Biomimetic matrix material may be suitable for the long-term repair of inguinal hernia.
Keywords: Revive, biomimetic, synthetics, biologics, hernia
Abstract
Background and Objectives:
Materials utilized for the repair of hernias fall into 2 broad categories, synthetics and biologics. Each has its merits and drawbacks. The synthetics have a permanent, inherent strength but are associated with some incidence of chronic pain. The biologics rely on variable tissue regeneration to give strength to the repair, limiting their use to specific situations. However, thanks to their transient presence and tissue ingrowth, the biologics do not result in a significant incidence of chronic pain. We studied the use of a biomimetic (REVIVE, Biomerix Corporation, Fremont, CA) in this setting in an attempt to obviate the disadvantages of each material.
Methods:
Fourteen patients underwent laparoscopic repair by totally extraperitoneal and transabdominal preperitoneal techniques of 16 inguinal hernias. Follow-up was as long as 19 mo, and 8 patients were followed for > 12 mo. There were no recurrences and a 5% incidence of functionally insignificant discomfort.
Results:
REVIVE is shown in histology and in vivo to demonstrate regeneration and tissue ingrowth into the polycarbonate/polyuria matrix similar to that in the biologics rather than scarring or encapsulation. There were no recurrences, indicating its strength and resilience as a permanent repair similar to that in the synthetics.
Conclusion:
This is proof of the concept that a biomimetic may bridge the gap between the biologics and synthetics and may be able to be utilized on a regular basis with the benefits of both materials and without their drawbacks.
INTRODUCTION
The repair of an inguinal hernia is the most commonly performed abdominal general surgery procedure.1 Over time, many aspects of the surgical technique to repair inguinal hernias have evolved, namely the approach and type of mesh material used to reinforce the repair. Surgical technique has progressed from a primary repair with sutures to the tension-free Lichtenstein repair. In recent years, laparoscopic surgery has emerged as a viable approach, further advancing the treatment of inguinal hernia. Today, the types of mesh material available to surgeons are numerous, with offerings ranging from synthetics like polypropylene or polyester to biologics derived from human and animal tissue. While the type of mesh material has evolved, there is no gold standard, and the best mesh material for use in the repair of an inguinal hernia remains a source of debate amongst surgeons.
Central to a physician's decision as to which mesh material to use is the consideration given to limiting chronic pain. The incidence of chronic pain in inguinal hernia repairs, a clinical outcome across all types of surgical approaches, ranges from 10% to 15%.2 It is believed that the type of mesh material used to reinforce an inguinal hernia repair plays a role in chronic pain.
Materials used in the repair of hernias fall into 2 broad categories, synthetics and biologics, each with its own advantages and disadvantages. Synthetics have a permanent degree of inherent strength but are believed to cause more chronic pain that results from the formation of scar plate and unorganized tissue healing response.3,4 Physicians looking for a more natural healing solution that is not a permanent implant have moved toward the use of biologics, in favor of a remodeling biomaterial that leaves the patient essentially de novo. Biologics facilitate a more natural pattern of host tissue infiltration. However, their use is limited, because they are costly, they have an inherent inability to bridge defects, and in some instances they may cause eventration and reherniation.5 Despite biologics' promise to restore the body's natural structure, the failure of these materials to provide a reliable repair across all types of hernias (i.e., direct and bridging repairs of ventral hernias) has prompted some surgeons to suggest that with improvements in tissue engineering, materials can be developed to eliminate these deficiencies, resulting in biomaterials that will become the standard of care in hernia repairs 10 y from now.6
This study evaluates REVIVE (Biomerix Corporation, Fremont, CA) surgical mesh, which consists of a novel biomaterial that combines the favorable properties of a biologic to support robust tissue ingrowth with the durability of a synthetic. The permanent structure of the mesh allows it to bridge defects, creating a strong mechanical repair, while its unique microarchitecture supports tissue ingrowth and formation (Figure 1).
Figure 1.
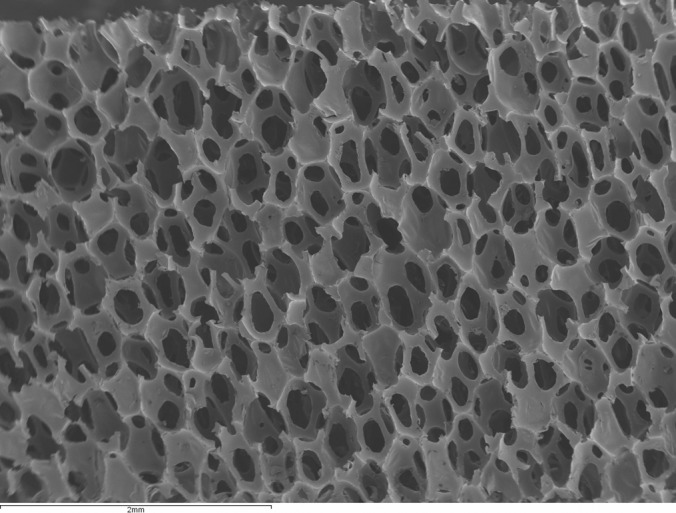
Scanning electron microscope image of the Revive matrix.
METHODS
In a single-arm evaluation study for the repair of inguinal hernias, 14 patients underwent laparoscopic repair with REVIVE. A total of 16 inguinal hernias were treated across the 14 patients.
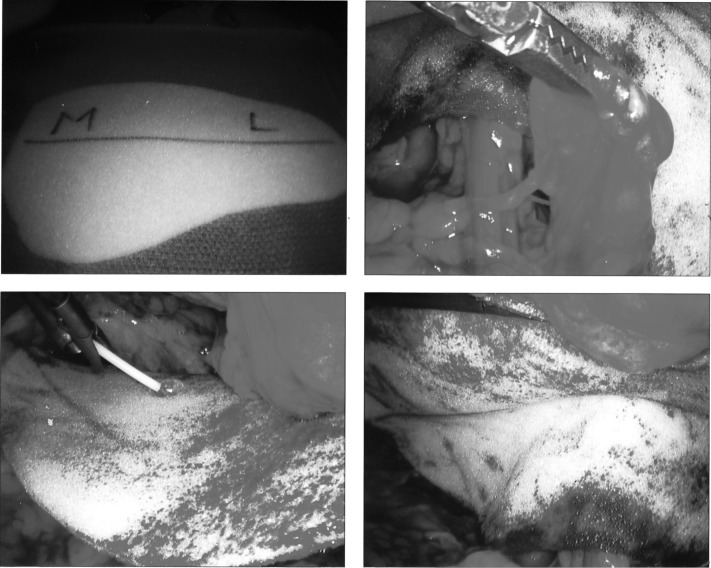
The only criterion for inclusion was the patient's suitability to undergo a laparoscopic inguinal hernia repair under general anesthesia. All procedures were elective and were performed with general anesthesia at an outpatient surgery center. Patients were informed of the intended use of an FDA-approved mesh, as well as the relative merits of the various materials that could be used. A preoperative antibiotic was administered to each patient: 2g of Ancef, or if the patient was allergic to penicillin, 900 mg of clindamycin. Repairs were accomplished laparoscopically using either a totally extraperitoneal or a transabdominal preperitoneal approach. A preshaped section of REVIVE mesh was placed from the midline of the pubis to the iliac crest. REVIVE is composed of a lightweight polypropylene mesh with 2 thin layers of a proprietary biomaterial bonded to both sides and designed to facilitate biointegration. The biomaterial is a nondegradable, reticulated polycarbonate-polyurethane-urea consisting of a 3-dimensional, open-cell, macroporous structure that selectively adsorbs plasma and extracellular matrix proteins, thus paving the way for cells to migrate, proliferate, attach, and regenerate new tissue. For totally extraperitoneal (TEP) repairs (Figure 2), fibrin glue (Tisseel, Baxter, Deerfield, IL) was used to fixate the mesh, while titanium tacks (ProTack, Covidien, Mansfield, MA) were used for (transabdominal preperitoneal) TAPP procedures (Figure 3). A Foley catheter was placed in the patients undergoing the TAPP repair but not in patients undergoing the TEP repair.
Figure 2.
The Revive matrix in a right inguinal Totally Extraperitoneal repair with fibrin tissue sealant fixation. Clockwise from the upper left; the Revive matrix, cut to shape, the reduced hernia sac held on the peritoneal side of the matrix repair, application of fibrin tissue sealant and, the matrix repair in position.
Figure 3.
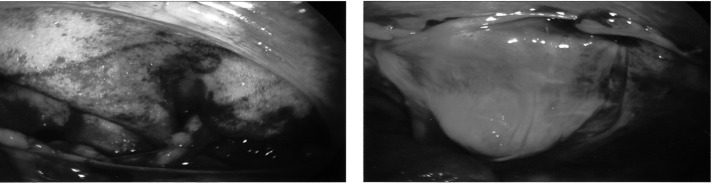
A Transabdominal Preperitonel repair. On the left, the matrix in the preperitoneum. On the right, the repair, reperitonealized.
Postoperative data included length of stay, infection, pain, and functional status. All patients were discharged the same day and re-evaluated at 2 wk. Outcomes were based on recurrence rates in the patients who were at least 12 mo out from their repair. Additionally, each patient was asked to fill out a simple questionnaire to evaluate the impact of any long-term pain. On the questionnaire, pain was characterized as either no pain, occasional twinges, or chronic daily pain that affects activities.
Seven patients were followed up for an average period of 25 mo (range, 18 to 33). Of the remaining patients, the follow-up period averaged 6 mo (range, 6 to 7).
RESULTS
Fourteen patients with a total of 16 inguinal hernia repairs were implanted with REVIVE. Of the 14 patients, half were followed for at least 18 mo, with an average follow-up of 25 mo. The average age of these 7 patients was 59 y, and the group consisted of 1 female and 6 males. These 7 patients had a total of 8 hernias, all of them indirect in nature.
Across all 14 patients, there were no (0%) hernia recurrences. Using a functional pain scale of no pain, pain with no limitations on activity, and pain that limits on activity, only 1 of 14 patients (93% No Pain, 7% Mild Pain) reported pain with limitations on activity at 6 mo. The one patient reported occasional pain that, in his words, resolved with activity and completely resolved at 24 mo. He had undergone a totally extraperitoneal repair with fibrin glue fixation, typical of many synthetics.
DISCUSSION
Since the introduction of the Bassini technique, characterized as a “tension” repair, numerous surgical methods have been developed and utilized in the repair of inguinal hernias. Most notable is the Lichtenstein “tension-free” open repair involving the placement of a synthetic mesh, such as a polypropylene, to strengthen the inguinal region. In the United States, as today's standard of care for the treatment of inguinal hernias, the Lichtenstein repair has resulted in low recurrence rates. However, one aspect of inguinal hernia surgeries that has not changed is the rate of chronic pain consistently reported across various surgical techniques and types of materials used as mesh reinforcement.7–9 To better understand the role of mesh and its potential influence on pain, it is important to understand the evolution of mesh materials and the perceived advantages and disadvantages of the various mesh materials available today.
For the past 20 y, advanced laparoscopic surgery techniques have been utilized for the repair of hernias, such as the use of plugs, intraperitoneal onlay patches, and totally extraperitoneal and transabdominal preperitoneal repairs. In laparoscopic repairs, surgeons have used polypropylene meshes and more recently polyester weaves. Initially, surgeons embraced the use of synthetic meshes that were heavy and bulky. However, over time, studies of heavyweight meshes have shown that these meshes can often result in the formation of a scar plate rather than production of healthy tissue incorporation, which may lead to more chronic pain.10 Although, synthetic meshes provide a durable repair at a low cost, the long-term potential for chronic pain is a significant drawback.
The introduction of biologics has provided surgeons with a permanent repair for the treatment of inguinal hernias without leaving a permanent implant. Biologics offer initial strength to the repair and are equivalent in strength to a synthetic prosthesis in the early stages of healing, and over time they are remodeled by the body creating a more natural healing response that may decrease the risk of long-term pain. However, the limitations of biologics make them a less desirable alternative for all inguinal hernia repairs. The high cost associated with biologics also makes them a less attractive option. Additionally, the lack of reliable ingrowth across the entire span of the matrix results in a failure to develop a permanent structure and maintain a durable repair, leading to eventration and in some cases reherniation, known as an inability to ‘bridge’ the defect in large ventral and direct inguinal hernias. There have, however, been few trials comparing the benefits of biologics and synthetics in this role.1
Manufacturers have tried to develop newer, more flexible polypropylene and polyester materials, with the promise of incorporation rather than encapsulation. The ideal material for the repair of inguinal hernias should result in a low rate of recurrence. Secondly, it should be well tolerated, causing little pain or limitation on a patient's activities. Lastly, it should be inexpensive. We believe the REVIVE surgical mesh demonstrates these ideal biomaterial attributes, making it a compelling alternative to existing synthetics and biologic mesh offerings.
REVIVE surgical mesh consists of an open-cell, 3-dimensional, interconnecting macroporous structure designed to play a role similar to the extracellular matrix. Preclinical research shows that REVIVE leads to excellent tissue regeneration and tissue ingrowth into the polycarbonate polyurethane-urea, as opposed to scarring or encapsulation typically seen with other synthetic alternatives. Its permanent structure offers the mechanical support of synthetics, while being highly permeable (average 380, Darcy's permeability constant). This makes it an ideal scaffold to support predictable organized fibrovascular tissue ingrowth. Further, other studies have shown that at 6 mo REVIVE demonstrates minimal shrinkage, a demonstrated trait of synthetics that can result in recurrence and pain.
Early outcomes of laparoscopic inguinal hernia repair utilizing REVIVE were promising. Despite the small sample size, we believe the results of this study are a directional indication of REVIVE's clinical performance and, while not a large enough sample for a definitive conclusion, does represent proof of concept justifying further evaluation. The absence of recurrences indicates its strength and resilience as a permanent repair similar to other synthetics. Overall, a favorable outcome was reported for chronic pain with this sample population. At 6 mo, only 1 out of 14 patients (7%) reported pain that resolved with activity. While the precise incidence of chronic pain after hernia repair is unknown, select studies suggests 20% of patients are affected, and an estimated 12% report the intensity of pain impairs some aspects of their daily activity.12 The low rate of pain reported in this study calls for broader analysis to study the benefits of REVIVE and how it could lead to a reduction in pain following an inguinal hernia repair.
CONCLUSION
These surgical cases provide a proof of concept that a REVIVE mesh may be utilized on a regular basis, leveraging the benefits associated with synthetics and biologics without their inherent drawbacks.
References:
- 1. Laparoscopic v. open repair of groin hernia: a randomized comparison. The MRC Laparoscopic Hernia Trial Group. Lancet. 1999;354(9174):185–190 [PubMed] [Google Scholar]
- 2. Fine AP. Laparoscopic repair of inguinal hernia using Surgisis mesh and fibrin sealant, JSLS. 2006;10(4):461–465 [PMC free article] [PubMed] [Google Scholar]
- 3. Schumpelick V, Fitzgibbons R. Hernia Repair Sequelae. 1st Edition, NY, NY: Springer; 2010 [Google Scholar]
- 4. Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12(1):63–69 [DOI] [PubMed] [Google Scholar]
- 5. Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix – an expensive hernia sac. Am J Surg. 2008;196(1):47–50 [DOI] [PubMed] [Google Scholar]
- 6. The Use of Biologic Materials for Hernia Repair. General Surgery News. March 2008;. 35(3) [Google Scholar]
- 7. Dennis R, O'Riordan D. Risk factors for chronic pain after inguinal hernia repair. Ann R Coll Surg Engl. 2007;89(3):218–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leardi S. Postoperative pain as complication of hernia surgical repair with mesh and plug. Chir Ital. 2003;55(60):887–891 [PubMed] [Google Scholar]
- 9. Piccio M. Randomized controlled trial of preservation or elective division of illioinguinal nerve on open inguinal hernia repair with polypropylene mesh. Arch Surg. 2004;139(7):755–758 [DOI] [PubMed] [Google Scholar]
- 10. Earle D, Romanelli J. Prosthetic materials for hernia: what's new. Contemp Surg. 2007;63(2)63–69 [Google Scholar]
- 11. Ansaloni L, Catena F, D'Alessandro L. Prospective randomized, double-blind, controlled trial comparing Lichtenstein's repair of inguinal hernia with polypropylene mesh versus urgisis gold soft tissue graft: preliminary results. Acta Biomed. 2003;74(Suppl 2):10–14 [PubMed] [Google Scholar]
- 12. Aasvang E, Kehlet H. Surgical management of chronic pain after inguinal hernia repair. Br J Surg. 2005;92:795–801 [DOI] [PubMed] [Google Scholar]