Abstract
Objective
The arcus tendineus fascia pelvis (ATFP) and arcus tendineus levator ani (ATLA) are elements of anterior vaginal support. This study describes their geometry in women with unilateral levator ani muscle defects and associated “architectural distortion.”
Study Design
Fourteen subjects with unilateral defects underwent MRI. 3-D models of the arcus were generated. Locations of these relative to an ilial reference line were compared between unaffected and affected sides.
Results
Pronounced changes occurred on the defect sides’ ventral region. The furthest point of the ATLA lay up to a mean 10.2mm (p=0.01) more inferior and 6.5mm (p=0.02) more medial than that on the intact side. Similarly, the ATFP lay 6 mm (p=0.01*) more inferior than on the unaffected side.
Conclusion
The ventral arcus anatomy is significantly altered in the presence of levator defects and architectural distortion. Alterations of these key fixation points will change supportive force direction along the lateral anterior vaginal wall, increasing the risk for anterior vaginal wall prolapse.
Keywords: pelvic organ prolapse, cystocele, paravaginal defect, arcus tendineus fascia pelvis, arcus tendineus levator ani, levator ani muscle
Introduction
Some 11% of U.S. women require surgical repair for their pelvic floor dysfunction, and this number will likely increase as the population ages [1–3]. The anterior vaginal wall is both the most common site of pelvic organ prolapse [1], and the most frequent site of operative failure [4–9]. An improved understanding of the pathomechanics of anterior vaginal wall support is needed to understand the causes of anterior wall prolapse and operative failure.
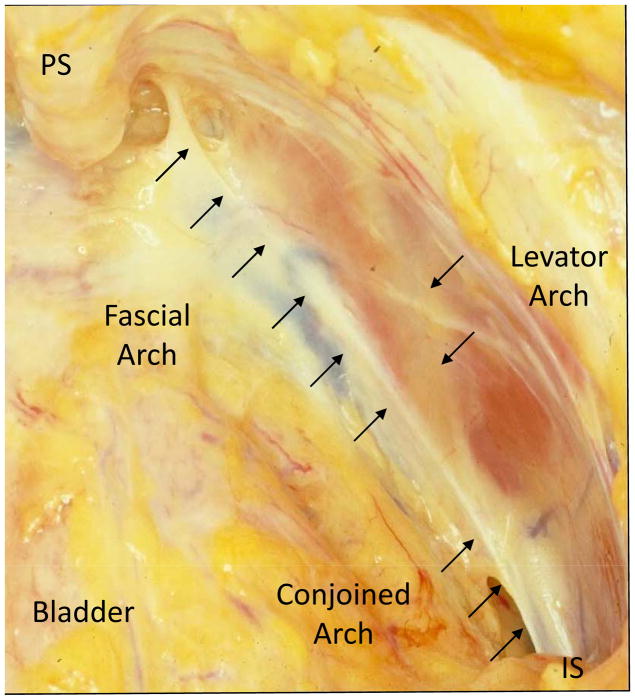
Magnetic resonance imaging (MRI) resolution has improved to the point that it is possible to examine the geometry of the individual structures and combinations of structures involved in anterior vaginal wall support. For example, a pattern of soft tissue abnormality termed “architectural distortion,” or AD, was recently described in which the lateral vaginal wall is seen to extend beyond its normal location, appearing to contact the obturator internus muscle [10]. Women with AD are more likely (78%) to have anterior wall prolapse than both women with levator defects but no AD (61%), as well as those with normal levators and no AD (31%) [10]. This distortion occurs in a structurally important region of anterior vaginal wall support at the interconnection between three key support structures: 1) the arcus tendineus fascia pelvis, or fascial arch; 2) arcus tendineus levator ani, or levator arch; and 3) the pubic portion of the levator ani muscle. (Figure 1) We have observed dislocation of these two arches in women with architectural distortion [10].
Fig 1. Right pelvic sidewall.
View looking down into Space of Retzius from above towards right pelvic sidewall. PS-pubic symphysis, IS-ischial spine.
Lack of a reliable method has hitherto precluded making measurements to test hypotheses concerning the status of these arches. This paper describes a technique using 3-D models to establish fascial and levator arch locations and to compare each structure’s location relative to a reference line in a unique group of women. Each of these women had a unilateral levator defect and architectural distortion on one side, but intact anatomy on the contralateral side. This offers a unique opportunity to compare normal and distorted pelvic sidewall anatomy in the same individual. The study goal was therefore to compare normal and distorted pelvic sidewall anatomy in women with unilateral defects in order to better understand why this population is more predisposed to anterior wall prolapse.
Materials and Methods
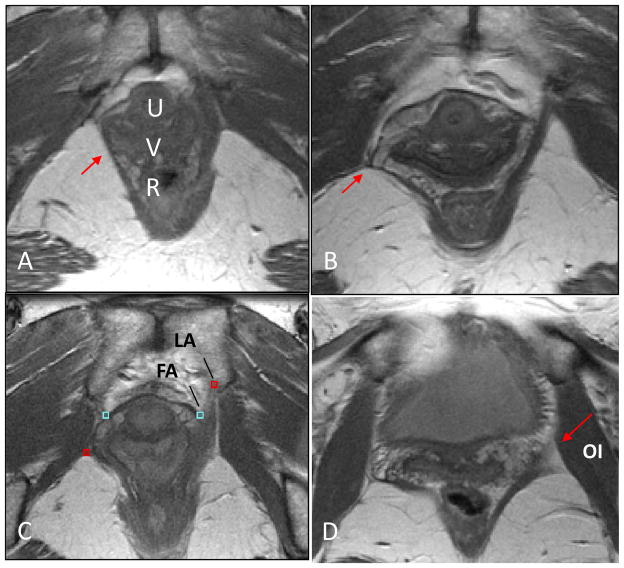
MRI scans from 14 women with unilateral levator defects and architectural distortion were selected from University of Michigan institutional review board-approved (IRB # 1999–0395, # 2002–0636, # 1995–0477) case-control studies of pelvic organ prolapse and urinary incontinence. All 14 subjects had unilateral levator defects that were classified as “major defects” on MR imaging, meaning more than half the levator ani muscle was involved [11].(Figure 2A) Architectural distortion was deemed present using earlier published criteria by Huebner, “lateral or posterior spill of the vagina from its normal position and posterior extension of the space of Retzius beyond the superior lateral sulcus of the vagina until it comes in contact with the superior surface of the iliococcygeal portion of the levator ani muscle.”[10] (Figure 2B)
Fig 2. Axial MR image of pelvis.
(A) Unilateral levator defect (red arrow). (B) Unilateral levator defect and architectural distortion (red arrow). (C) Levator arch (LA) at the ventral insertion of the levator muscle and fascial arch (FA) at lateral end of pubovisceral muscle labeled on non-defect side. (D) Red arrow indicates position of placement of levator arch with bulkier portion of levator ani muscle converging with the obturator internus muscle. U- urethra, V-vagina, R-rectum, OI – obturator internus.
All 14 subjects underwent MR imaging of the pelvis in the supine position at rest. MR imaging was performed on either a 1.5 Tesla superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI) or a 3 Telsa Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands) with a 6-channel, phased array, coil being available after 2006. Visibility of the structures was not altered by this transition. Ultrasound gel was placed in the vagina in later scans to better outline its contour. For these standard anatomical scans made at rest, turbo spin echo (TSE) techniques were used to image the sagittal, coronal, and axial planes. At rest, 30 images were obtained in each plane (repetition time (TR) range 2,300 – 3,000 ms, echo time (TE) 30 ms, 4 mm slice thickness, 1 mm gap, number of signal averages - NSA 2, 256 × 255 voxels).
The original axial, sagittal and coronal DICOM (Digital Imaging and Communications in Medicine) static images were imported into the 3-D Slicer® software program (version 2.1b1, Brigham and Women’s Hospital, Boston, MA) and aligned, ensuring that structures co-localized in all 3 axes by simultaneous review of 3-D scan planes in the viewer. 3-D models were made of the pelvic bones, fascial and levator arches on axial images by tracing their outlines (pelvic bones) or placing points on structure locations (arches). (Figure 3)
Fig 3. Building 3-D Model.
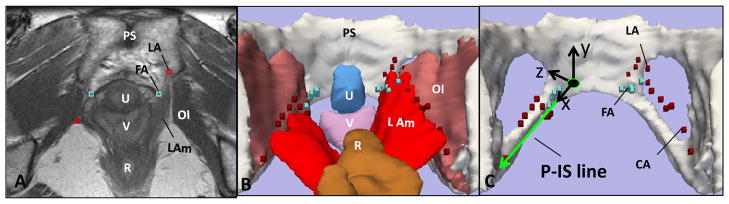
(A) Axial image showing FA (fascial arch) and LA (levator arch). 3D models made from MRI showing arches with (B) and without (C) adjacent structures. P-IS reference line as X-axis in local pelvic sidewall coordinate system visible in (C) on defect side. LAm-levator ani; OI-obturator internus; PS- pubic symphysis; R-rectum; U-urethra; V-vagina.
The actual fascial and levator arches are too small to be seen with standard MR techniques. However their location can be determined by the anatomical fact that they occur at the intersections of visible structures, thereby allowing their position to be marked in individual images. Ventrally, near the pubic symphysis, the levator arch was identified either on the medial aspect of the convergence of the levator ani and obturator internus muscles, or at the ventral insertion of the levator muscle when this was not present.(Figure 2C) At the dorsal location near the ischial spine, where the levator muscle is often “split”, the levator arch was identified along with the bulkier portion.(Figure 2D) The most dorsal aspect of the levator arch approached the ischial spine cranial to where the obturator internus wraps around the spine and in the plane where the sacrospinous ligament attachment is visible.
The fascial arch is identified ventrally at the lateral aspect of the pubovesical muscle based on previous anatomical and MRI observations [12,13].(Figure 2C) In the more dorsal region, it is no longer identified as a separate structure, but fuses to become one with the dorsal portion of the levator arch, also known as the conjoined arch (CA). For the remainder of this paper, this conjoined arch will still be referred to as the levator arch. The 3-D MR coordinates of each point along the arches were recorded using fiducial markers within the Slicer® software program. Figure 3C illustrates the relationship of these models to the obturator internus, levator ani, bladder, vagina and rectum.
To analyze the location of these structures both within a single subject comparing the normal and abnormal anatomic sides of the pelvis and between subjects, the coordinates of our models were then translated, or reassigned coordinates within local pelvic sidewall systems. In order to create this local system, 3-D MRI coordinates of the fascial arch insertion into the pubic symphysis and location of the ischial spine were identified to generate a reference line, the Pubis to Ischial Spine or P-IS line (Figure 3C). This line became the x-axis of a new pelvic sidewall coordinate system. It was then divided into ten equal deciles in each subject to normalize the distance along the reference line. Using a best fit curve technique, the fascial and levator arches were re-sampled at each decile along X-axis for comparison. X, Y and Z values for the levator and fascial arches were then recorded in this new system for comparison.
Blinded inter-rater reliability testing was performed on point placement with the senior and first author and found to have Pearson correlation coefficients ranging from 0.92–0.99 depending upon which arch and which axis were compared. In addition, the mean differences between raters indicated no systematic rater bias. Statistical analyses were performed using paired t-tests to compare distances above and lateral to the reference line at each decile in normal (non-defect side) and abnormal (defect side) anatomy.
Results
The mean age of the 14 study participants was 47 ± 12 (SD) years, median parity two and mean body mass index 25.6 ± 4.2 kg/m2. All subjects were Caucasian and only one had undergone hysterectomy. None had prior surgery for pelvic organ prolapse. Six (43%) subjects had prolapse defined as at least one POP-Q (Pelvic Organ Prolapse Quantification system) point at least 1 cm beyond the hymenal ring on clinical examination. The predominant prolapse was located in the anterior compartment in four individuals, the apical compartment in one individual, and equally in the anterior and posterior compartment in the final individual. All subjects had major, unilateral defects and architectural distortion as defined in the Methods. Right-sided defects predominated (79% vs. 21%). Table 1.
Table 1.
| Characteristics | Study Population (n=14) |
|---|---|
| Age (yrs)* | 47 ± 12 |
| BMI (kg/m2)* | 25.6 ± 4.2 |
| Median parity | 2 |
| Race* | |
| Caucasian | 14 (100%) |
| Hysterectomy* | 1 (7%) |
| Levator Defects* | |
| Right side | 11 (79%) |
| Left side | 3 (21%) |
mean ± standard deviation or n (%)
The most pronounced differences in arch location when comparing normal with architectural distortion sidewalls occurred in the ventral region, in the craniocaudal direction. Figure 4 depicts the craniocaudal relationships of the fascial and levator arches relative to the P-IS line, showing their locations at each decile. The x-axis is oriented along the P-IS line where “0” depicts the pubic insertion and “100” is the ischial spine.
Fig 4. Cranio-caudal position relative to P-IS Line.
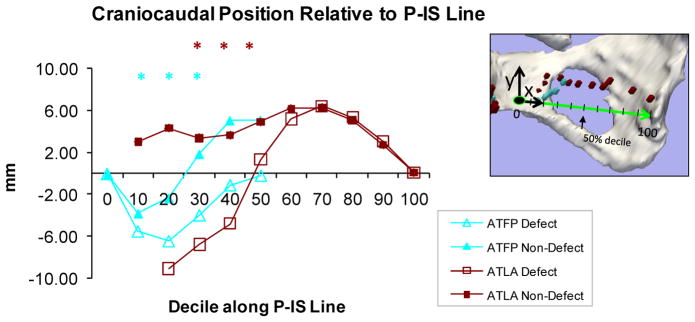
View of Y axis looking at pelvic sidewall. “0” is pubic end and “100” is at ischial spine. Asterisks reveal statistically significant differences between normal and abnormal sides.
Significant differences in location occurred at 10, 20 and 30 percentile for the fascial arch (p = 0.03, 0.001, and 0.012 respectively) and 30, 40, and 50 percentile for the levator arch (p = 0.008, 0.002, and 0.046 respectively). The most statistically significant differences for both arches occurred at the 30 percentile position. At this point, the fascial arch on the defect side was located 6 mm inferior to its position on the normal side (i.e., 4 mm below vs. 1.8 mm above, p=0.01) while the levator arch was located more than 1 cm inferior to its normal position (i.e., 6.8 mm inferior to the P-IS line for the defect side, and 3.4 mm superior to the P-IS line on the intact side).
Figure 5 depicts the mediolateral relationships of the arches relative to the P-IS line. Again, the most pronounced changes occurred in the ventral region. The ventral levator arch was more medially located on the defect side, most significantly at the third decile (2 mm vs. 8.5 mm lateral to P-IS line at 30 percentile, p=0.02). For the fascial arch there was no difference in the mediolateral location when comparing intact and defect sides; in both the fascial arch moved from medial to lateral as it fused with the levator arch.
Fig 5. Medio-lateral Position relative to P-IS Line.
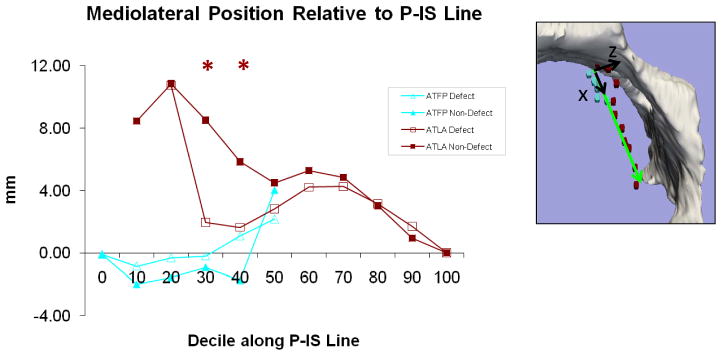
View of Z-axis looking into pelvis from above. “0” is pubic end and “100” is at ischial spine. Asterisks reveal statistically significant differences between normal and abnormal sides.
Comment
This study describes the development of a novel 3-D geometric approach for comparing of the anatomy of the fascial arches on the pelvic sidewall in the presence or absence of levator defects and architectural distortion. It also reports early observations using this technique. The method allows the location of the fascial and levator arches to be determined so that specific structural hypotheses can be tested in living women with known characteristics (i.e. architectural distortion). Using this measurement technique we have found that fascial and levator arch anatomy is significantly altered in the presence of levator defects and architectural distortion. The levator arch lies 10.2 mm more caudally, 6.5 mm more medially and does not come as close to the pubis along pelvic sidewalls on the side where a levator defect is present. In the upright posture it could be said to have “fallen” downward and medially from its usual location. Similarly, the fascial arch extends more caudally as well on the defect side. These changes are most pronounced in the ventral region near the symphysis.
The fascial and levator arches form part of a larger structural complex involved in anterior vaginal wall support that we refer to as the “paravaginal complex”. It consists of the two arches, the fascial connections between the vaginal wall and the arches, and the pubovisceral portion of the levator ani muscle. Together these structures provide support to the mid and distal anterior vaginal wall. Richardson was the first to call attention to abnormalities between the lateral vaginal margin and the arcus tendineus in this region, noting detachment of the anterior vaginal wall’s pubocervical fascia from the arcus tendineus fascia pelvis in women with cystourethrocele [14,15]. Later, muscle defects in this area were seen intraoperatively in approximately half of women with paravaginal defects and these have later been objectively confirmed on MRI images [16]. Blinded comparison of normal and abnormal anatomy is not possible in the operating room, so assessment of these hypotheses has awaited the advent of modern imaging techniques.
Alterations in these key fixation points will change the supportive force vectors along the anterior vaginal wall. With levator defects, the levator arch and levator ani fall inferior to the fascial arch thereby not being able to provide upward support in response to increased intra-abdominal pressure. In addition, this distal support loss changes the distribution of stress along the length of the vaginal wall and increases the load on intact proximal structures. This then begs the question as to why only 43% of the subjects in this particular study have prolapse even though they all have a unilateral levator defect and distortion? Perhaps the other 57% have compensated with other intact support structures, suggesting that maybe Knudson’s “two-hit hypothesis” applies in pelvic organ prolapse as well cancer [17]. Our prior studies included a high percentage of women with bilateral defects that may not have been able to compensate [10].
Our findings support anatomical descriptions in the literature that note two arches which fuse to a single arch in the ventral region of the pelvis. Ocelli and Albright both visualized these features in cadaver dissections [18,19]. Albright divides the fascial arch into three regions with the levator arch joining the fascial arch in the proximal portion of the middle region, a little more dorsal than our findings; he also references an arcus tendineous rectovaginalis that is not included in our observations [19]. Pit observed the levator and fascial arches merging in only four out of 10 of their cadaver dissections; in the remaining six it appeared to fuse with the ischial spine more laterally than the fascial arch [20]. This discrepancy may be due to distortion of these fine structures during the embalming process.
Findings from the present study extend current observations of normal paravaginal anatomy in the literature by defining the normal locations and providing a more detailed picture of the interactions occurring between two important elements of the paravaginal complex and their distortion due to levator defects. It also introduces a quantitative system where measurements of location can objectively be recorded to compare measurements in normal and abnormal anatomy. Most of the literature is based upon cadaver studies in which patient status is rarely known in regard to normal or abnormal levator muscles, nor was there any emphasis on the locations of these structures relative to an anatomic reference system. Huddleston did publish an MRI study examining what was labeled as paravaginal defects and describes three characteristic findings in 12 patients with cystourethroceles: upper defects causing the chevron sign, middle defects causing saddlebag sign, and a lower defect causing the mustache sign [21]. Review of the images in this article, given more recent insights indicates that these are patients with levator ani muscle defects.
Several factors must be considered when interpreting the results of this study. This study includes a modest number of unique subjects with unilateral defects, thus introducing the possibility that we are not capturing all the nuances of these unilateral defects. A strength of the study is that normal and abnormal anatomy can be compared within the same individual. In doing so, we make the assumption that the changes observed on the defect side do not influence arch geometry of the intact side. This seems reasonable because the anatomy on the unaffected side seems normal, and because if such changes were present, they would be expected to diminish the side-to-side differences, not exaggerate them. Although the normality of the side without architectural distortion was established by experienced examiners, it is possible that there may be subtle changes in this side when compared to individuals with no architectural distortion. This is under investigation at present. The modest sample size means that it is possible that some of the relationships that currently did not reach statistical significance in the ventral regions would do so in a larger sample. Another limitation is that a continuous structure was modeled using discrete points from serial MR images, then requiring a best fit curve to recreate this structure and possibly introducing error. We attempted to minimize this error by utilizing the best fit curve approach, incorporating our known 3-D coordinates into our final curve. In addition, we inspected the resulting lines and confirmed their fidelity using the original model. Finally, structures near the ischial spine are difficult to identify on the MR scans; however our data appear reproducible given inter-rater reliability correlation coefficients between 0.92 and 0.99.
We also acknowledge that this is a somewhat uniform population (all Caucasian) and that this may limit the generalizability of our results. Further studies that compare the findings in these unilateral defects with those seen in women with bilateral defects are also needed but our experience in looking at these defects suggests that these studies will be confirmatory. Finally, acquiring high resolution MRI images with sufficient detail for the present analysis can currently only be carried out in the supine position. It would be ideal to have images in the upright posture or during straining, but images of sufficient quality for such an analysis are not at present available.
The present technique to create and quantify 3-D models of the fascial and levator arches in the presence of unilateral levator defects and architectural distortion greatly enhances our ability to study anatomic alterations affecting mechanisms of pelvic floor prolapse. Our results suggest alterations in the anatomy within the paravaginal complex that may contribute to the increased rates of prolapse that we see among this population. Not only do our efforts bring us closer to understanding the normal locations of paravaginal structures, they also provide data to more accurately refine our biomechanical model of the pelvis to better understand the complex interactions between these structures and their roles in prolapse. In addition, now that we have a technique to establish the presence of architectural distortion and the geometric location of specific structures, it will be possible in the future to conduct studies to compare the treatment outcomes of women with and without architectural distortion.
Acknowledgments
We gratefully acknowledge support from the National Institute of Child Health and Human Development Grants R01 HD 38665 and the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health 1 P50 HD044406
Footnotes
Presented at the 30th Annual Scientific Meeting for the American Urogynecologic Society, Hollywood, Florida, September 2009.
Author Contributions:
KA LARSON: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing
J LUO: Protocol/project development, Data collection or management, Data analysis, Manuscript editing
A YOUSUF: Data collection or management
JA ASHTON-MILLER: Protocol/project development, Data analysis, Manuscript editing
JOL DeLANCEY: Protocol/project development, Data collection or management, Data analysis, Manuscript editing
Financial Disclaimers:
Dr. John OL DeLancey and Dr. James Ashton-Miller receive research support from American Medical Systems and Kimberly Clark. Dr. DeLancey has been a consultant for Johnson and Johnson. Jiajia Luo’s doctoral studies are partially funded by American Medical Systems. The other authors have no disclosures to report.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–38. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 3.He W, Sengupta M, Velkoff V, DeBarros K. 65+ in the US: 2005, current population report, special studies. United States Government Printing Office; Washington DC: 2005. Dec, Available from: www.census.gov. [Google Scholar]
- 4.Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: A prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175(6):1418–21. doi: 10.1016/s0002-9378(96)70084-4. discussion 1421–2. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: A randomized controlled trial. Obstet Gynecol. 2008;111(4):891–8. doi: 10.1097/AOG.0b013e31816a2489. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen JK. Current concepts in the diagnosis and surgical repair of anterior vaginal prolapse due to paravaginal defects. Obstet Gynecol Surv. 2001;56(4):239–46. doi: 10.1097/00006254-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Maher C, Baessler K. Surgical management of anterior vaginal wall prolapse: An evidencebased literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):195–201. doi: 10.1007/s00192-005-1296-3. [DOI] [PubMed] [Google Scholar]
- 8.Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98(1):40–4. doi: 10.1016/s0029-7844(01)01378-3. [DOI] [PubMed] [Google Scholar]
- 9.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–73. doi: 10.1067/mob.2000.110910. discussion 1373–4. [DOI] [PubMed] [Google Scholar]
- 10.Huebner M, Margulies R, DeLancey JOL. Pelvic architectural distortion is associated with pelvic organ prolapse. Int Urogynecol J. 2008;19:863–867. doi: 10.1007/s00192-007-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JOL. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107(1):144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLancey JOL. Pubovescial ligament: A separate structure from the urethral supports (“pubo-urethral ligaments”) Neurourology and Urodynamics. 1989;8:53–61. [Google Scholar]
- 13.Chou Q, DeLancey JOL. A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J Obstet Gynecol. 2001;185:44–50. doi: 10.1067/mob.2001.116368. [DOI] [PubMed] [Google Scholar]
- 14.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 15.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–62. [PubMed] [Google Scholar]
- 16.DeLancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93–8. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 17.Knudson AG. Mutation and Cancer: Statistical Study of Retinoblastoma. Proc Nat Acad Sci. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocelli B, Narducci F, Hautefeuille J, Francke JP, Querleu D, Crepin G, Cosson M. Anatomic study of arcus tendineus fasciae pelvis. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2001;97:213–219. doi: 10.1016/s0301-2115(00)00527-3. [DOI] [PubMed] [Google Scholar]
- 19.Albright TS, Gehrich AP, Davis GD, Sabi FL, Buller JL. Arcus tendineus fascia pelvis: A further understanding. Am J Obstet Gynecol. 2005;193:677–681. doi: 10.1016/j.ajog.2005.02.129. [DOI] [PubMed] [Google Scholar]
- 20.Pit MJ, De Ruiter MC, Nijeholt A, Marani E, Zwartendijk J. Anatomy of the arcus tendineus fascia pelvis in females. Clinical Anatomy. 2003;16:131–137. doi: 10.1002/ca.10102. [DOI] [PubMed] [Google Scholar]
- 21.Huddleston HT, Dunnihoo DR, Huddleston PM, Meyers PC. Magnetic resonance imaging of defects in DeLancey’s vaginal support levels I, II, and III. Am J Obstet Gynecol. 1995;172:1778–1784. doi: 10.1016/0002-9378(95)91411-0. [DOI] [PubMed] [Google Scholar]