Abstract
Brain aging is characterized by considerable heterogeneity, including varying degrees of dysfunction in specific brain systems, notably a medial temporal lobe memory system, and a frontostriatal executive system. These same systems are also affected by neurodegenerative diseases of late life. Recent work using techniques for presymptomatic detection of disease in cognitively normal older people has shown that some of the late life alterations in cognition, neural structure and function attributed to aging probably reflects early neurodegeneration. However, it has become clear that these same brain systems are also vulnerable to aging itself in the absence of even subtle disease. Thus, fundamental systemic limitations appear to confer vulnerability of these neural systems to a variety of insults, including those recognized as typical disease and those that are attributed to age. By focusing on the fundamental causes of neural system vulnerability, the prevention or treatment of a wide range of late-life neural dysfunction might be possible.
Keywords: Aging, Alzheimer's, Parkinsons's, cerebrovascular disease, Amyloid, Imaging
How does the brain age? The causes of the changes in cognition, neural function, and brain structure experienced by many older people are debated. Specifically, do the neurodegenerative dieases of older age often contribute to these problems, or is there a “pure” age-related form of neural decline that is clearly separate from disease? This question has occupied scientists involved in the study of aging for decades, but is now becoming more tractable because of advances in techniques for studying the human brain during life.
While heuristically useful, separating age and disease also creates problems. First, what is “aging”? There is no single mechanism that underlies age-related change other than the passage of time. Aging uncovers systemic limitations of human biology that may be susceptible to a variety of deleterious processes, some of which are related to frank disease and others not. Distinguishing age and disease has lead to the concept of “normal aging”, which may be defined as what is left when disease is excluded. However, individuals in their 7th – 9th decades without significant disease (vascular, metabolic, or degenerative) are not statistically normal and are more aptly described as “supernormal” or optimally aged (Rowe and Kahn, 1987). Studies of such individuals may provide insight into the intrinsic potential of human neural systems, and could lead to ways of optimizing function in aging that are completely different from the methods for treating age-related disease.
Is our conceptualization of normal aging in fact contaminated by the study of normal older people who are experiencing neurodegeneration? While for decades many studies of older people may have excluded those with manifest disease, it has become increasingly clear that neurodegeneration exists in subtle preclinical forms for many years prior to symptom onset. The use of norms to exclude individuals does not ameliorate these problems since many presymptomatic individuals will test in a normal range, and the norms themselves may have been contaminated by participants with preclinical symptoms. In fact, in-depth examination of many neural systems in older people reveals alterations that have been attributed to normal aging. But are such individuals “normal” in the sense of being free of disease, or do age-related deficits simply reflect undetected neurodegeneration?
What is the behavioral phenotype of cognitive aging?
Over decades, considerable effort has been devoted to defining the cognitive profile of healthy (neurologically disease-free) older people. A frequent shortcoming of these studies is the use of cross-sectional as opposed to longitudinal data. Cross sectional data do not permit the differentiation of lifelong or developmental subject characteristics from those that actually decline in older age. Nevertheless, many studies have characterized older individuals using two general approaches. One can be defined in terms of cognitive processes, while the other is more related to neural systems; these are not mutually incompatible, and both rely on examination of cognitive test performance. Regardless of the approach there is wide agreement that older people are heterogeneous in their cognitive performance. Some older individuals have abilities typical of much younger people, while others show marked decrements (Wilson et al., 2002). Furthermore, there is good agreement that some cognitive abilities, particularly semantic memory or knowledge, are relatively preserved with age (Park et al., 2002). The cognitive abilities that most consistently decline with age are processing speed, working memory, and episodic memory CE (Schaie, 1996; Park et al., 2002). Models of cognitive aging that attempt to define change in terms of fundamental cognitive processes that drive general decline propose a small number of underlying cognitive processes that are responsible for decline in multiple areas; such models generally include processing speed (Salthouse and Ferrer-Caja, 2003) or executive function (Hasher and Zachs, 1988) as key factors. A major theory of cognitive aging attributes many aspects of cognitive decline to loss of prefrontal cortical function (West, 1996), although some aspects of episodic memory are not well accounted for by this model. Neural system-based conceptualizations define age-related cognitive decline in terms of two fundamental neural systems that support episodic memory and executive function (Buckner, 2004; Hedden and Gabrieli, 2004). Differential decline of these cognitive processes reflects varying degrees of involvement of the medial temporal lobe memory system and a prefrontal cortex/striatal executive system. The major late-life neurodegenerative disorders affect these 2 neural systems differently and thereby may explain some of the heterogeneity in brain aging. In addition, there are other neural systems that are affected by age and disease that will be discussed, and there are numerous other systems that are not the focus of this review.
Late-life Neurodegenerative diseases
Three common age-related diseases are the most likely culprits in producing neural alterations attributed to normal aging: Alzheimer's disease, cerebrovascular disease, and Parkinson's disease. Relationships between these disorders and aging could represent several different situations: manifest neurodegenerative disease, pre-symptomatic neurodegenerative disease, or a “phenocopy” of a neurodegenerative disease that is actually based in a different mechanism.
Alzheimer's disease (AD)
Alzheimer's disease is crucial to discussions of cognitive aging simply because it is so prevalent; at least 1% of people at age 65 have AD, with prevalence rising at least to 30% by age 80 (Hofman et al., 1991). These figures reflect the presence of dementia, a progressive diminution of cognitive abilities incompatible with independent functioning. AD usually begins with anterograde amnesia, although other cognitive disturbances can occur in the initial stage. AD evolves from more subtle syndromes, often referred to as mild cognitive impairment (MCI). While the strong age-association of AD has at times resulted in the belief that it represented normative aging, it is amply clear that many individuals live to late life without dementia.
Many recent reviews have summarized the molecular pathology, clinical features, and therapeutic approaches to AD (Querfurth and LaFerla, 2010; Golde et al., 2011). The disease is defined by the association of cognitive symptoms with the neuropathological findings of neuritic or senile plaques and neurofibrillary tangles (NFTs) revealed on histological examination of the brain (figure 1). Plaques are composed of the aggregated β-amyloid (Aβ) protein surrounded by dystrophic and degenerating neurites, while NFTs are composed of the microtubule-associated protein tau, which is hyperphosphorylated and aggregated as paired helical filaments. A major theory of AD causation holds that soluble forms of Aβ initiate the disease leading to alterations in synaptic structure and function, followed by Aβ aggregation into plaques (Hardy, 2009). This is consistent with evidence that reveals limited associations between plaque Aβ and dementia severity, but stronger associations between cognition and NFTs and synaptic number and size (DeKosky and Scheff, 1990; Nelson et al., 2012). In its full form this amyloid cascade hypothesis holds that early soluble Aβ unleashes a chain of events causing alterations in synapses and tau, and a host of downstram structural and functional neural changes that are closely related to cognitive decline.
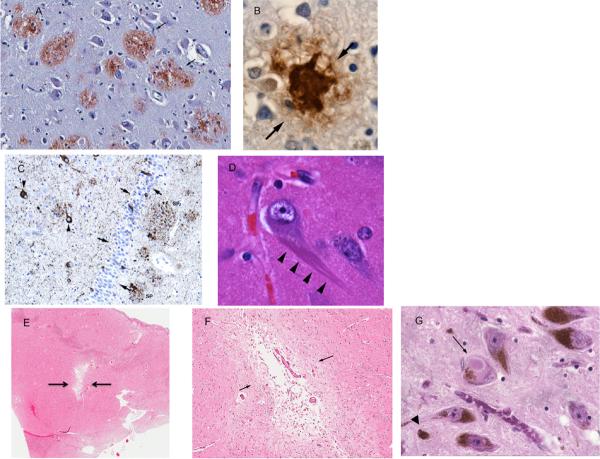
Figure 1. Neuropathology of common age-related diseases.
(A) Aβ-immunostained senile plaques (arrows) (B) Magnified view of an Aβ-immunoreactive cortical senile plaque (C) Tau-immunostained section of the hippocampus showing phospho-tau immunoractive, NFT-bearing neurons (arrowheads) and the granule cell layer of the hippocampus (arrows) with some tau-immunoreactive neurons (D) Magnified view of neuron with NFTs (arrow) (E) Low magnification view of a small-vessel infarct in the basal ganglia (F) Magnified basal ganglia infarct (G) Brainstem Lewy body (arrow) and neuromelanin, some of which is extraneuronal (arrowhead).
Several issues complicate the differentiation of AD from normal aging. First, the relationship between fundamental features of AD pathology and cognition are tenuous. Recent neuropathological criteria recognize that AD pathological change can exist in the absence of symptoms (Hyman et al., 2012), raising the likelihood that some individuals may be resistant to the pathology. This is a particularly confusing situation in the very old, where the relationship between AD pathological change and cognition is particularly weak (Savva et al., 2009; Balasubramanian et al., 2012). Second, the pathology associated with AD occurs in partial forms. NFTs, without Aβ plaques, are seen in many cognitively normal people, usually in medial temporal lobe structures, and they increase exponentially with advancing age (Price and Morris, 1999). These NFTs particularly affect entorhinal cortical neurons that project to dentate gyrus as the perforant pathway, functionally disconnecting the hippocampus (Hyman et al., 1984). Aβ plaques also occur in “diffuse”, as opposed to cored or neuritic forms, and these too are commonly seen in cognitively intact older people (Knopman et al., 2003). Whether these pathologies reflect early AD or processes related to aging itself remains debated.
Cerebrovascular disease (CVD)
Stroke, or cerebral infarction, is also associated with both advancing age and cognitive decline (Seshadri et al., 2006). The most fulminant effect of stroke on cognition appears as “multi-infarct dementia” (Hachinski et al., 1974), but the prevalence of this disorder is argued. At least as important are many other forms of CVD seen in aging, including asymptomatic disease detected on imaging (O'Brien et al., 2003). Other manifestations of CVD include alterations in subcortical white matter involving demyelination, rarefaction, and high signal intensity on magnetic resonance images, thinning of cerebral cortex and cerebral atrophy, and subclinical, silent infarction related to small vessel occlusion (DeCarli et al., 1999; Vermeer et al., 2003) (figure 1). All of these forms of CVD have been linked with cognitive dysfunction, are subtle or clinically undetectable in onset and progression, and may play a role in age related decline.
Parkinson's disease (PD)
Parkinson's disease is a disorder of the motor system with cardinal manifestations of slowing of motion (bradykinesia), tremor, rigidity, and gait and postural instability. The characteristic neuropathology involves loss of dopaminergic neurons in the pars compacta of the substantia nigra (SN). These neurons, which project to the striatum as the nigro-striatal pathway, also contain Lewy bodies, an abnormally aggregated form of the protein α-synuclein (figure 1). PD has a strong age-associated prevalence and is accompanied by cognitive dysfunction. Both dementia (Aarsland et al., 1996), and mild cognitive impairment (Litvan et al., 2012) are common in PD patients. This type of major cognitive dysfunction is associated with widespread limbic and neocortical Lewy body pathology as well as concomittant AD pathology (Compta et al., 2011). However, PD is also associated with a range of more subtle cognitive manifestations that may reflect dopamine deficiency.
Effects of AD pathology in normal aging
Substantial AD pathology is consistently reported in postmortem studies of older individuals who were cognitively normal prior to death. About 20–40% of cognitively normal individuals in their 8th to 9th decades have at least intermediate levels of Aβ and NFT-tau pathology on autopsy examination (Bennett et al., 2006; Kok et al., 2009); many of these people meet pathological criteria for AD. This evidence has been used to assail the amyloid cascade hypothesis, but adherents hold that such individuals were in a stage of preclinical AD that would have progressed to dementia had they lived longer. These people may remain normal because of neural compensation or reserve, which has been conceptualized in two ways: a static or passive form of reserve, often referred to as “brain reserve”, and a more dynamic form of reserve, described as “cognitive reserve” or compensation (Stern, 2002). Brain reserve implies that individuals differ in baseline neural resources at the onset of age-related pathology, perhaps because of developmental factors or the balance of lifelong positive and negative exposures. The dynamic concept of reserve implies a more active compensatory process that could represent functional reorganization that recruits additional neural resources to maintain task performance. Individuals with AD pathology who maintain normal cognition are the focus of intense investigation because they may represent the earliest phase of AD, and thus the types of individuals most amenable to therapy. Such people clearly reside on the border of normal cognitive aging and neurodegeneration.
A major technological advance for studying such individuals is the development of radiolabeled tracers that bind to Aβ which can be imaged with positron emission tomography (PET) (figure 2). A number of such PET amyloid imaging agents are currently available; the ligand [11C]PiB (or Pittsburgh compound B) has been the most widely used (Klunk et al., 2004), with longer-lived [18F]-labeled imaging agents recently available (Clark et al., 2011). PET amyloid imaging detects only the fibrillar aggregated forms of Aβ, which is probably a later phenomenon and less pathogenic than the soluble forms. In studies of cognitively intact elderly, proportions of subjects with PET evidence of fibrillar Aβ deposition are congruent with the 20–30% rates seen with autopsy examination (Morris et al., 2010). These values also agree with measurements of Aβ obtained in cerebrospinal fluid (CSF) (Jagust et al., 2009), where lower levels of CSF Aβ probably reflect increased deposition in insoluble forms in the amyloid plaque. Although both CSF and PET imaging show similar proportions of older people with evidence of brain Aβ, the majority of older people do not have evidence of brain Aβ deposition. Nevertheless, this technology makes it possible to evaluate living people for the effects of Aβ on neural systems.
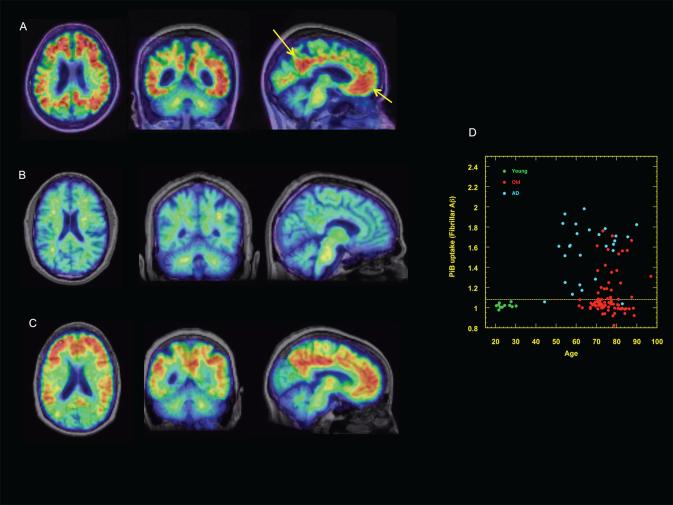
Figure 2. PET evidence of fibrillar brain Aβ deposition in normal aging.
[11C]PiB-PET scans (A) A patient with AD. Hotter colors indicate tracer retention, or binding to Aβ, throughout cortex. Aβ deposition is seen throughout cortex but particularly in medial parietal and frontal regions (arrows), areas associated with the default mode network. (B) A normal older person with no evidence of brain Aβ. Cool colors indicate non-specific uptake primarily in white matter (C) A normal older person with extensive Aβ deposition in a pattern identical to that seen in AD. (D) Quantitative tracer retention by age in a group of young subjects (green), AD patients (blue), and 95 cognitively normal older people (red) from the Berkeley Aging Cohort. Y-axis is the distribution volume ratio (DVR), a quantitative index of total brain tracer retention indicating the brain fibrillar Aβ load. Dotted yellow line indicates 2 SD above the mean of young normal subjects DVR, a threshold surpassed by 28% of the older normal subjects.
The ability to detect brain Aβ deposition in older healthy adults has resulted in intensive efforts to define cognitive performance in such people, with somewhat conflicting results. While many studies have failed to find any evidence of lower cognitive performance (Aizenstein et al., 2008), a number have reported subtle decrements in a host of cognitive abilities in those with either imaging or pathological evidence of Aβ. Most often this is seen in episodic memory (Bennett et al., 2006; Pike et al., 2011; Perrotin et al., 2012) although other cognitive domains may be affected (Rodrigue et al., 2012). Longitudinal memory decline also appears to be greater in those with evidence of Aβ (Storandt et al., 2009; Resnick et al., 2010). In studies that report such cross-sectional or longitudinal decline, deficits are quite small.
Preservation of cognitive ability could be due to reserve, or it could reflect the fact that the full cascade of negative events has not yet occurred. These downstream pathological alterations appear to follow Aβ in a sequence of events with prototypical ordering (Jack et al., 2010). Brain atrophy, for example, is associated with Aβ deposition in normals (Jack et al., 2009) and hippocampal atrophy may mediate effects of Aβ on cognition (Mormino et al., 2009) so that individuals with Aβ who have not yet developed these downstream changes could be spared symptoms. Thus, older people with evidence of Aβ may remain normal because they have not expressed the full phenotype of AD – they have amyloid pathology without clear evidence of neurodegeneration and will look like all other older individuals on every measure except Aβ. However, mounting evidence suggests that very subtle alterations in brain function may also be present in such individuals.
Aβ deposition is associated with disruption of large-scale neural networks. These have been studied in the resting state using the technique of functional connectivity MRI (fcMRI), which makes use of the observation that spontaneous fluctuations in the MRI signal occur synchronously within neural systems (Biswal et al., 1995). These networks reflect sensory and motor systems as well as ensembles of brain regions brought online during tasks requiring memory, attention, or executive function (Damoiseaux et al., 2006). These may be “task positive” networks activated by externally-directed cognitive tasks, or “task negative” networks that are deactivated during externally driven cognition. The primary task negative network, the default mode network (DMN) (Raichle et al., 2001) is intrinsically disconnected in patients with AD (Greicius et al., 2004); a particularly interesting observation since the pattern of Aβ deposition in the brain overlaps spatially with the topography of the DMN (Buckner et al., 2005). The major nodes of this network include precuneus/posterior cingulate, lateral parietal, and medial prefrontal cortices, some of which also participate in memory retreival 2005). This network is also functionally disrupted in cognitively intact older people with Aβ (Hedden et al., 2009), and also shows signs of atrophy (Becker et al., 2011; Oh et al., 2011). During fMRI experiments of memory encoding, older individuals deactivate the DMN less than younger individuals and the degree of deactivation is inversely proportional to the amount of Aβ deposition (Sperling et al., 2009) (figure 3).
Figure 3. Brain activation and deactivation during memory encoding.
(A) Map of the topographic distribution of PiB retention in a group of older subjects. (B) Deactivation in young subjects during the succesful encoding of memories. (C) Deactivation in older individuals without PiB retention is reduced in comparison to young (D) Deactivation in older individuals with PiB retention is further reduced. The patterns of PiB retention and deactivation reflect the default-mode network (DMN). A–D from Sperling et al, 2009, with permission. (E) Pattern of activation (yellow/orange) and deactivation (blue) across all young and old subjects during encoding of scenes (F) Differences between young and old subjects in hippocampal brain activation (arrow) during succesful memory encoding, indicating reduced activation with aging (G) Same data as in (F), but shown as a function of PiB retention in the elderly, demonstrating higher activation in PiB+ elderly than young. E–G from Mormino et al, 2012, with permission.
Increased fMRI brain activation in task positive networks during the performance of cognitive tasks has also been reported in patients with AD (Grady et al., 2003), MCI (Dickerson et al., 2004) and those with genetic risks for AD (Bookheimer et al., 2000). Associations between increased activation and better performance have lead to the interpretation that such changes are compensatory in nature. However, reduced activation has also been reported (Johnson et al., 2006) and studies may vary considerably by disease stage of participants, the cognitive task and its difficulty, and the particular brain regions examined. Two studies reported increased activation during memory encoding in older people who are amyloid positive compared to those who are amyloid negative (Sperling et al., 2009; Mormino et al., 2012), while one showed reduced activation (Kennedy et al., 2012). Interestingly, looking at older individuals as a group suggests that aging is associated with diminished activation; differentiating older people by Aβ deposition shows that older people who deposit Aβ activate more than young (figure 3).
These studies point out the difficulty in generalizing about “aging” without also examining individuals for subtle signs of brain disease. Many features seen with Aβ deposition – decline in episodic memory, brain atrophy, altered resting state functional connectivity, and changes in brain activation – are commonly ascribed to normal aging. Epidemiologic cohort studies (Elias et al., 2000; Amieva et al., 2008) show that subtle cognitive decline is seen in some normal individuals up to 12 years before the diagnosis of dementia, and studies of individuals with autosomal dominant genetic causes of AD show alterations in brain structure and function 10 to 15 years before expected symptom onset (Bateman et al., 2012; Reiman et al., 2012). Longitudinal studies of aging have also shown that individuals who remain cognitively stable over time have less evidence of brain atrophy at baseline than those who subsequently decline, pointing out that studies of normal aging contain at least some individuals destined for dementia (Burgmans et al., 2009). Taken together, these observations make a strong case that many of the cognitive and neural effects ascribed to normal aging could be related to presymptomatic AD. A closer look, however, suggests that the situation is not so clear.
Does presymptomatic AD explain cognitive aging?
Anterograde amnesia is an early manifestation of AD, a subtle feature of Aβ deposition, and a common finding in normal aging. With amyloid imaging we can now exclude people with fibrillar Aβ to examine independent effects of age on cognition. Even older individuals with no evidence of fibrillar brain Aβ show decline in visual memory and executive function compared to young people (Oh et al., 2012), a strong point in favor of the argument that presymptomatic AD does not explain all frontal/executive and medial temporal/episodic memory dysfunction. As amyloid imaging becomes more widely applied it is likely that we will see more cognitive investigations of normal older people who have been screened for Aβ.
There are also important differences between the patterns of atrophy seen in aging and presymptomatic AD. Cross-sectional and longitudinal studies reveal whole brain volume loss in normal indviduals that includes grey and white matter and begins in middle age, with rates of loss approximately doubled in AD patients (Fotenos et al., 2005). While this global volume loss in AD is dramatic, investigation of individuals in early stages is more revealing of specific regional vulnerabilities. Studies of either patients with MCI or normal older people who subsequently develop AD show atrophy or cortical thinning in specific regions including the entorhinal cortex (Stoub et al., 2005), hippocampus (Jack et al., 1999) and precuneus, angular, and supramarginal gyri (Davatzikos et al., 2009; Dickerson et al., 2011). These regions overlap with the brain regions that are atrophic in cognitively normal people with evidence of Aβ (Becker et al., 2011; Oh et al., 2011). However, cross-sectional and longitudinal studies of aging show prominent volume loss in both prefrontal cortex (PFC) (Raz et al., 1997; Resnick et al., 2003; Fjell et al., 2009) and striatum (Raz et al., 2003b), which are neither associated with Aβ nor implicated in the subsequent development of AD. This fronto-striatal atrophy, which has been conceptualized as the substrate for the “frontal hypothesis” of cognitive aging, must have other explanations than AD or Aβ. Furthermore, studies are beginning to appear that screen individuals for factors related to AD (such as genetic risks and Aβ) and nevertheless show substantial age-related brain atrophy, even in AD-related areas like entorhinal cortex (Fjell et al., 2012). In the aggregate, these data do not support a unitary etiology of age-related atrophy that is caused by preclinical AD.
Dysfunction of the DMN, while associated with Aβ deposition, has also been seen in studies of normal aging both as disconnection in resting state studies (Damoiseaux et al., 2008) and as reduced deactivation during tasks requiring semantic classification (Lustig et al., 2003) and episodic memory encoding (Miller et al., 2008). While the similarities between these aging studies and Aβ-related findings are striking, older individuals with no evidence of brain Aβ also show reduced deactivation of the DMN during memory encoding (Vannini et al., 2012) and reductions in resting state DMN connectivity (Andrews-Hanna et al., 2007). Thus, older people both with and without evidence of fibrillar Aβ share a fundamental disruption in the function of the major task negative network. This mechannism underlying this neural “phenocopy” of AD in Aβ-free aging is unexplained.
A variety of cognitive tasks have been associated with increased fMRI evidence of neural activation in PFC in normal older adults compared to younger adults. This is often accompanied by reduced activation elsewhere in the brain and a tendency for older people to activate both hemispheres when younger people acivate unilaterally (Cabeza et al., 1997). A widely hypothesized explanation for this effect involves PFC compensation for failing neural function elsewhere, such as the hippocampus (Daselaar et al., 2006). A recent meta-analysis that considered both task demands and performance reported significant trends for increases in activation in dorsolateral PFC in older compared to younger individuals, with greater activation in the young in more ventral PFC, occipital cortex (particularly during perceptual tasks) and hippocampus (Spreng et al., 2010). These types of observations have resulted in cognitive models that have stressed compensation, particularly in PFC, as a response to age-related change (Reuter-Lorenz and Lustig, 2005; Park and Reuter-Lorenz, 2009). At this point, it is difficult to assess the extent to which such activation-related changes might be due to presymptomatic AD. The constellation of reduced activation in a typically AD-affected region, the hippocampus, with increased age-related activation in a typically AD-spared region, the PFC, suggests that preclinical AD could underlie some of these findings. However, there are as yet too few studies of normal older people with measured brain Aβ to draw substantial conclusions. Increases in activation in aging are unlikely to have a single explanation. Future studies will need to examine interactions between task difficulty, behavioral performance, brain regions, and biomarkers of presymptomatic AD.
Normal aging, AD, and preclinical AD share the interesting features of anterograde amnesia, brain atrophy, network dysfunction, and increased cognitive activation. Based on these similarities, as well as the likelihood that normal cognitive aging studies to date have included a fair proportion of people with substantial brain Aβ accumulation, it is tempting to consider that many of the features of normal aging represent subtle forms of AD. However, it is important to keep in mind that most older people do not express fibrillar Aβ deposition. Furthermore, a small number of recent studies that compare older people without brain evidence of Aβ to young people show age-related alterations in cognition, atrophy, resting state networks, and brain activation. This could reflect our inability to fully exclude AD, or it could be that the identical neural systems show the same kind of vulnerability to the different processes of aging and disease.
Cerebrovascular disease and Cognitive Aging
Studies of cognitively normal older individuals can exclude participants with overt or symptomatic CVD by careful clinical examination since such individuals usually have neurological symptoms affecting motor and sensory systems that are straightforward to detect. However, 20–30% of older people also have asymptomatic cerebrovascular disease such as infarction, microbleeds, and microinfarcts (Vermeer et al., 2003; Jeerakathil et al., 2004). Another category of vascular pathology frequently seen on MR scans of older individuals is white matter hyperintensities (WMH) (figure 4). These areas of increased MR signal seen on T2 and FLAIR images have been strongly associated with a host of vascular risk factors and the pathological changes of white matter rarefaction and demyelination (Breteler et al., 1994; Jagust et al., 2008). Although MR scanning could exclude most such individuals from studies of normal aging, microinfarcts pose challenges. Many normal aging studies do not exclude such individuals because WMH and similar findings might be considered part of normal aging, because microinfarcts are undetectable, or because MR scans may not have been performed.
Figure 4. Pathological and imaging evidence of white matter disease.
Normal white matter seen on gross inspection (A) and microscopically (B). Severe white matter pallor (C) indicating rarefaction and demyelination microscopically (D). These findings are consistent with white matter hyperintensities (E) which characteristically surround the ventricles throughout subcortical white matter (arrow). These WMHs also disrupt fiber tracts, seen in (F) using diffusion tensor imaging tractography.
There is ample evidence that these vascular pathologies affect cognition in normal older people without dementia or MCI. WMH predominate in the frontal lobes (Wen and Sachdev, 2004) and regardless of their location tend to have effects on glucose metabolism that preferentially affect prefrontal cortex, and behavioral effects on executive function (Gunning-Dixon and Raz, 2003; Tullberg et al., 2004; Marchant et al., 2012). WMH are associated with a typical age-related cognitive deficit, failure of cognitive control, as well as with failure to activate PFC during tasks requiring cognitive control (Mayda et al., 2011). Even the risk factors for CVD affect PFC structure and function. Higher scores on the Framingham Cardiovascular Risk Profile are associated with reductions in glucose metabolism in PFC (Kuczynski et al., 2009) and hypertension is associated with reductions in prefrontal cortical volume, and decline in executive function tasks (Raz et al., 2003a). A strong chain of evidence thus links CVD and cerebrovascular risk, PFC atrophy and decline in prefrontal cognition in aging, suggesting that at least part of the frontal hypothesis of aging could be mediated by cerebrovascular disease.
It is likely, however, that the neural mechanisms linking CVD to behavior are even more pervasive, as WMH are not only associated with decline in executive function, but also memory (Gunning-Dixon and Raz, 2000). While memory decline in aging could reflect involvement of PFC in strategic processing there is ample evidence for direct hippocampal effects. Hypertension is associated with hippocampal atrophy (Raz et al., 2005), and WMH are associated with reduced activation in the medial temporal lobes during memory encoding (Nordahl et al., 2006). CVD thus affects both major neural systems that have been associated with cognitive aging: the PFC/executive system and the medial temporal lobe memory system.
Manifestations of cerebrovascular disease can be generalized, subtle, and temporally remote. For example, levels of blood pressure at midlife are associated with greater cerebral atrophy in late life (DeCarli et al., 1999). The precise mechanisms mediating relationships between vascular disease or risk factors and volume loss are unknown, but could involve loss of white matter volume, microbleeds or microinfarcts. A role for chronic ischemia in the etiology of WMH or cortical atrophy has been proposed but never proven. Reduced white matter integrity, measured with the MR technique of diffusion tensor imaging (DTI) is also related to a crucial cognitive phenomenon in aging, loss of processing speed (Burgmans et al., 2011). Decreases in functional connectivity within the DMN in older subjects are also related to loss of white matter integrity revealed by DTI (Andrews-Hanna et al., 2007). However, the biological basis of these DTI findings is unclear. Associations between DTI changes and WMH, and between DTI changes and some measures of cerebrovascular disease (Nitkunan et al., 2008) suggest that vascular factors may play a role. Whether these DTI changes reflect a vascular etiology of age-associated cognitive decline responsible for processing speed and network alterations, or whether they reflect disease-free aging remains to be determined.
The evidence is thus overwhelming that CVD, like AD, exists in subtle forms in many older people. Interestingly, exclusion of normal subjects with either cerebrovascular risk factors or overt cerebrovascular disease attenuates (though does not remove) both age-related cognitive decline and longitudinal brain atrophy (Head et al., 2002; Resnick et al., 2003). CVD affects both neural systems that decline with age and is thereby implicated in decline of executive and memory function. Based on this evidence, it is reasonable to conclude that subtle forms of cerebrovascular disease have contributed to our conceptualization of aging, and that this group of disorders and risk factors may be at least partly responsible for both frontal and medial temporal theories of cognitive aging. Most importantly, many factors associated with CVD such as hypertension, obesity, and diabetes are treatable, an argument against the inevitability of at least some aspects of cognitive aging.
Parkinson's disease and Cognitive Aging
While PD is characteristically a disorder of movement, cognitive decline and dementia are common symptoms in PD patients that are most often associated at postmortem with widely distributed Lewy bodies or concommitant AD pathology (Jellinger, 2012). However, these pathological processes fail to account for many cases of PD-associated cognitive decline (Libow et al., 2009). In fact, the essential neurochemical disturbances associated with PD provide a compelling explanation for some forms of age-related cognitive decline. In addition to degeneration of the SN, neurons in the ventral tegmental area (VTA) also degenerate in PD. Both the VTA and SN contain neurons that project to limbic and cortical regions involved in cogntion (Bjorklund and Dunnett, 2007). Cognitive deterioration in PD is associated with loss of both VTA neurons, and neurons in the medial part of the SN which project to the dorsal caudate (Rinne et al., 1989; Zweig et al., 1993). These findings have implications for a neurochemical basis of age-related cognitive decline.
The dopaminergic deficits of PD provide a useful model for understanding the neurochemical changes that underlie cognitive aging. PD patients show dysfunction in cognitive processes that rely upon dorsolateral PFC abilities and include planning, temporal sequencing, delayed response, and set-shifting (Pillon et al., 1986; Postle et al., 1997). These neuropsychological deficits have been investigated using PET to study in-vivo measures of neurochemistry. Such studies have made use of dopamine precursor molecules such as 18F labeled Fluorodopa (FDOPA) and fluorometatyrosine (FMT, figure 5) to study dopamine synthesis in the presynaptic neuron, and also a variety of radiolabeled ligands that bind to the dopamine transporter (DAT) on the presynaptic neuron. Such studies have shown relationships between lower caudate FDOPA uptake (Bruck et al., 2001), reduced DATs (Marie et al., 1999) and impaired cognition in PD patients. The loss of nigral dopaminergic input to the dorsolateral caudate likely plays a key role in prefrontal cognitive dysfunction because caudate is anatomically linked to PFC through the striato-thalamo-cortical functional loops (Alexander et al., 1986). There is also in vivo PET evidence for loss of extrastriatal dopamine (Klein et al., 2010) and for reduced PFC glucose metabolism (Polito et al., 2012) and neural activation (Carbon et al., 2004) that correlates with dopamine loss.
Figure 5. In vivo dopamine imaging and relationships to brain function.
(A) Uptake of the dopamne synthesis tracer [18F]-fluorometatyrosine (FMT) in a normal individual. Hotter colors indicate tracer uptake in presynaptic neurons in striatum (yellow arrows) and brainstem (red arrow). (B) Correlation between performance on the listening span test, a test of working memory, and dopamine synthesis measured with FMT. Hot-colored voxels (indicated with red arrow) are regions in which greater dopamine function is associated with better working memoryAC performance in a group of older individuals. (C) Correlation of caudate dopamine synthesis with brain activation in the left middle frontal gyrus. Higher dopamine synthesis was associated with greater activation during the delay phase of a working memory task. (B and C from Landau et al, 2009, with permission). (D) Regions in which binding potential at the D1 receptor was reduced in young people performing the multi-source interference task. Reduced binding potential reflects release of endogenous dopamine. (E) Changes in binding potential by age in the same experiment as D. Younger individuals show evidence of dopamine release, while older individuals do not. D and E from Karlsson et al, 2009, with permission.
Could these strong links between midbrain dopaminergic neurodegeneration, striatal dopamine loss, and failure on prefrontal cognitive tasks in PD find a correlate in normal cognitive aging? The link between dopamine and prefrontal cognition is well established through decades of human and animal research (Arnsten, 2011). In addition, there are compelling similarities between the neurochemical and motoric aspects of PD and aging. Nigral dopaminergic loss is a feature of aging itself. Studies of postmortem tissue have revealed loss of nigral dopaminergic neurons and DATs at a rate of 5–8%/decade (Fearnley and Lees, 1991; Ma et al., 1999). These post-mortem measures are paralleled by multiple in vivo PET studies showing age-associated loss of of DATs (Volkow et al., 1994), vesicular monoamine transporters (a marker of presynaptic dopaminergic neurons) (Frey et al., 1996), and D2 dopamine receptors (Volkow et al., 1998) in the striatum. Although the high density of dopaminergic terminals and receptors in the striatum make that region most amenable to reliable PET measurement, recent methods for investigating this system in extrastriatal regions have shown age-associated loss of D2 receptors in PFC and MTL (Kaasinen et al., 2000) consistent with degeneration of mesocortical and mesolimbic projections. Community studies that have examined the prevalence of parkinsonism note that at least 15–30% of older individuals show motor impairment similar to but less severe than those with full-fledged PD (Bennett et al., 1996; Uemura et al., 2011). A community-based study with autopsy follow up, in fact, linked the presence of such parkinsonian symptoms in normal older people to loss of nigral neurons (Ross et al., 2004). Finally, in addition to the well-recognized association between frontal atrophy and aging, one of the brain regions showing the strongest and most consistent age-associated shrinkage is the striatum (Raz et al., 2003b). Although this has not been directly related to loss of dopaminergic input, the rate of striatal volume loss with advancing age parallels the rate of dopamine loss in aging.
Straightforward evidence of a dopamine-deficiency substrate of cognitive aging comes from studies that have used PET measurements of dopamine function to explain individual differences in the cognitive performance of older people. This work has shown associations between D2 receptors or DATs and a range of cognitive abilities, including executive function, episodic memory, semantic memory, perceptual speed, and spatial cognition (Volkow et al., 1998; Backman et al., 2000; Erixon-Lindroth et al., 2005; Reeves et al., 2005). There are strong relationships between dopamine synthesis and working memory (Landau et al., 2009), and between D1 receptors and variability in cognitive performance in aging (MacDonald et al., 2012). Some evidence suggests that older people upregulate dopamine synthesis in the striatum as a potential compensatory mechanism (Braskie et al., 2008). Another powerful approach involves measuring actual dopamine release by using a PET receptor ligand that can be displaced by endogenous dopamine. In one such study younger individuals showed evidence of dopamine release in the striatum during an interference task, while dopamine release was not detectable in the older group (Karlsson et al., 2009)(figure 5).
A few studies have paired PET measurement of dopamine with fMRI measures of brain activation to link neurochemistry to neural activity in aging. Caudate dopamine synthesis is associated with activation in PFC in older people during the delay period of a working memory task (Landau et al., 2009), findings that parallel dopamine-dependent PFC delay period neural activity in primate electrophysiological studies of working memory (Goldman-Rakic, 1996). Functional connectivity between PFC and caudate during the delay period was greater in younger than older subjects, and greater connectivity was associated with both better working memory performance and lower dopamine synthesis, supporting the observation that increased synthesis might reflect compensatory processes in a deficient system (Klostermann et al., 2012). Dopamine may also affect allocation of cognitive resources by modulating deactivation during cognition. Greater dopamine synthesis is associated with more DMN deactivation in young people, but this relationship is lost in older individuals (Braskie et al., 2011). Similar relationships between dopamine, brain activation and cognitive performance in aging have been seen in studies that used PET to examine dopamine receptors. Numerous studies have shown an age-related blunting of the load-related increase in brain activation during working memory tasks that could indicate failure of neural recruitment in the face of a more demanding task. Dopamine D1 receptor density in the caudate explains a substantial proportion of the variance in BOLD response in PFC in this situation (Backman et al., 2011). This under-recruitment could be reproduced in younger subjects given a D1 receptor antagonist (Fischer et al., 2010).
Does PD explain cognitive aging? From the perspective of typical PD – that is, a movement disorder associated with the deposition of brainstem Lewy bodies and dopamine deficiency – PD seems an unlikely explanation for much of cognitive aging. However, subtle changes in motor function are common in aging as is loss of dopaminergic neurons and many dopaminergic neurochemical measures. There is also strong evidence linking these dopamine losses to decline in cognitive function with age, and specifically to alterations in both the fronto-striatal executive system and the DMN. While this dopamine deficiency probably does not necessarily reflect incipient PD, it shares behavioral and neural similarities implicating dopamine loss as a fundamental mechanism of cognitive aging.
Differentiating age and disease in the brain: animal models
If studies of cognitive aging have included people with subtle forms of AD, CVD, and PD is there any way to define mechanisms of brain aging that are independent of these disorders? While the careful application of techniques for characterizing older subjects, such as amyloid imaging or structural brain imaging offers considerable promise, animal models of naturally-occurring aging phenomena can also be helpful in establishing aging phenotypes. Although some old animals, including dogs and monkeys, show evidence of amyloid plaque accumulation (Selkoe et al., 1987), they do not show full blown evidence of AD or other neurodegenerative disorders that complicate the study of aging. Rodents and primates both exhibit signs of aging that are similar to those seen in humans including behavioral deficits, structural and ultrastructural alterations, and changes in electrophysiology (Yeoman et al., 2012). These deficits may or may not be driven by mechanisms identical to those that underlie human aging, as there is no a priori theory that necessitates common cross-species mechanisms of aging. Nevertheless, these animal models may generate approaches to human aging that offer the potential for revealing fundamental age-associated mechanisms of decline.
Studies of aged monkeys have conclusively demonstrated age-associated performance decrements on a variety of behavioral tasks that reflect function of both dorsolateral PFC and medial temporal lobe systems. Modern anatomic studies have failed to detect substantial neuronal loss in either of these regions (Amaral, 1993; Peters et al., 1994; Hof et al., 2000). In the dlPFC the morphological changes that appear best correlated to declining performance with age involve alterations in synapses (Peters et al., 2008). This includes loss of synapse density and apical dendritic branching that particularly affects the small, thin spines most involved in plasticity (Dumitriu et al., 2010). These spines may be affected by disinhibition of cAMP signaling; a recent study indicated that inhibition of cAMP signaling in old monkeys restored neuronal activity during the delay period of a working memory task (Wang et al., 2011). These findings unify structural and neurochemical theories of PFC aging using animal models that do not reflect the common neurodegenerative diseases like AD and PD.
Age related change in the primate medial temporal lobe involves loss of spine and synapse density in the subiculum (Uemura, 1985a; Uemura, 1985b), as well as alterations in the proportions of multiple-synapse boutons and non-synaptic boutons in the outer molecular layer of the dentate gyrus (DG) (Hara et al., 2011). In rodents, aging is also associated with loss of synapses in DG (Geinisman et al., 1992) and loss of synaptophysin in DG and CA3 (Smith et al., 2000) that is attributable to loss of DG input via the perforant pathway. A multimodal/multispecies imaging approach used fMRI in monkeys to measure cerebral blood volume (CBV), a correlate of neuronal function, and Arc gene expression in rats, an immediate early gene whose expression is related to neuronal activity (Small et al., 2004). Results indicated age-associated loss of CBV and arc gene expression in only the DG in both species. Furthermore, CBV was related to behavioral performance on the delayed non-match to sample task, a test of memory that draws upon medial temporal lobe resources.
Similarities between human brain aging and the nonhuman primate are clear. There is extensive evidence for synaptic changes throughout neocortex in association with human aging. This includes reduction in dendritic spine density, volume and length seen across the lifespan in multiple cortical regions (Jacobs et al., 1997; Benavides-Piccione et al., 2012). Neuronal loss, historically considered a major aspect of brain aging, is probably minimal, while the synaptic alterations predominate. Nevertheless, loss of specific neuronal types may play important roles. This is particularly true in the medial temporal lobe, where differential vulnerability to age and disease has been noted. The entorhinal cortex is susceptible to AD pathology, with involvement by NFTs and loss of the perforant pathway providing input from the entorhinal cortex to the dentate gyrus (Hyman et al., 1984). Age, in contrast, appears to be associated with neuronal loss in the hilus of the dentate gyrus and the subiculum (West et al., 1994), while notably sparing CA1–CA3. Although AD is also associated with neuronal loss in the hilus and subiculum, CA1 is an interesting region in demonstrating considerable AD-related neuronal loss without showing age effects (West et al., 1994; Price et al., 2001).
Taken together, these data suggest two types of age-related morphological alterations distinct from neurodegeneration that occur in animals and humans. One is loss of synaptic density particularly in PFC. The other is neuronal loss in the DG that is related to age and not disease, and which may reflect loss of synaptic input, via the perforant pathway. Conversely, loss in CA1 and entorhinal cortex is more likely to reflect the presence of AD. These findings can inform investigations of age-related cognitive decline in humans that may reflect aging, as opposed to neurodegenerative, phenotypes.
Are there non-degenerative aging phenotypes of neural function?
While standard clinical approaches to the investigation of memory in aging have been very helpful in defining relationships between age and disease, more mechanistic behavioral approaches offer additional promise in this regard. For example, the dual-process theory of episodic memory contrasts recollection as a relatively precise reconstruction of the details of an event, while familiarity is conceptualized as a more vague sense of “knowing” or previous experience. Studies consistently find age-related failure of recollection, but less consistent failure of familiarity (Jennings and Jacoby, 1993), while patients with AD show failure in both processes. One controversial model posits that recollection is related to hippocampal function and familiarity to extrahippocampal medial temporal lobe structures. This dissociation has been seen in studies that have used MRI to measure the volumes of these structures in aging and dementia (Yonelinas et al., 2007; Wolk et al., 2011). These studies provide some evidence for a distinction between aging and AD based in hippocampal anatomy, since AD effects on both hippocampus (CA1) and entorhinal cortex may have their behavioral correlate in loss of familiarity and recollection, while aging is better related to hippocampally mediated (ie, DG) deficits in recollection.
Another behavioral approach to the study of memory has relied upon computational models of hippocampal function emphasizing pattern separation and pattern completion. Reviewed elsewhere (Yassa and Stark, 2011), this approach compares how the hippocampus manages representations that share features to either match degraded representations to stored templates (ie, pattern completion), or to differentiate representations with similar features from previously stored information (ie, pattern separation). These functions permit, respectively, the processes of generalization, and the ability to encode novel information as distinct from prior, similar information. Considerable evidence links the DG to performance of pattern separation, and predictably, older adults show deficits on a task requiring the separation of spatial patterns (Stark et al., 2010). However, such deficits share features of AD since they occur in individuals with evidence of greater age-related impairment, and because they are also seen in patients with MCI (Yassa et al., 2010).
Studies using imaging approaches have recently begun to investigate subregions of the hippocampus with both structural and functional methods. High-field MRI permits higher resolution and contrast and therefore differentiation of hippocampal fields. In general AD tends to involve volume loss in the subiculum and CA1 (Fouquet et al., 2012), and age affects volume in CA3/DG (which cannot be differentiated on these images) (Mueller and Weiner, 2009) (figure 6), although some studies have found that age may also be associated with loss in CA1 (Mueller and Weiner, 2009) and subiculum (La Joie et al., 2010). Memory performance in older people has been linked to CA3/DG volume, while atrophy in CA1 was related to the presence of cerebrovascular risk factors (Shing et al., 2011). This again suggests a relative specificity of some subregions for age, and others for disease, in this case of a vascular nature. It is important to keep in mind the limitation of these studies; particularly limits in resolution that may make independent measures of neighboring regions difficult, and the fact that older individuals could still be in preclinical stages of AD. However, a search for regional specificity in the hippocampus, especially if paired with measures of Aβ and cerebrovascular disease, offers potential for defining age-specific morphological alterations.
Figure 6. Human hippocampus in cross section and MR images.
(A) Coronal cross-section of human temporal lobe. DG = dentate gryrus, S=subiculum, PrS=presubiculum, EC=entorhinal cortex, cs=collateral sulcus, FG=fusiform gyrus, its=inferior temporal sulcus, ITG=inferior temporal gyrus, mts=middle temporal sulcus, MTG=middle temporal gyrus, sts=superior temporal sulcus, STG=superior temporal gyrus. (B) Coronal MRI view indicating location of hippocampus (arrow). (C, D) High field MRI images showing hippocampus and medial temporal lobe segmented into CA3/DG (brown), CA1–2 transition (yellow), CA1 (blue), Subiculum (green) and entorhinal cortex (red).
These morphological alterations have functional correlates as well. Initial studies used a form of resting fMRI data acquisition to investigate steady-state blood flow as an index of basal hippocampal function (Small et al., 2002), demonstrating age-related decline in hippocampal function in the DG and subiculum as distinct from entorhinal cortex. Application of the pattern separation paradigm has also produced interesting results; older individuals show greater activation in CA3/DG than younger subjects performing this task while also showing a tendency for pattern completion, rather than separation (Yassa et al., 2011a). These findings have been linked to a loss of DG input via the perforant pathway, seen using DTI techniques (Yassa et al., 2011b). It is interesting to note that functional loss in the DG related to perforant pathway damage is seen in pathological processes common to animal models of aging, older humans, and the NFT pathology of AD. This is an excellent example of how a single neural system is vulnerable to a variety of insults that may reflect both age-related and neurodegenerative processes.
Older adults also show reductions in dorsolateral PFC activation compared to younger adults during the response phase of a working memory task (Rypma and D'Esposito, 2000), with faster response time in the older subjects associated with greater activation. Studies of cognitive control have also been productive in establishing unique age-related mechanisms of impairment. Such studies have shown reductions in suppression of task-irrelevant information by PFC in older, compared to younger people (Gazzaley et al., 2005), as well as failure to shift between functional brain networks following interruption (Clapp et al., 2011). These functional alterations reflect predominant age-related effects on PFC, which could reflect some of the synaptic alterations seen in aging, although the actual mechanism of these changes is unknown.
Summary and Conclusions
The behavioral and neural alterations associated with aging affect the medial temporal and fronto-striatal systems that underlie episodic memory and executive function. In addition, there is considerable evidence for age-related disruption of the DMN, and, within the medial temporal lobe, dysfunction in the DG/perforant pathway system. Deposition of brain Aβ has substantial effects on both medial temporal and neocortical systems that are involved in memory function. NFT pathology affects the entorhinal cortex and perforant pathway. Loss of dopamine has effects on the frontostriatal/executive system. CVD affects both medial temporal and frontostriatal systems. The DMN is affected by age, Aβ, CVD, and dopamine deficiency. Is cognitive aging just the aggregation of these disorders, or something else?
We can draw important inferences from the small number of studies that find many of the AD-like age-related changes persist in older people screened for fibrillar Aβ. It remains possible that such subjects have still not been adequately screened to rule out early neurodegeneration like AD. This is an important concept that reflects our current ignorance about what truly constitutes AD. Is AD simply fibrillar Aβ that can be detected with PET? Or does it reflect soluble Aβ, diffuse Aβ plaques, NFTs (without Aβ) or even synaptic loss? It is impossible to completely differentiate age and AD until we know what AD is. Similar concerns apply to the other age-related neurodegenerations.
However, the observation that the same neural systems can be affected by more than one process also raises fundamental questions about our understanding of aging itself. If the episodic memory system is affected in individuals with and without Aβ, and the frontostriatal system is affected by conditions as varied as CVD, dopamine deficiency and age-related synaptic loss, perhaps the question is not whether disease affects these systems, but rather how such systems are vulnerable to so many different processes. In other words, these neural systems are highly susceptible to multiple factors, including those that can be clearly ascribed to our current conceptualizations of disease, and those that do not fit such neat categories. This explanation highlights aging as a process that reflects systemic vulnerabilities to a variety of deleterious factors that accumulate with the passage of time. Some of these factors are disease-related and others are not.
The important question therefore is not whether a disease accounts for the change we see with age, but whether we can unravel the fundamental mechanisms that produce changes in the neural systems under discussion. If we can indeed identify fundamental mechanisms responsible for the degeneration of these systems, we may be on the road to developing methods that increase brain health in the face of many different diseases and even age itself. Answering these questions will no doubt be enhanced by the dramatic improvement in our ability to detect biomarkers of disease in asymptomatic people so that we may define how our current understanding of disease relates to our current understanding of aging. While this may not provide an ultimate accounting of mechanisms underlying age-related change, it may speed therapeutic approaches not only to what we now consider overt disease, but to age-related decline that is based in fundamental mechanisms of neural vulnerability.
Acknowldegments
This work was partially supported by NIH grant AG034570. I thank Susan Landau, Gil Rabinovici, John Flannery, and Mike Yassa for critical feedback. Figures were generously contributed by Harry Vinters, Owen Carmichael, Susanne Mueller and David Amaral.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Tandberg E, Larsen JP, Cummings JL. Frequency of dementia in Parkinson disease. Arch Neurol. 1996;53:538–542. doi: 10.1001/archneur.1996.00550060082020. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLongh RM, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amaral DG. Morphological analyses of the brains of behaviorally characterized aged nonhuman primates. Neurobiol Aging. 1993;14:671–672. doi: 10.1016/0197-4580(93)90066-k. [DOI] [PubMed] [Google Scholar]
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Backman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Nyberg L. Dopamine D1 receptors and age differences in brain activation during working memory. Neurobiol Aging. 2011;32:1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79:915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Piccione R, Fernaud-Espinosa I, Robles V, Yuste R, Defelipe J. Age-Based Comparison of Human Dendritic Spine Structure Using Complete Three-Dimensional Reconstructions. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs154. DOI 10.1093/cercor/bhs154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie MN, Landau SM, Wilcox CE, Taylor SD, O'Neil JP, Baker SL, Madison CM, Jagust WJ. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp. 2011;32:947–961. doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie MN, Wilcox CE, Landau SM, O'Neil JP, Baker SL, Madison CM, Kluth JT, Jagust WJ. Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci. 2008;28:14320–14328. doi: 10.1523/JNEUROSCI.3729-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MMB, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JHW, van Harskamp F, Tanghe HLJ, de Jong PTVM, van Gijn J, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Bruck A, Portin R, Lindell A, Laihinen A, Bergman J, Haaparanta M, Solin O, Rinne JO. Positron emission tomography shows that impaired frontal lobe functioning in Parkinson's disease is related to dopaminergic hypofunction in the caudate nucleus. Neurosci Lett. 2001;311:81–84. doi: 10.1016/s0304-3940(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmans S, Gronenschild EH, Fandakova Y, Shing YL, van Boxtel MP, Vuurman EF, Uylings HB, Jolles J, Raz N. Age differences in speed of processing are partially mediated by differences in axonal integrity. Neuroimage. 2011;55:1287–1297. doi: 10.1016/j.neuroimage.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Vuurman EF, Smeets F, Gronenschild EH, Uylings HB, Jolles J. The prevalence of cortical gray matter atrophy may be overestimated in the healthy aging brain. Neuropsychology. 2009;23:541–550. doi: 10.1037/a0016161. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004;21:1497–1507. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci U S A. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C, Williams DR, de Silva R, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer's-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain. 2009;132:2026–2035. doi: 10.1093/brain/awp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Erixon-Lindroth N, Farde L, Wahlin TB, Sovago J, Halldin C, Backman L. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res. 2005;138:1–12. doi: 10.1016/j.pscychresns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Aging and Parkinson's disease:substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fischer H, Nyberg L, Karlsson S, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Backman L. Simulating neurocognitive aging: effects of a dopaminergic antagonist on brain activity during working memory. Biol Psychiatry. 2010;67:575–580. doi: 10.1016/j.biopsych.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB. Accelerating Cortical Thinning: Unique to Dementia or Universal in Aging? Cereb Cortex. 2012 doi: 10.1093/cercor/bhs379. DOI 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Fouquet M, Desgranges B, La Joie R, Riviere D, Mangin JF, Landeau B, Mezenge F, Pelerin A, de La Sayette V, Viader F, et al. Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic Mild Cognitive Impairment. Neuroimage. 2012;59:3309–3315. doi: 10.1016/j.neuroimage.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE. Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia: a cause of mental deterioration in the elderly. Lancet. 1974;2:207–209. doi: 10.1016/s0140-6736(74)91496-2. [DOI] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, Morrison JH. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci. 2011;31:7737–7744. doi: 10.1523/JNEUROSCI.0822-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zachs RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of Learning and Motivation. Vol. 22. Academic Press; New York: 1988. pp. 193–225. [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: the role of regional cortical shrinkage and cognitive resources. Psychol Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind:a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Nimchinsky EA, Young WG, Morrison JH. Numbers of meynert and layer IVB cells in area V1: a stereologic analysis in young and aged macaque monkeys. J Comp Neurol. 2000;420:113–126. doi: 10.1002/(sici)1096-9861(20000424)420:1<113::aid-cne8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hofman A, Rocca WA, Brayne C, Breteler MM, Clarke M, Cooper B, Copeland JR, Dartigues JF, da Silva Droux A, Hagnell O, et al. The prevalence of dementia in Europe: a collaborative study of 1980–1990 findings. Eurodem Prevalence Research Group. Int J Epidemiol. 1991;20:736–748. doi: 10.1093/ije/20.3.736. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, Ellis WG, Zarow C, Mungas D, Reed BR, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Mild cognitive impairment in Parkinson disease: heterogenous mechanisms. J Neural Transm. 2012 doi: 10.1007/s00702-012-0771-5. DOI 10.1007/s00702-012-0771-5. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychol Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Nyberg L, Karlsson P, Fischer H, Thilers P, Macdonald S, Brehmer Y, Rieckmann A, Halldin C, Farde L, et al. Modulation of striatal dopamine D1 binding by cognitive processing. Neuroimage. 2009;48:398–404. doi: 10.1016/j.neuroimage.2009.06.030. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Devous MD, Sr., Hebrank AC, Bischof GN, Park DC. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage. 2012;62:1–8. doi: 10.1016/j.neuroimage.2012.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, Baudrexel S, Diederich NJ, Heiss WD, Hilker R. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- Klostermann EC, Braskie MN, Landau SM, O'Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol Aging. 2012;33:623.e15–24. doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology. 2009;72:738–743. doi: 10.1212/01.wnl.0000343005.35498.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mezenge F, Landeau B, Villain N, Mevel K, Pelerin A, Eustache F, Desgranges B, Chetelat G. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage. 2010;53:506–514. doi: 10.1016/j.neuroimage.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libow LS, Frisina PG, Haroutunian V, Perl DP, Purohit DP. Parkinson's disease dementia: a diminished role for the Lewy body. Parkinsonism Relat Disord. 2009;15:572–575. doi: 10.1016/j.parkreldis.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Cilliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol. 1999;409:25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Karlsson S, Rieckmann A, Nyberg L, Backman L. Aging-related increases in behavioral variability: relations to losses of dopamine D1 receptors. J Neurosci. 2012;32:8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, DeCarli CS, Madison CM, Weiner MW, Chui HC, Jagust WJ. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging. 2012;33:1006.e25–36. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie RM, Barre L, Dupuy B, Viader F, Defer G, Baron JC. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson's disease. Neurosci Lett. 1999;260:77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- Mayda AB, Westphal A, Carter CS, DeCarli C. Late life cognitive control deficits are accentuated by white matter disease burden. Brain. 2011;134:1673–1683. doi: 10.1093/brain/awr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Abeta Deposition in Aging Is Associated with Increases in Brain Activation during Successful Memory Encoding. Cereb Cortex. 2012;22:1813–1823. doi: 10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkunan A, Charlton RA, McIntyre DJ, Barrick TR, Howe FA, Markus HS. Diffusion tensor imaging and MR spectroscopy in hypertension and presumed cerebral small vessel disease. Magn Reson Med. 2008;59:528–534. doi: 10.1002/mrm.21461. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, et al. Vascular cognitive impairment. Lancet Neurology. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- Oh H, Madison C, Haight TJ, Markley C, Jagust WJ. Effects of age and beta-amyloid on cognitive changes in normal elderly people. Neurobiol Aging. 2012;33:2746–2755. doi: 10.1016/j.neurobiolaging.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. beta-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;4:621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Ellis KA, Villemagne VL, Good N, Chetelat G, Ames D, Szoeke C, Laws SM, Verdile G, Martins RN, et al. Cognition and beta-amyloid in preclinical Alzheimer's disease: data from the AIBL study. Neuropsychologia. 2011;49:2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Lhermitte F, Agid Y. Heterogeneity of cognitive impairment in progressive supranuclear palsy, Parkinson's disease, and Alzheimer's disease. Neurology. 1986;36:1179–1185. doi: 10.1212/wnl.36.9.1179. [DOI] [PubMed] [Google Scholar]
- Polito C, Berti V, Ramat S, Vanzi E, De Cristofaro MT, Pellicano G, Mungai F, Marini P, Formiconi AR, Sorbi S, et al. Interaction of caudate dopamine depletion and brain metabolic changes with cognitive dysfunction in early Parkinson's disease. Neurobiol Aging. 2012;33:206.e29–39. doi: 10.1016/j.neurobiolaging.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Postle BR, Jonides J, Smith EE, Corkin S, Growdon JH. Spatial, but not object, delayed response is impaired in early Parkinson's disease. Neuropsychology. 1997;11:171–179. doi: 10.1037//0894-4105.11.2.171. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003a;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: longitudinal evidence. Am J Neuroradiol. 2003b;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Reeves SJ, Grasby PM, Howard RJ, Bantick RA, Asselin MC, Mehta MA. A positron emission tomography (PET) investigation of the role of striatal dopamine (D2) receptor availability in spatial cognition. Neuroimage. 2005;28:216–226. doi: 10.1016/j.neuroimage.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, et al. 2012. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurology. 11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in Parkinson's disease is related to neuronal loss in the medial substantia nigra. Ann Neurol. 1989;26:47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr., Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, et al. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237 doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychol Aging. 2003;18:91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Intellectual development in adulthood: The Seattle Longitudinal Study. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987;235:873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- Shing YL, Rodrigue KM, Kennedy KM, Fandakova Y, Bodammer N, Werkle-Bergner M, Lindenberger U, Raz N. Hippocampal subfield volumes: age, vascular risk, and correlation with associative memory. Front Aging Neurosci. 2011;3:2. doi: 10.3389/fnagi.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. JINS. 2002;8:448–460. [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, deToledo-Morrell L. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in the subiculum of Macaca mulatta: dendritic branching pattern. Exp Neurol. 1985a;87:412–427. doi: 10.1016/0014-4886(85)90172-4. [DOI] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in the subiculum of Macaca mulatta: synaptic density. Exp Neurol. 1985b;87:403–411. doi: 10.1016/0014-4886(85)90171-2. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Wada-Isoe K, Nakashita S, Nakashima K. Mild parkinsonian signs in a community-dwelling elderly population sample in Japan. J Neurol Sci. 2011;304:61–66. doi: 10.1016/j.jns.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Huijbers W, Ward A, Johnson KA, Sperling RA. The Ups and Downs of the Posteromedial Cortex: Age- and Amyloid-Related Functional Alterations of the Encoding/Retrieval Flip in Cognitively Normal Older Adults. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs108. DOI 10.1093/cercor/bhs108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Logan J, Schlyer D, MacGregor R, Hitzemann R, Wolf AP. Decreased dopamine transporters with age in healthy human subjects. Ann Neurol. 1994;36:237–239. doi: 10.1002/ana.410360218. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22:144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal aging and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, DeKosky ST. A medial temporal lobe division of labor: insights from memory in aging and early Alzheimer disease. Hippocampus. 2011;21:461–466. doi: 10.1002/hipo.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman M, Scutt G, Faragher R. Insights into CNS ageing from animal models of senescence. Nat Rev Neurosci. 2012;13:435–445. doi: 10.1038/nrn3230. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig RM, Cardillo JE, Cohen M, Giere S, Hedreen JC. The locus ceruleus and dementia in Parkinson's disease. Neurology. 1993;43:986–991. doi: 10.1212/wnl.43.5.986. [DOI] [PubMed] [Google Scholar]