Abstract
Regulatory T cells play a critical role in maintaining immune tolerance and preventing autoimmune disease. Treg cells express the transcription factor Foxp3, which acts as a master regulator of their differentiation and controls their capacity to suppress T cell responses. Treg cells have an intrinsically anergic phenotype and do not produce IL-2 or proliferate upon stimulation ex vivo. Recent reports have identified that Helios, a member of the Ikaros family of transcription factors, is expressed in Treg cells. However, its specific function is not yet fully understood. In this study, we show that Helios regulates IL-2 production in Treg cells by suppressing the Il2 gene transcription. Loss of Helios in Treg cells breaks their anergic phenotype and results in de-repression of the Il2 locus, allowing Treg cells to display increased baseline proliferation and to produce IL-2 following stimulation. Conversely, forced expression of Helios in CD4+Foxp3− T cells results in a loss of their normal ability to produce IL-2. Helios acts by binding to the Il2 promoter and inducing epigenetic modifications that include histone deacetylation. We also show that loss of Helios in Treg cells results in decreased Foxp3 binding to the Il2 promoter, indicating that Helios promotes binding of Foxp3 to the Il2 promoter. Interestingly, the loss of Helios in Treg cells also causes a decrease in suppressive capacity. Our results identify Helios as a key regulator of Il2 expression in Treg cells, contributing to the maintenance of the anergic phenotype.
Introduction
Regulatory T (Treg) cells are a subset of CD4+ T cells essential for the maintenance of immune homeostasis and the suppression of T cell responses (1). Thymically derived natural Treg cells are characterized by elevated expression of CD25, the alpha chain of the high-affinity IL-2 receptor, GITR, and CTLA-4 Critical to the establishment of the Treg phenotype is expression of the transcription factor Foxp3, which regulates Treg cell generation, phenotype and function (2, 3). Underscoring the importance of Foxp3 are the clinical features of Immunodysregulation, Polyendocrinopathy, and Enteropathy, X-linked syndrome, where a loss-of-function mutation in Foxp3 causes a deficiency in Treg generation. The resulting generalized autoimmunity manifests as polyendocrinopathy, enteropathy, and dermatitis, and is usually fatal within the first few years of life (4, 5).
Treg cells can suppress T cells responses by modulating the activity of antigen-presenting cells, by directly suppressing T cells or by secreting immune-regulatory cytokines (6–10). A defining feature of Treg cells is their intrinsically anergic phenotype. Upon stimulation with cognate antigen, Treg cells do not proliferate or produce IL-2, rather, they depend on TCR activation and signals from local activated immune cells to be activated, proliferate and exert their suppressive function (11, 12). The lack of IL-2 production by Treg cells might be linked to their suppressive function, as addition of IL-2 to co-cultures of Treg cells and responder T cells abrogates the Treg cells suppressive effects (13, 14). Interestingly, the chromatin of the Il2 promoter in Treg cells is maintained in a closed state, indicating suppression of Il2 expression at the epigenetic level (15, 16). Furthermore, Foxp3 has been proposed to mediate this silencing effect (17). This inhibition of the Il2 gene expression is similar to what has been described in anergic effector T cells, where the transcription factor Ikaros induces Il2 gene silencing (18, 19). Helios, a member of the Ikaros family of transcription factors that is normally expressed during thymocyte development, is also highly upregulated in Treg cells, but not in other mature T cell populations (20). Helios has a high level of sequence and structural homology to Ikaros and contains four amino-terminal zinc finger DNA-binding domains, as well as two carboxy-terminal zinc fingers, which mediate homo- and heterodimerization with other Ikaros family proteins (21, 22). Despite the high levels of Helios expression in Treg cells, Helios-null mice do not show any defect in Treg cells development, which appear to maintain their suppressive capacity (20, 23). However human Treg cells in which Helios expression has been knocked down show reduced suppressive activity and the expression of Helios may identify Foxp3+ Treg populations with high suppressive capacity (24, 25).
In this study, we intended to determine the function of Helios in Treg cells. Our results show that Helios is necessary for the suppression of IL-2 production in Treg cells. Helios binds to the Il2 promoter to maintain it in a deacetylated state, rendering it transcriptionally inactive. Our results also indicate that Helios regulates Foxp3 binding to the Il2 promoter, suggesting that the two transcription factors cooperate to enforce silencing of Il2 transcription in Treg cells. Our findings provide a novel specific role for Helios in Treg cells, and help to expand our understanding of the interconnected mechanisms of gene regulation and function in these cells.
Materials and Methods
Mice
Wild-type C57BL6/J, OT-II TCR-transgenic C57BL/6-Tg(TcraTcrb)425Cbn/J, Rag1-deficiet B6.129S7-Rag1tm1Mom/J and Foxp3-RFP C57BL/6-Foxp3tm1Flv/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained under specific pathogen-free conditions. All animal work was performed according to guidelines established by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee.
Cell Culture
Primary CD4+ T cells were isolated from the spleens and lymph nodes of 4–6 week old mice using anti CD4-conjugated magnetic beads (Invitrogen, Carlsbad, CA). Cells were activated with 0.5 μg/ml of plate bound anti-CD3 (clone 145-2C11) and 0.5 μg/ml of anti-CD28 (clone 37.51) antibodies (eBioscience, San Diego, CA). To differentiate these cells to a Th1 phenotype, cells were activated for 6 days in the presence of 10 ng/ml recombinant mIL-12 (eBioscience), 10 μg/ml blocking anti-IL-4 antibody (clone 11B.11) and 10 U/ml recombinant human IL-2 (NCI BRB Preclinical Repository, Frederick, MD). Cells were cultured in DMEM supplemented with 10% FCS, 2 mM GlutaMAX (Life Technologies, Grand Island, NY), nonessential amino acids (Lonza, Walkersville, MD), essential vitamins (Lonza), and 50 μM 2-mercaptoethanol. To generate RFP+ Tregs, primary CD4+ T cells were isolated as described above from 4–6 week old Foxp3-RFP mice, and RFP+ cells purified by cell sorting. Cells were activated with 0.5 μg/ml of plate bound anti-CD3 and 0.5 μg/ml of anti-CD28 antibodies and cultured in supplemented DMEM as described above, with 100 U/ml recombinant murine IL-2 (mIL-2) (eBioscience).
ELISA
Treg or Th1 cells (2.5–5×104) were stimulated with 0.5 μg/ml plate-bound anti-CD3 and anti-CD28 for 16 h. Supernatants were collected, and IL-2 levels were measured in a sandwich ELISA following the manufacturer’s recommendations (BD Biosciences, San Jose, CA).
Real-Time PCR (qRT-PCR)
RNA was isolated from cells using the RNeasy RNA Isolation Kit (Qiagen, Valencia, CA). cDNA was synthesized, and expression levels were measured using PpwerSYBR PCR mix (Applied Biosystems, Carlsbad, CA) in a StepOne Plus thermocycler (Applied Biosystems). mRNA induction was calculated as 2−ΔΔCt, using primers for actin as control. Melting curves established the specificity of the amplified band. Primers used are as follows: Il2 Forward: 5′TCTGCGGCATGTTCTGGATTT; Il2-Reverse: 5′ATGTGTTGTCAGAGCCCTTTAG; Helios-Forward: 5′ACACCTCAGGACCCATTCTG; Helios-Reverse: 5′CCATGCTGACATTCTGGAG; Eos-Forward: 5′ CACCGGCAAGGGAAGGATAAT; Eos-reverse: 5′TGAGTCCCCGCTACTTTCACA; Ikaros-Forward: 5′ GCTGGCTCTCAAGGAGGAG; Ikaros-Reverse: 5′ CGCACTTGTACACCTTCAGC; Aiolos-Forward: 5′CTGAATGACTACAGCTTGCCC; Aiolos-Reverse: 5′GCTCCGGCTTCATAATGTTCT; Ctla4 Forward: 5′CATGGTGTCGCCAGCTTTC; Ctla4-Reverse: 5′AGTCACCCGGACCTCATCA; Il2ra-Forward: 5′CTCCCATGACAAATCGAGAAAGC; Il2ra-Reverse: 5′TCTCTTGGTGCATAGACTGTGT; Tgfb1-Forward: 5′TCATGTCTCAGTTCCCATCTAGT;Tgfb1-Reverse: 5′GAGAGCGAGGCCATCAGTC; Tnfrsf18-Forward: 5′GAGCAATACGGCCATTTGACT; Tnfrsf18-Reverse: 5′GAGCTGGACTGTGGTTAGGAA.
Intracellular staining
Cells were stimulated with either 0.5 μg/ml of plate-bound anti-CD3 and anti-CD28 antibodies, or PMA (20 nM) and Ionomycin (500 nM) for 3 hours. Brefeldin A was added at 5 μg/ml for an additional 3 hours. Cells were then washed, fixed and permeabilized using the Foxp3 Buffer Staining Set (eBioscience). Cells were incubated with 0.1 μg of APC-conjugated anti-IL-2 (clone JES6-5H4), APC-conjugated anti-Foxp3 (clone FJK-16s) (eBioscience), or with 0.05 μg of PE-conjugated anti-Helios (clone 22F6) (Biolegend) antibodies for 45 minutes, and then washed and analyzed on a FACScan DxP5 flow cytometer (Becton Dickinson, Cytek Development, Fremont, CA). Analysis was performed using FlowJo Software (Tree Star).
Chromatin Immunoprecipitation (ChIP)
Histone acetylation, Helios, and Foxp3 binding were assayed using the EZ-ChIP assay kit (EMD-Millipore, Billerica, MA) following the manufacturer’s instructions. Nuclear lysates from 5–8×106 cells were subjected to immunoprecipitation overnight at 4°C with anti-acetyl-H3 (Millipore), anti-Helios or anti-Foxp3 antibodies (G20 and V17, respectively; Santa Cruz Biotechnology, Santa Cruz, CA) or with isotype or polyclonal type control antibodies. Specific primer pairs were designed to amplify the −232bp to +99bp region of the Il2promoter (Forward: 5′TAAGTGTGGGCTAACCCGA; Reverse: 5′TTGAGGTCACTGTGAGGAGT), the Cd3ε promoter (Forward: 5′TTCCTGCCTCCGCTGGAGGG; Reverse: 5′GGCAGAAGCCTCCGCCTTGG), and the Foxp3 promoter (Forward: 5′GCAGCTTCTGGGAGCCAGCC; Reverse: 5′TGGCAGAGCTGGCCACTCCT). Purified DNA was subjected to quantitative PCR analysis. Data was analyzed by adjusting input samples to 100%, calculating the percent return on input samples, and then subtracting the percent return on input of the immunoprecipitation from the isotype control antibody.
Lentiviral transduction of T cells
To generate shHelios-expressing lentiviral particles, HEK293T cells were transfected using TransIT-LT1 (Mirus Bio LLC, Madison, WI) with the pCCL-cPPT-PGK-EGFP-WPRE backbone plasmid into which one of two short hairpin-forming sequences (shHelios1: Sense: 5′CACCTACCTTGGAGCTGATTCAAGAGATCAGCTCCAAGGTAGGTGA; Antisense: 5′TCACCTACCTTGGAGCTGATCTCTTGAATCAGCTCCAAGGTAGGTGA; or shHelios2: Sense: 5′TGCAACATCTGTGGCTACATCTCTTGAATGTAGCCACAGATGTTGCA; Antisense: 5′TGCAACATCTGTGGCTACATCTCTTGAATGTAGCCACAGATGTTGCA) were inserted. Additionally, cells were transfected with lentiviral packaging plasmids expressing Gag/Pol (pMDLg/pRRE), Rev (pRSV-Rev), and VSV-G (pMD2.G). Supernatants were harvested from cells every 12 hours for a period of 3 days. Following each harvest, supernatants added to target cells in media containing 6 μg/ml of hexadimethrene bromide (Sigma-Aldrich, St. Louis, MO).
Transfection of primary T cells
4 days post-activation under Th1-skewing conditions as described above, cells were transduced with pHAGE-CMV-dsRedExpress-IRES-GFP-W (a gift from S. Horwitz) into which full-length Helios had been cloned, using an Amaxa Nucleofector and Mouse T Cell Nucleofection Kit (Lonza) according to the manufacturer’s protocol. 24 hours following transduction, cells were stimulated and prepared for intracellular staining as described above.
In vitro suppression assay
Wild-type, scramble-shRNA or shHelios-transduced purified RFP+ Treg cells were co-cultured with 2.5×105 splenocytes isolated from C57BL6/J mice and 5×104 naïve or Th1 cells from OT-II mice, in the presence of 1μM OVA peptide. Cells were incubated for 48h, and supernatants were collected. IL-2 production was assayed with ELISA as described above. Alternatively, CD4 T cells from OT-II mice were labeled with CFSE and activated with 1 μg/ml plate-bound anti-CD3 in the presence of equal numbers of Treg cells (control and lentiviral transduced). Four days later cells were harvested and CFSE dilution measured using a FACScan and FlowJo software. Where indicated 5 μg/ml of neutralizing anti-IL-2 antibody (clone JES6-1A12 (e-Bioscience) were added to the suppression reaction.
Inflammatory bowel disease (IBD) induction
Rag1−/− mice were injected intravenously with 5×105 Treg-depleted naïve CD4+ T cells isolated from C57BL6/J mice. Twenty-four hours later, mice were injected intraperitoneally with 2.5×105 of either wild type RFP+ Treg cells isolated from RFP-Foxp3 mice or with Treg cells transduced with lentivirus expressing a scramble control shRNA or Helios-specific shRNA. Mice were monitored over a period of 6–7 weeks for the development of colitis by weekly monitoring of body weight. Mice were then sacrificed and colons were isolated, fixed and stained with hematoxylin and eosin for histopathological evaluation at the Histopatholoy Core of the Albert Einstein College of Medicine. Colitis was graded using the Maggio-Price colitis index by adding scores (inflammation, mucosal hyperplasia and extension of lesion) from cecum, and ascending, transverse, descending and distal colon for each sample as described (26).
Results
Regulation of Helios expression in Treg cells
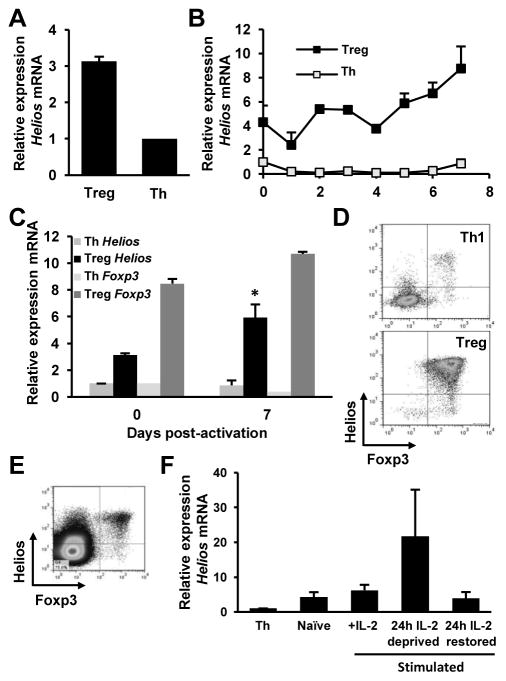
Previous reports have underlined the essential role of Ikaros in the regulation of cytokine gene expression in anergic effector Th1 cells (18, 19). Given the intrinsic anergic status of Treg cells, we chose to investigate the potential role of Ikaros family members in the regulation of Il2 expression in Treg cells. First, we assessed the relative gene transcription of Ikaros family members with qRT-PCR in isolated CD4+CD25+ T cells from the spleen and lymph nodes of mice compared to naïve CD4+ T cells or in vitro differentiated effector Th1 cells, using actin as a housekeeping control transcript. Consistent with previous reports (20, 24, 27), we found a marked upregulation (>3 fold) of Helios in freshly isolated naïve Treg cells as compared to naïve or effector Th1 cells (Fig. 1A and B). To gain insight into the possible regulation of Helios transcription, we followed the kinetics of Helios transcription in Treg cells activated in vitro for one week using anti-CD3 and anti-CD28 antibodies. We found that Helios mRNA levels were significantly upregulated in activated Treg cells compared with the basal level of expression found in naïve Treg cells. Foxp3 expression remained high in those cells, and only a small increase was detected, though, following activation. No significant increase in Helios expression was found in CD4+CD25- T cells at any time point following activation (Figs. 1B and C). These results indicated specific expression of Helios in Treg cells that was upregulated following to activation.
Figure 1. Regulation of Helios expression in Treg cells.
A. CD4+CD25+ Treg cells were purified from C57BL6/J mice and mRNA was isolated. qRT-PCR analysis was performed to detect Helios mRNA. Results are shown as fold induction of Helios mRNA over the levels detected in naïve CD4+CD25− T helper cells (Th). β-actin was used as a control housekeeping transcript. Results shown are the average of 3 independent experiments+SEM. B and C. Purified CD4+CD25+ Treg and CD4+CD25− Th cells were activated with 0.5 μg/ml of plate-bound anti-CD3 and anti-CD28 antibodies and mRNA was isolated at time points shown. qRT-PCR analysis was used to detect Helios and Foxp3 mRNA. Results are shown as fold induction of Helios mRNA over the levels detected in naïve CD4+CD25− Th cells. Th cells were activated under Th1 skewing conditions. Results shown are the average of 3 independent experiments+SEM. *p<0.05 when comparing Helios expression in naive and activated Treg cells. D. CD4+RFP+ Treg cells and CD4+RFP− Th cells were isolated from Foxp3-RFP mice and activated as in B for 6 days. Helios and Foxp3 expression were detected by flow cytometry on cells gated as CD4+. Data is shown from one representative experiment out of 3 independent experiments. E. Helios and Foxp3 expression detected by flow cytometry in thymocytes. F. CD4+RFP+ Treg cells were isolated from Foxp3-RFP mice and stimulated as in B for 5 days in the presence of 100U/ml mIL-2 (IL-2). Cells were then were deprived of mIL-2 for 24 hours, after which mIL-2 was restored to the media for another 24 hours. Samples were taken from fresh Treg cells and following deprivation or restoration of IL-2 and the expression of Helios mRNA determined by qRT-PCR. Expression of Helios (from 2 independent experiments showing mean+SEM) is represented as fold-increase over the Helios mRNA levels in CD4+RFP− Th cells.
Recently, several reports have shown the expression of Helios in T cell subsets other than Treg cells, such as Th2 and T follicular helper cells, activated T cells and peripherally-induced Treg cells (28–30). To confirm we were working with a pure expanded Treg population, and that our subsequent analysis of Helios expression and function was restricted to Treg cells, we isolated CD4+RFP+ cells from 5 week old C57BL/6-Foxp3tm1Flv/J mice (Foxp3-RFP), which contain an IRES-RFP cassette inserted between the translation stop codon and the poly-A tail on the 3′ end of the Foxp3 locus (31). The use of very young mice minimizes also the possibility that the RFP+ cells isolated might not be Treg cells. Six days post activation, cells were fixed and stained for Helios and Foxp3, and analyzed by flow cytometry. As a control, we used effector CD4+ T cells that were cultured under Th1-skewing conditions for 6 days. As expected, more than 95% of the Th1 control cells expressed neither Foxp3 nor Helios; by contrast, more than 95% of RFP+ Treg cultures expressed both Helios and Foxp3 (Fig. 1D), indicating that Helios expression was mostly restricted to Foxp3+ Treg cells. Confirming the expression of Helios in extrathymic Treg cells, similar results were obtained when thymocytes were from the thymi of RFP-Foxp3 mice, where more than 90% of FoxP3+ cells also express Helios (Fig. 1E).
Previously we had shown that IL-2 signaling was able to prevent the induction of T cell anergy by inhibiting the expression of several anergy-associated genes, including Ikaros (32). Furthermore, our initial analysis of Helios expression in Treg cells revealed that following addition of IL-2 in the Treg culture, we could observe a transient downregulation of the expression of Helios (Fig. 1B). We therefore sought to determine whether IL-2 signaling could regulate Helios expression in Treg cells. CD4+RFP+ Treg cells were activated with anti-CD3 and anti-CD28 antibodies in the presence of IL-2. After 5 days, IL-2 was removed from the culture media for 24h. Following this period of cytokine deprivation, IL-2 was replenished in the media and cells were cultured for an additional 24h. qRT-PCR analysis was performed at points before IL-2 deprivation, after 24h of IL-2 deprivation, and after 24h of IL-2 replenishment to determine levels of Helios transcription. Results showed that lL-2 signaling appeared to downregulate Helios expression, as increased in Helios transcription could be detected upon IL-2 withdrawal, and a subsequent decrease in Helios transcription was evident following replenishment of IL-2 (Fig 1F).
Helios inhibits Il2 expression in Treg cells
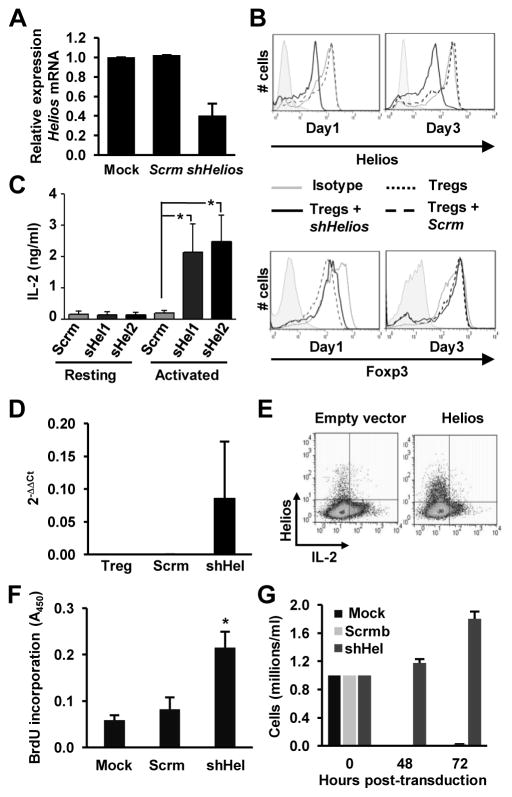
While several groups have reported the expression of Helios in Treg cells, its exact function remains unclear. Given Ikaros’ established role in silencing transcription of the Il2 gene during T cell anergy (18, 19), we hypothesized that Helios might have a similar role in Treg cells; to suppress the expression of Il2. To investigate this possibility, we generated lentiviral vectors expressing shRNAs directed against Helios (shHelios) and used them to transduce CD4+RFP+ Treg cells. After transduction, reduction of Helios expression was assessed by both qRT-PCR and flow cytometry. We found approximately a 60% decrease of Helios mRNA levels, which correlated with a clear decrease in protein expression levels (Fig. 2A and B). To ensure that knockdown of Helios was stable over the course of our experiments, we cultured Treg cells for an additional 3 days post-transduction and assessed Helios expression levels by flow cytometry. After 3 days of culture post-transduction, suppression of Helios expression was maintained at a level similar to that seen 24h post transduction (Fig. 2B). One of the defining features of Treg cells is their intrinsically anergic phenotype. Unlike other types of effector T cells, they do not proliferate or produce IL-2 upon stimulation with a cognate antigen (13). This lack of IL-2 expression appears to be enforced at the transcriptional level, as analysis of the Il2 promoter in Treg cells revealed a closed, heterochromatic-like configuration when compared with IL-2-producing effector T cells (16). To examine the role of Helios in the regulation of IL-2 production in Treg cells, we transduced isolated CD4+RFP+ Treg cells with lentiviral vectors expressing two different shRNAs specific for Helios and measured IL-2 production following re-stimulation with anti-CD3 and anti-CD28 antibodies. As expected, scramble control Treg cells did not produce IL-2 when re-stimulated. However, Treg cells in which Helios expression had been knocked down, began to produce IL-2 upon re-stimulation, indicating that the loss of Helios expression had caused the loss of their anergic phenotype (Fig. 2C). The increase in IL-2 expression appeared to be a consequence of increased Il2 gene transcription, as we could also detect increased expression of Il2 mRNA in those Treg cells where the expression of Helios had been suppressed (Fig. 2D). There have been conflicting reports on whether or not loss of Helios might alter expression levels of Foxp3 in Treg cells. Therefore, we also examined Foxp3 expression in cells transduced with the lentiviral vectors expressing shRNA against Helios by qRT-PCR and flow cytometry. Despite the loss of Helios, we did not observe any significant changes in the levels of Foxp3 mRNA or protein expression (Fig. 2B and data not shown).
Figure 2. Helios-deficient Treg cells express IL-2 upon stimulation.
A. Purified CD4+RFP+Treg cells were mock transduced or transduced with lentivirus expressing either a control scramble shRNA (Scrm) or a specific shRNA for Helios (shHelios). Helios expression was measured by qRT-PCR analysis. Results shown are the average+SEM of 6 independent experiments. B. Purified CD4+RFP+ Treg cells were transduced as in A and intranuclear staining was performed to detect Helios and Foxp3. Cells were analyzed by flow cytometry 1 and 3 days after completion of the transduction protocol. Results are representative of 3 independent experiments. C and D. Treg cells were transduced with lentivirus expressing either a control scramble shRNA (Scrm) or one of two specific shRNA for Helios (shHel). Cells were then stimulated with 0.5 μg/ml of plate-bound anti-CD3 and anti-CD28 antibodies for 24 hours and IL-2 production was measured by ELISA (C) or qRT-PCR (D). Results shown are the average of 3 independent experiments+SEM. *p<0.05. E. CD4+ T cells were isolated and cultured for 5 days under Th1-skewing conditions. Cells were then transfected with either an empty-vector or a Helios-expression construct. After 24h, cells were re-stimulated for 6 hours with 0.5 μg/ml of plate-bound anti-CD3 and anti-CD28 antibodies and intracellular staining was performed to detect Helios and IL-2. Cells were analyzed by flow cytometry. Results shown are representative of 3 independent experiments. F and G: CD4+RFP+ Treg cells were activated and either mock transduced or transduced with lentivirus expressing either a control scramble shRNA (Scrm) or a specific shRNA for Helios (shHel). After transduction, cells were cultured in fresh media without IL-2 for 60 hours. BrdU was then added and cells cultured for an additional 12 hours. Proliferation was monitored by measurement of BrdU incorporation (F) or by counting the number of trypan-blue excluded viable cells (G). Results shown are average of 2 independent experiments with 3 different samples in each. *p<0.05 (shHelios vs. Mock).
The previous experiments identified a role for Helios as a repressor of IL-2 expression in Treg cells. To further confirm that Helios had this activity, we next examined if Helios was able to exert its repressive effect on IL-2 in Th1 effector cells, which are normally able to produce IL-2 upon activation. Purified CD4+ T cells from C57BL6 mice were activated and cultured under Th1-skewing conditions for 6 days. We then transfected them with a CMV-driven Helios overexpression construct. Twenty-four hours post-transfection, we recovered Helios- and control empty-vector-transfected T cells and stimulated them with anti-CD3 and anti-CD28 antibodies for 6 hours to measure IL-2 production by intracellular cytokine staining and flow cytometry. As expected, almost all IL-2 producing cells in the empty-vector transduced population were effector Helios− T cells. A sub-population of Helios+ cells could also be seen in control cells, likely representing endogenous Treg cells present in the culture, which were unable to produce IL-2 (Fig 2E). Interestingly, the population of Helios+ cells in the Helios-transduced Th1 cultures were also mostly IL-2−, whereas almost all IL-2+ cells within the culture represented the untransfected Helios− cell population, confirming that expression of Helios is sufficient to suppress IL-2 production. Taken together with our shRNA knockdown findings in Treg cells, these results identify a role for Helios in the regulation of IL-2 production in T cells.
We also analyzed if the loss of Helios would affect the proliferative capacity of Treg cells. We found that in the absence of exogenous IL-2, control Treg cells and Treg cells transfected with scramble shRNA failed to proliferate and eventually died. However, knock-down of Helios expression allowed Treg cells to survive and proliferate (Fig. 2 F and G). It is important to note that these experiments were performed in resting Treg cells. In the absence of TCR engagement, even Treg cells in which Helios expression had been knocked down did not produce any IL-2 (Fig 2C, second and third bars), indicating that the effect Helios deficiency had on the regulation of Treg proliferation and survival was likely independent of its ability to suppress activation-induced IL-2 production in Treg cells.
Helios binds to the Il2 promoter and maintains epigenetic silencing of the Il2 gene in Treg cells
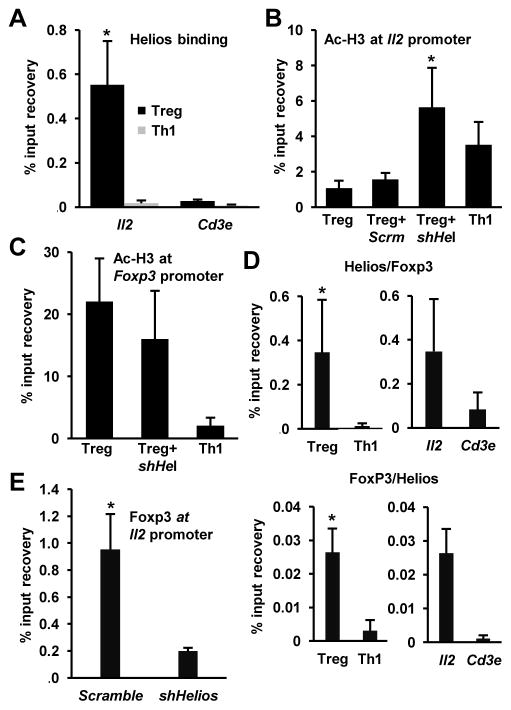
While we had identified a role of Helios in the repression of Il2 expression in Treg cells, we sought to further clarify the mechanism by which Helios exerted its regulatory effects. Several binding sites for Ikaros have been identified in the Il2 promoter (18, 19). Given Helios’ homology to Ikaros and that both target the same consensus sequence for DNA binding (33), we determined whether Helios would bind the Il2 promoter in Treg cells to silence it. To examine this, we performed ChIP using an antibody directed against Helios, and assessed its binding to the Il2 promoter in CD4+RFP+ Treg cells. We used CD4+ T cells cultured under Th1-skewing conditions as a control population of cells that express much lower levels of Helios (Fig. 1A) and actively transcribe Il2. We found a clear enrichment of Helios association with the Il2 promoter in Treg cells that was not detected in IL-2-producing Th1 cells (Fig. 3A), indicating that Helios’ regulatory effects on the Il2 locus involves recruitment to its promoter. This association was specific for the Il2 locus, as we did not detect any significant increase in Helios binding to the Cd3e promoter, a gene not expected to be regulated by Helios in Treg cells (Fig. 3A).
Figure 3. Helios binds to the Il2 promoter in Treg cells and regulates histone acetylation.
A. Binding of Helios to the Il2 promoter was detected by ChIP using anti-Helios antibodies in Treg and effector Th1 cells. Binding to the Cd3e promoter was used as control for specificity. Results are the mean+SEM of 3 independent experiments. * p<0.05 (Treg vs. Th1 at the Il2 promoter). B and C: Changes in the histone acetylation at the Il2 (B) and Foxp3 (C) promoters were determined by ChIP using antibodies against acetylated H3 (Ac-H3) in Th1 cells and control Treg cells (Treg) or Treg cells transduced with lentivirus expressing a scramble shRNA (Scrm) or a specific shRNA for Helios (shHel). Results are the average+SEM of 3 independent experiments. * p<0.05 (shHel vs. Treg). D. Concomitant binding of Helios and Foxp3 to the Il2 promoter was detected by sequential ChIP using anti-Helios (1st) and anti-Foxp3 (2nd) (top panels) or anti-Foxp3 (1st) and anti-Helios (2nd) (bottom panels) antibodies in nuclear lysates from Treg and Th1 cells. Binding to the Cd3e promoter in Treg cells was used as control to prove specificity. Results are the average+SEM of 3 independent experiments. E. Binding of Foxp3 to the Il2 promoter was determined by ChIP using antibodies against Foxp3 in Treg cells transduced with lentivirus expressing a scramble shRNA or a specific shRNA for Helios. Results are the average+SEM of 3 independent experiments. * p<0.05.
It has been previously shown that regulation of the Il2 gene expression in Treg cells takes place at the epigenetic level (16). Deacetylation of core histones mediated by histone deacetylases (HDACs) is associated with decreased locus accessibility by the transcriptional machinery and silencing of gene expression. We analyzed the acetylation status of core histones at the Il2 promoter in CD4+RFP+ Treg cells by ChIP using anti-acetylated H3 antibodies. These experiments confirmed that histone acetylation in the Il2 promoter in Treg cells was much lower than in effector T cell controls, which maintain the Il2 locus in a transcriptionally active state (18) (Fig 3B). We and others have previously shown that Ikaros enforces T cell anergy by recruiting HDACs and inducing deacetylation of core histones in the Il2 promoter (18, 19, 34). Helios, like Ikaros, has been also shown to associate with components of the Nucleosome Remodeling and Deacetylase (NurD) complex and recruit HDACs (35). Therefore, we hypothesized that Helios could be responsible for maintaining the Il2 promoter in a deacetylated state in a similar manner. To investigate this, we used specific shRNAs to inhibit the expression of Helios in CD4+RFP+ Treg cells and examined the acetylation status of histone H3 at the Il2 promoter. Control Treg cells and scramble shRNA-transduced Treg cells displayed a low level of acetylation, consistent with their inability to produce IL-2. By contrast, shHelios-expressing Treg cells showed a significant increase in H3 acetylation at the Il2 promoter (Fig 3B), indicating that epigenetic modifications associated with active transcription had occurred. We also examined H3 acetylation at the Foxp3 promoter, a gene that is transcriptionally active in Treg cells. As expected, H3 acetylation at the Foxp3 promoter was much higher in Treg cells than in effector T cells. However, inhibition of the expression of Helios did not cause a change in the levels of histone acetylation in the Foxp3 promoter (Fig. 3C). These findings show that Helios is required to suppress Il2 production in Treg cells by maintaining the Il2 promoter in a transcriptionally inactive state.
FoxP3 can also bind the Il2 promoter and participate in the inhibition of the Il2 gene transcription cooperating with other binding partners in Treg cells (17). We then determined if Helios might also cooperate with Foxp3 to regulate the Il2 promoter activity in Treg cells. To examine this possibility, we performed a sequential ChIP analysis to determine if both Helios and Foxp3 were localized simultaneously to the Il2 promoter in Treg cells. Interestingly, we found that Foxp3 and Helios were found to localize simultaneously on the Il2 promoter, indicating that they might act in concert to regulate its activity (Fig. 3D). Our sequential ChIP findings led us to question whether Helios would be required for Foxp3 binding to the Il2 promoter. To examine this possibility, we transduced either a scramble shRNA, or a specific shRNA for Helios in CD4+RFP+ Treg cells, and performed ChIP analysis to determine Foxp3 binding to the Il2 promoter. As previously reported in Treg cells (17), FoxP3 recruitment to the Il2 promoter was clearly detected in control scramble shRNA-transduced Treg cells. However, Treg cells expressing a shRNA specific for Helios showed a marked loss of Foxp3 association with the Il2 promoter (Fig. 3E). These results indicate that in the absence of Helios, Foxp3 has a reduced ability to bind the Il2 promoter, and therefore, that Helios promotes Foxp3 binding to that locus.
Helios regulates Treg-mediated suppression
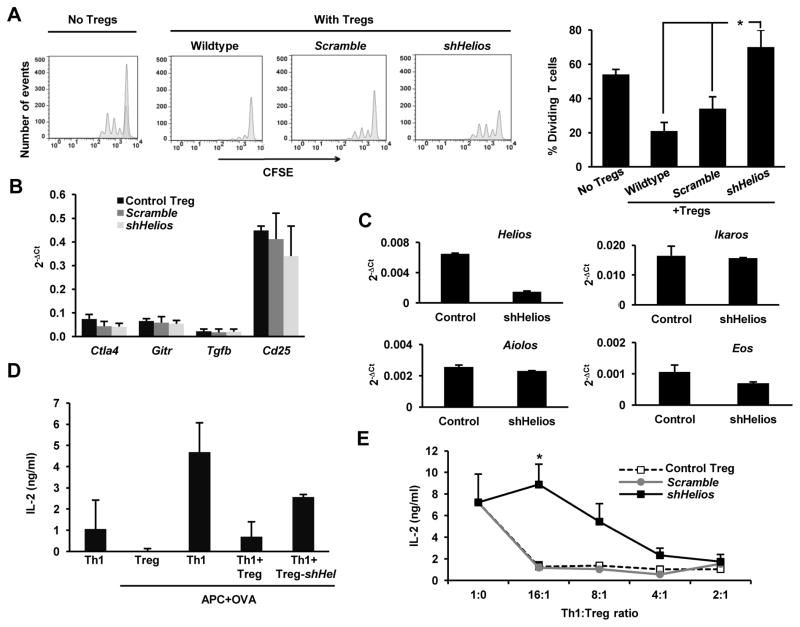
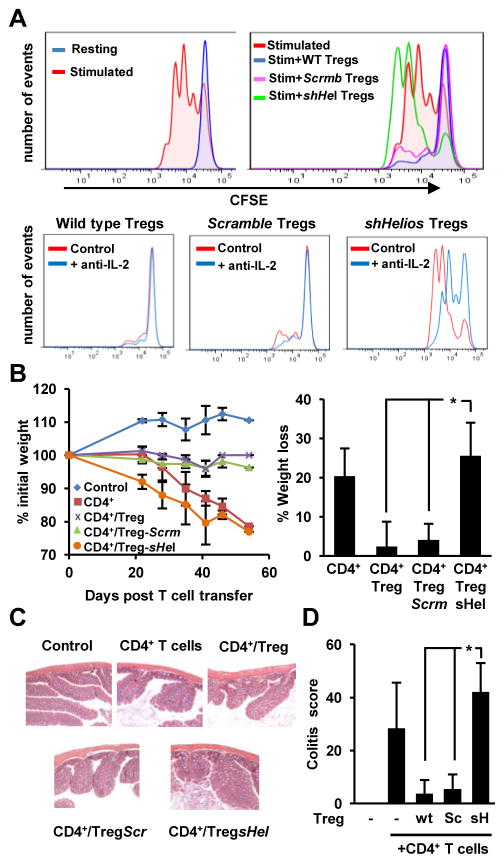
One of the primary functions of Treg cells in the immune system is to suppress other T cell responses. Because we observed that loss of Helios led to increased IL-2 expression in Treg cells, we investigated whether the loss of an anergic phenotype would also affect the suppressive capacity of these cells. Purified CD4+RFP+ Treg cells were activated, transduced with either scramble or Helios-specific shRNAs, and co-cultured with CFSE-labeled CD4+CD25− T cells, and activated with plate-bound anti-CD3 for 72 hours. Analysis of CFSE dilution by flow cytometry indicated that knocking down Helios expression decreased the suppressive activity of Treg cells (Fig. 4A). The loss of suppressive activity was not due to loss of Foxp3, as we did not observe changes in the levels of Foxp3 expression in Treg cells where Helios expression had been knocked down (Fig. 2B). Interestingly, the expression of other genes also involved in Treg-mediated suppression, including Ctla4, Gitr, Tgfb and Cd25, was not altered either by the reduction in Helios expression (Fig. 4B). Knock down of Helios did not induce any compensatory upregulation of any other member of the Ikaros family of transcription factors either (Fig. 4C). These results suggested that the loss of Helios would cause a breakdown of the anergic phenotype of Treg cells, resulting in increased proliferation and production of IL-2, which could likely be the cause for the loss of suppressive activity in Treg cells with reduced Helios expression.
Figure 4. Helios regulates suppressive capacity of Treg cells.
A. Proliferation of naïve CD4+ Th cells was assessed by CFSE dilution in resting cells, and cells activated with 0.5 μg/ml of plate-bound antiCD3 (left panel) or cells activated in the presence of equal numbers of control Treg cells or Treg cells transduced with a lentivirus expressing a scramble shRNA or a shRNA specific for Helios (right panels). The percentage of Th cells undergoing more than two divisions was calculated for each condition (right graph) Data shown is from three independent experiments. *p<0.05 B and C. Expression of Ctla4, Gitr, Tgfb and Cd25 (B) or different Ikaros family members (C) was determined by qRT-PCR in control Treg cells or Treg cells expressing a scramble shRNA or a Helios specific shRNA (shHelios). Results show average+SEM of 3 independent experiments. D and E. Treg-mediated suppression of Th1 cells cytokine production was measured by ELISA. IL-2 production was measured in cultures of Th1 OT-II cells activated with OVA323–339-loaded (1μm) T cell-depleted splenocytes in the presence or absence of Treg cells at different Th1/Treg ratios (2:1 in D and from 2:1 to 16:1 in E) using control Treg cells or Treg cells transduced with a lentivirus expressing a scramble shRNA or a specific shRNA for Helios (shHel). Results are average+SEM of 4–6 independent experiments. *p<0.05. (shHelios vs. control or scramble shRNA-expressing Treg cells).
To confirm these results, Treg cells isolated from Foxp3-RFP mice were transduced with either scramble or Helios-specific shRNAs were co-cultured for 48 hours with Ova323–339-peptide loaded APCs and TCR-transgenic, OVA-specific CD4+ Th1 cells derived from OT-II mice. Under these conditions, the Treg cells, which did not express the transgenic TCR, did not produce any IL-2 (Fig. 4D, second bar). However, we still found that Treg cells in which the expression of Helios was inhibited showed reduced suppressive capacity, measured as inhibition of Il-2 production, compared to uninfected and scramble control-transduced Treg cells, which effectively suppress responder T cells even at low Treg to Th1 ratios (Fig. 4D and E). To further understand the possible role of the IL-2 that may be produced by Treg cells during a suppression reaction, we assessed the effect of neutralizing IL-2 in a suppression reaction were T cells were polyclonally activated, and therefore Helios-deficient Treg cells would secrete IL-2 (Fig. 2C). As expected, Treg cells expressing a Helios specific shRNA, fail to efficiently suppress proliferation of CD4+CD25− T cells activated with plate bound antiCD3, whereas control untransduced Treg cells, or Treg cells infected with a virus expressing a scramble shRNA blocked activation-induced T cell proliferation (Fig. 5A upper panels). Addition of a blocking anti-IL-2 antibody to the culture did not affect control Treg cells, but partially restored the suppressive capacity of Helios-deficient Treg cells (Fig. 5A lower panels). Together, these results indicate that Helios regulates different aspects of Treg biology that modulate their suppressive function, including the inhibition of IL-2 expression and the regulation of their anergic status.
Figure 5. Helios regulates the suppressive capacity of Treg cells in vitro and in vivo.
A. Proliferation of naïve CD4+ Th cells was assessed by CFSE dilution in resting cells and cells activated with 0.5 μg/ml of plate-bound antiCD3 alone (upper left panel) or in the presence of equal numbers of control Treg cells or Treg cells transduced with a lentivirus expressing a scramble shRNA or a shRNA specific for Helios (upper right panel). The effect on Treg-mediated suppression of blocking IL-2 using a neutralizing anti-IL2 antibody during the suppression reactions for different Treg groups was also analyzed (lower panels). Data shown is from two independent experiments performed in triplicate. B: IBD was induced in Rag1−/− mice as described in the material and methods section. The protective effect of cotransferring naïve CD4+ T cells and control Treg cells or Treg cells transduced with a lentivirus expressing a scramble shRNA (Scrm) or a shRNA specific for Helios (sHel) was assessed by monitoring total body weight, expressed as percentage of the initial weight for each animal (B), of through histopathological analysis of the colon in mice sacrificed 50 to 55 days post-adoptive T cell transfer. (C–D). Control untouched mice were also analyzed. Percentage of body weight loss at time of sacrifice was calculated (B, right panel). Histopathological score was calculated for each sample as previously described (28). Data are presented as average+SD from three independent experiments, in which one mouse per condition per experiment were analyzed. *p<0.05.
To determine if Helios would also regulate Treg function in vivo we measured the impact of knocking down Helios expression in a mouse model of IBD. IBD was induced by injection of naïve CD4+CD25− T cells into Rag1−/− mice, which caused weight loss and colon inflammation that extended from the cecum to the distal colon (Fig. 5B–D). As expected, transfer of control or scramble shRNA-expressing Treg cells protected against IBD, and mice showed no weight loss or any signs of mucosal inflammation in the colon. Supporting the in vitro data, Treg cells that expressed a shRNA for Helios, failed to protect against IBD and mice showed in some cases even more marked weight loss and higher colitis scores that mice receiving just naïve T cells (Fig. 5 B–D).
Discussion
Helios is a member of the Ikaros family of transcription factors whose expression appears to be restricted primarily to the lymphoid compartment (21). Confirming previous reports (20, 24, 30, 36), we have seen that Treg cells express much higher levels of this transcription factor than naïve T helper or effector Th1 cells. Though originally identified as a possible target of Foxp3, the regulation of Helios expression in Treg cells has not been fully characterized yet. It was suggested that Helios could be a target of Foxp3 in Treg cells (24, 27). It has also been reported that Helios expression can be maintained during Treg expansion by the presence of oligodeoxynucleotides in a TLR-independent manner, suggesting that oligodeoxynucleotide-sensing pathways may participate in the regulation of Helios expression in Treg cells (37). Our results show that signals transduced through the TCR can also regulate Helios expression in Treg cells, as upregulation of Helios mRNA was clearly detected in Treg cells up to one week following activation through engagement of the TCR and CD28. Our results also indicate that IL-2R mediated signaling can inhibit the expression of Helios, which may reflect a negative feedback regulatory mechanism to downregulate Helios expression during Treg cell expansion. Interestingly, IL-2 has also been shown to inhibit the expression of Ikaros in Th1 cells, preventing it from repressing Il2 transcription in activated T cells (32).
Although most Ikaros family members have important roles during hematopoietic cell development, expression of these transcription factors can also be detected in differentiated cells, where they regulate different processes. Ikaros has been previously identified as a factor required to suppress Il2 transcription in anergic CD4+ T cells (18, 19) and Aiolos has recently shown to participate in the inhibition of Il2 expression in developing Th17 cells (38). Interestingly, Eos was also shown to cooperate with Foxp3 in the suppression of the expression of several genes, including Il2, in Treg cells (15). Our results support that Helios is also responsible for the repression of Il2 transcription in Treg cells, and therefore for the maintenance of the anergic phenotype of this T cell population, as shRNA-mediated inhibition of Helios expression allowed Treg cells to produce IL-2 following stimulation. Interestingly, while lack of Il2 expression in specific Treg populations has been correlated with decreased levels of several transcription factors, those Treg cells that failed to produce IL-2 also express the highest levels of Helios (39). The Ikaros family proteins can bind the same DNA motifs (33), several of which have been identified at the Il2 promoter, where they recruit Ikaros to regulate Il2 transcription in Th1 cells (18, 19). Our results show that the effect of Helios on Il2 transcription appears also to be direct, as specific recruitment of Helios to the Il2 promoter occurs in Treg cells. Transcription factors of the Ikaros family frequently interact and have been shown to associate with one another to form heterodimers (22). It would be interesting to explore if Helios might also cooperate with other Ikaros proteins to silence Il2 expression. This possibility could explain why silencing of either Helios or Eos results in de-repression of Il2 expression in Treg cells (15). Alternatively Helios might act independently of Eos, which would be supported by our finding that upon knockdown of Helios, there is decreased association of Foxp3 with the Il2 promoter in Treg cells, which does not occur when Eos expression is inhibited (15). One of the targets of Foxp3 in Treg cells is the Il2 promoter, where Foxp3 has been shown to cooperate with other transcription factors to inhibit the expression of this cytokine gene (15, 17). Our analyses using sequential ChIP show that both Foxp3 and Helios bind simultaneously to the proximal Il2 promoter, and that Foxp3 binding is reduced when Helios expression is inhibited. These results indicate that Helios may cooperate with Foxp3 to suppress Il2 expression, and that binding of Helios could facilitate binding of Foxp3 to the Il2 promoter.
Our results add to the overall model that Ikaros proteins may have common and overlapping roles, acting individually or coordinately, to repress Il2 expression in different T cell populations; Ikaros in anergic Th1 cells, Aiolos in Th17 cells and Eos and Helios in Treg cells. The specific role of each of those proteins may be determined by their pattern of expression or their ability to interact with different partners. Recently it has been shown that Helios is also expressed in T cells that differentiate into Th2 cells (29). We can speculate that Helios may also downregulate Il2 expression in this T helper cell population that does not produce significant amounts of IL-2. However, as shown for Ikaros in Th2 cells, Helios may also regulate the expression or other lineage determining proteins or cytokines in those T cells (34, 40).
Helios has the ability to interact with histone remodeling complexes, including NuRD, which contain HDACs and contribute to silencing of gene expression by inducing core histone deacetylation on regulatory elements of specific genes (35). Although, as it has been shown for Ikaros (41), we cannot rule out that Helios may also exert its repressive effect independently of its ability to recruit HDACs, our results support that in Treg cells Helios contributes to silencing Il2 expression by inducing deacetylation of histones at the Il2 promoter. We detected decreased levels of H3-acetylation in Treg cells compared with effector Th1 cells, which present high levels of histone acetylation at the Il2 locus (18). These results are consistent with lack of activation-induced IL-2 production in Treg cells and high levels of IL-2 expression in effector Th1 cells. However, suppression of Helios expression in Treg cells caused increased activation-induced expression of IL-2 and resulted in increased levels of acetylated H3, similar to the ones found in effector Th1 cells.
Treg cells present an anergic phenotype when stimulated ex vivo and are not capable of proliferating when stimulated in the absence of IL-2 (11). Our results indicate that Helios not only maintains Il2 gene silencing in Treg cells, but it also appears to control the proliferative responses of this T cell population, as inhibition of Helios expression led to increased proliferation of Treg cells. It is possible that derepression of Il2 expression in the absence of Helios might be responsible, via an autocrine loop, for increasing Treg cell division. However, it is important to note that IL-2 expression in Helios knock-down Treg cells required engagement of the TCR, whereas baseline proliferation was detected in Helios-deficient Treg cells even in the absence of re-stimulation. Interestingly, Helios inhibition also protected Treg cells from cell death, a process that has been recently proposed to be independent of IL-2 signaling in this T cell population (42). Supporting the notion that Helios may regulate T cell survival, overexpression of Helios in naive CD4+CD25− T cells and some T cell lines has been reported to induce cell death (24, 36). The downregulation of Helios expression that we detected in response to IL-2 signaling might, therefore, represent a pro-survival pathway activated by this cytokine in proliferating Treg cells. Our results support, thus, that Helios can control Treg function at different levels: by suppressing Il2 transcription and maintaining a non-proliferative anergic state, and by regulating Treg survival.
Although IL-2 receptor signaling is required for the maintenance and activation of Treg cells (42–44), Treg-mediated suppression is inhibited in vitro in the presence of IL-2 (10, 13, 14). Our results show that breaking the anergic phenotype of Treg cells by inhibiting Helios expression reduces their suppressive activity in vitro and in vivo. This effect, although likely mediated in part by the expression of IL-2 in Treg cells and therefore partially inhibited using anti-IL-2 blocking antibodies, cannot be completely explained by that fact. In conditions where Helios-null Treg cells did not produce IL-2, we still detected a clear loss of suppressive function in those Treg cells that had reduced expression of Helios. We cannot rule out that that Helios may also regulate the expression of other genes required to confer suppressive activity to Treg cells, however, we did not detect any changes in the levels of expression of several Foxp3 target genes associated with suppression, such as Cd25 or Ctla4, Gitr, or in Foxp3 itself. Similarly, a recent report has failed to identify a role for Helios in the regulation of a Treg gene signature, which appears to be redundantly regulated by a different set of transcription factors (45). Treg cells develop normally in Helios-deficient mice and appear to suppress with a similar activity as their wild type counterparts (20, 23). However, experiments performed with human Treg cells in which Helios expression had been knocked down showed reduced suppressive activity in those cells (24). Differences observed between knock-out cells and knocked-down Treg cells may be explained by the presence of active redundant mechanisms in Treg cells from Helios−/− mice, as Ikaros-family proteins recognize the same consensus biding sequences and can cooperate with each other forming heterodimers (22). In this sense, it has been reported that in some genetic backgrounds Helios-deficient mice are not viable, which may indicate that those redundant mechanisms may be active only in certain strains or under specific conditions (23). We did not observe any compensatory increase in the expression of Ikaros, Aiolos or Eos in Treg cells where Helios expression was knocked down, which might explain why the acute loss of this transcription factor might have resulted in a clear alteration of the phenotype of those Treg cells.
The expression of Helios has been proposed to identify the population of thymically derived natural Treg cells (20). Additionally, a recent report shows that naïve Helios+ cells likely represent thymically-derived Treg cells, which contain a completely de-methylated TSDR locus (37). However, recent reports have suggested that this transcription factor might also be expressed in peripherally induced Treg cells (30). We performed our experiments using Treg cells isolated from young specific pathogen-free Foxp3-RFP mice, which contain mostly natural Treg cells. However, since Helios may also be expressed in induced Treg cells (30), it is possible that it might also play a similar role in those cells, silencing the expression of Il2 and regulating proliferation and survival of induced Treg cells.
Acknowledgments
This work was supported by NIH grants AI079363 and AI059738 (to FM). IB was supported by training grant T32GM007288.
Abbreviations used
- ChIP
chromatin immunoprecipitation
- qRT-PCR
quantitative real time PCR
- RFP
red fluorescent protein
- sh
small hairpin RNA
- Th
T helper cell
- Treg
regulatory T cell
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 6.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–8. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–11. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–30. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 12.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 14.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D, Drake CG, Liu JO, Ostrowski MC, Pardoll DM. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–6. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su L, Creusot RJ, Gallo EM, Chan SM, Utz PJ, Fathman CG, Ermann J. Murine CD4+CD25+ regulatory T cells fail to undergo chromatin remodeling across the proximal promoter region of the IL-2 gene. J Immunol. 2004;173:4994–5001. doi: 10.4049/jimmunol.173.8.4994. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–86. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179:7305–15. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 20.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, Akashi K, Weissman IL, Fisher AG, Smale ST. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 1998;12:782–96. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, Morgan BA. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol. 1998;8:508–15. doi: 10.1016/s0960-9822(98)70202-7. [DOI] [PubMed] [Google Scholar]
- 23.Cai Q, Dierich A, Oulad-Abdelghani M, Chan S, Kastner P. Helios deficiency has minimal impact on T cell development and function. J Immunol. 2009;183:2303–11. doi: 10.4049/jimmunol.0901407. [DOI] [PubMed] [Google Scholar]
- 24.Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, Pan F, Pardoll DM, Drake CG. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47:1595–600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, Tam AJ, Getnet D, Drake CG. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am J Pathol. 2002;160:739–51. doi: 10.1016/S0002-9440(10)64894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 28.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R, Chan S, Kastner P, Cunningham AF, Maclennan IC, Mohr E. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One. 2011;6:e20731. doi: 10.1371/journal.pone.0020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottschalk RA, Corse E, Allison JP. Expression of helios in peripherally induced foxp3+ regulatory T cells. J Immunol. 2012;188:976–80. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 31.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–31. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dure M, Macian F. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol Immunol. 2009;46:999–1006. doi: 10.1016/j.molimm.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rebollo A, Schmitt C. Ikaros, Aiolos and Helios: transcription regulators and lymphoid malignancies. Immunol Cell Biol. 2003;81:171–5. doi: 10.1046/j.1440-1711.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, Wells AD. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. J Biol Chem. 2010;285:2545–53. doi: 10.1074/jbc.M109.038794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem. 2007;282:30227–38. doi: 10.1074/jbc.M702541200. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 37.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, Shevach EM. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–8. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK. Aiolos promotes T(H)17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13:770–7. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendfeldt H, Benary M, Scheel T, Steinbrink K, Radbruch A, Herzel H, Baumgrass R. IL-2 Expression in Activated Human Memory FOXP3(+) Cells Critically Depends on the Cellular Levels of FOXP3 as Well as of Four Transcription Factors of T Cell Activation. Front Immunol. 2012;3:264. doi: 10.3389/fimmu.2012.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umetsu SE, Winandy S. Ikaros is a regulator of Il10 expression in CD4+ T cells. J Immunol. 2009;183:5518–25. doi: 10.4049/jimmunol.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koipally J, Georgopoulos K. Ikaros-CtIP interactions do not require C-terminal binding protein and participate in a deacetylase-independent mode of repression. J Biol Chem. 2002;277:23143–9. doi: 10.1074/jbc.M202079200. [DOI] [PubMed] [Google Scholar]
- 42.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–30. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–9. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 45.Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS, Gazit R, Adoro S, Glimcher L, Chan S, Kastner P, Rossi D, Collins JJ, Mathis D, Benoist C. A multiply redundant genetic switch ‘locks in’ the transcriptional signature of regulatory T cells. Nat Immunol. 2012;13:972–80. doi: 10.1038/ni.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]