Abstract
Multidrug resistance–associated protein 3 (Mrp3; Abcc3) expression and activity are up-regulated in rat liver after in vivo repeated administration of ethynylestradiol (EE), a cholestatic synthetic estrogen, whereas multidrug resistance-associated protein 2 (Mrp2) is down-regulated. This study was undertaken to determine whether Mrp3 induction results from a direct effect of EE, independent of accumulation of any endogenous common Mrp2/Mrp3 substrates resulting from cholestasis and the potential mediation of estrogen receptor (ER). In in vivo studies, male rats were given a single, noncholestatic dose of EE (5 mg/kg s.c.), and basal bile flow and the biliary excretion rate of bile salts and glutathione were measured 5 hours later. This treatment increased Mrp3 mRNA by 4-fold, detected by real-time polymerase chain reaction, despite the absence of cholestasis. Primary culture of rat hepatocytes incubated with EE (1–10 µM) for 5 hours exhibited a 3-fold increase in Mrp3 mRNA (10 µM), consistent with in vivo findings. The increase in Mrp3 mRNA by EE was prevented by actinomycin D, indicating transcriptional regulation. When hepatocytes were incubated with an ER antagonist [7α,17β-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI182/780), 1 µM], in addition to EE, induction of Mrp3 mRNA was abolished, implicating ER as a key mediator. EE induced an increase in ER-α phosphorylation at 30 minutes and expression of c-Jun, a well-known ER target gene, at 60 minutes, as detected by Western blotting of nuclear extracts. These increases were prevented by ICI182/780. In summary, EE increased the expression of hepatic Mrp3 transcriptionally and independently of any cholestatic manifestation and required participation of an ER, most likely ER-α, through its phosphorylation.
Introduction
Multidrug resistance–associated protein 3 (Mrp3; ABCC3), is expressed in different cell types, including hepatocytes, where it is localized to the basolateral membrane (König et al., 1999a). Mrp3 extrudes anionic substrates, such as sulfated bile salts, bilirubin glucuronides, 17β-glucuronosyl estradiol, and some anticancer drugs (König et al., 1999b; Kool et al., 1999; Hirohashi et al., 2000). In normal conditions, these substrates are preferentially excreted across the apical domain into the canaliculus via multidrug resistance-associated protein 2 (Mrp2; ABCC2). The extent of constitutive Mrp3 expression is normally low in rats but is markedly up-regulated in Mrp2-deficient rats (Johnson et al., 2006) and in rats with obstructive cholestasis (Donner and Keppler 2001; Soroka et al., 2001). Human Mrp3 was also found to be inducible, as shown in patients with Dubin-Johnson syndrome (König et al., 1999b). As a result, this transporter has been postulated to protect the liver by extruding bilirubin and bile salt conjugates from the hepatocytes when bile secretion is impaired.
Estrogens are involved in the pathogenesis of both oral contraceptive–induced cholestasis and cholestasis of pregnancy (Vore 1987; Reyes and Simon 1993). Ethynylestradiol (EE), a synthetic estrogen, is known to reduce bile flow after chronic treatment in experimental animals. In the rat, decreased bile flow after EE administration (5 mg/kg body weight for 5 consecutive days) has been associated with decreased expression and activity of Mrp2 (Trauner et al., 1997; Lee et al., 2000). We demonstrated that Mrp3 is up-regulated in liver from EE-induced cholestatic rats (Ruiz et al., 2006, 2007), but the mechanisms involved remain uncertain. In other models of cholestasis, such as in bile duct ligated or lipopolysaccharide-treated rats, accumulation of common endogenous Mrp2/Mrp3 substrates or increased production of cytokines, such as tumor necrosis factor-α or interleukin-6, have been proposed to be responsible for Mrp3 up-regulation (Ogawa et al., 2000; Bohan et al., 2003; Vee et al., 2009). We previously reported that spironolactone counteracted the decrease in bile flow and biliary excretion of the Mrp2 substrates glutathione and acetaminophen-glucuronide in EE-treated cholestatic rats through up-regulation of Mrp2 (Ruiz et al., 2007). Rats treated simultaneously with spironolactone and EE exhibit up-regulation of both Mrp2 and Mrp3 (Ruiz et al., 2007) and are not cholestatic, implying that accumulation of endogenous Mrp2/Mrp3 substrates is unlikely to be responsible for Mrp3 up-regulation. We hypothesized that a more direct action of EE on Mrp3 gene regulation is involved.
The physiologic actions of estrogens are mediated by estrogen receptors (ER)–α and β, belonging to the superfamily of steroids/nuclear receptors (Kuiper et al., 1996). Various aspects of ER-α transcriptional activation are dependent on phosphorylation of the receptor. Coactivator recruitment, subcellular localization, receptor dimerization, ligand binding, and posttranslational modifications are regulated through the phosphorylation of individual sites of ER-α (Williams et al., 2009). Mutations of Ser118 to alanine caused, in a number of cell types, a significant reduction in transcriptional activation by ER from reporter gene containing estrogen response elements, with no effect on DNA binding properties or nuclear localization of ER (Ali et al., 1993). The liver expresses predominantly ER-α (Alvaro et al., 2000), and its expression is under multi-hormonal regulation. By using knockout mice, Yamamoto et al. (2006) demonstrated that ER-α mediates the repression of hepatic transporters and alterations of bile acid biosynthesis, contributing to development of EE-induced cholestasis/hepatotoxicity. It is not known, however, whether ER activation is responsible for Mrp3 up-regulation.
In the present study, we investigated whether a direct action of EE, requiring participation of an ER, is involved in up-regulation of Mrp3 in the rat. The data indicate that ER-α likely mediates transcriptional Mrp3 up-regulation in response to EE treatment and that this can occur in the absence of cholestasis.
Materials and Methods
Chemicals.
EE, leupeptin, phenylmethylsulfonyl fluoride, glutathione, glutathione reductase, 3α-hydroxysteroid dehydrogenase, β-nicotinamide adenine dinucleotide, and NADPH were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical grade purity and used as supplied.
Animals.
Adult male Wistar rats weighing 300–350 g were used. They had free access to food and water and were maintained on an automatically timed 12-hour light/12-hour dark cycle. All procedures involving animals were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
In Vivo Experiments: Administration of a Single Dose of EE and Assessment of Biliary Secretory Function.
Rats were randomly divided in two experimental groups (n = 5). EE-treated rats were administered EE at a single dose of 5 mg/kg body weitht subcutaneously, equivalent to the first dose of the usual cholestatic protocol (Trauner et al., 1997). EE was dissolved in propylene glycol as a vehicle at a final concentration of 33.7 mM. Control rats were injected with propylene glycol (0.5 ml/kg body weight subcutaneously). Five hours later, bile was collected for determination of bile flow and biliary excretion of bile salts and glutathione as described (Ruiz et al., 2005). At the end of the collection period, animals were sacrificed by exsanguination, the livers removed, washed with cold saline, and snap frozen in liquid nitrogen and preserved at −70°C until use for total RNA isolation.
In Vitro Experiments: Isolation, Culture, and Treatment of Rat Hepatocytes.
Hepatocytes were isolated from normal livers of male Wistar rats by collagenase perfusion and mechanical disruption (Seglen, 1973). Cell viability, assessed by trypan blue exclusion, was greater than 86%. Freshly isolated hepatocytes were plated onto collagen-coated glass plates at 3.8 × 104 cells/cm2, containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 µg/ml) (Invitrogen, Carlsbad, CA). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 for 3 hours, allowing cell attachment. Media then were changed to new media containing DMEM phenol red-free supplemented with 10% charcoal-dextran treated FBS (Hyclone Laboratories, Logan, UT), penicillin (100 U/ml), and streptomycin (100 µg/ml) (Invitrogen), and hepatocytes were incubated for 5 hours in the presence of EE (1, 10, or 100 µM) or its vehicle (DMSO 0.08% v/v). In some incubations, cells were pretreated for 30 minutes with 5 µg/ml actinomycin D (Fluka, St. Louis, MO). Alternatively, cells were pretreated for 30 minutes with the ER antagonist 7α,17β-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI182/780, 1 µM) (Tocris Cookson Inc., Ellisville, MO) before the addition of EE. The inhibitors were present during the treatment with EE. At the end of the incubation period, total RNA was prepared for real-time polymerase chain reaction (PCR) analysis. In a different set of incubations, cells were incubated with ICI182/780 (1 µM) for 30 minutes and then with EE (10 µM) for 30, 60, or 120 minutes. At the end of incubation periods, cells were harvested to prepare nuclear extracts.
None of the treatments affected cell viability (data not shown), as determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltretazoliumbromide assay (Rigalli et al., 2011), when EE was added to incubations at a 1 or 10 μM concentration. Addition of EE at a 100 μM concentration induced a significant decrease in cell viability, and thus, this concentration was not further used in subsequent experiments.
Quantitative Real-Time PCR and Western Blot Studies.
Total RNA was isolated from liver samples or cultured hepatocytes with use of TRIzol (Invitrogen), according to the manufacturer’s protocol. Real-time PCR was performed on a Mx3000P System (Stratagene, La Jolla, CA) with use of the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen), as described previously (Rigalli et al., 2011). Sequences of primer pairs for rat Mrp3, Mrp2, and 18S and reaction conditions were as previously described (Ruiz et al., 2007).
Phosphorylation of ER-α was studied by Western blotting of nuclear extracts. In brief, cultured hepatocytes were washed with ice-cold PBS and scrapped with sucrose 0.3 M and protease inhibitors (35 nM leupeptin and 0.1 µM phenylmethylsulfonyl fluoride) and phosphatase inhibitor cocktail (1/100 v/v; Sigma-Aldrich). After sonicating the samples, they were centrifuged at 1000g for 10 minutes at 4°C.The pellets then were washed twice with 0.3 M sucrose and protease and phosphatase inhibitors and centrifuged at 1000g for 10 minutes. The nuclei-enriched pellets were lysed with radioimmunoprecipitation assay buffer for 1 hour at 4°C and centrifuged for 15 minutes at 8000g. Supernatants were subjected to protein concentration determination (Sedmak and Grossberg, 1977) and 12% SDS-polyacrylamide gel electrophoresis. Western blot analysis was performed as described elsewhere (Ruiz et al., 2005), using anti-phospho-ER-α (Ser118), anti-ER-α, and anti-histone (SC-12915, H-184 and AE-4, respectively; Santa Cruz Biotechnology Inc., Santa Cruz, CA). The effect of EE on expression of c-Jun, a known ER target gene (Hyder et al., 1995), was also examined. Nuclear extracts were obtained as described above, and c-Jun expression was evaluated by Western blotting using anti-c-Jun (SC-1694; Santa Cruz Biotechnology Inc.), as described elsewhere (Ruiz et al., 2005).
Statistical Analysis.
Results are expressed as mean ± S.D. Statistical analysis was performed using Student’s t test or one-way analysis of variance, followed by Newman Keuls test. Values of P < 0.05 were considered to be statistically significant.
Results
Mrp3 Up-Regulation by EE Is Independent of Cholestasis and Mediated by ER.
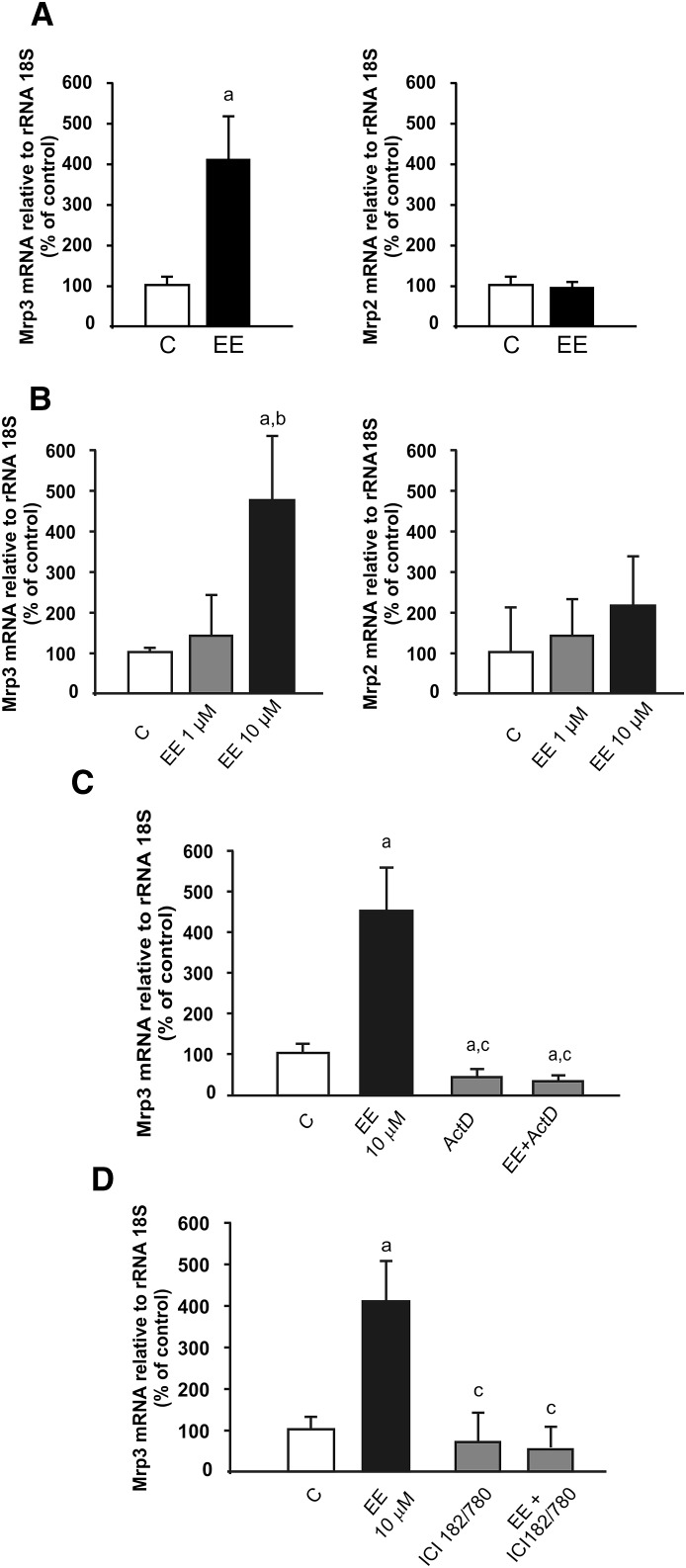
To determine whether the effect of EE is independent of cholestasis and the consequent accumulation of endogenous Mrp2/Mrp3 substrates, two different experiments were performed. As a first approach, basal bile flow, biliary excretion of total bile salts and glutathione, and serum alkaline phosphatase were measured 5 hours after a single dose of EE. These cholestatic markers remained unchanged when compared with vehicle-treated controls (Table 1), indicating that cholestasis was not yet established. Under this condition, we observed that the levels of hepatic Mrp3 mRNA were increased by 330% (Fig. 1A), to a similar extent as previously reported after repeated administration with EE (Ruiz et al., 2007). As a second approach, primary culture of rat hepatocytes was used as an experimental model. We found that 10 µM EE produced an increase in Mrp3 mRNA (350%) (Fig. 1B) similar to that found in vivo, whereas 1 µM EE induced no changes. As a negative control, we evaluated Mrp2 mRNA levels and found no changes at either 1 or 10 µM EE (Fig. 1B).
TABLE 1.
Effect of EE on markers of cholestasis
Rats were injected with a single dose of EE (5 mg/kg s.c. body weight) or vehicle (control), and bile and serum samples were collected 5 hours later. Data represent mean ± S.D. (n=5).
| Marker | Control | EE |
|---|---|---|
| Bile flow (μl/min g liver) | 1.40 ± 0.14 | 1.35 ± 0.19 |
| Biliary excretion of total bile salts (nmol/min g liver) | 50 ± 6 | 47 ± 5 |
| Biliary excretion of total glutathione (nmol/min g liver) | 2.00 ± 0.79 | 1.80 ± 0.72 |
| Serum ALP activity (U/l) | 256 ± 10 | 269 ± 12 |
Fig. 1.
Effect of EE on Mrp3 mRNA expression. (A) In vivo studies: analysis of RNA obtained from liver of rats 5 hours after administration of a single 5 mg/kg body weight dose of EE. (B) In vitro studies: analysis of RNA obtained from primary culture of hepatocytes incubated with EE (1 or 10 µM) for 5 hours. Mrp3 and Mrp2 mRNA levels were detected by real-time PCR. (C) Analysis of RNA obtained from cultured hepatocytes incubated with actinomycin D (ActD) for 30 minutes and with EE (10 µM) for 5 hours. (D) Analysis of RNA obtained from cultured hepatocytes incubated with ICI182/780 for 30 minutes and with EE (10 µM) for 5 hours. Mrp3 mRNA levels were detected by real-time PCR. Data are presented as a percentage of control (C; 100%) and were expressed as means ± S.D. (n = 5). Statistical analysis was performed using Student’s t test (A) or one-way analysis of variance, followed by Newman Keuls test (B and C). Values of P < 0.05 were considered to be statistically significant. 18S rRNA was used as internal control. a: significantly different from (C), P < 0.05. b:significantly different from EE 1 µM, P < 0.05. c: significantly different from EE 10 µM, P < 0.05.
To study the mechanism involved in the induction of Mrp3 mRNA levels, we used the RNA polymerase II inhibitor actinomycin D. Fig. 1C shows that the action of EE on Mrp3 expression in primary culture of hepatocytes was prevented by actinomycin D, providing support for the transcriptional effect of the estrogen.
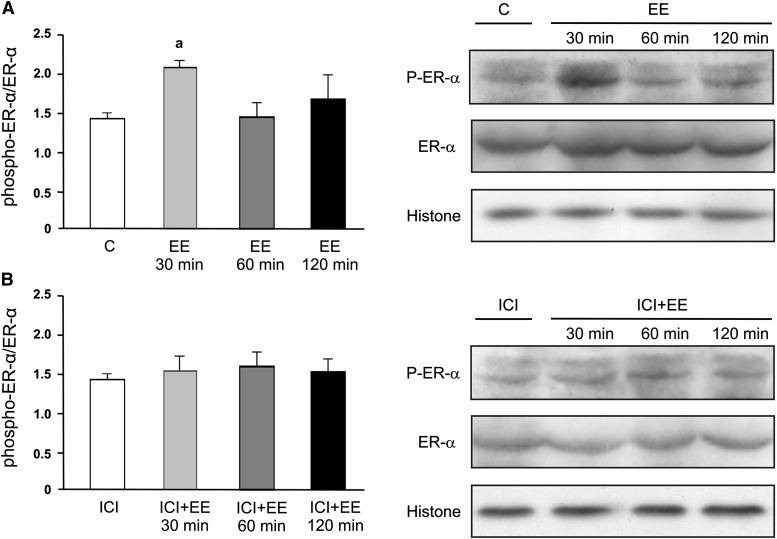
To evaluate whether ER mediates the action of EE, we measured Mrp3 mRNA expression in the presence or absence of ICI 182/780 and found that Mrp3 mRNA up-regulation was abolished in the presence of the ER antagonist (Fig. 1D). Further analysis of ER-α phosphorylation by Western blotting of nuclear extracts at 30, 60, or 120 minutes after addition of EE to the culture medium showed increased detection of phospho-ER-α at 30 minutes but not at 60 or 120 minutes (Fig. 2A). Increased phosphorylation of ER-α by EE was absent when culture cells were preincubated with ICI182/780 (Fig. 2B). The two bands for phospho-ER-α likely represent degrees of phosphorylation and/or alternative sites of phosphorylation different from Ser118 and were quantified together. The total levels of nuclear ER remained unchanged among groups.
Fig. 2.
Effect of EE on nuclear content of phospho-ER-α (Ser118) in cultured hepatocytes. (A) Hepatocytes were incubated with EE (10 µM; 30, 60, or 120 minutes) or vehicle (C, control). (B) Hepatocytes were preincubated with ICI182/780 (1 µM; 30 minutes) and with EE (10 µM; 30, 60, or 120 minutes). Equal amounts of nuclear extract protein (15 µg) were loaded in all lanes. Uniformity of protein loading and transfer from gel to nitrocellulose membrane were controlled with Ponceau S and histone detection. Densitometric analyses were performed separately for phospho-ER-α and for total ER-α, and the phospho-ER-α/ER-α densitometry ratio was calculated and expressed as means ± S.D. (n = 4). Statistical analysis was performed using one-way analysis of variance, followed by Newman Keuls test. Values of P < 0.05 were considered to be statistically significant. a: significantly different from C, 60 minutes and 120 minutes, P < 0.05.
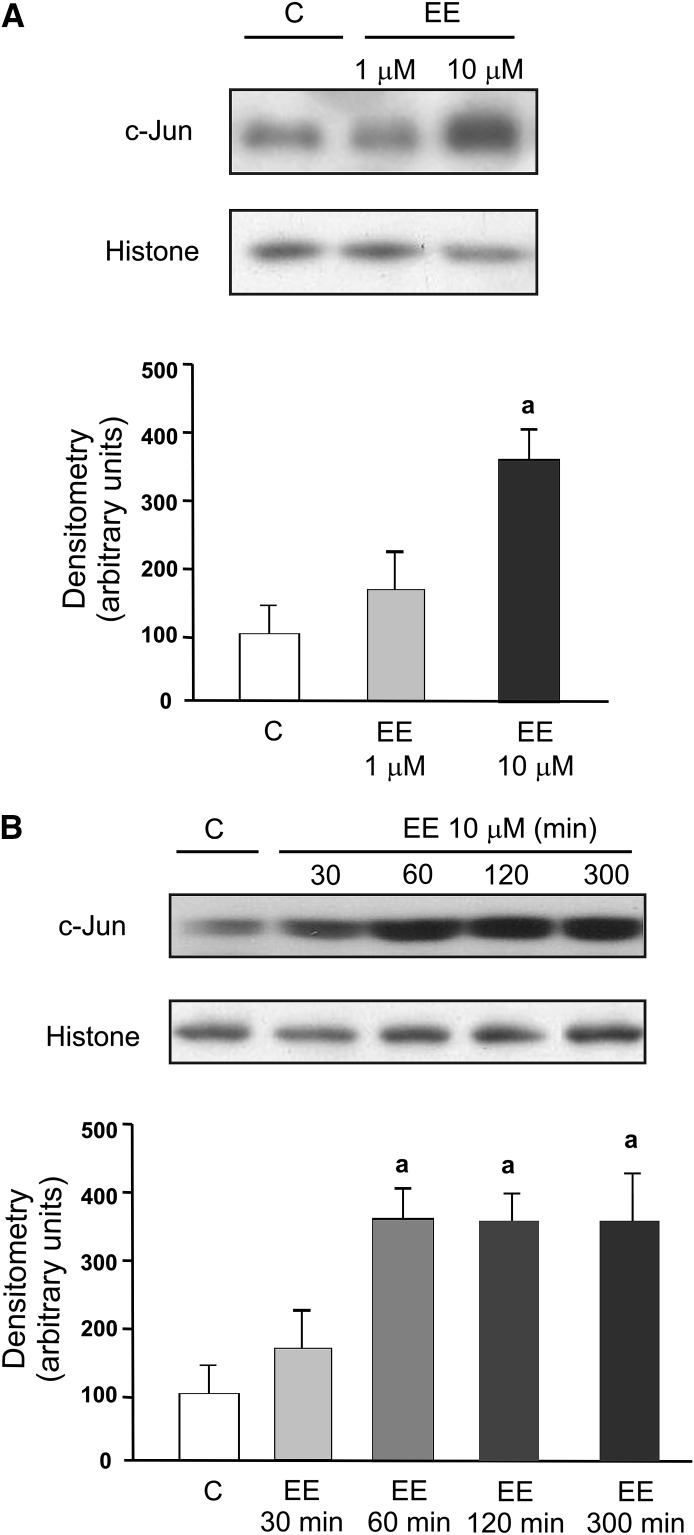
To further implicate ER in up-regulation of Mrp3 mRNA expression observed under current experimental conditions, we evaluated the effect of EE on the expression of c-Jun, a well-known ER target gene (Hyder et al., 1995). We found an increase in c-Jun protein expression at the same EE concentration able to increase Mrp3 mRNA levels (Fig. 3A). This effect was evident as early as 60 minutes after addition of EE to the culture medium (Fig. 3B). Induction of c-Jun expression was also prevented by ICI182/780 (data not shown).
Fig. 3.
Concentration- and time-dependent effect of EE on expression of c-Jun in cultured hepatocytes. (A) Hepatocytes were incubated with EE (1 or 10 µM for 5 hours) or vehicle (C, control). a: significantly different from C and 1 µM P < 0.05. (B) Hepatocytes were incubated with EE (10 µM for 30, 60, 120, or 300 minutes) or vehicle (C). a: significantly different from C and 30 minutes, P < 0.05. Equal amounts of nuclear extract protein (15 µg) were loaded in all lanes. Uniformity of protein loading and transfer from gel to nitrocellulose membrane were controlled with Ponceau S and histone detection. Data were expressed as means ± S.D. (n = 4). Statistical analysis was performed using one-way analysis of variance, followed by Newman Keuls test. Values of P < 0.05 were considered to be statistically significant.
Discussion
Various studies have shown that EE decreases Mrp2 and increases Mrp3 expression in rat liver (Trauner et al., 1997; Lee et al., 2000; Kamisako and Ogawa, 2005; or Ruiz et al., 2006). Under cholestatic conditions, down-regulation of Mrp2 leads to intrahepatic accumulation of its substrates, such as bilirubin conjugates and conjugated bile salts. These substrates, common to other Mrps, are postulated to act as inducers of Mrp3 (Ogawa et al., 2000). However, the present data, based on results after administration of a single, noncholestatic dose of EE to rats, showed an increase in Mrp3 mRNA expression within 5 hours, suggesting a more direct regulation of Mrp3 gene. This hypothesis is further supported by a similar induction of Mrp3 mRNA in primary cultures of rat hepatocytes, where conjugated bilirubin, a potential inducer of Mrp3, is absent. We cannot rule out the possibility that additional endogenous compounds (e.g., newly synthesized bile salts) participate in the regulation of Mrp3 mRNA. However, we found, in this same model, that an ER inhibitor prevented Mrp3 mRNA up-regulation, further supporting a causal EE-ER interaction. This induction was observed at a 10 µM concentration of EE. The volume of distribution for EE in rats was estimated to be 2 l/kg (Zamek-Gliszczynski et al., 2011), and in consequence, a concentration of EE of 10 µM would be compatible with administration of a 5–6 mg/kg birth weight dose of EE, which is equivalent to the single dose currently administered in vivo. ER-α was also implicated in repression of hepatic transporters and alteration of bile acid biosynthesis using a dose of EE that is even higher than the dose commonly used in rats (Yamamoto et al., 2006). Although the observed increase in mRNA levels after 5 hours of treatment in vivo and in hepatocytes might be caused by ER as a common mediator, further studies would be necessary to demonstrate that the increased Mrp3 mRNA levels occurring in the isolated hepatocytes model results in induction of Mrp3 protein, as previously seen after repeated treatment in vivo (Ruiz et al., 2007).
The data from actinomycin D experiments indicate that Mrp3 was transcriptionally up-regulated by EE. Similar experiments in the presence of the ER antagonist ICI182/780 unambiguously demonstrate the participation of an ER in Mrp3 up-regulation. ER-α is the predominant ER subtype present in liver (Alvaro et al., 2000), and its activation by ligand can regulate genes by either a transcriptional or a posttranscriptional manner (i.e., mRNA stabilization) (Ing, 2005). In addition, it is well demonstrated that ER-α becomes predominantly phosphorylated on Ser118 in response to treatment with estradiol, which further results in increased association with known ER-α coactivators (Lannigan, 2003). Our data demonstrate increased phosphorylation of ER-α by EE, as detected in the nucleus of cultured hepatocytes. Of interest, preincubation of the cells with an ER antagonist, shown to prevent induction of Mrp3 (Fig. 1), was also able to prevent ER-α phosphorylation (Fig. 2) and induction of the expression of c-Jun, a well-known ER target gene. Taken together, these findings suggest that EE induces ER-α phosphorylation, followed by transcriptional regulation of Mrp3.
ER regulates transcription processes through its binding to the ER element in the promoter region (classic way). Alternatively, many estrogen-responsive genes are regulated by sequences that are not bound directly by ER, such as activating protein-1 (AP-1) and specificity protein–1 binding sites (Marino et al., 2006). To gain insight into the mechanisms mediating Mrp3 induction by EE, we performed an in silico analysis using the TFSEARCH database (http://www.cbrc.jp/research/db/TFSEARCH.html). We could not identify any ER element in the Mrp3 promoter sequence. However, the analysis located several binding sites for AP-1 and for specificity protein–1 for Mrp3 gene, suggesting an indirect action of the ER-α involving these transcription factors. Of interest, EE induced an increase in the expression of c-Jun, a major component of AP-1, at the same concentration that could induce Mrp3 mRNA. Further studies are necessary to implicate c-Jun or an alternative transcription factor in Mrp3 induction by EE downstream ER activation.
Altered disposition of compounds that are common substrates for Mrp2 and Mrp3 could result from differential modulation of both transporters. In this regard, the preferential biliary versus basolateral disposition of acetaminophen-glucuronide observed in normal rats is reversed in rats treated with EE because of simultaneous up-regulation of Mrp3 and down-regulation of Mrp2 (Ruiz et al., 2007). In addition, Mrp3 exhibits a higher affinity for acetaminophen-glucuronide than does Mrp2 (Xiong et al., 2000; Manautou et al., 2005). Our current data clearly demonstrate that EE can induce Mrp3 in the absence of any cholestatic manifestations. It is not known whether human hepatic Mrp3 is induced by EE at the pharmacological doses used in oral contraceptive treatment. However, in earlier studies, women taking oral contraceptive steroids demonstrated a greater plasma clearance of acetaminophen together with increased elimination of acetaminophen-glucuronide in urine (Miners et al., 1983). The induction of Mrp3 might lead to increased basolateral rather than canalicular excretion of acetaminophen glucuronide, thus leading to increased urinary excretion of this metabolite. In conclusion, the present data demonstrate, for the first time to our knowledge, that induction of Mrp3 by EE in rat liver is independent of cholestasis and occurs via activation of ER.
Acknowledgments
The authors thank Dr. J. Elena Ochoa for excellent technical assistance in hepatocytes isolation.
Abbreviations
- AP-1
activating protein-1
- EE
ethynylestradiol
- ER
estrogen receptor; ICI182/780, 7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
- Mrp3
multidrug resistance–associated protein 3
- Mrp2
multidrug resistance–associated protein 2
Authorship Contributions
Participated in research design: Ruiz, Mottino, Catania.
Conducted experiments: Ruiz, Rigalli, Arias, Villanueva.
Contributed analytic tools: Banchio, Vore.
Performed data analysis: Ruiz, Rigalli, Mottino, Catania.
Wrote or contributed to the writing of the manuscript: Ruiz, Rigalli, Vore, Mottino, Catania.
Footnotes
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica [PICT 2007 04-1637 and PICT 2010-1072]; Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 112-2008-01-00029/00691]; Universidad Nacional de Rosario [BIO 214], and Fundación Alberto J. Roemmers, Argentina; and by the National Institutes of Health The Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD58299].
This work was previously presented as a poster presentation at the following workshop: Ruiz ML, Rigalli JP, Arias A, Villanueva SS, Banchio C, Vore M, Mottino AD, and Catania VA (2012) Molecular basis for hepatic multidrug resistance-associated protein 3 induction by ethynylestradiol. 47th Annual Meeting of the European Association for the Study of the Liver: International Liver Congress; 2012 April 18–22; Barcelona, Spain.
References
- Ali S, Metzger D, Bornert JM, Chambon P. (1993) Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J 12:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F, Gaudio E. (2000) Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 119:1681–1691 [DOI] [PubMed] [Google Scholar]
- Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. (2003) Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem 278:36688–36698 [DOI] [PubMed] [Google Scholar]
- Donner MG, Keppler D. (2001) Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology 34:351–359 [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. (2000) ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem 275:2905–2910 [DOI] [PubMed] [Google Scholar]
- Hyder SM, Nawaz Z, Chiappetta C, Yokoyama K, Stancel GM. (1995) The protooncogene c-jun contains an unusual estrogen-inducible enhancer within the coding sequence. J Biol Chem 270:8506–8513 [DOI] [PubMed] [Google Scholar]
- Ing NH. (2005) Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod 72:1290–1296 [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KL. (2006) Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab Dispos 34:556–562 [DOI] [PubMed] [Google Scholar]
- Kamisako T, Ogawa H. (2005) Alteration of the expression of adenosine triphosphate-binding cassette transporters associated with bile acid and cholesterol transport in the rat liver and intestine during cholestasis. J Gastroenterol Hepatol 20:1429–1434 [DOI] [PubMed] [Google Scholar]
- König J, Rost D, Cui Y, Keppler D. (1999 a) Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 29:1156–1163 [DOI] [PubMed] [Google Scholar]
- König J, Nies AT, Cui Y, Leier I, Keppler D. (1999b) Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta 1461:377–394 [DOI] [PubMed] [Google Scholar]
- Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, Elferink RP, Baas F, Borst P. (1999) MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA 96:6914–6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannigan DA. (2003) Estrogen receptor phosphorylation. Steroids 68:1–9 [DOI] [PubMed] [Google Scholar]
- Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. (2000) Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology 118:163–172 [DOI] [PubMed] [Google Scholar]
- Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, Elferink RO. (2005) Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology 42:1091–1098 [DOI] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P. (2006) Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JO, Attwood J, Birkett DJ. (1983) Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol 16:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. (2000) Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol 278:G438–G446 [DOI] [PubMed] [Google Scholar]
- Reyes H, Simon FR. (1993) Intrahepatic cholestasis of pregnancy: an estrogen-related disease. Semin Liver Dis 13:289–301 [DOI] [PubMed] [Google Scholar]
- Rigalli JP, Ruiz ML, Perdomo VG, Villanueva SS, Mottino AD, Catania VA. (2011) Pregnane X receptor mediates the induction of P-glycoprotein by spironolactone in HepG2 cells. Toxicology 285:18–24 [DOI] [PubMed] [Google Scholar]
- Ruiz ML, Villanueva SS, Luquita MG, Sánchez-Pozzi EJ, Crocenzi FA, Pellegrino JM, Ochoa JE, Vore M, Mottino AD, Catania VA. (2005) Mechanisms involved in spironolactone-induced choleresis in the rat. Role of multidrug resistance-associated protein 2. Biochem Pharmacol 69:531–539 [DOI] [PubMed] [Google Scholar]
- Ruiz ML, Villanueva SSM, Luquita MG, Vore M, Mottino AD, Catania VA. (2006) Ethynylestradiol increases expression and activity of rat liver MRP3. Drug Metab Dispos 34:1030–1034 [DOI] [PubMed] [Google Scholar]
- Ruiz ML, Villanueva SS, Luquita MG, Ikushiro S, Mottino AD, Catania VA. (2007) Beneficial effect of spironolactone administration on ethynylestradiol-induced cholestasis in the rat: involvement of up-regulation of multidrug resistance-associated protein 2. Drug Metab Dispos 35:2060–2066 [DOI] [PubMed] [Google Scholar]
- Sedmak JJ, Grossberg SE. (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79:544–552 [DOI] [PubMed] [Google Scholar]
- Seglen PO. (1973) Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res 82:391–398 [DOI] [PubMed] [Google Scholar]
- Soroka CJ, Lee JM, Azzaroli F, Boyer JL. (2001) Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33:783–791 [DOI] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. (1997) The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology 113:255–264 [DOI] [PubMed] [Google Scholar]
- Vee ML, Lecureur V, Stieger B, Fardel O. (2009) Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab Dispos 37:685–693 [DOI] [PubMed] [Google Scholar]
- Vore M. (1987) Estrogen cholestasis. Membranes, metabolites, or receptors? Gastroenterology 93:643–649 [PubMed] [Google Scholar]
- Williams CC, Basu A, El-Gharbawy A, Carrier LM, Smith CL, Rowan BG. (2009) Identification of four novel phosphorylation sites in estrogen receptor alpha: impact on receptor-dependent gene expression and phosphorylation by protein kinase CK2. BMC Biochem 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Turner KC, Ward ES, Jansen PL, Brouwer KL. (2000) Altered hepatobiliary disposition of acetaminophen glucuronide in isolated perfused livers from multidrug resistance-associated protein 2-deficient TR(-) rats. J Pharmacol Exp Ther 295:512–518 [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess HA, Guo GL, González FJ, Korach KS, Maronpot RR, Negishi M. (2006) Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem 281:16625–16631 [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Day JS, Hillgren KM, Phillips DL. (2011) Efflux transport is an important determinant of ethinylestradiol glucuronide and ethinylestradiol sulfate pharmacokinetics. Drug Metab Dispos 39:1794–1800 [DOI] [PubMed] [Google Scholar]