Abstract
Two ladybeetles, Cycloneda sanguinea and Harmonia axyridis, were exposed in the laboratory to eight fungicide formulations commonly used in citrus production in Florida. Both benomyl and the combination of copper and petroleum oil proved toxic to larvae of C. sanguinea that were exposed to concentrations corresponding to recommended field rates, either as leaf residues or in topical spray applications. Larvae of C. sanguinea also suffered significant mortality when exposed to neem oil as a leaf residue, but not after topical application. Larvae of H. axyridis exposed to these compounds completed development with the same success as control larvae in all trials. However, H. axyridis larvae exhibited slower development following exposure to leaf residues of ferbam applied at twice the recommended rate. Exposure to azoxystrobin as a leaf residue at twice the recommended concentration resulted in accelerated larval development in both species. No compounds appeared repellent to adult beetles of either species. Adult beetles of both species were observed resting on portions of filter paper treated with fosetyl-Al more often than on untreated, control portions. Azoxystrobin, ferbam and mefenoxam similarly arrested the movement of adult C. sanguinea, whereas benomyl and the copper and petroleum oil combination arrested the movement of adult H. axyridis. The differential sensitivity of the two coccinellid species is discussed in the context of the potential displacement of the indigenous C. sanguinea by the invasive H. axyridis.
Keywords: Cycloneda sanguinea, development, Harmonia axyridis, repellency, toxicity
Introduction
The ‘blood-red’ ladybeetle, Cycloneda sanguinea, an indigenous species, and the multi-colored Asian ladybeetle, Harmonia axyridis, an introduced species, are both important generalist predators in Florida citrus. Both species are important predators of the brown citrus aphid, Toxoptera citricida (Michaud, 1999), and the Asian citrus psyllid, Diaphorini citri, (Michaud, 2000), although the full range of their diets is not known. C. sanguinea has been observed to consume immature stages of Florida red scale, Chrysomphalus aonidum (van Brussel & Bhola, 1970), Australian red scale, Aonidiella aurantii (de Crouzel et al., 1979), green scale, Coccus viridis (Sousa & Perez, 1977), whitefly, Bemisia tabaci (Link et al., 1980), eggs of Heliothis virescens (McDaniel & Sterling, 1979), Empoasca sp. (Cotte & Cruz, 1989) and eggs of the phytophagous coccinellid Epilachna varivestis (Chicas & Maes, 1993). H. axyridis originates in China, although insects released in the USA were imported from Japan (McClure, 1986a). This species is reported to prey on various scale families (McClure, 1983; 1986b; Shi et al., 1997), Lepidoptera (Kim & Noh, 1968), Heteroptera (Fujisaki, 1975), and even mites (Lucas et al., 1997). These two generalist predators likely contribute significant biological control to a wide range of pest insects in citrus. Despite the fact that C. sanguinea and H. axyridis are the most abundant coccinellid species present in Florida citrus, their sensitivity to most of the agrochemicals frequently applied in citrus has never been tested.
Citrus in Florida suffers from many diseases caused by fungal pathogens including the citrus scab (Elsinoe fawcetti), post-bloom fruit drop (Colletotrichum acutatum), melanose (Diaprthe citri), greasy spot (Mycosphaerella citri), alternaria (Alternaria alternata), and phytophthora (Phytophthora nicotianae). Consequently a wide range of fungicides are employed to protect seedlings, foliage and developing fruit during various periods. Windows of particular susceptibility to disease include flowering and periods of leaf expansion. These periods are also times of peak activity for insects such as aphids and psyllids (which feed exclusively on new citrus growth) and the coccinellids that feed on them. This study examines how eight fungicides frequently employed in Florida citriculture can affect the developmental success of larvae, and the behavioral responses of adults, of C. sanguinea and H. axyridis.
Materials and Methods
Stock Colonies
Larvae of Cycloneda sanguinea L., and Harmonia axyridis Pallas were reared on a diet of frozen Ephestia sp. eggs, bee pollen, water and a liquid diet formulation (Entomos Inc., 4445 SW 35th Terrace, Gainesville, Fla, 32608) in individual plastic Petri dishes (5.5 cm × 1.0 cm). The water and liquid diet were encapsulated in polymer beads (Entomos) that permitted both larvae and adults to access the contents, while maintaining a low relative humidity in the dishes. Adult beetles of both species were maintained in 1 L ventilated glass mason jars (∼ 50 – 90/jar) filled with strips of shredded wax paper for their first 9–12 days of life following eclosion. The jars were kept in a greenhouse maintained at 24 ± 2°C, 60 ± 5% RH, with natural lighting and provisioned with water (on a wick), the liquid diet (presented in stretched Parafilm® domes), bee pollen, and frozen eggs of Ephestia sp. on a daily basis. Adult females were transferred to individual plastic Petri dishes (5.5 cm × 1.0 cm) for oviposition. Ovipositing females were provided with water beads, diet beads, frozen Ephestia sp. eggs, and bee pollen as necessary. Eggs were harvested daily and held in an incubator at 24°C, 60 ± 5 % RH under fluorescent light (L:D 16:8) and hatched ca. 3.5 ± 0.5 days later under these conditions. Larvae were provided with Ephestia sp. eggs for their first 24 h of life and were used for experiments on the morning of the second day when they were 24 ± 6 h old.
Fungicides
Eight different fungicidal formulations were selected for testing against both coccinellid species. These were: (1) fosetyl-Al at concentrations of 0.05 and 0.1 % (Aliette® WDG, Rhône-Poulenc Ag. Co.) recommended for control of brown rot caused by Phytophthora spp.; (2) azoxystrobin at concentrations of 0.025 and 0.05 % (Abound® 2.08F, Zeneca Ag. Products) recommended for control of Alternaria, citrus scab, melanose and greasy spot; (3) benomyl at concentrations of 0.12 and 0.24 % (Benlate 50 WP, Dupont, Inc.) recommended for control of citrus scab, post-bloom fruit drop; (4) Copper at concentrations of 0.12 and 0.24 % (40% atofap, Elf Atochem Corporation) + petroleum oil (Sunspray® 9E, Sunoco Inc. [R & M], Ten Penn Center, 1801 Market St., Philadelphia, PA, 19103-1699) at concentrations of 1.0 and 2.0%, recommended for control of Alternaria, brown rot, citrus scab, greasy spot and melanose; (5) fenbuconazole at concentrations of 0.0125 and 0.025 % (Enable® 4F, Rohm & Haas Co.) recommended for control of greasy spot; (6) ferbam at concentrations of 0.18 and 0.36 % (Ferbam®, UCB Chemicals Corporation) recommended for control of Alternaria, citrus scab and post-bloom fruit drop; (7) neem oil at the concentration of 1.0 % (Trilogy Oil 70 EC, Thermo-Trilogy Corporation) recommended for control of Alternaria; (8) mefenoxam at concentrations of 0.24 and 0.47 % (Ridomil® Gold 4 EC, Ciba-Geigy Corporation) recommended for control of foot and root rots caused by Phytophthora spp. Fungicide concentrations were calculated on a weight-to-weight basis for wettable powders, and a volume-to-volume basis for liquid formulations. The initial fungicide concentrations tested approximated field rates as recommended in the 2000 Florida Citrus Pest Management Guide (Knapp, 2000), assuming application in 935 L of water per ha (100 gallons of water per acre). When no significant toxicity was observed at the recommended rate, an additional test was performed at double that concentration. Since materials are typically applied on citrus trees in volumes ranging from 100 to 300 gallons per acre (depending on available equipment and the nature of tank mixes) the initial concentrations employed in these experiments represented the ‘high end’ of the concentration range to which beetles would likely be exposed under field conditions.
Topical Spray Toxicity Assays
In topical spray assays (hereafter, topical assays) coccinellid larvae 24 (± 6) h-old were placed into plastic Petri dishes (5.5 cm × 1.0 cm) in groups of eight. Larvae in control groups (n = 16) were sprayed with 1 ml of distilled water in a Potter Precision Spray Tower (Burkard Manufacturing Co. Ltd., Rickmansworth Herts, UK); larvae in treatment groups (n = 16) were sprayed with 1 ml aqueous solution of the test material. Larvae were then individually transferred to clean plastic Petri dishes and provisioned with Ephestia sp. eggs, water beads, diet beads and bee pollen. Dishes were checked for mortality after 4 and 24 h, and daily thereafter. Larvae were provided with fresh food every 3 days until pupation or death. Dates of pupation and adult eclosion were recorded for each replicate. Mortality data were corrected using Abbott's formula (Abbott, 1925) and analyzed using a Chi-square Goodness-of-fit test. Data for larval developmental time were analyzed by one-way ANOVA (SPSS, 1998).
Residue Toxicity Assays
Freshly picked grapefruit leaves were washed in a 0.005% solution of Chlorox® bleach, rinsed in distilled water, and dried on paper towels. Leaf disks 3 cm in diameter were punched from the leaves using a sharpened piece of steel pipe. Leaf disks in treatment series (n = 16) were sprayed with 1 ml of aqueous solution of the test material in a Potter Precision Spray Tower. Leaf disks in control series (n = 16) were sprayed with 1 ml distilled water. Both treated and control leaf disks were placed into numbered plastic Petri dishes (3.5 cm × 1.0 cm) and a small measure of Ephestia sp. eggs (ca. 0.05 g) was placed in the center of each disk. Single, 24 (± 6) h-old coccinellid larvae were then transferred to each dish. Larvae were transferred to clean plastic Petri dishes (5.5 cm × 1.0 cm) after 24 h exposure to residues and provisioned with Ephestia sp. eggs, bee pollen, diet beads and water beads. All subsequent maintenance, monitoring and analytical procedures were identical to those employed in the topical assays.
Repellency Assays
Repellency assays were carried out using adult beetles of both species from stock colonies. Circular filter papers (9.0 cm diameter) were divided in half by a pencil line. The treatment half of each filter disk was sprayed with 1 ml aqueous solution of the test material in a Potter Precision Spray Tower while the other (control) half was covered with a half piece of filter disk. Filter disks were then placed individually into plastic Petri dishes (9.0 cm × 1 cm), the walls of which were coated with a fluon® solution (Australian Entomological Supplies, PO Box 250, Bangalow, NSW 2479, Australia) applied to prevent climbing of the insects and oblige them to remain on the paper surface. Twenty adult beetles were then transferred to the dishes, one per dish, and observed periodically at intervals of 10 to 15 minutes for a total of 12 observations. Only beetles that were resting on either the treated or untreated side of the filter disks were tallied for their position during each observation; beetles that were moving or that straddled the boundary were excluded from tallies during a particular observation. Following each observation, beetles were gently disturbed with a sable hair brush to cause them to change position prior to the subsequent observation. The data were analyzed using a paired t-test (SPSS, 1998).
Results and Discussion
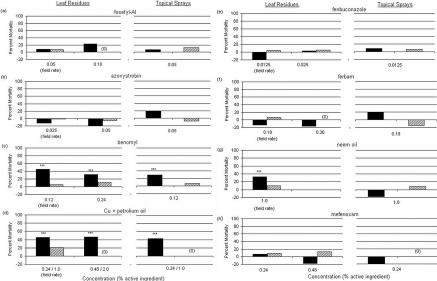
Benomyl and the combination of copper and petroleum oil both resulted in significant mortality to C. sanguinea larvae at all concentrations tested (Fig. 1c & d), but were not toxic to H. axyridis larvae. Larvae of C. sanguinea suffered significant mortality when exposed to neem oil as a leaf residue applied at the recommended rate, but not when it was topically applied at the same rate. The sensitivity of C. sanguinea to benomyl is cause for concern since this compound is frequently applied during flowering to protect against post-bloom fruit drop (Knapp, 2000). Many species of coccinellids, including C. sanguinea and H. axyridis, are attracted to blooming citrus groves and can be found feeding on nectar and pollen within citrus flowers.
Figure 1.
(Left and continued above) Percent mortality (corrected using Abbott's formula) of coccinellid larvae (n = 16 in all trials; solid bars, Cycloneda sanguinea; hatched bars, = Harmonia axyridis) exposed to different concentrations of fungicides as leaf residues and in topical spray applications: (a) fosetyl-Al, (b) axoxystrobin, (c) benomyl, (d) copper + petroleum oil, (e) fenbuconazole, (f) ferbam, (g) neem oil, (h) mefenoxam. Fungicide concentrations were calculated on a weight-to-weight basis for wettable powders, and a volume-to-volume basis for liquid formulations. ‘Field rate’ indicates the concentration corresponding to the recommended field rate assuming application in 935 L water per ha. ‘(0)’ indicates identical survival between treatment and controls; blanks indicate experiments were not performed at that concentration. Asterisks indicate level of significance: * P < 0.05, ** P < 0.01, *** P < 0.001.
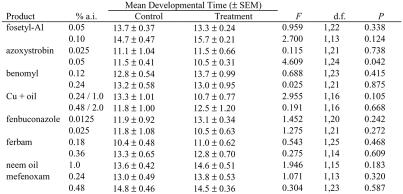
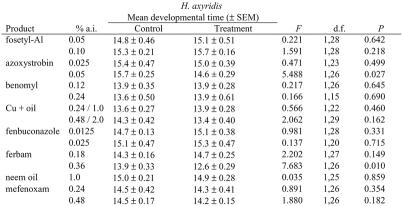
Exposure to azoxystrobin as a leaf residue at twice the recommended concentration resulted in significantly faster larval development in both coccinellid species (Tables 1 & 2). Larvae of H. axyridis displayed significantly slower larval development when exposed to leaf residues of ferbam applied at twice the recommended rate (Table 2). Slower development can be interpreted as a detrimental side-effect of exposure to the material, but the faster development of larvae exposed to azoxystrobin treatment is more difficult to interpret. Developmental rate is affected by diet in both species and faster development is sometimes associated with larger body size in the resulting adults (Michaud, 2000). Since we did not compare either the weights of eclosing beetles in these experiments, or their fecundity or fertility, we cannot conclude whether the faster development induced by exposure to abnormal chemical stimuli was either beneficial or detrimental.
Table 1.
Mean larval developmental times (± SEM) for Cycloneda sanguinea larvae exposed to leaf residues of various fungicides for 24 h. Data were compared by one-way ANOVA.
Table 2.
Mean larval developmental times (± SEM) for Harmonia axyridis larvae exposed to leaf residues of various fungicides for 24 h.
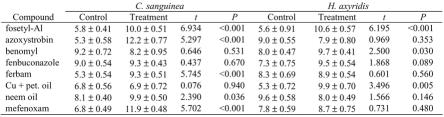
No fungicide formulations appeared repellent to adults of either species of beetle and no mortality of beetles occurred during any trial (Table 3). Five of the eight compounds, fosetyl-Al, azoxystrobin, ferbam, neem oil and mefenoxam, acted to retain adult C. sanguinea on treated surfaces, rather than repel them, whereas fosetyl-Al, benomyl and copper + petroleum oil had a similar effect on adult H. axyridis. It should be noted that this bioassay was designed to test the contact repellency of materials and that the arrested movement of beetles over treated surfaces should not be equated with ‘attraction’, i.e. there is no implication that beetles would orient toward such treated surfaces from any distance. Because the bioassay did not contrast bout lengths and bout frequencies of walking, standing, and feeding within the experimental arenas it is not possible to distinguish between repellency, attraction, and excitatory behaviors or interactions of these outcomes.
Table 3.
Mean numbers (± SEM) of Cycloneda sanguinea and Harmonia axyridis adults (n = 20) observed on treated versus control surfaces of Petri dish arenas over a series of 12 observations. Data were compared using a paired t-test.
All of the fungicides tested appeared to be generally innocuous to H. axyridis. Hassan et al. (1991) tested eight fungicides for toxicity to H. axyridis and found all of them to be harmless or only slightly harmful. Fosetyl-Al, azoxystrobin, ferbam and mefenoxam had no apparent detrimental effects on the survival or development of larvae of either species. Bartlett (1963) found ferbam to be non-toxic to six species of coccinellids, but only adult stages were exposed to residues in this study and larval stages are likely to be far more sensitive.
Larvae of C. sanguinea experienced significant mortality following exposure to three of the eight compounds, a possible indication that this species may be more sensitive than H. axyridis to a range of agrochemicals. Neem oil has been shown to be toxic to other Coleoptera (Sharma 1999; Reddy et al., 1999) and Heteroptera (Anjaneyulu et al., 1999). Villanueva-Jimenez & Hoy (1998) ranked neem as ‘semi-compatible’ with biological control of citrus leaf miner, Phyllocnistis citrella, by the parasitoid Ageniaspis citricola. However, neem oil has limited applications in citrus and is therefore unlikely to have much impact on C. sanguinea populations. The toxicity resulting from exposure to the copper and petroleum oil combination is of greater concern. According to the State Statistical Report on citrus chemical usage (Florida Agricultural Statistics Service, 2000) more acreage was treated with copper in Florida between 1993 and 1997 than with any other fungicide (62% of orange groves and 66% of grapefruit groves received at least one spray). However, the primary use of copper in citrus for processing is to protect trees against defoliation by greasy spot and the majority of these sprays are applied during summer months when most coccinellids are aestivating. Negative impacts on C. sanguinea populations are more likely to result from the copper applied to specialty citrus such as hybrid tangelo and mandarin varieties that require protection from alternaria. Applications in citrus for fresh market are more often applied during periods of leaf expansion in spring and fall, periods when coccinellids are active in groves.
It should be noted that exposure to residues created in a spray tower represents a worst-case scenario for toxicity testing. In order to obtain realistic estimates of their negative impacts on natural enemies, materials demonstrating toxicity under these conditions should be subjected to field trials where other variables such as uneven deposition patterns and weathering come into play.
Prior to 1998, C. sanguinea was the most abundant aphidophagous coccinellid in Florida citrus state-wide (Michaud, 2000), but recent observations suggest that H. axyridis is now the dominant species and field observations are on-going to monitor the relative abundance of both species. H. axyridis appears to be a strong competitor and may be in the process of displacing the native C. sanguinea. If this is true, the widespread use of copper and/or benomyl could serve to accelerate the disappearance of C. sanguinea from citrus since it is far more sensitive to these materials than is the invasive foreigner.
Acknowledgments
This work was made possible by grants from USDA, APHIS, PPQ and Funds for Rural America. The author wishes to thank J. Pohle, M. Baker and R. Villanueva for technical support. Florida Agricultural Experiment Station Journal Series No.R-08033.
References
- Abbott W.S.. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. [Google Scholar]
- Anjaneyulu GVSR, Varsha N, Pulla-Rao UDVP, Sateesh TVR, Mishra KD, Nayak V. Acute toxicity of neem oil to aquatic hemipteran predatory insect Notonecta sp. Environ Ecol. 1999;17:57–61. [Google Scholar]
- Bartlett B.R.. The contact toxicity of some pesticide residues to hymenopterous parasites and coccinellid predators. J Econ Entomol. 1963;56:694–698. [Google Scholar]
- Chicas JMS, Maes JM. 1993 Natural enemies of Epilachna varivestis Mulsant in El Salvador. Bol Inform CATIE. No. 28:1–4. [Google Scholar]
- Cotte O, Cruz C. Natural enemies of leafhopper of the genus Empoasca (Homoptera: Cicadellidae) in pigeon peas. J Agric Univ Puerto Rico. 1989;73:161–163. [Google Scholar]
- de Crouzel IS, Botto EN, Zanelli M, Vetrano A. Biological control experiment against the Australian red scale, Aonidiella aurantii (Maskell) on the citrus farm Ana-Cua in San Roque, Corrientes. Preliminary report. Revista Soc Entomol Argentina. 1979;38:47–61. [Google Scholar]
- Florida Agricultural Statistics Service. 2000 State Statistical Report. 1222 Woodward St., Orlando, Fla. 32803. [Google Scholar]
- Fujisaki K. Breakup and re-formation of colony in the first-instar larvae of the winter cherry bug, Acanthocoris sordidus Thunberg (Hemiptera: Coreidae), in relation to the defence against their enemies. Res Pop Ecol. 1975;16:252–264. [Google Scholar]
- Kim CW, Noh YT. Study on the natural enemies proper in Korea attacking fall webworm, Hyphantria cunea Drury. Entomol Res Bull. 1968;4:17–36. [Google Scholar]
- Knapp J. 2000 2000 Florida Citrus Pest Management Guide. University of Florida, Cooperative Extension Service, Institute of Food and Agricultural Sciences. [Google Scholar]
- Link D, Costa EC. Occurrence of natural enemies of the whitefly, Bemisia tabaci (Gennadius, 1889), on soybean crops. Rev Cen Cien Rur 1980. 1980;10:111–113. [Google Scholar]
- Lucas E, Coderre D, Vincent C. Voracity and feeding preferences of two aphidophagous coccinellids on Aphis citricola and Tetranychus urticae. Entomol Exp Appl. 1997;85:151–159. [Google Scholar]
- McClure MS. Lady beetle attacks red pine scale. Front Plant Sci. 1983;35:3–4. [Google Scholar]
- McClure MS. Importing ladybird beetles to control red pine scale. Front Plant Sci. 1986a;39:5–7. [Google Scholar]
- McClure MS. Role of predators in regulation of endemic populations of Matsucoccus matsumurae (Homoptera: Margarodidae) in Japan. Environ Entomol. 1986b;15:976–983. [Google Scholar]
- McDaniel SG, Sterling WL. Predator determination and efficiency on Heliothis virescens eggs in cotton using 32P2. Environ Entomol. 1979;8:1083–1087. [Google Scholar]
- Michaud JP. Sources of mortality in colonies of the brown citrus aphid, Toxoptera citricida. Biocontrol. 1999;44:347–367. [Google Scholar]
- Michaud JP. 2000 The Asian citrus psyllid, Diaphorini citri, and its natural enemies. Citrus Industry, August issue pp. 42–44. [Google Scholar]
- Reddy MU, Bharati SR, Reddy DDR. Efficacy of some vegetable oils as protectants against the pulse beetle (Callosobruchus chinensis) in green gram (Phaseolus aureus) during storage. Ind J Nutr Diet. 1999;36:436–442. [Google Scholar]
- Sharma RK. Efficacy of neem products against storage pests in maize. Ann Agr Res. 1999;20:198–201. [Google Scholar]
- Shi GL, Liu XQ, Li J, Li LC, Yang FD. Study on the bionomics of Quadraspidiotus perniciosus and its infestation pattern. Sci Silv Sin. 1997;33:161–167. [Google Scholar]
- Sousa JG, Perez PG. Preliminary results on the population dynamics of Coccus viridis (Green), Aphis spiraecola (Patch) and Toxoptera aurantii (Boyer) in an orange grove. Rev Cien Fac Cien Agric. 1977;4:43–56. [Google Scholar]
- SPSS. 1998 SPSS 8.0 for Windows. SPSS Inc., Chicago, Illinois. [Google Scholar]
- van Brussel EW, Bhola B. The biology and control of the Florida red scale, Chrysomphalus ficus Ashm. Surinaamse Landbouw. 1970;18:64–76. [Google Scholar]
- Villanueva-Jimenez JA, Hoy MA. Toxicity of pesticides to the citrus leafminer and its parasitoid Ageniaspis citricola evaluated to assess their suitability for an IPM program in citrus nurseries. BioControl. 1998;43:357–388. [Google Scholar]