supernatant material in the uncentrifuged pool. The samples were compared in neighboring lanes of pre-cast 4–15% gradient mini gels from Bio-Rad®. Electrophoresis was conducted at constant voltage (100 v), and terminated after 1 hr and 12 min. Coomassie Brilliant Blue staining was used to reveal the banding patterns.
Results
1. Vitellogenin
Cecropia produced Vg earlier than the Monarch (Figure 3). Like many other saturniids, Cecropia synthesizes part of its Vg, in this case about 30 mg per 6 g female, during the larval-pupal molt, and stores it in the hemolymph during the months of diapause (Telfer, 1954). Beginning 10 days before eclosion, when yolk formation begins, there is a second period of Vg synthesis (Pan, 1971) (Figure 3) that draws on precursors released from the hexamerins present at that time (Pan and Telfer, 1996, 1999). In Monarchs, Vg was not detected in either pupae or short day adults. In females raised under long day conditions it was first detected 2–3 days after eclosion (Figure 3) and it continued to accumulate until day 7 when eggs began to be laid.
Figure 3.
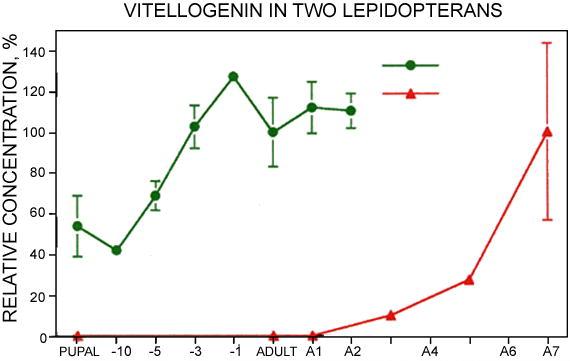
Vg content of abdominal extracts of pharate and post-eclosion adults of Cecropia, and of long-day Monarch adults. A1 to A7 designate days after eclosion in Monarchs. Days before eclosion in Cecropia are indicated by −10 to −1. P indicates diapausing pupae of Cecropia. Relative concentrations were estimated by Oudin tests using an antiserum produced in rabbits injected with isolated Cecropia Vg. Standard curves were produced by serial dilutions of extracts of day 0 adult Cecropia, and day 7 adult Monarchs. Error bars indicate standard errors; when not shown they were too small to be graphed. N = 6 for Cecropia and 5 for Monarchs.
In SDS-PAGE, Coomassie-stained Vg subunits were identified at 180 and 40 kDa by their presence in females and absence from males (Figure 4, Vg). They were found in pupal and adult extracts of Cecropia and in extracts of long day Monarch adults, the same samples in which Vg had been detected by immunodiffusion.
Figure 4.
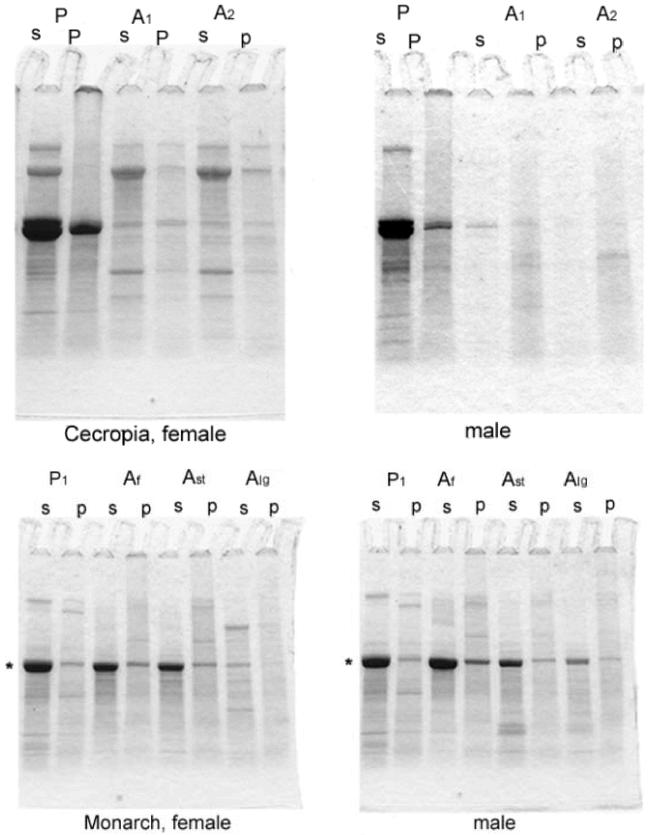
SDS-PAGE of centrifugal pellets (p) and supernatants (s) of Cecropia (A) and Monarch extracts (B). For Cecropia, P = pupa and A1,2 indicate days 1, and 2 after eclosion. For Monarchs, Af = adults freshly caught in the field; Ast = adults fed for four weeks under short day conditions; Alg = adults kept for two weeks in short days and then transferred to long days for an additional two weeks. The extract in each lane was a pool of the extracts from the indicated species and stage analyzed in Fig. 3. Asterisks indicate the 80 kDa level of the gels.
Vg is stored as a solute in the hemolymph of female Cecropia pupae and accordingly centrifugal pellets of extracts at that stage did not produce detectable 180 and 40 kDa bands. In adults most Vg has been transferred to the yolk; pellets at this stage produced weak Vg bands due, presumably, to small yolk spheres that are not readily disrupted by homogenization.
2. Hexamerin utilization in females
Cecropia
All three Cecropia hexamerins were consumed during the first 13 days of somatic tissue metamorphosis, 55% of the soluble MtH's and 33% of soluble ArH disappeared before VG began its second rise (Figure 5A). The last eight days of adult development encompass late somatic tissue development as well as the second rise in Vg content, and during this period the three hexamerins fell to less than 2% of pupal levels.
Figure 5.
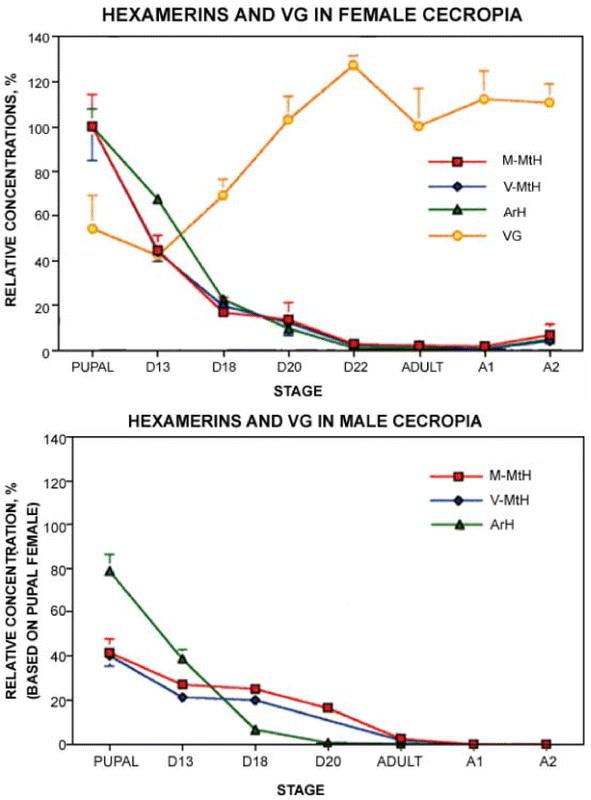
Changes in hexamerin contents in pharate and post-eclosion adults of Cecropia females (A) and males (B). D13–20 = days of adult development; A0–2 = days after eclosion. Error bars indicate standard errors; when not shown they were too small to be graphed. N = 6 for females and 4–6 for males.
Coomassie staining of SDS-PAGE gels of pupal extracts was concentrated in a cluster of circa 80 kDa bands (Figure 4A, asterisks). Mini gels resolved the hexamerin subunit bands in the clusters poorly, but for present purposes that was not a problem. Low 80 kDa staining in centrifugal pellets of adult Cecropia extracts (Figure 4A, lanes A1 and A2) showed that PBS-insoluble reserves, like the soluble reserves assayed by immunodiffusion, were essentially negligible after eclosion.
Monarch
Nearly all of the soluble ArH stored in Monarch pupae was consumed during metamorphosis, but substantial MtH reserves remained. This was first seen in individuals that had been collected in the field in October and then fed as captives for four weeks under short day conditions (Figure 6A). At the end of the four weeks, short-day females still contained about 20% as much M-MtH and 30% as much V-MtH as female pupae. When captive females were held for two weeks under short day conditions and then for two additional weeks in the long days that promote egg formation, these figures were much lower—1.5% for M-MtH (p < 0.01) and 0.7% for V-MtH (p < 0.02) (Figure 6A). These results were confirmed by SDS-PAGE, which detected a strong 80 kDa band in short day adults (Figure 4B, lanes Ast) and very little staining in long-day adults (lanes Alg). The time-course of the decrease was examined in a set of females that had been raised from eggs in the laboratory under long day conditions (Figure 7). In this case 32% of extractable pupal M-MtH and 47% of V-MtH remained at the time of eclosion. During one week of feeding as adults under continuing long day conditions three quarters of the reserves present at eclosion were consumed.
Figure 6.
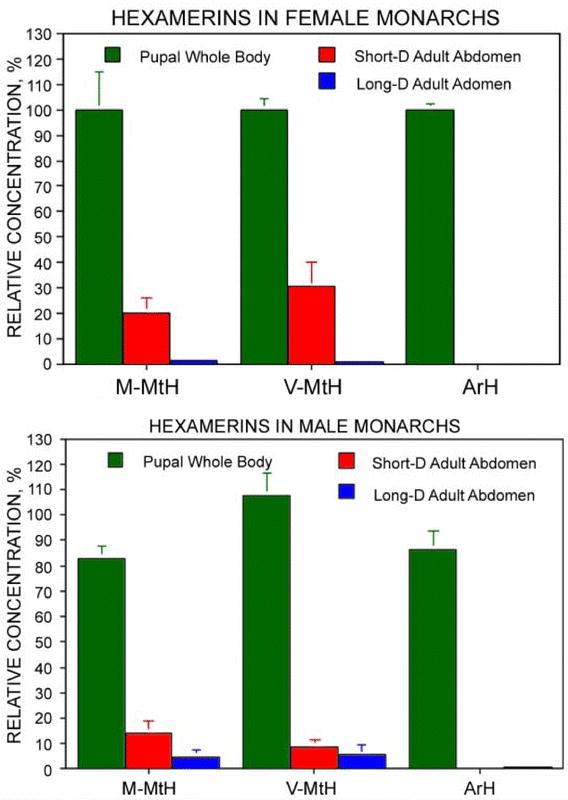
Changes in soluble hexamerin contents in Monarch females (A) and males (B). Short-D adults had been fed daily for four weeks under short day light conditions. Long-D adults had been fed daily for two weeks under short day conditions and for two additional weeks under long day conditions. Contents are expressed as percentages of those in female pupae. Error bars indicate standard errors; when not shown they were too small to be graphed. N = 7 for females and 5 for males. Differences between male and female pupae were not significant (p > 0.2 for all three proteins) as determined by a standard unpaired t-test.
Figure 7.
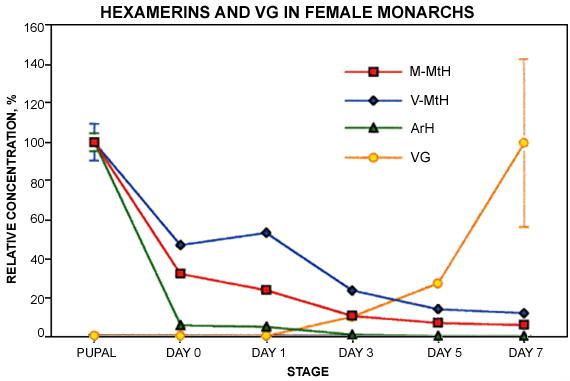
Time course of hexamerin depletion in adult Monarch females raised from eggs under long day conditions and kept under long day conditions for 7 days after eclosion. Vg results were taken from Fig. 3. N = 5
3. Sexual Differences.
While males contained roughly as much soluble ArH as females in Cecropia pupae, their contents of V-MtH and M-MtH were significantly lower (Figure 5B). A sexual difference was also seen during adult development: from days 13 to 21, when vitellogenesis and chorion deposition occur, utilization of V-MtH and M-MtH was more rapid in females than in males (Figure 5A and B). In males, as described above for females, immunodiffusion failed to detect more than 1–2% of the pupal levels of any of the three hexamerins at the time of eclosion (Figure 5B, Adult). In confirmation of this, SDS-PAGE revealed only traces of 80 kDa material in centrifugal pellets (Figure 4A, stages A1 and A2, lanes P).
Monarchs differed from the lepidopteran norm in showing no sexual differences in the amounts of soluble V- and M-MtH stored in pupae (Figure 6B, green bars). In four week old adults raised under short day conditions, however, females retained larger reserves than males (Figure 6A and B, red bars), but had consumed more of these two proteins under conditions permitting egg formation (Figure 6A and B. blue bars).
Discussion
1. Egg formation versus metamorphosis
The results are clear in showing that methionine-rich hexamerins in the absence of ArH support egg formation in Monarchs. Short-day adults, whose ovaries do not produce eggs, retained 20–30% of their pupal reserves of the MtH's, but only 0.3% of ArH (Figure 6A). Reduction of V-and M-MtH to only 1–2% of pupal levels in long-day females that had begun to lay eggs confirms that adult MtH's are in fact consumed during egg formation. The Monarch results also show that V- and M-MtH support somatic tissue development, as 50% of pupal V-MtH and 70% of M-MtH disappeared during adult development (Figure 7). The depletion of pupal V- and M-MtH in males (Figure 6B) also indicates functions other than the support of egg formation.
ArH, too, is multi-functional. It is known to support larval molting in the absence of the MtH's in Manduca sexta (Kramer et al., 1980; Riddiford and Hice, 1985; Webb and Riddiford, 1988) and Cecropia (Telfer et al., 1983). This situation differs in the dictyopteran, Blatta orientalis, whose two hexamerins are both present in larval hemolymph and cycle together during molting (Duhamel and Kunkel, 1978). ArH can also support reproductive functions, for it was as effective as M-MtH in providing precursors for the synthesis of Vg and chorions in pharate adult Luna moths (Pan and Telfer, 1996).
Selectivity in the consumption of the multiple hexamerins within a lepidopteran species is shown by these results to be related to availability: the MtH's are not used in the larval molts because they are not synthesized until late in the last instar (Tojo et al., 1978; Riddiford and Hice, 1985; Jones et al, 1993; Memmel et al, 1994); and pupal reserves of ArH do not support egg formation in adult Monarchs because they are fully consumed during metamorphosis. When both are at hand, however, both contribute to whatever metamorphic and reproductive functions are under way.
Two kinds of processes are apparent that affect availability. Most obvious is the early synthesis of ArH that makes it uniquely available for the support of larval molting. The second involves differences in mode of storage. In insects generally, storage hexamers are secreted into larval hemolymph, where they can attain extraordinary concentrations. When metamorphosis approaches they are reclaimed to varying degrees by the fat body, and packaged in cytoplasmic storage granules. In an earlier study over 99% of injected M-MtH was cleared from the hemolymph of pharate pupae, compared with only 35% of ArH (Pan and Telfer, 1992). A consequence of this in both Luna and Cecropia pupae is that the MtH's are the most prominent proteins of fat body extracts while ArH is the most prominent protein of hemolymph (Figure 2 in Pan and Telfer, 1996, and Figure 17 in Tojo et al, 1978). There can also be differences in the disposition of proteins within the fat body, for in Cecropia the hexamerin storage granules contain protein crystals embedded in an amorphous material (Tojo et al., 1978). Differences in accessibility provide a plausible speculation on how V- and M-MtH, but not ArH, might survive metamorphosis in sufficient amounts to support post-eclosion egg formation.
2. How hexamerins support egg formation
Hexamerins presumably support egg formation primarily by providing precursors for protein synthesis, for the synthesis of yolk proteins by fat body and of chorion proteins by the follicle cells both entail transcription and the incorporation of free amino acids (Wyatt, 1991; Paul and Kafatos, 1975; Nadel and Kafatos, 1980). Cases of intact hexamerin incorporation by ovaries have been described, but these are idiosyncratic rather than general. Antigenic epitopes of all four lepidopteran hexamerins are present among yolk proteins of saturniid moths, but they are only 0.2% as concentrated as those of Vg (Telfer and Pan, 1988). In Hyphantria cunea antigenic activity of an M-MtH-like protein was detected in intercellular spaces and yolk during vitellogenesis, but was absent from mature eggs (Seo et al., 1998). The authors speculated that this protein provides an internal source of amino acids for protein synthesis in the maturing egg. A more striking exception occurs in Riptortus clavatus, a hemipteran whose yolk contains approximately equal amounts of Vg and a 500 kDa, biliverdin-binding hexamerin (Chinzei et al., 1990; Miura et al., 1998). The yolk form of this otherwise conventional storage protein is secreted by the fat body of adult females in synchrony with Vg and selectively endocytosed by the vitellogenic oocyte. In another unusual case, the principal hemolymph storage proteins of last instar larvae and pupae of Bombyx mori are not hexamerins but a family of 30 kDa proteins that persist into the adult stage and are accumulated during vitellogenesis as major constituents of yolk (Zhu et al., 1986; Chen and Yamashita, 1990). But immunodiffusion and SDS-PAGE revealed nothing comparable to either the Riptortus or Bombyx storage proteins in egg-filled abdomens of long-day Monarchs.
3. Hexamerin synthesis in adults?
There are other cases of the resumption of hexamerin synthesis in eclosed adults (e.g., Kunkel and Pan, 1976; Wyatt et al., 1992), but these occur in males as well as females and are thus not necessarily related to egg formation. Post-eclosion synthesis is not ruled out as a source of V- and M-MtH in adult Monarchs, but if it occurs it is not sufficient to prevent the depletion that accompanies egg formation. It would not, in any case, affect the argument that the consumption of residual pupal V- and M-MtH, rather than ArH, is correlated with post-ecdysial egg formation.
4. V-MtH versus M-MtH
The two methionine-rich hexamerins are electrophoretically distinct and show no detectable similarities in precipitation by polyclonal rabbit antibodies. Yet they behaved here identically in the timing of their disappearance during adult development and in the fraction of their pupal reserves that survived metamorphosis. Cladistic analyses of amino acid sequences deduced from cDNA clones suggest that they are descended from products of a gene duplication occurring early in lepidopteran evolution (Burmester et al., 1998). But we are as yet left with no clue as to why natural selection has favored a pair of diverging MtH's over a single protein that might have averaged their amino acid compositions.
Glossary
| Abbreviation: | |
|---|---|
| V-MtH | Hexamerins high in methionine |
| M-Mth | Hexamerins with moderate levels of methionine |
| Vg | vitellogenin |
References
- Ackery P, Vane-Wright R. 1984 Milkweed Butterflies:their Cladistics and Biology. British Museum, London. [Google Scholar]
- Bean D, Silhacek D. Changes in titer of the female-predominant storage protein (81k) during larval and pupal development of the wax moth, Galleria mellonella. Arch Insect Biochem Physiol. 1988;10:333–348. [Google Scholar]
- Burmester T, Massey H, Zakharkin S, Benes H. The evolution of hexamerins and the phylogeny of insects. J Mol Evol. 1998;47:93–108. doi: 10.1007/pl00006366. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yamashita O. Nonselective uptake of different 30 kDa plasma proteins by developing ovaries of the silkworm, Bombyx mori. J Seri Sci Japan. 1990;59:202–209. [Google Scholar]
- Chinzei Y, Haruna T, Miura K, Numata H, Nakayama S. Purification and characterization of biliverdin-associated cyanoprotein from eggs and hemolymph of the bean bug, Riptortus clavatus (Heteroptera: Alydidae) Insect Biochem. 1990;20:545–555. [Google Scholar]
- Duhamel R, Kunkel J. A molting rhythm for serum proteins of the cockroach, Blatta orientalis. Comp Biochem Physiol. 1978;60B:333–337. doi: 10.1016/0305-0491(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. 1988 Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory: Chapter 5. [Google Scholar]
- Jones G, Brown N, Manczak M, Hiremath S, Kafatos F. Molecular cloning, regulation and complete sequence of a hemocyanin-related, juvenile hormone-suppressible protein from insect hemolymph. J Biol Chem. 1990;25:8596–8602. [PubMed] [Google Scholar]
- Jones G, Manczak M, Horn M. Hormonal regulation and properties of a new group of basic hemolymph proteins expressed during insect metamorphosis. J Biol Chem. 1993;268:12284–12291. [PubMed] [Google Scholar]
- Kanost M, Kawooya J, Law J, Ryan R, Van Heusden M, Ziegler R. Insect Hemolymph Proteins. Adv Insect Physiol. 1990;22:299–396. [Google Scholar]
- Kramer S, Mundall E, Law J. Purification and properties of manducin, an amino acid storage protein of the haemolymph of larval and pupal Manduca sexta. Insect Biochem. 1980;10:279–288. [Google Scholar]
- Kunkel J, Pan M. Selectivity of yolk protein uptake: Comparison of vitellogenins of two insects. J Insect Physiol. 1976;22:809–818. doi: 10.1016/0022-1910(76)90248-1. [DOI] [PubMed] [Google Scholar]
- Magee J, Kraynack N, Massey H, Telfer W. Properties and significance of a riboflavin-binding hexamerin in the hemolymph of Hyalophora cecropia. Arch Insect Biochem Physiol. 1994;25:137–157. doi: 10.1002/arch.940250206. [DOI] [PubMed] [Google Scholar]
- Massey H. 1995 The Evolution of Insect Storage Proteins: Driven by Composition and Constrained by Sequence. Ph.D dissertation, University of Pennsylvania Philadelphia, PA. [Google Scholar]
- Memmel N, Trewitt P, Grzelak K, Rajaratnam V, Kumaran A. Nucleotide sequence, structure and developmental regulation of LHP82, a juvenile hormone-suppressible hexamerin gene from the waxmoth, Galleria mellonella. Insect Biochem Mol Biol. 1994;24:133–144. doi: 10.1016/0965-1748(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Miller S, Silhacek D. Binding of riboflavin to lipophorin and a hexameric protein in the haemolymph of Heliothis virescens. Insect Biochem Mol Biol. 1992;23:571–583. [Google Scholar]
- Miura K, Shinoda T, Yura M, Nomura S, Kamiya K, Yuda M, Chinzei Y. Two hexameric cyanoprotein subunits from an insect, Riportus clavatus. Sequence, phylogeny and developmental and juvenile hormone regulation. Eur J Biochem. 1998;258:929–940. doi: 10.1046/j.1432-1327.1998.2580929.x. [DOI] [PubMed] [Google Scholar]
- Munn E, Greville G. The soluble proteins of developing Calliphora erythrocephala, particularly calliphorin, and similar proteins in other insects. J Insect Physiol. 1969;15:1935–1950. [Google Scholar]
- Nadel M, Kafatos F. Specific protein synthesis in cellular differentiation. IV. The chorion proteins of Bombyx mori and their program of synthesis. Dev Biol. 1980;75:26–40. doi: 10.1016/0012-1606(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Oudin J. L'analyse immunochimique qualitative: methods par diffusion des antigenes au sein de l'immunserum precipitant gelose. Ann Inst Pasteur. 1948;75:30–52. 109–130. [PubMed] [Google Scholar]
- Pan M. The synthesis of vitellogenin in the Cecropia silkworm. J Insect Physiol. 1971;17:677–689. [Google Scholar]
- Pan M, Telfer W. The use of rocket immunoelectrophoresis in the study of insect haemolymph proteins. Bull Inst Zool, Academia Sinica. 1990;15:109–124. [Google Scholar]
- Pan M, Telfer W. Selectivity in storage hexamerin clearing demonstrated with hemolymph transfusions between Hyalophora cecropia and Actias luna. Arch Insect Biochem Physiol. 1992;19:203–221. doi: 10.1002/arch.940190306. [DOI] [PubMed] [Google Scholar]
- Pan M, Telfer W. Methionine-rich hexamerin and arylphorin as precursor reservoirs for reproduction and metamorphosis in female Luna moths. Arch Insect Biochem Physiol. 1996;32:149–162. doi: 10.1002/(SICI)1520-6327(1996)33:2<149::AID-ARCH5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pan M, Telfer W. Equivalence of riboflavin-binding hexamerin and arylphorin as reserves for adult development in two saturniid moths. Arch Insect Biochem Physiol. 1999;42:138–146. doi: 10.1002/(SICI)1520-6327(199910)42:2<138::AID-ARCH4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Paul M, Kafatos F. Specific protein synthesis in cellular differentiation. II. The program of protein synthetic changes during chorion formation by silkmoth follicles, and its implementation in organ culture. Dev Biol. 1975;42:141–159. doi: 10.1016/0012-1606(75)90320-6. [DOI] [PubMed] [Google Scholar]
- Riddiford L, Hice R. Developmental profiles of the mRNAs for Manduca arylphorin and two other storage proteins during the final larval instar of Manduca sexta. Insect Biochem. 1985;15:489–502. [Google Scholar]
- Riddiford L, Law J. 1983 Larval serum proteins of Lepidoptera. The Larval Serum Proteins of Insects. K. Scheller. NY, Thieme-Stratton. 75–86. [Google Scholar]
- Ryan R, Keim P, Wells M, Law J. Purification and properties of a predominantly female-specific protein from the hemolymph of the larva of the tobacco hornworm, Manduca sexta. J Biol Chem. 1985;260:782–787. [PubMed] [Google Scholar]
- Seo S, Kang Y, Cheon H, Ki H. Distribution and accumulation of storage protein-1 in ovary of Hyphantria cunea Drury. Arch Insect Biochem Physiol. 1998;37:115–128. doi: 10.1002/(SICI)1520-6327(1998)37:2<115::AID-ARCH1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Telfer W. Immunological studies of insect metamorphosis. II. The role of a sex-limited blood protein in egg formation by the Cecropia silkworm. J Gen Physiol. 1954;37:539–558. doi: 10.1085/jgp.37.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer W, Keim P, Law J. Arylphorin, a new protein from Hyalaphora cecropia: Comparisons with calliphorin and manducin. Insect Biochem. 1983;13:601–613. [Google Scholar]
- Telfer W, Kunkle J. The function and evolution of insect storage hexamers. Ann Rev Entomol. 1991;36:205–228. doi: 10.1146/annurev.en.36.010191.001225. [DOI] [PubMed] [Google Scholar]
- Telfer W, Massey H. 1987 A storage hexamer from Hyalophora that binds riboflavin and resembles the apoprotein of hemocyanin. Molecular Entomology. J. Law. NY, Alan R Liss. 305–314. [Google Scholar]
- Telfer W, Pan M. Adsorptive endocytosis of vitellogenin, lipophorin, and microvitellogenin during yolk formation in Hyalophora. Arch Insect Biochem Physiol. 1988;9:339–355. [Google Scholar]
- Telfer W, Pan M, Law J. Lipophorin in developing adults of Hyalophora cecropia: support of yolk formation and preparation for flight. Insect Biochem. 1991;21:653–663. [Google Scholar]
- Tojo S, Betchaku T, Ziccardi V, Wyatt G. Fat body protein granules and storage proteins in the silkmoth, Hyalophora cecropia. J Cell Biol. 1978;78:823–838. doi: 10.1083/jcb.78.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo S, Nagata M, Kobayashi M. Storage proteins of the silkworm, Bombyx mori. Insect Biochem. 1980;10:289–303. [Google Scholar]
- Tojo S, Morita M, Agui N, Hiruma K. Hormonal regulation of phase polymorphism and storage protein fluctuation in the common cutworm, Spodoptera litura. J. Insect Physiol. 1985;31:283–292. [Google Scholar]
- Webb B, Riddiford L. Synthesis of two storage proteins during larval development of the tobacco hornworm, Manduca sexta. Dev Biol. 1988;130:671–681. doi: 10.1016/0012-1606(88)90359-4. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Buck N. A role for storage proteins in autogenous reproduction in Aedes atropalpus. J Insect Physiol. 1996;42:961–966. [Google Scholar]
- Wheeler D, Liebig J, Holldobler B. Atypical vitellogenins in ponerine ants (Hymenoptera: Formicidae) J Insect Physiol. 1999;45:287–293. doi: 10.1016/s0022-1910(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Wyatt G. Gene regulation in insect reproduction. Invert Reprod Dev. 1991;20:1–35. [Google Scholar]
- Wyatt G, Kanost M, Chin B, Cook K, Kawasoe B, Zhang J. Juvenile hormone analog and injection effects on locust hemolymph protein synthesis. Arch Insect Biochem Physiol. 1992;20:167–180. [Google Scholar]
- Wyatt G, Pan M. Insect plasma proteins. Ann Rev Biochem. 1978;47:779–817. doi: 10.1146/annurev.bi.47.070178.004023. [DOI] [PubMed] [Google Scholar]
- Zhu J, Indrasith L, Yamashita O. Characterization of vitellin, egg-specific protein, and 30 kDa protein from Bombyx eggs, and their fates during oogenesis and embryogenesis. Biochim Biophys Acta. 1986;882:427–436. [Google Scholar]


