Laparoscopy is technically feasible and can be utilized to optimally cytoreduce recurrent ovarian, fallopian, or primary peritoneal cancers in a well-selected patient population.
Keywords: Operative laparoscopy, recurrent ovarian cancer, fallopian tube cancer, Primary peritoneal cancer, secondary and tertiary cytoreduction
Abstract
Background and Objective:
Studies on the role of laparoscopy in secondary or tertiary cytoreduction for recurrent ovarian cancer are limited. Our objective is to describe our preliminary experience with laparoscopic secondary/tertiary cytoreduction in patients with recurrent ovarian, fallopian, and primary peritoneal cancers.
Methods:
This is a retrospective analysis of a prospective case series. Women with recurrent ovarian, fallopian tube, or primary peritoneal cancers deemed appropriate candidates for laparoscopic debulking by the primary surgeon(s) were recruited. The patients underwent exploratory video laparoscopy, biopsy, and laparoscopic secondary/tertiary cytoreduction between June 1999 and October 2009. Variables analyzed include stage, site of disease, extent of cytoreduction, operative time, blood loss, length of hospital stay, complications, and survival time.
Results:
Twenty-three patients were recruited. Only one surgery involved conversion to laparotomy. Seventeen (77.3%) of the patients had stage IIIC disease at the time of their initial diagnosis, and 20 (90.9%) had laparotomy for primary debulking. Median blood loss was 75 mL, median operative time 200 min, and median hospital stay 2 d. No intraoperative complications occurred. One patient (4.5%) had postoperative ileus. Eighteen (81.8%) of the patients with recurrent disease were optimally cytoreduced to < 1cm. Overall, 12 patients have no evidence of disease (NED), 6 are alive with disease (AWD), and 4 have died of disease (DOD), over a median follow-up of 14 mo. Median disease-free survival was 71.9 mo.
Conclusions:
In a well-selected population, laparoscopy is technically feasible and can be utilized to optimally cytoreduce patients with recurrent ovarian, fallopian, or primary peritoneal cancers.
INTRODUCTION
Ovarian carcinoma is the second most common gynecologic malignancy, and the most common cause of death among women with gynecologic malignancies.1 In the United States, ovarian cancer affected approximately 21,550 women in 2009 with 14,600 estimated deaths.2 Surgery, along with adjuvant or neoadjuvant chemotherapy, has been the cornerstone of management of ovarian carcinoma, as well as primary peritoneal and fallopian tube carcinomas.3 Optimal maximum cytoreductive surgery has been consistently shown to have a survival benefit in the setting of primary advanced ovarian cancers.4–5 Laparoscopy, in some patients, is being used for the primary surgical approach, as well as for second-look assessment after adjuvant chemotherapy.6 Since the first case report of successful laparoscopic management of early ovarian cancer,7,8 multiple studies have demonstrated the feasibility and efficacy of complete laparoscopic staging in early stage ovarian cancer.9–13 In advanced stage ovarian cancer, the role of laparoscopy has been described as a tool for diagnosis and determining feasibility of resectability, with limited studies on its role in cytoreductive procedures.14 Video laparoscopy offers several advantages over laparotomy: optical magnification of abdominal and pelvic anatomy, ease in visualization of the diaphragm and peritoneal surfaces, shorter postoperative recovery, smaller incisions with subsequently fewer postoperative wound infections and herniations, and decreased length of hospital stay.
The benefits of tumor reductive surgery for recurrent ovarian carcinoma are still under evaluation.15 However, most series of secondary cytoreduction have utilized the open surgical approach. Even most of the recent series on secondary, tertiary, and quaternary cytoreduction for gynecologic carcinoma recurrence, do not focus on or utilize a laparoscopic approach.16
We describe our preliminary experience with laparoscopic secondary and tertiary cytoreduction in patients with recurrent ovarian, fallopian tube, or primary peritoneal cancers.
MATERIALS AND METHODS
Patients
The women selected for this study had confirmed or suspected recurrent ovarian, fallopian tube, or primary peritoneal epithelial type cancers and presented to authors FN, HG, LC at their affiliated medical centers, between June 1999 and October 2009. All patients had previously undergone surgical cytoreduction and chemotherapy and had evidence of recurrence. Recurrence of disease was defined as recurrence after a period of at least 6 mo with no clinical evidence of disease after their initial treatment. The included women were specifically medically stable to undergo surgery and, based on their history, clinical and imaging studies; were candidates for surgical exploration; and were judged to be suitable candidates for laparoscopic debulking by the primary surgeon(s). All patients underwent preoperative evaluation including history, physical examination, medical assessment, computed tomography (CT) imaging of the chest, abdomen and pelvis, and tumor marker assays, and were counseled extensively preoperatively and appropriate informed consent was obtained. During the initial intraoperative assessment, the decision to proceed with a total laparoscopic approach was made by the treating physician, based on the extent of disease and the surgeon's ability to perform the necessary resections by laparoscopy. During the laparoscopic evaluation, the extent of disease was evaluated to be either disseminated peritoneal carcinomatosis and not amenable to cytoreduction either by laparoscopy or laparotomy, or localized disease. In the first group, after obtaining biopsies to test for tumor sensitivity, the procedure was terminated and the patient was treated with chemotherapy. In the localized group, the decision was made to perform laparoscopic or laparatomic cytoreduction based on the extent of the disease. For the purpose of this study, only patients who were cytoreduced laparoscopically were included. Patients were discharged from the hospital when they were medically stable and able to tolerate oral intake. Intravenous or intraperitoneal standard chemotherapy was started as soon as the patients were able to tolerate it, as appropriate. All patients were followed every 3 mo with complete physical examination, measurement of tumor markers, and imaging studies as needed. Subsequent surgery and chemotherapy were initiated according to standard practice.
Outcome variables analyzed included histology, stage, site of disease, extent of debulking, operative time (ORT), blood loss (EBL), length of hospital stay (HS), complications, follow-up duration, and disease-free survival time. Pathology, operative reports, and hospital and office charts were reviewed upon approval by the institutional review board. Postoperative complications were defined as adverse events occurring within 30 d of surgery. Optimal cytoreduction was defined as residual disease < 1cm. Disease-free survival (DFS) was defined as time from surgical secondary/tertiary cytoreduction to time of recurrence as determined by physical examination, laboratory, imaging, or pathological modalities. Patients who had no disease-free interval were, by definition, excluded from DFS analysis.
Surgical Technique
All procedures were performed using a multiple puncture operative laparoscopy with the patient under general anesthesia.17 Initial closed transumbilical or left upper quadrant (Palmer's point) entry using Veress needle was utilized in most patients. Alternatively, periumbilical cutdown and balloon trocar was utilized. Between 3 and 4 lower abdominal ancillary trocars, 5cm to 12cm, were placed in the lower and/or upper abdomen when needed for thorough exploration and treatment of pathological findings. All patients received one dose of preoperative antibiotic therapy. The surgical principles used for tumor cytoreduction during laparotomy were followed. The procedure began with careful inspection of the abdomen and pelvis. Following the initial survey of the abdomen and pelvis, aspiration of any pelvic ascites or peritoneal washings was performed, and the aspirates were prepared for cytologic evaluation. Appropriate biopsies were performed and sent for frozen section evaluation. Resection of bulky and metastatic tumor (including bowel resection, diaphragm ablation or resection, and resection of bulky metastatic pelvic and/or paraaortic lymph nodes) was performed with a goal of maximal cytoreduction and preferably to no visible disease. Different laparoscopic instruments and techniques were used to achieve optimal cytoreduction, including sharp scissors, electrosurgery, (Ethicon Endo-Surgery, Cincinnati, Ohio), CO2 laser, PlasmaJet (Plasma Surgical Limited, Oxfordshire, UK), and argon beam coagulator.18 Rectosigmoid colon resection, when necessary, was performed first by mobilizing the descending colon to the level of the splenic flexure. A low anterior rectosigmoid resection was performed proximally and distally utilizing a 60-mm GIA stapler. The mobilized proximal sigmoid colon was brought out through the widened lower middle or left quadrant laparoscopic incision, and an anvil was placed and secured using a purse string suture. The anvil was then placed back into the peritoneal cavity. A 33-mm end-to-end anastomosis (EEA) stapler was passed into the rectum, and the spike was deployed, connected to the anvil and activated, creating an end-to-end anastomosis. By clamping the proximal colon with a bowel grasper, the anastomosis site was tested by filling the pelvis with lactated Ringer's solution and, under observation with the laparoscope, insufflating the rectum with air to check for leakage. The site was also tested by filling the rectum with indigo carmine and examining for leakage.17 Small bowel resection was mobilized laparoscopically, and resection and anastomosis was performed either in situ or through a mini laparotomy. Liver resection was performed using ultrasonic Harmonic shears for initial resection and bipolar electro-desiccation for further hemostasis. Splenectomy was performed by placing the patient in a slight reverse Trendelenburg position, turning the operating table to left side up for better access to the spleen. Using ultrasonic Harmonic shears, short gastric vessels were secured using clips, and after entry to the lesser sack obtained, the spleen was mobilized from its attachments and ligaments. The laparoscopic stapling device (Ethicon Endo-Surgery, Cincinnati, Ohio) was used to staple and cut the splenic vessels and remove the spleen. Diaphragmatic metastatic disease was addressed by appropriate placement of upper abdominal trocars and using stripping or ablating techniques for different modalities, such as CO2 laser, PlasmaJet energy, or argon beam coagulator. At times, transection of the falciform and partial liver mobilization is required so as to have complete access to the entire surface of the diaphragm.
All specimens were placed in endoscopic bags and removed from the abdomen prior to terminating the procedure. The port sites were irrigated, and a full-thickness closure was performed to possibly decrease trocar-site metastasis. Chemotherapy was started as soon as the patient was stable and able to tolerate oral intake.
RESULTS
A total of 23 cases were included in this study. Twenty-two of the 23 cases were completed laparoscopically. One case was converted to laparotomy. The patient had a history of stage IIIC papillary serous ovarian cancer that was cytoreduced by laparotomy. She had previously undergone laparotomy with rectosigmoid colon resection for diverticulitis. This patient underwent exploratory laparoscopy, which revealed extensive intraabdominal and pelvic adhesions with loops of small and large bowel matted together and attached to the abdominal wall, pelvic sidewall, and vaginal apex. The decision was made to convert to laparotomy to perform optimal cytoreduction. This patient is excluded from the statistical analyses. The remaining patients were subsequently divided into 2 groups: 1) Nineteen patients underwent secondary cytoreduction 6 mo or more after completion of primary cytoreduction and chemotherapy; and 2) Three patients presented for tertiary cytoreduction for second recurrence.
Overall, most of the initial cancers were stage III (77.3%) and serous histology (40.9%), followed by adenocarcinoma not otherwise specified (NOS; 36.3%) (Table 1). Most the patients, 20 out of 22 (90.9%), had prior laparotomy debulking. Eighteen of the 22 patients (81.8%) were optimally cytoreduced to < 1cm. Cytoreductive procedures included resection of tumor in the vaginal cuff, pelvis, abdominal wall, diaphragm, liver, spleen, omentum, mesentery, and small and large bowel. Among the 22 patients, no intraoperative complications occurred, but one postoperative complication did occur.
Table 1.
Surgical Outcomes
| Variables | Group 1 Secondary Cytoreduction | Group 2 Tertiary Cytoreduction | All Patients |
|---|---|---|---|
| Age (years) | 57.9 ±12.5 | 57.7 ±20.6 | 57.6 ±13.1 |
| BMI (kg/m2) | 28.0 ±3.9 | 23.8 ±3.7 | 27.7 ±4.7 |
| Histologic Type | |||
| Serous | 9 (47.4%) | 0 | 9 (40.9%) |
| Adenocarcinoma | 5 (26.3%) | 3 (100%) | 8 (36.3%) |
| Endometrioid | 2 (10.5%) | 0 | 2 (9.1%) |
| Clear cell | 1 (5.3%) | 0 | 1 (4.5%) |
| Mixed | 2 (10.5%) | 0 | 2 (9.1%) |
| Initial Stage | |||
| I | 1 (5.3%) | 0 | 1 (4.5%) |
| II | 4 (21%) | 0 | 4 (18.2%) |
| III | 14 (73.7%) | 3 (100%) | 17 (77.3%) |
| OR Time (min) | 180.5 (60–335) | 281 (200–480) | |
| EBL (mL) | 50 (10–200) | 100 (50–300) | |
| HS (days) | 2 (0–7) | 2 (1–5) | |
| Complications (%) | |||
| Intraoperative | 0 | 0 | 0 |
| Postoperative | 1 (7.1%) | 0 | 1 (4.5%) |
Group 1: Secondary Cytoreduction
Nineteen patients had suspicious recurrence based on symptoms, imaging and/or elevated CA-125 levels. One patient had a false-negative laparoscopy. She had rising CA-125, pain, and a possible retroperitoneal mass on CT scan. Multiple dense adhesions were noted during laparoscopy; however, thorough exploration and surgical pathology of biopsies taken were negative for metastasis. One month later, repeat CT scan showed an enlarged mass and clinical evidence of recurrence. The metastatic lesion was apparently retroperitoneal in the pelvis and was likely missed during laparoscopy due to dense adhesions and fibrotic peritoneum. The patient responded to subsequent intravenous chemotherapy.
Most of the initial cancers were stage III (73.7%); the majority had serous histology (47.4%), followed by adenocarcinoma NOS (26.3%). Eight of the patients had disease confined to the pelvis, 5 had disease in the upper abdomen, and 5 had disease in the pelvis and upper abdomen. Median estimated blood loss (EBL) was 50 mL (range, 10 to 200), median operating room time (ORT) was 180.5 min (range, 60 to 335), and median hospital stay (HS) was 2 d (range, 0 to 7). Fifteen of these patients were optimally cytoreduced (78.9%). Specifically, 4 patients were cytoreduced to microscopic disease, and the remainder to residual disease < 0.5cm. The 4 patients with suboptimal cytoreduction underwent second-line chemotherapy. No intraoperative complications and one postoperative complication occurred. One patient developed an ileus with resolution after bowel rest. All patients with optimal cytoreduction underwent subsequent mostly platinum based chemotherapy.
Median follow-up was 14 mo (range 2 mo to 119 mo). Median time to recurrence after initial treatment with primary cytoreduction was 24 mo (range 6 mo to 276 mo). After secondary cytoreduction, 5 of the 19 patients had progressive disease within 6 mo after their procedures, whereas the remaining 14 (74.7%) had at least 6 mo of disease-free survival. Of the 5 patients with progressive disease, 3 were alive with disease (AWD), and 2 were dead of disease (DOD). These 2 patients died at 2 mo and 14 mo after secondary cytoreduction. Median DFS for the 14 patients is 78.5 (74.7%) months.
Group 2: Tertiary Cytoreduction
Three patients underwent tertiary cytoreduction: 2 for recurrence and 1 for symptomatic persistent disease. All of the initial cancers were stage III. One patient had disease in the upper abdomen, and 2 had disease in both the pelvis and upper abdomen. Histology demonstrated adenocarcinoma NOS for all 3 patients, and 1 patient also had gastrointestinal stromal tumor. Median EBL was 100 mL (range, 50 to 300), median ORT was 281 min (range, 200 to 480), and median hospital stay was 2 d (range, 1 to 5). All of these patients were optimally cytoreduced. No intraoperative or postoperative complications occurred (Table 1).
Median follow-up was 14 mo (range, 4 to 36). Median time to recurrence after secondary cytoreduction was 12 mo. After tertiary cytoreduction, 1 of the 3 patients had evidence of disease shortly after cytoreduction despite optimal cytoreductive surgery. She died of disease 14 mo after tertiary cytoreduction. The other 2 patients had at least 6 mo of DFS. Mean DFS for these 2 patients was 17 mo. At the current time, 1 is AWD and 2 are DOD. For the two patients that are DOD, the mean time to death was 25 mo (14 mo, and 36 mo). No patients had port-site metastasis.
DISCUSSION
From our experience, in selected patients, optimal laparoscopic cytoreduction is feasible not only for disease in the pelvis but also for upper abdominal diseases. Recently, we reported our experience utilizing laparoscopic surgery in assessing the resectability and its ability to cytoreduce advanced ovarian, fallopian, and primary peritoneal cancers.14,18 Moreover, in our series we observed no compromise of recurrence-free interval in the group of patients who underwent laparoscopic debulking as compared with those that had laparotomy, 31.7 mo versus 21.5 mo. In this study, we show that secondary and tertiary cytoreduction of lower and upper abdominal disease is possible. We have performed 2 sigmoid resections, small bowel resection, 3 liver wedge resections, splenectomy, and destruction of diaphragmatic lesions. We report a complication rate of 4.5% and median length of hospital stay of 2 d.
The theoretic benefit from cytoreductive surgery relates to removing large tumor volumes that have a decreased growth fraction, thereby improving the efficacy of chemotherapeutic agents.19 Despite achieving clinical remission after completion of initial treatment, most patients (60%) with advanced epithelial ovarian cancer will ultimately develop recurrent disease.20 Other factors that have been evaluated but have shown little or no effect on survival include histology, tumor grade, serum CA-125 level, and preoperative radiographic and physical findings.21–24 The management of recurrent ovarian cancer is less clear than that of primary disease. The current body of literature regarding secondary cytoreductive surgery (SCR) is mainly composed of retrospective studies and a few prospective studies.
SCR in recurrent ovarian cancer was first described by Berek et al. It is suggested that optimal cytoreduction, defined as < 1cm of gross residual disease, should be the goal of all cytoreductive surgical procedures as the literature has clearly shown the median survival improves from 38 mo in patients with residual disease > 1 cm, to 72 to 106 mo in those with no gross residual disease.25 Furthermore, it has been shown that salvage chemotherapy prior to cytoreduction may adversely affect survival.26 Although strict guidelines for eligibility for secondary cytoreductive surgery are yet undefined, current data suggest that SCR may benefit only a highly select group of patients.26 Those that may benefit are patients with localized recurrent disease, a disease-free interval > 6 mo, good performance status, and those that have the potential for optimal secondary cytoreduction.
Applications of laparoscopy in advanced ovarian cancer have been described in triage for resectability, second-look assessment, and in select cases, primary and secondary cytoreduction.6 Thorough staging procedures, and optimal cytoreduction, provide both essential prognostic information and confer a survival benefit to those who have microscopic postoperative disease.19 Laparoscopy offers multiple advantages over traditional laparotomy including smaller incisions, improved visualization, less blood loss, reduction in the need for analgesics, decreased morbidity, and a more rapid recovery.9,11,27 An additional advantage for patients with ovarian cancer requiring adjuvant therapy includes a shorter interval to the initiation of adjuvant therapy.
Benedetti et al.28 performed a prospective observational study of 48 patients with recurrent epithelial ovarian cancer as evidenced by pelvic/aortic lymph node relapse. Twenty-nine patients were amenable to cytoreductive surgery. Postoperatively, all patients received adjuvant treatment. After clinical and laparoscopic staging, SCR including systematic lymphadenectomy, were performed. After a median follow-up of 26 mo, among cytoreduced patients, 18 women were alive with no evidence of disease and 9 were alive with disease. Estimated 5-y overall survival and disease-free interval for women operated on were 87% and 31%, respectively.
Tebes et al.29 in their study of 85 patients with recurrent epithelial ovarian cancer who underwent SCR by laparotomy, showed median survival of 30 mo for patients who were optimally cytoreduced and 17 mo for patients with residual disease. Tian et al.30 retrospectively reviewed 123 patients with recurrent epithelial ovarian cancer who underwent SCR. Median survival was 31.7 mo overall, 63.2 mo for patients with complete resection of all visible disease, 31.1 mo for patients with 0.1cm to 1cm of residual disease, and 15.6 mo for those with > 1cm of residual disease. They report 22.8% NED, 26% AWD, and 51.2% DOD with 26.1-mo median follow-up time.
Recently, Shih et al.31 reported on their series of 77 patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancers who underwent tertiary cytoreduction. Median disease-specific survival for the entire cohort was 47.7 mo. Forty-nine percent of the patients have DOD during the follow-up time of 28.9 mo. Residual disease after tertiary cytoreduction was been shown to have prognostic significance alone. Our study shows a median DFS of 17 mo, with 66.6% DOD over the follow-up time of 14 mo. Our numbers in this study, however, are very small to suggest any recommendations or conclusions.
Advances in laparoscopic instrumentation and technique have made a laparoscopic approach to surgical cytoreduction possible in select patients. The first report of successful laparoscopic cytoreduction in advanced ovarian cancer was described by Amara et al.27 This case series included 5 patients who underwent successful total laparoscopic primary staging or SCR. All patients did well postoperatively. One patient subsequently died due to recurrent disease, declining further intervention.
Trinh et al.32 recently reported a series of 36 patients with evidence of recurrent ovarian cancer as determined by elevated CA-125. The patients were asymptomatic and had no radiologic evidence of tumor. Thirty-four of the 36 underwent laparoscopic SCR with an electrosurgical loop excision procedure and argon beam coagulator. Ninety-four percent of the patients were optimally cytoreduced, with a 6% complication rate and median progression-free survival of 1.1 y. We report here 81.8% optimal cytoreduction in our series, with one conversion to laparotomy (4.3%). Our study shows a median DFS of 71.9 mo. We report 54.5% NED, 27.3% AWD, and 18.2% DOD with median follow-up of 14 mo.
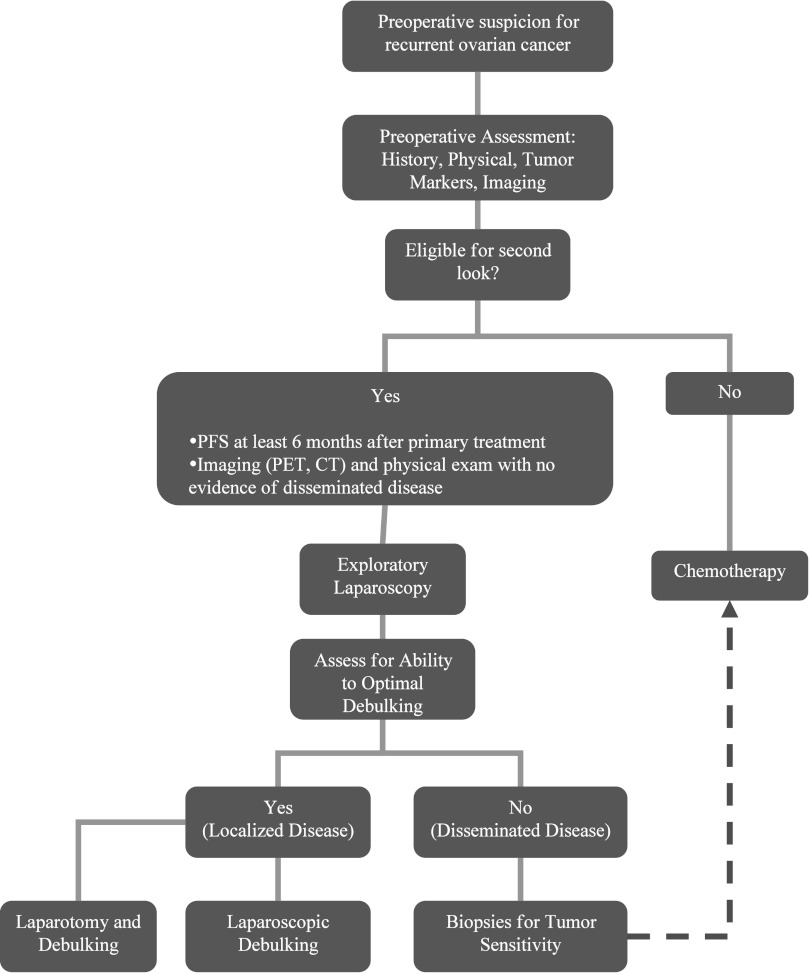
The high success rate in our study is partly due to selection of patients who were likely to be optimally debulked. This was based on preoperative imaging and diagnostic laparoscopy. We proceeded with debulking if the patient did not have disseminated disease and the disease was localized. We suggest that prior to starting any operation, all patients should be assessed laparoscopically to see whether or not they could be optimally debulked. To decrease the selection bias, we will prospectively assess all patients with a first recurrence to determine what percentage actually undergo chemotherapy versus laparoscopy with biopsies for diagnosis, suboptimal cytoreduction, optimal cytoreduction, and conversion to laparotomy (Figure 1).
Figure 1.
Algorithm for assessment and treatment of the recurrent ovarian cancer.
CONCLUSION
Most recurrent patients have little chance for a long disease-free survival; however, less invasive treatments may improve the quality of their lives while not compromising their survival. Our cohort suggests that laparoscopic optimal SCR in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer is technically feasible with acceptable morbidity in a well-selected population. Moreover, complete SCR combined with further adjuvant therapy at the time of relapse may improve clinical outcome in select patients. When selecting patients for SCR, the most significant preoperative factors are disease-free interval and success of a prior cytoreductive effort. Once the decision is made to pursue a SCR, the most important factor for improved survival is optimal secondary cytoreduction.
Laparoscopic secondary and tertiary cytoreduction is safe to perform and with comparable outcomes. Larger studies are needed to further evaluate the role of laparoscopic cytoreduction in recurrent ovarian cancers.
Contributor Information
Farr R. Nezhat, Department of Obstetrics and Gynecology, Columbia University, and Department of Obstetrics and Gynecology, St. Luke's-Roosevelt Medical Center, NY, New York, USA.; Safety and Efficacy of Video Laparoscopic Surgical Debulking of Recurrent Ovarian, Fallopian Tube, and Primary Peritoneal Cancers
Shaghayegh M. Denoble, Department of Obstetrics and Gynecology, Chilton Memorial Hospital, Pompton Plains, NJ, USA..
Jennifer E. Cho, Department of Obstetrics and Gynecology, North Shore University Hospital, Manhasset, NY, USA..
Douglas N. Brown, Department of Obstetrics and Gynecology, Walter Reed Army Medical Center, WA, DC, USA..
Enrique Soto, Department of Obstetrics, Gynecology, and Reproductive Science, The Mount Sinai Medical Center, NY, New York, USA..
Linus Chuang, Department of Obstetrics, Gynecology, and Reproductive Science, The Mount Sinai Medical Center, NY, New York, USA..
Herbert Gretz, Department of Obstetrics, Gynecology, and Reproductive Science, The Mount Sinai Medical Center, NY, New York, USA..
Prakash Saharia, Department of General Surgery, Winthrop University Hospital, Mineola, NY, New York, USA..
References:
- 1. Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249 Epub 2009 May 27 [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute: Ovarian Cancer. U.S National Institutes of Health. Available at http://www.cancer.gov/cancertopics/types/ovarian Accessed May 28, 2009
- 3. Cho JE, Liu C, Gossner G, Nezhat FR. Laparoscopy and gynecologic oncology. Clin Obstet Gynecol. 2009;52(3):313–326 [DOI] [PubMed] [Google Scholar]
- 4. Winter WE, III, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;20(25):3621–3627 [DOI] [PubMed] [Google Scholar]
- 5. Winter WE, III, Maxwell GL, Tian C, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;1(26):83–89 [DOI] [PubMed] [Google Scholar]
- 6. Liu CS, Nagarsheth NP, Nezhat FR. Laparoscopy in ovarian cancer: a paradigm change in the management of ovarian cancer. J Minim Invasive Gynecol. 2009;16:250–262 [DOI] [PubMed] [Google Scholar]
- 7. Reich H, McGlynn F, Wilkie W. Laparoscopic management of stage I ovarian cancer. J Reprod Med. 1990;35:601–605 [DOI] [PubMed] [Google Scholar]
- 8. Nezhat F, Nezhat C, Burrell M. Laparoscopically assisted hysterectomy for the management of a borderline ovarian tumor: a case report. J Laparoendosc Surg. 1992;2:167–169 [DOI] [PubMed] [Google Scholar]
- 9. Nezhat FR, Ezzati M, Chuang L, Shamshirsaz AA, Rahaman J, Gretz H. Laparoscopic management of early ovarian and fallopian tube cancers: surgical and survival outcome. Am J Obstet Gynecol. 2009;200(83):e1–e6 [DOI] [PubMed] [Google Scholar]
- 10. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629 [DOI] [PubMed] [Google Scholar]
- 11. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol. 2005;192:1614–1619 [DOI] [PubMed] [Google Scholar]
- 12. Park JY, Kim DY, Suh DS, et al. Comparison of laparoscopy and laparotomy in surgical staging of early-stage ovarian and fallopian tubal cancer. Ann Surg Oncol. 2008;15:2012–2019 [DOI] [PubMed] [Google Scholar]
- 13. Pomel C, Provencher D, Dauplat J, et al. Laparoscopic staging of early ovarian cancer. Gynecol Oncol. 1995;58:301–306 [DOI] [PubMed] [Google Scholar]
- 14. Nezhat F, DeNoble SM, Liu CS, et al. The safety and efficacy of laparoscopic surgical staging and debulking of apparent advanced stage ovarian, fallopian tube and primary peritoneal cancers. JSLS. 2010;14:155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bristow RE. Surgical standards in the management of ovarian cancer. Curr Opin Oncol. 2000;12(5):474–480 [DOI] [PubMed] [Google Scholar]
- 16. Leitao MM, Jr., Kardos S, Barakat RR, Chi DS. Tertiary cytoreduction in patients with recurrent ovarian carcinoma. Gynecol Oncol. 2004;95:181–188 [DOI] [PubMed] [Google Scholar]
- 17. Nezhat C, Nezhat F, Nezhat C. Nezhat's Operative Gynecologic Laparoscopy and Hysteroscopy. 3rd ed New York, NY: Cambridge University Press; 2008 [Google Scholar]
- 18. Nezhat FR, Datta MS, Lal N, et al. Laparoscopic cytoreduction for primary advanced or recurrent ovarian, fallopian tube, and peritoneal malignancies. Gynecol Oncol. 2008;108:S60 [Google Scholar]
- 19. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–979, discussion 979–980 [DOI] [PubMed] [Google Scholar]
- 20. Burke TW, Morris M. Secondary cytoreductive surgery for ovarian cancer. Obstet Gynecol Clin North Am. 1994;21:167–178 [PubMed] [Google Scholar]
- 21. Janicke F, Holscher M, Kuhn W, et al. Radical surgical procedure improves survival time in patients with recurrent ovarian cancer. Cancer. 1992;70:2129–2136 [DOI] [PubMed] [Google Scholar]
- 22. Vaccarello L, Rubin SC, Vlamis V, Wong G, Jones WB, Lewis JL. Cytoreductive surgery in ovarian carcinoma patients with a documented previously complete surgical response. Gynecol Oncol. 1995;57:61–65 [DOI] [PubMed] [Google Scholar]
- 23. Zang RY, Zhang ZY, Li ZT, et al. Effect of cytoreductive surgery on survival of patients with recurrent epithelial ovarian cancer. J Surg Oncol. 2000;75:24–30 [DOI] [PubMed] [Google Scholar]
- 24. Ayhan A, Gultekin M, Taskiran C, et al. The role of secondary cytoreduction in the treatment of ovarian cancer: Hacettepe University experience. Am J Obstet Gynecol. 2006;194(1):49–56 [DOI] [PubMed] [Google Scholar]
- 25. Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564 [DOI] [PubMed] [Google Scholar]
- 26. Munkarah A, Levenback C, Wolf JK, et al. Secondary cytoreductive surgery for localized intraabdominal recurrences in epithelial ovarian cancer. Gynecol Oncol. 2001;81:237–241 [DOI] [PubMed] [Google Scholar]
- 27. Amara DP, Nezhat C, Teng NN, Nezhat F, Rosati M. Operative laparoscopy in the management of ovarian cancer. Surg Laparosc Endosc. 1996;6:38–45 [PubMed] [Google Scholar]
- 28. Benedetti Panici P, Scambia G, Baiocchi G, et al. Technique and feasibility of systematic para-aortic and pelvic lymphadenectomy for gynecological malignancies. Int J Gynecol Cancer. 1991;1:133–140 [Google Scholar]
- 29. Tebes SJ, Sayer RA, Palmer JM, et al. Cytoreductive surgery for patients with recurrent epithelial ovarian carcinoma. Gynecol Oncol. 2007;106(3):428–427 [DOI] [PubMed] [Google Scholar]
- 30. Tian WJ, Jiang R, Cheng X, et al. Surgery in recurrent epithelial ovarian cancer: benefits on Survival for patients with residual disease of 0.1–1cm after secondary cytoreduction. J Surg Oncol. 2010;101(3):244–250 [DOI] [PubMed] [Google Scholar]
- 31. Shih KK, Chi DS, Barakat RR, Leitao MM., Jr Tertiary cytoreduction in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer: an updated series. Gynecol Oncol. 2010;117(2):330–335 [DOI] [PubMed] [Google Scholar]
- 32. Trinh H, Ott C, Fanning J. Feasibility of laparoscopic debulking with electrosurgical loop excision procedure and argon beam coagulator at recurrence in patients with previous laparotomy debulking. Am J Obstet Gynecol. 2004;190(5):1394–1397 [DOI] [PubMed] [Google Scholar]
- 33. Berek JS, Hacker NF, Lagasse LD. Survival of patients following secondary cytoreductive surgery in ovarian cancer. Obstet Gynecol., 61(2): pp. 189–193, 1983 [PubMed] [Google Scholar]