Abstract
Pregnancy increases the urinary excretion of chemicals in women and rodents. It is unknown whether the enhanced clearance of drugs during pregnancy involves changes in the expression of transporters that mediate chemical secretion and reabsorption. The purpose of this study was to quantify the mRNA and protein expression of efflux transporters in kidneys from virgin and pregnant mice on gestational days 7, 11, 14, and 17 and postnatal days 1, 15, and 30 with use of quantitative polymerase chain reaction, Western blot, and immunofluorescence. Multidrug resistance protein (Mdr) 1b mRNA, multidrug resistance-associated protein (Mrp) 4 mRNA, and protein levels decreased significantly by 25–75% throughout pregnancy and lactation. Similarly, Mrp2 and multidrug and toxin extrusion transporter (Mate) 1 mRNA expression were down-regulated 20–40% during mid to late gestation but returned to control levels by postnatal day 15. In contrast, Mrp3 mRNA and protein increased 225% and 31%, respectively, at gestational day 14. Coordinated down-regulation of brush border transporters Mate1, Mrp2, and Mrp4 and up-regulation of the basolateral Mrp3 transporter would reduce chemical secretion into urine.
Introduction
Pregnancy-induced hormonal and hemodynamic changes significantly alter renal function. In humans and rodents, increases in cardiac output and renal vasodilation enlarge the size of the kidneys, elevate renal plasma flow by 50–85%, and increase the rate of glomerular filtration by 40–65% (Chapman et al., 1998; Conrad, 2004; Maynard and Thadhani, 2009; Cornelis et al., 2011). Clinically, pregnant women exhibit proteinuria, glucosuria, and aminoaciduria, which have been attributed to alterations in renal function (Davison and Dunlop, 1980; Cornelis et al., 2011). Together, hyperfiltration and increased blood flow to the kidneys alter maternal pharmacokinetics by elevating the renal clearance of chemicals during pregnancy.
Renal transporters are membrane-spanning proteins that play a critical role in facilitating the movement of toxins and drugs into the urine via secretion and into the blood via reabsorption. To enable these processes, transporters are prominently localized on both the apical (brush border) and basolateral membranes of proximal tubule epithelial cells. Transporters expressed within the kidneys are members of one of the two superfamilies: the solute carrier (SLC) and ATP-binding cassette (ABC) families. Efflux transporters that extrude chemicals from cells are members of both the ABC and the SLC families and include the multidrug resistance–associated proteins (Mrps), multidrug resistance proteins (Mdrs), breast cancer resistance protein (Bcrp), and multidrug and toxin extrusion proteins (Mates) (reviewed in Klaassen and Aleksunes, 2010).
Recent studies have begun to explore transporter regulation in the kidneys during pregnancy and the subsequent postpartum period. In one study, expression of renal Mdr1b mRNA in mice decreased between mid and late gestation, with little to no change at the protein level (Zhang et al., 2008). In addition, expression of Bcrp in kidneys of mice was generally unchanged throughout pregnancy, with an increase in mRNA and protein only during mid-gestation (Wang et al., 2006). Despite these prior investigations, there are no studies that have systematically investigated the coordinated expression of apical and basolateral renal transporters in pregnant rodents during different periods of pregnancy and lactation.
Studies investigating the influence of pregnancy on renal clearance have focused predominantly on overall maternal pharmacokinetic parameters. Clinically, the urinary clearance of drugs, such as ceftazidime, atenolol, and amoxicillin, is often elevated during pregnancy (Hebert et al., 2005; Nathorst-Boos et al., 1995; Andrew et al., 2007). The renal secretion and urinary clearance of the cardiac glycoside digoxin has also been shown to increase in a study of 13 pregnant patients (Hebert et al., 2008). Because digoxin is a substrate of the MDR1 transporter, the study by Hebert et al. (2008) indirectly suggested that elevated renal MDR1 activity may participate in the increased tubular secretion and clearance of digoxin during pregnancy (Kawahara et al., 1999; Hebert et al., 2008).
Because of the enhanced urinary clearance of chemicals in humans and rodents during pregnancy, we hypothesized that expression of efflux transporters would favor renal secretion and limit reabsorption. Therefore, the purpose of the current study was to quantify the mRNA and protein expression of efflux transporters in the kidneys of mice during pregnancy and lactation. Mice were selected for this analysis because of their increasing use in developmental toxicology studies. The results obtained from this study will provide new insight into the potential contribution of renal transporters to drug clearance during pregnancy in mice.
Materials and Methods
Animals.
Adult female and male C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA) and mated overnight [denoting gestation day (GD) 0]. Virgin control mice were not mated. Mice were provided food and water ad libitum. At GDs 7, 11, 14, and 17 and postnatal days (PNDs) 1, 15, and 30, kidneys were collected from pregnant and time-matched virgin mice (n = 3–4). Pups were housed with dams until weaning at PND21. Kidneys were snap frozen and stored at −80°C until use. The Institutional Animal Care and Use Committees at Rutgers University and the University of Kansas Medical Center approved these studies.
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction.
Total RNA was isolated from kidneys by phenol-chloroform extraction using RNA-Bee (Fisher, Pittsburgh, PA) and was purified using the RNeasy mini kit (Qiagen, Valencia, CA). RNA concentrations were determined at 260 nm on a Nanodrop ND 2000 spectrophotometer (Thermoscientific, Wilmington, DE). Specific forward and reverse primers for each gene were added to 1 μg of cDNA from each sample (Integrated DNA Technologies, Coralville, IA). Primer sequences are provided in Supplementary Table 1. Sybr Green was used to detect amplified products on an ABI 7900HT PCR system (Applied Biosystems, Carlsbad, CA). Ct values were converted to Δ Δ Ct values by comparing to a reference gene, ribosomal protein 13a (Rpl13a). An example of Ct values for Rpl13a mRNA in virgin, pregnant, and lactating mice is included in Supplementary Fig. 1.
Western Blot Analysis.
Kidney protein homogenates were prepared in 500 μl of Sucrose-Tris buffer composed of 250 mM sucrose and 10 mM Tris-base at pH 7.5 and protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO). Forty micrograms of protein homogenates were electrophoretically separated on 4–12% BisTris gels and transferred to polyvinylidene fluoride membranes (Invitrogen, Carlsbad, CA). Equal protein loading was confirmed using β-actin (Ab8227; Abcam, Cambridge, MA). Antibodies included Mrp1 (MRPr1; Alexis, Farmingdale, NY), Mrp2 (M2III-5; Alexis), Mrp3 (M3II-2; G. Scheffer, VU Medical Center, Amsterdam, The Netherlands), Mrp4 (M41-10; Alexis), Mrp5 (M51-10; G. Scheffer), Mrp6 (M6II-68; G. Scheffer), Bcrp (BXP-53; Alexis), Mdr1a/b (C219; Abcam), and Mate1 (sc-138983; Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with secondary antibodies followed by SuperSignal West Dura chemiluminescent substrate (ThermoScientific, Pittsburgh, PA), antibody-protein complexes were detected and quantified using a Fluorchem imager (Alpha Innotech, San Leandro, CA).
Immunofluorescence Staining.
Indirect immunofluorescence staining of efflux transporters on frozen mouse kidney sections was performed as previously described (Aleksunes et al., 2006, 2008). Images were acquired on a Zeiss Observer D1 microscope with a x-cite series 120Q fluorescent illuminator (Zeiss Inc., Thornwood, NY) and a Jenoptik camera with ProgRes CapturePro 2.8 software (Jenoptik, Easthampton, MA). Images were cropped, and brightness and contrast were adjusted equally for each slide in Adobe Photoshop CS2 (San Jose, CA). All sections were both stained and imaged under uniform conditions for each antibody used. Negative controls without primary antibody were included in the analysis (unpublished data).
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism version 5 software (GraphPad, La Jolla, CA). Messenger RNA and protein expression were normalized to Rpl13A and β-actin, respectively. Data were then normalized to virgin controls at each time point (set to 1.0) and presented as mean ratios ± S.E.M. Differences among groups were evaluated using unpaired t tests at each time point, and P values ≤0.05 were considered to be statistically significant.
Results
Kidney Weights and Kidney to Body Weight Ratios in Pregnant and Lactating Mice.
Pregnancy had little effect on the absolute weight of the kidneys but did reduce the kidney to body weight ratio on GD7 and 17 (Supplementary Fig. 2). Compared with virgins, lactation increased kidney weights on PND1 and 15; however, the relative kidney weights were unchanged.
Renal mRNA Expression of Efflux Transporters in Pregnant and Lactating Mice.
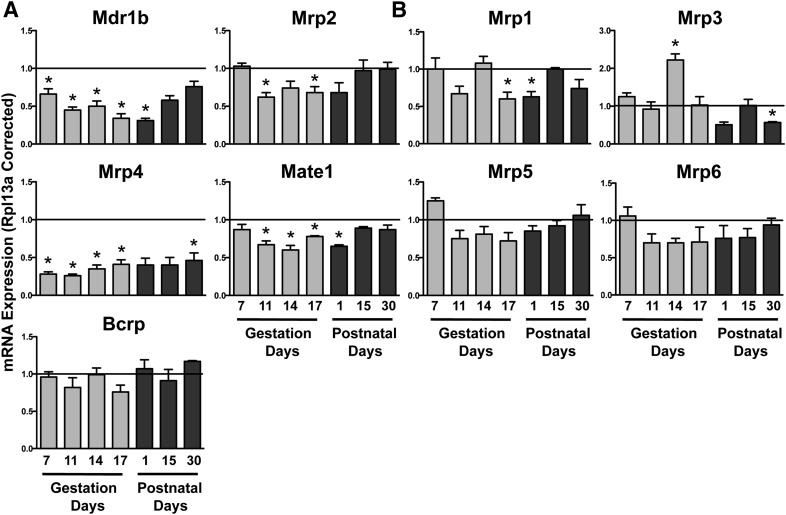
Efflux transporters are localized on the apical (Mdr1b, Mrp2, Mrp4, Mate1, and Bcrp) (Fig. 1A) and basolateral (Mrp1, 3, 5, and 6) (Fig. 1B) membranes of proximal tubule cells. Mdr1b and Mrp4 decreased as early as GD7 (Fig. 1A). By GD11, Mate1 and Mrp2 mRNA also decreased by 25–40%. Expression of Mdr1b, Mrp2, Mrp4, and Mate1 mRNA remained reduced at PND1. The basolateral transporter Mrp1 mRNA decreased modestly on GD17 and PND1 (Fig. 1B). Of interest, Mrp3 mRNA increased by 2.3-fold on GD14. Most transporter mRNA returned to virgin control levels by PND15, with the exception of Mrp3 and 4, which were still reduced at PND30. No change in Bcrp, Mrp5, or Mrp6 mRNA was observed during pregnancy and lactation.
Fig. 1.
Renal mRNA expression of efflux transporters in pregnant and lactating mice. Total RNA was isolated from kidneys, and mRNA levels were quantified using quantitative PCR. mRNA expression of (A) apical and (B) basolateral efflux transporters is shown. Data were normalized to virgin controls at each time point (set to 1.0) and presented as mean relative expression ± S.E.M. Light gray bars represent pregnant mice and dark gray bars represent lactating mice. *Statistical difference, compared with time-matched virgin mice (P ≤ 0.05).
Renal Protein Expression of Efflux Transporters in Pregnant Mice.
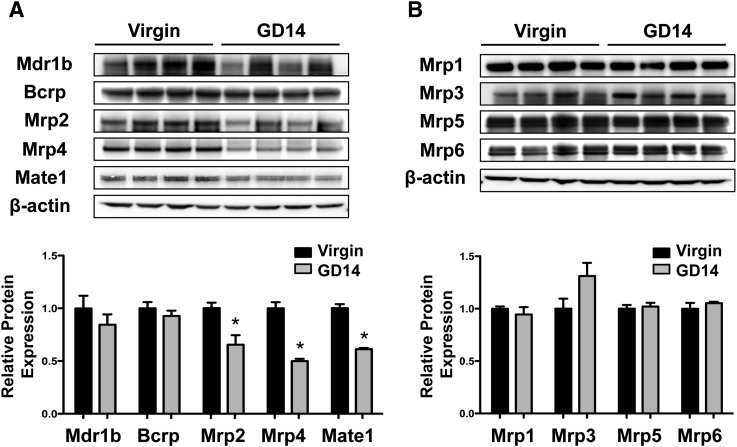
The majority of mRNA changes occurred at GD14. Therefore, this time point was selected for Western blot and immunofluorescence analysis. Similar to mRNA expression, protein levels of Mate1, Mrp2, and Mrp4 decreased significantly by 35–50% on GD14 (Fig. 2A). Mrp3 protein expression increased by 31% in kidneys of pregnant mice, although the increase was not statistically significant (Fig. 2B). Bcrp, Mdr1b, Mrp1, 5, and 6 protein levels were unchanged at GD14 (Fig. 2B).
Fig. 2.
Renal protein expression of efflux transporters in pregnant mice. Protein expression of (A) apical and (B) basolateral efflux transporters on GD14 is shown. Immunoblots are presented in the upper portion, and equal protein loading was confirmed using β-actin. Protein band intensity was quantified using densitometry. Data were normalized to virgin controls and presented as mean relative protein expression ± S.E.M. *Statistical difference, compared with time-matched virgin mice (P ≤ 0.05).
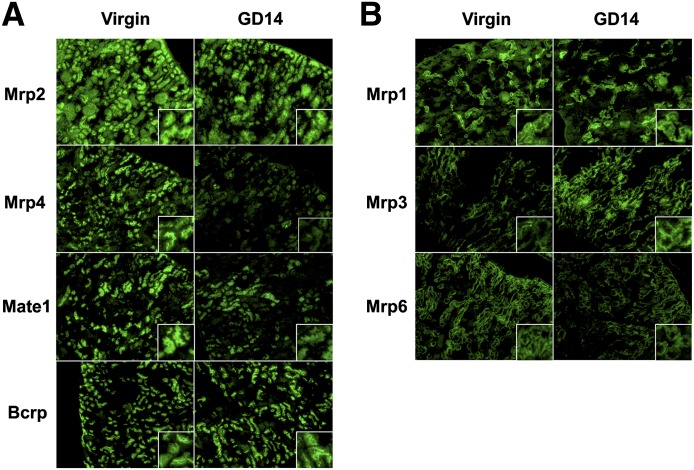
Immunofluorescent staining of apical and basolateral efflux transporters in renal tissue sections are shown in Fig. 3, A and B, respectively. Mrp2, Mrp4, Mate1, and Bcrp staining was localized to the apical membranes of proximal tubule cells located in the S1 and S2 segments of the cortex (Fig. 3A). Apical staining of Mrp2, Mrp4, and Mate1 proteins, but not Bcrp, was reduced in tissue sections from pregnant mice on GD14 (Fig. 3A). Mrp1 and Mrp6 immunofluorescence was observed in the S1 or S2 segments of proximal tubule cells (Fig. 3B). Staining of Mrp1, Mrp3, and Mrp6 was localized to the basolateral membrane (Fig. 3B). Contrary to the uniform distribution of Mrp6 across the cortex, Mrp1 staining was isolated in individual tubules and glomeruli. Of interest, Mrp3 staining was observed in tubules at the corticomedullary junction. Compared with virgin controls, staining of Mrp3 and Mrp6 was increased and modestly decreased, respectively, in tissue sections from pregnant mice (Fig. 3B). No change in the intensity of Mrp1 staining was observed. There are no commercial antibodies that selectively stain Mdr1 protein in mouse tissues.
Fig. 3.
Immunofluorescent detection of renal efflux transporters in pregnant mice. Immunofluorescent detection of (A) apical and (B) basolateral efflux transporters in renal tissues of virgin and pregnant mice at GD14. Indirect immunofluorescence of transporters (green) was conducted on kidney cryosections (6 μm) obtained from GD14 pregnant mice and time-matched virgin controls. Images of cortical regions are shown at 10× magnification. Images were cropped, enlarged, and provided as insets.
Discussion
The current study explored the renal mRNA and protein expression and localization of efflux transporters in pregnant and lactating mice. Pregnancy decreased Mdr1b, Mrp1, Mrp2, Mrp4, and Mate1 mRNAs and increased Mrp3 mRNA. Down-regulation of apical transporter mRNAs occurred at different time points in pregnancy and, in general, returned to baseline levels between PND1 and 30, with the exceptions of Mrp3 and Mrp4. Of interest, Mrp3 up-regulation was limited to GD14 only. Protein analysis revealed reduced Mate1, Mrp2, and Mrp4 protein and enhanced Mrp3 protein on GD14. Likewise, Mrp6 immunofluorescence also revealed a trend for reduced Mrp6 protein in cortical proximal tubules. The findings of this study were opposite of the initial hypothesis that transporter expression patterns would favor secretion during pregnancy. Instead, significant reductions in the expression of efflux transporters on the apical membrane of proximal tubule cells would likely impair chemical secretion during pregnancy. Thus, the contribution of transporters to the elevated urinary clearance of compounds in mice during pregnancy appears to be minimal, based on mRNA and protein expression patterns in this study. In fact, the substantial down-regulation of efflux transporters on the brush-border membrane could be a compensatory change to prevent additional loss of nutrients and endogenous chemicals through the urine. Prevention of proximal tubule secretion via transporters may be a mechanism to counterbalance the physiologic increases in glomerular filtration that occur during pregnancy. However, further investigation is needed to determine the exact reason for the down-regulation of apical afflux transporters during pregnancy, which may be unrelated to physiologic changes.
In general, renal excretion of drugs is increased in pregnancy. Bcrp is a key transporter for the antidiabetic drug glyburide (Zhou et al., 2008). Physicians are increasingly prescribing glyburide for pregnant women with diabetes, because it does not appear to accumulate in the fetal compartment to a great extent, in large part because of Bcrp in the placenta (Pollex et al., 2008; Zhou et al., 2008). The systemic clearance of glyburide increases during pregnancy in rodents (Zhou et al., 2008). Subsequent work in knockout mice revealed minimal contribution of apical Bcrp to the elevated renal clearance of glyburide during pregnancy, a finding consistent with the largely unchanged expression of Bcrp in this study (Zhou et al., 2010). This suggests a more dominant role for glomerular filtration in elevating overall renal clearance of glyburide during pregnancy (Zhou et al., 2010). Taken together, the apical efflux transporter patterns of expression do not appear to be a contributing factor to the elevated renal excretion of drugs commonly seen during pregnancy. Alternatively, pregnant mice may not be a suitable model for humans in investigating the renal clearance of some chemicals. Nonetheless, translational studies are needed to determine whether human transporters are altered in a similar fashion to mice during pregnancy.
Uptake transporters are also important in urinary secretion and reabsorption. The mRNA expression of various SLC transporters was also quantified in this study (Table 1). In general, there were modest changes in expression of the organic anion transporters (Oats) and the organic cation and carnitine transporters (Oct/Octns). Similar to efflux transporters, mRNA expression of several uptake transporters was lower in pregnant mice. Oat1, Oat3, Oatp1a6, and Octn1 mRNA decreased modestly throughout pregnancy. In addition, a decrease in mRNA expression was noted at PND1 for Oat3, Oat5, Oatp1a6, Oatp3a1, Octn1, and Octn2 similar to efflux transporters. Because these transporters are expressed on the apical and basolateral membranes, there may be a concerted effort to reduce not only secretion, but also reabsorption, during pregnancy. However, some uptake transporters, including Oct1, Oat2, peptide transporter 1, and apical sodium-dependent bile acid transporter, were elevated during pregnancy, suggesting that the regulation of uptake transporters may be more complicated than efflux transporters.
TABLE 1.
Renal Efflux and Uptake Transporter mRNA Changes During Gestational and Postnatal Periods
Data were normalized to Rpl13a mRNA expression and normalized to virgin controls at each time point (set to 1.0; n= 3–4 animals). Data are presented as mean ratios ± S.E.M. Oatp1a1, Mdr1a, Mate2, and Cnt3 were too low to be quantified.
| Transporter mRNA |
Gestational Day |
Postnatal Day |
|||||
|---|---|---|---|---|---|---|---|
| 7 | 11 | 14 | 17 | 1 | 15 | 30 | |
| Efflux | |||||||
| Abca1 | 1.06 ± 0.07 | 0.72 ± 0.05 | 0.80 ± 0.07 | 0.80 ± 0.04 | 1.20 ± 0.37 | 1.46 ± 0.24 | 1.15 ± 0.05 |
| Ostα | 0.99 ± 0.12 | 1.15 ± 0.17 | 0.70 ± 0.19 | 0.81 ± 0.05 | 1.31 ± 0.35 | 0.56 ± 0.03* | 1.24 ± 0.20 |
| Ostβ | 0.77 ± 0.07* | 0.89 ± 0.06 | 0.73 ± 0.04* | 1.64 ± 0.22 | 1.02 ± 0.21 | 0.76 ± 0.01 | 0.93 ± 0.05 |
| Uptake | |||||||
| Oat1 | 0.76 ± 0.01* | 0.66 ± 0.09 | 0.73 ± 0.08 | 0.66 ± 0.07* | 0.86 ± 0.09 | 1.02 ± 0.12 | 0.86 ± 0.17 |
| Oat2 | 0.79 ± 0.08 | 1.19 ± 0.16 | 1.88 ± 0.37 | 1.25 ± 0.07* | 1.38 ± 0.08 | 1.07 ± 0.18 | 1.05 ± 0.14 |
| Oat3 | 0.74 ± 0.05* | 0.88 ± 0.02 | 0.75 ± 0.13 | 0.74 ± 0.02* | 0.83 ± 0.01 | 0.70 ± 0.08 | 1.02 ± 0.18 |
| Oat5 | 1.31 ± 0.12 | 1.33 ± 0.23 | 1.46 ± 0.18 | 0.95 ± 0.10 | 0.67 ± 0.04 | 1.21 ± 0.13 | 0.93 ± 0.09 |
| Urat1 | 0.73 ± 0.06 | 0.56 ± 0.06* | 1.22 ± 0.36 | 0.83 ± 0.19 | 0.70 ± 0.14 | 1.17 ± 0.04 | 1.15 ± 0.09 |
| Oatp1a6 | 0.65 ± 0.05* | 0.78 ± 0.05 | 0.75 ± 0.08 | 0.60 ± 0.04* | 0.43 ± 0.06* | 1.16 ± 0.07 | 1.19 ± 0.15 |
| Oatp2b1 | 0.89 ± 0.04 | 0.77 ± 0.10 | 1.34 ± 0.12 | 0.95 ± 0.06 | 0.65 ± 0.19 | 1.46 ± 0.07 | 0.91 ± 0.06 |
| Oatp3a1 | 1.19 ± 0.07 | 0.98 ± 0.05 | 1.11 ± 0.06 | 0.84 ± 0.03* | 0.68 ± 0.04 | 1.07 ± 0.10 | 0.90 ± 0.09 |
| Oatp4c1 | 1.09 ± 0.16 | 0.76 ± 0.16 | 0.58 ± 0.06* | 0.76 ± 0.07 | 1.04 ± 0.15 | 1.24 ± 0.27 | 1.21 ± 0.06 |
| Oct1 | 0.79 ± 0.03* | 1.08 ± 0.04 | 1.04 ± 0.05 | 1.14 ± 0.01* | 1.06 ± 0.05 | 0.74 ± 0.03 | 1.21 ± 0.05* |
| Oct2 | 0.86 ± 0.01 | 0.85 ± 0.10 | 1.11 ± 0.10 | 0.81 ± 0.04 | 0.83 ± 0.03 | 0.94 ± 0.22 | 0.97 ± 0.04 |
| Oct3 | 0.91 ± 0.24 | 0.81 ± 0.20 | 0.69 ± 0.10 | 0.86 ± 0.15 | 1.04 ± 0.33 | 0.44 ± 0.17 | 1.13 ± 0.06 |
| Octn1 | 0.61 ± 0.04* | 0.80 ± 0.09 | 0.67 ± 0.04* | 0.94 ± 0.07 | 0.67 ± 0.04* | 0.57 ± 0.04* | 0.97 ± 0.04 |
| Octn2 | 0.71 ± 0.05* | 1.26 ± 0.11 | 0.92 ± 0.10 | 1.27 ± 0.15 | 0.79 ± 0.04* | 0.81 ± 0.03 | 0.91 ± 0.04 |
| Pept1 | 1.18 ± 0.08 | 1.18 ± 0.10 | 1.52 ± 0.12* | 0.83 ± 0.25 | 1.30 ± 0.18 | 1.10 ± 0.14 | 1.06 ± 0.18 |
| Pept2 | 1.27 ± 0.07 | 1.03 ± 0.15 | 0.98 ± 0.13 | 0.70 ± 0.12 | 0.94 ± 0.21 | 1.03 ± 0.21 | 0.94 ± 0.08 |
| Ent1 | 0.55 ± 0.05* | 0.89 ± 0.17 | 0.93 ± 0.08 | 1.24 ± 0.28 | 0.95 ± 0.10 | 1.25 ± 0.25 | 0.80 ± 0.11 |
| Ent2 | 1.32 ± 0.10 | 1.05 ± 0.07 | 0.84 ± 0.08 | 1.36 ± 0.47 | 0.72 ± 0.08* | 0.98 ± 0.26 | 0.80 ± 0.05 |
| Cnt1 | 1.20 ± 0.06 | 0.94 ± 0.06 | 0.80 ± 0.11 | 0.78 ± 0.03 | 0.70 ± 0.15 | 0.84 ± 0.06 | 0.99 ± 0.04 |
| Cnt2 | 1.06 ± 0.09 | 0.56 ± 0.08 | 0.58 ± 0.10* | 0.58 ± 0.02* | 0.86 ± 0.46 | 0.63 ± 0.20 | 0.99 ± 0.13 |
| Asbt | 1.26 ± 0.22 | 1.50 ± 0.07 * | 1.26 ± 0.10 | 1.12 ± 0.29 | 0.67 ± 0.02 | 0.96 ± 0.18 | 0.71 ± 0.17 |
Differences among groups by unpaired t tests at each time point (P ≤ 0.05).
Sex hormones, such as estradiol and testosterone, have been shown to participate in the regulation of efflux transporter expression. Administration of 17β-estradiol to gonadectomized or hypophysectomized mice increases Mrp3 mRNA expression in kidneys (Maher et al., 2006). In silico analysis of the 5′ flanking region of the mouse Mrp3 promoter has identified two putative binding sites for estrogen receptors, although the functional activity of these sites is unknown (Maher et al., 2006). Likewise, administration of the semisynthetic estrogen ethinylestradiol to rats increases Mrp3 and reduces Mrp2 protein expression in the liver (Ruiz et al., 2006). In addition to estradiol, levels of testosterone are also increased in mice during pregnancy (Barkley et al., 1979). This may be important in the down-regulation of Mrp4 mRNA and protein in pregnant mice. Exogenous administration of 5α-dihydroxytestosterone to gonadectomized mice markedly represses Mrp4 mRNA expression, possibly through an androgen response element in the 5′ flanking region (Maher et al., 2006). These studies support a role for elevated estrogen levels during pregnancy to up-regulate Mrp3 expression and, possibly, repress Mrp2 and for testosterone to repress Mrp4 levels.
Pregnancy alters the expression of transporters not only in the kidneys, but also in other tissues, such as the maternal liver. During late pregnancy, the liver expression of Mrp2 protein and Oatp1a4 mRNA and the bile acid transporters bile salt export pump and sodium-taurocholate cotransporting polypeptide are decreased in rats (Cao et al., 2001). Likewise, we have observed down-regulation of hepatic uptake (Oatps and Oct1) and efflux (Mrp2, Mate1, Abcg5/8) transporters during pregnancy in mice (unpublished data). Of interest, Mrp4 and Oat2 mRNA were increased in the livers of pregnant mice. Although Bcrp mRNA is decreased in the livers of pregnant C57BL/6 mice, no change in protein expression can be observed (unpublished data). These data are in conflict with a report by Wang et al. (2006), in which they observed up-regulation of Bcrp mRNA and protein in the livers and kidneys of pregnant FVB mice (Wang et al., 2006). These divergent findings may reflect strain differences in the regulation of transporters during pregnancy.
In conclusion, the mRNA and protein expression of renal efflux transporters in mice was altered during pregnancy. Significant down-regulation in mRNA expression of the apical efflux transporters Mdr1b, Mrp2, Mrp4, and Mate1 was observed. Marked reduction in protein expression of Mate1, Mrp2, and Mrp4 suggested minimal involvement in the elevated urinary clearance of compounds typically observed in pregnancy. Further functional studies in brush border membrane preparations for substrates of these transporters, such as metformin (Terada et al., 2006) and antiretroviral drugs (Schuetz et al., 1999; Huisman et al., 2002), should be performed to determine the relevance of the observed protein changes. These chemicals are renally eliminated and have been under-investigation for prescribing during pregnancy (Unadkat et al., 2007; Weinberg et al., 2011; Silva et al., 2012). Likewise, circulating bile acids are increased during pregnancy, and their renal secretion may be reduced during pregnancy because of decreased Mrp4 expression (Rius et al., 2006). Nonetheless, this study suggests that changes in the expression of renal transporters are unlikely to contribute to the enhanced urinary clearance of chemicals in rodents during pregnancy.
Supplementary Material
Acknowledgments
The authors thank Dr. Curtis Klaassen for providing kidneys for the mRNA time-course experiments, Drs. Bruno Steiger and George Scheffer for antibodies, Drs. Angela Slitt and Jason Richardson for quantitative polymerase chain reaction primer sequences, and graduate students and fellows of the Aleksunes laboratory for their contributions to this project.
Abbreviations
- Abc
ATP-binding cassette
- Bcrp
breast cancer resistance protein
- GD
gestational day
- Mate
multidrug and toxin extrusion transporter
- Mdr
multidrug resistance protein
- Mrp
multidrug resistance-associated protein
- Oat
organic anion transporter
- Oatp
organic anion transporting polypeptide
- Oct
organic cation transporter
- Octn
organic cation/carnitine transporter
- PCR
polymerase chain reaction
- PND
postnatal day
- Slc
solute carrier
Authorship Contributions
Participated in research design: Yacovino, Aleksunes.
Conducted experiments: Yacovino, Gibson.
Contributed new reagents or analytic tools: Aleksunes.
Performed data analysis: Yacovino, Gibson.
Wrote or contributed to the writing of the manuscript: Yacovino, Aleksunes.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK080774], and the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES020522, ES005022, and ES007148].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Aleksunes LM, Cui Y, Klaassen CD. (2008) Prominent expression of xenobiotic efflux transporters in mouse extraembryonic fetal membranes compared with placenta. Drug Metab Dispos 36:1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. (2006) Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89:370–379 [DOI] [PubMed] [Google Scholar]
- Andrew MA, Easterling TR, Carr DB, Shen D, Buchanan ML, Rutherford T, Bennett R, Vicini P, Hebert MF. (2007) Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther 81:547–556 [DOI] [PubMed] [Google Scholar]
- Barkley MS, Geschwind II, Bradford GE. (1979) The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod 20:733–738 [DOI] [PubMed] [Google Scholar]
- Cao J, Huang L, Liu Y, Hoffman T, Stieger B, Meier PJ, Vore M. (2001) Differential regulation of hepatic bile salt and organic anion transporters in pregnant and postpartum rats and the role of prolactin. Hepatology 33:140–147 [DOI] [PubMed] [Google Scholar]
- Chapman AB, Abraham WT, Zamudio S, et al. (1998) Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54:2056–2063 [DOI] [PubMed] [Google Scholar]
- Conrad KP. (2004) Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Investig 11:438–448 [DOI] [PubMed] [Google Scholar]
- Cornelis T, Odutayo A, Keunen J, Hladunewich M. (2011) The kidney in normal pregnancy and preeclampsia. Semin Nephrol 31:4–14 [DOI] [PubMed] [Google Scholar]
- Davison JM, Dunlop W. (1980) Renal hemodynamics and tubular function normal human pregnancy. Kidney Int 18:152–161 [DOI] [PubMed] [Google Scholar]
- Hebert MF, Carr DB, Anderson GD, Blough D, Green GE, Brateng DA, Kantor E, Benedetti TJ, Easterling TR. (2005) Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol 45:25–33 [DOI] [PubMed] [Google Scholar]
- Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, Thummel KE, Fishbein DP, Unadkat JD. (2008) Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther 84:248–253 [DOI] [PubMed] [Google Scholar]
- Huisman MT, Smit JW, Crommentuyn KM, Zelcer N, Wiltshire HR, Beijnen JH, Schinkel AH. (2002) Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS 16:2295–2301 [DOI] [PubMed] [Google Scholar]
- Kawahara M, Sakata A, Miyashita T, Tamai I, Tsuji A. (1999) Physiologically based pharmacokinetics of digoxin in mdr1a knockout mice. J Pharm Sci 88:1281–1287 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. (2006) Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem Pharmacol 71:1470–1478 [DOI] [PubMed] [Google Scholar]
- Maynard SE, Thadhani R. (2009) Pregnancy and the kidney. J Am Soc Nephrol 20:14–22 [DOI] [PubMed] [Google Scholar]
- Nathorst-Böös J, Philipson A, Hedman A, Arvisson A. (1995) Renal elimination of ceftazidime during pregnancy. Am J Obstet Gynecol 172:163–166 [DOI] [PubMed] [Google Scholar]
- Pollex E, Lubetsky A, Koren G. (2008) The role of placental breast cancer resistance protein in the efflux of glyburide across the human placenta. Placenta 29:743–747 [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. (2006) Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol 290:G640–G649 [DOI] [PubMed] [Google Scholar]
- Ruiz ML, Villanueva SS, Luquita MG, Vore M, Mottino AD, Catania VA. (2006) Ethynylestradiol increases expression and activity of rat liver MRP3. Drug Metab Dispos 34:1030–1034 [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. (1999) MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med 5:1048–1051 [DOI] [PubMed] [Google Scholar]
- Silva JC, Fachin DR, Coral ML, Bertini AM. (2012) Perinatal impact of the use of metformin and glyburide for the treatment of gestational diabetes mellitus. J Perinat Med 40:225–228 [DOI] [PubMed] [Google Scholar]
- Terada T, Masuda S, Asaka J, Tsuda M, Katsura T, Inui K. (2006) Molecular cloning, functional characterization and tissue distribution of rat H+/organic cation antiporter MATE1. Pharm Res 23:1696–1701 [DOI] [PubMed] [Google Scholar]
- Unadkat JD, Wara DW, Hughes MD, et al. (2007) Pharmacokinetics and safety of indinavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother 51:783–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. (2006) Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab 291:E1295–E1304 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Forster-Harwood J, Davies J, et al. (2011) Safety and tolerability of antiretrovirals during pregnancy. Infect Dis Obstet Gynecol 2011:867674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. (2008) Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol 74:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. (2008) The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 73:949–959 [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang Y, Hebert MF, Unadkat JD, Mao Q. (2010) Increased glyburide clearance in the pregnant mouse model. Drug Metab Dispos 38:1403–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.