A laparoscopic transperitoneal approach allowed for resection of a pulmonary sequestration avoiding an open thoracoabdominal incision.
Keywords: Laparoscopy, Bronchopulmonary sequestration
Abstract
Pulmonary sequestration is a rare cystic malformation composed of bronchopulmonary tissue that is discontinuous from the tracheobronchial tree and has an anomalous systemic blood supply. We present a case of a 40-y-old male who presented with an extralobar pulmonary sequestration and underwent a laparoscopic retroperitoneal mass excision. Preoperative imaging revealed a large 11.3-cm retroperitoneal tumor consisting of a multiloculated cystic lesion. The patient was discharged home, and at 3-mo follow-up no complaints were reported.
INTRODUCTION
Pulmonary sequestration (PS) can be divided into an intralobar variant (75% to 86%) and an extralobar variant (14% to 25%).1 Only 8% of extralobar PSs are intraabdominal or retroperitoneal, and most are identified in the first decade of life in association with other anomalies.2 The definitive diagnosis is usually confirmed by pathological examination of a surgically removed specimen, but a wide incision is required. We report a case of a large extralobar PS that was laparoscopically treated.
CASE REPORT
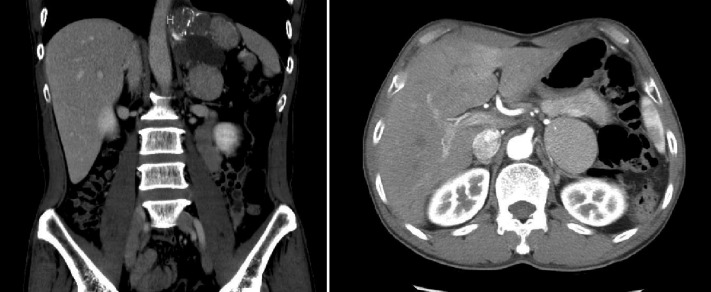
A 40-y-old man presented with left flank pain of 1-wk duration. He had no significant past medical history and no other urologic symptoms. Physical examination was normal. Laboratory studies that included a complete blood count, blood chemistries, urinalysis, and urine culture were within normal limits. A computed tomography (CT) scan revealed a large 11.3-cm tumor in the left retroperitoneum bordered by the upper-medial side of the kidney and the left subdiaphragmatic area (Figure 1). It appeared as a multiloculated cystic mass with a 5.5-cm solid component. The solid portion of the mass was in contact with the left adrenal gland, primarily in the superior portion. The mass did not enhance with contrast media. The cystic portion of the mass was multiseptated with multiple calcifications. Adrenal function testing, including 24-h hormonal testing, was performed, and all results were within normal limits. A decision was made to remove the mass via a laparoscopic approach.
Figure 1.
Computed tomography (CT) shows a tumor approximately 11.3 cm in size in the left retroperitoneum. The mass was multiloculated, containing cystic and solid component.
The laparoscopic procedure was performed using the standard retroperitoneal approach for left-sided laparoscopic adrenalectomy. Four trocars were used. The mass was densely adherent to the surrounding structures but was separate from the adrenal gland. Several feeding vessels were encountered at the medial side of the mass, suggestive of a systemic blood supply. The mass was resected completely and retrieved through an extension of the first port incision with a bag device.
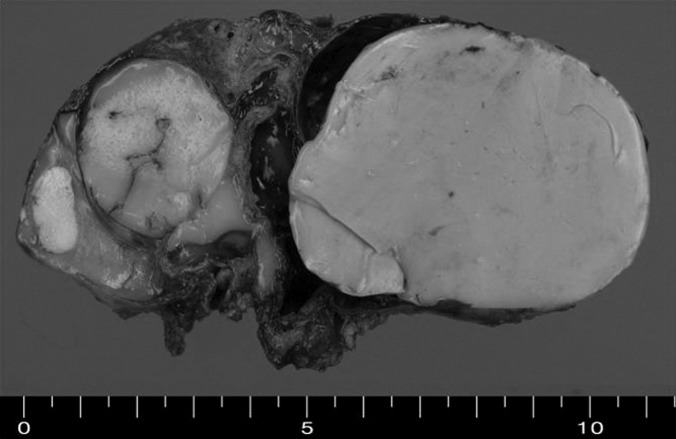
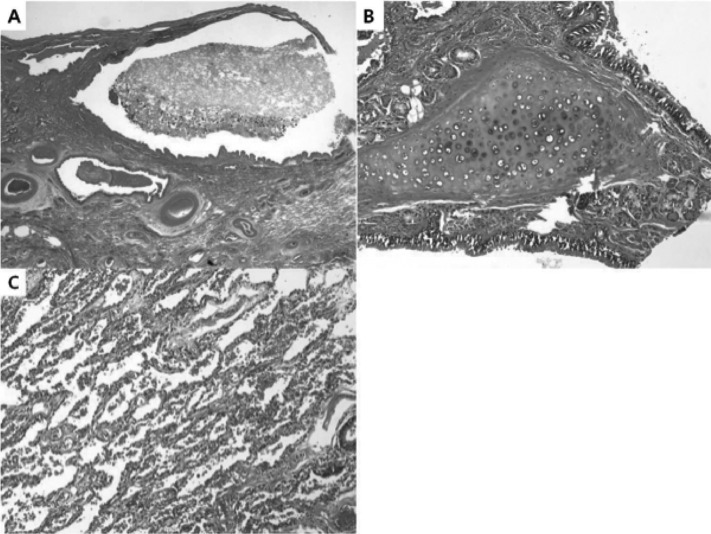
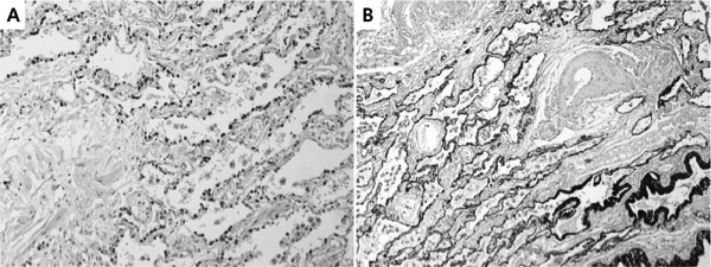
Grossly, the tumor was a multilocular solid-cystic mass measuring 10.5 cm×5.8 cm×4.9 cm. The multilocular cystic spaces ranged from 0.5 cm to 5 cm in diameter and were filled with homogenous rubbery brown solid clusters (Figure 2). Histologically, the cysts were lined by pseudostratified ciliated columnar epithelium (Figure 3). The underlying loose fibrovascular tissue showed hyaline cartilage, grouped seromucous glands, thick-walled vessels, strips of smooth muscles and focal alveolar structures that were positive for thyroid transcription factor-1 (TTF-1) and cytokeratin 7 (CK 7) on immunohistochemistry (Figure 4). The mass was covered by its own pleura. The cystic dilated bronchial structures contained hard mucous secretions, thereby giving the appearance of a multicystic solid mass. The final pathologic diagnosis was a retroperitoneal pulmonary extralobar sequestration.
Figure 2.
Gross photograph of the resected retroperitoneal extrapulmonary lobar sequestration. Macrocopic examination reveals a large encapsulated multilocular solid and cystic mass.
Figure 3.
Histological examination reveals dilated bronchial structures (A), lined by ciliated pseudostratified columnar epithelium, underlying loose fibrovascular tissue with hyaline cartilage and grouped seromucous glands (B). Alveolar structures are also present (C).
Figure 4.
Immunohistochemical finding shows alveolar structures positive for thyroid transcription factor–1 (TTF-1) (A) and cytokeratin 7 (CK 7) (B).
DISCUSSION
Pulmonary sequestration (PS) is a cystic or solid mass composed of nonfunctioning primitive tissue that does not communicate with the tracheobronchial tree. It is a type of congenital thoracic malformation. Its blood supply is from the systemic circulation (thoracic or abdominal aorta) rather than the pulmonary circulation. There are 2 forms of PS. One is intrapulmonary, which is surrounded by normal lung tissue. The other is extrapulmonary, which has its own pleural investment and is outside of the lung.3
The theory of pulmonary sequestration involves an accessory lung bud that develops from the ventral aspect of the primitive foregut. Early embryologic development of the accessory lung bud results in the sequestration forming within the normal lung tissue. This is the intrapulmonary variant, and the sequestration is encased within the same pleural covering of the normal lung. In contrast, later embryologic development of the accessory lung bud results in the extrapulmonary variant.4
Extralobar PS affects males approximately 4 times more often than females. It occurs on the left in 95% of cases. Of these, 75% are found in the costophrenic sulcus. They may also be found in the mediastinum, pericardium, and within or below the diaphragm. They are associated with other congenital malformations in more than 50% of cases, such as congenital diaphragmatic hernias, congenital adenomatoid malformation type II, and congenital heart disease. Due to the associated congenital malformation, more than 60% of patients present in the first 6 mo of life with respiratory distress, feeding difficulty, and congenital cardiac failure.1
In adulthood, subdiaphragmatic extralobar PS is often found as an incidental retroperitoneal mass on imaging studies, and accompanying symptoms are less common. Serum hormones should be examined if there is difficulty distinguishing the mass from an adrenal tumor.5,6 In our case, the hormonal tests were within normal limit.
The definitive diagnosis is usually confirmed by pathological examination of a surgically removed specimen. A diagnosis can be made without surgery by percutaneous tissue biopsy and imaging.6 However, usually the mass is located in an area not easily amenable to percutaneous biopsy.7
While the differential diagnosis of a suprarenal mass includes variable developmental anomalies and tumors, including neuroblastoma, the presence of systemic feeding vessels on color Doppler in the absence of raised urinary catecholamines narrows the differential.2 CT or magnetic resonance imaging (MRI) facilitates identification of the anatomical relationships of the lesion, including the origin of the systemic feeding vessels, allowing surgeons to plan for an optimal surgical approach.8 However, CT/MRI does not provide a conclusive diagnosis of extralobar PS,9 and a systemic supply to a subdiaphragmatic mass is not a definitive finding in establishing the diagnosis of PS. We could not find any evidence of systemic blood supply on CT, but it was visualized during surgery.
Management of an asymptomatic PS with no connection to the surrounding lung is controversial. At present, open surgery remains the best approach for definitive resection of intralobar or extralobar sequestration.10 Open surgery allows complete removal of the mass but has the disadvantage of requiring an invasive surgical incision (usually a thoracoabdominal incision). Laparoscopic removal of PS is a good alternative treatment in some cases. Although the size of the mass was large, we were able to successfully remove the tumor by a laparoscopic retroperitoneal approach.
CONCLUSION
We present a case of a large extralobar pulmonary sequestration that was difficult to diagnose with radiography alone. Laparoscopic management of the mass was successful despite its unusual size.
Contributor Information
Hee Jo Yang, Department of Urology, College of Medicine, Soonchunhyang University, Cheonan, Korea..
Seung Woo Lee, Department of Urology, College of Medicine, Soonchunhyang University, Cheonan, Korea..
Hyun Ju Lee, Department of Pathology, College of Medicine, Soonchunhyang University, Cheonan, Korea..
Ji Hye Lee, Department of Pathology, College of Medicine, Soonchunhyang University, Cheonan, Korea..
Youn Soo Jeon, Department of Urology, College of Medicine, Soonchunhyang University, Cheonan, Korea..
References:
- 1. Yucel O, Gurkok S, Gozubuyuk A, et al. Diagnosis and surgical treatment of pulmonary sequestration. Thorac Cardiovasc Surg. 2008;56:154–157 [DOI] [PubMed] [Google Scholar]
- 2. Laje P, Martinez-Ferro M, Grisoni E, Dudgeon D. Intraabdominal pulmonary sequestration. A case series and review of the literature. J Pediatr Surg. 2006;41:1309–1312 [DOI] [PubMed] [Google Scholar]
- 3. Flye MW, Conley M, Silver D. Spectrum of pulmonary sequestration. Ann Thorac Surg. 1976;22:478–482 [DOI] [PubMed] [Google Scholar]
- 4. Bruce MS. Pediatric pulmonary Sequestration [Internet]. Medscape Reference [updated 2007 Jul 7; cited 2011 Sep 10]. Available at: http://emedicine.medscape.com/article/1005815-overview.html
- 5. Damani MN, Ganem JP, Freeman JA. Intra-abdominal pulmonary sequestration: a benign suprarenal mass. Urology 1999;53:1228. [DOI] [PubMed] [Google Scholar]
- 6. Roberts WW, Nelson JB, Fishman EK, Jarrett TW. Diagnosis of retroperitoneal pulmonary sequestration using computerized tomography guided fine needle biopsy. J Urol. 2000;164:445. [PubMed] [Google Scholar]
- 7. Nelson JB, Blum MD, Cook WA. Retroperitoneal pulmonary sequestration: a rare congenital anomaly in a 71-year-old man. J Urol. 1994;152:2341–2343 [DOI] [PubMed] [Google Scholar]
- 8. Curtis MR, Mooney DP, Vaccaro TJ, et al. Prenatal ultrasound characterization of the suprarenal mass: distinction between neuroblastoma and subdiaphragmatic extralobar pulmonary sequestration. J Ultrasound Med. 1997;16:75–83 [DOI] [PubMed] [Google Scholar]
- 9. Amitai M, Konen E, Rozenman J, Gerniak A. Preoperative evaluation of pulmonary sequestration by helical CT angiography. Am J Roentgenol. 1996;167:1069–1070 [DOI] [PubMed] [Google Scholar]
- 10. Osaki T, Kodate M, Takagishi T, Nomi M, Murakami J, Yamamoto H. Unique extralobar sequestration with atypical location and aberrant vessels. Ann Thorac Surg. 2010;90:1711–1712 [DOI] [PubMed] [Google Scholar]