Abstract
Brain damage caused by intracerebral hemorrhage (ICH) is mediated in part by the toxicity of extravascular blood deposited in brain parenchyma during the hematoma formation. In this paper we discuss the therapeutic benefits and potential mechanisms associated with the activation of transcription factor Nrf2 regarding its role in defending brain tissue against toxicity of blood, a component of secondary injury. We emphasize the pleiotropic capacity of Nrf2 as it recruits multiple pathways aiming at reducing deleterious effects of blood lysis products.
Keywords: Intracerebral hemorrhage, Nrf2, oxidative stress, cytoprotection
Introduction
Hemorrhagic stroke (ICH) is the third leading cause of death in the US. An estimated 37,000 to 52,400 people in the US suffer an ICH each year. This rate is expected to double by 2050 as a result of an aging population and changing racial demographics, and to date there are no specific treatments for human ICH [1–3]. The strongest predictor of the poor clinical outcome after ICH is the volume of hematoma. The deposited blood is damaging initially via mass effect and then via blood (hemolysis products) toxicity and pro-inflammatory responses. These blood products, primarily through oxidative damage may contribute to delayed neuronal loss, vascular injury, blood brain barrier opening, edema, and neurological deficit or death [3–4].
Hemolysis product toxicity and detoxification mechanisms after ICH
There is an immediate mass effect with physical disruption and increased intracranial pressure. In addition, there is secondary brain injury due to clot-derived factors including hemoglobin (Hb) and Hb degradation products (heme and iron). Hb is the most abundant RBC protein. Normally, it takes approximately 1–4 days for RBC to undergo massive lysis, resulting in release of cell-free Hb [5–6]. The cell-free Hb is a potent oxidant which is toxic to all brain cells, neurons and oligodendrocytes in particular [1, 6–8]. The deposited RBC are considered to be benign to the brain tissue until the hemolysis begins. In agreement with this notion, intracranial injection of lysed RBC could within hours reproduce most of the pathological features normally found days after injection of whole (not-lysed) blood [9]. Since the onset of Hb-, heme-, or iron-mediated toxicity is significantly delayed apart from the ICH ictus, it is likely that the preconditioning therapies started hours after ICH could help brain to gain extra resistance to these toxic/pathogenic factors.
Normally, to neutralize the toxicity imposed by cell-free Hb, haptoglobin (Hp) (assuming its availability) tightly binds to free Hb, forming Hb-Hp complexes that are subsequently cleared by the microglia/macrophages (Mφ) expressing specialized Hb scavenger receptor, CD163 [10–11]. Analogous to Hp, hemopexin (Hx) [12–13] may bind heme and form chemically stable heme-Hx complexes that are cleared through Mφ scavenger receptor CD91 [14]. Once internalized by Mφ, heme is catabolized by heme oxygenases (HOs), enzymes that mediate breakdown of the porphyrin ring of heme into biliverdin, carbon monoxide (CO) and iron [15]. Iron must be immediately sequestered to prevent additional oxidative stress. Ferritin (Fx) is the most likely candidate for this function [16]. Fx synthesis normally occurs in microglia depending on the local concentration of iron and the presence of iron-regulatory proteins [17]. Thus, preconditioning approaches (treatment initiated prior to hemolytic process is initiated) allowing for: (1) boosting the scavenging/cleanup of hemolytic products (Hb, heme and iron) by Mφ, (2) improving production of Hp, Hx, Fx, and HO-1, as well as (3) improving overall resistance of brain cells to oxidative stress, could represent uniquely effective strategies allowing the brain to better withstand the ICH-induced secondary injury caused by hemolysis products. Interestingly, all the above mentioned pre-conditioning components can be achieved via activation of a ubiquitous pleiotropic transcription factor, nuclear factor-erythroid 2 p45-related factor 2 (Nrf2).
Nrf2
Nrf2 is a basic region-leucine zipper (bZIP) protein in the CNC-bZIP subfamily and master genomic regulator of the cellular antioxidant defense system and detoxification. In response to oxidative stress (and electrophilic compounds), Nrf2 is activated, which will boost the expression of cytoprotective and anti-oxidative target genes and prepare the cells to be more resistance to oxidative stress [18–19]. Normally, Nrf2 is sequestered in the cytoplasm by interacting with inhibitory protein Kelch-like ECH-associated protein 1(Keap1). In most cells, Nrf2 is present at low concentrations due to continuous Nrf2 degradation through proteasome pathway. Nrf2 degradation is Keap1-dependent via binding to the Cul3 E3 ligase complex to mediate a rapid ubiquitination and proteasome degradation. The t1/2 for Nrf2 is considerably short and proposed to be 10–40 min [19–20]. Keap1 contains 25 cysteine residues that likely represent a direct target for inducers of Nrf2 activation. Alkylation, oxidation or reduction of the sulfhydryl group on these cysteine residues of Keap1 may represent an initiating step in Nrf2 activation. Ultimately, Nrf2 forms the heterodimeric complexes with Mif family proteins, to transactivate the antioxidant response elements (ARE).
The beneficial effect of Nrf2 on ICH pathogenesis
Oxidative stress
As indicated above, one of the key factors linked to ICH pathogenesis is oxidative stress [3]. Taking this into account, we have recently shown that Nrf2 activation may play essential role in protecting the brain from oxidative damage imposed by ICH [21]. Nrf2 is known to control the expression of numerous enzymes constituting an antioxidant/cytoprotective defense system, e.g. gamma-glutamylcysteine synthetase (γGCS), superoxide dismutase (SOD; primarily SOD1), catalase, glutathione reductase (GR), thioredoxin reductase (TR), peroxiredoxins (Prxs) or glutathione (GSH) S-transferase (GSTs). By using sulforaphane (SF) to activate Nrf2 in rodents subjected to ICH injury, we have demonstrated that both the pre-treatment and, most importantly, post-treatment with SF were uniquely effective in upregulating the expression of many of these Nrf2-regulated anti-oxidative proteins, causing the reduction in oxidative burden to brain tissue, and ultimately improving neurological functional recovery. We and others also showed that Nrf2-deficient mice suffered from the more pronounced injury in response to ICH, suggesting loss of the self-protective effect via Nrf2 [21–22]. Furthermore, SF was no longer effective in Nrf2 deficient mice indicating that the protective effect of SF was indeed through Nrf2. At cellular level, existing studies suggest that Nrf2 activation may benefit neurons [23], astrocytes [24], oligodendrocytes [25] and microglia (our unpublished data) regarding susceptibility to oxidative damage, indicating high relevance of this therapeutic approach to the entire neurovascular unit.
Hemoglobin detoxification by Haptoglobin (Hp)
Hp is an acute-phase α2-acid response glycoprotein well-known to form highly stable Hb-Hp complexes with free Hb [26]. Because iron-rich Hb via Fenton oxidation reaction may cause robust lipid peroxidation, oxidative DNA damage, neuronal death and consequently inflammation [27–29], the neutralization of Hb by Hp may prevent Hb-mediated toxicity. Following hemolysis, Hp appears to act as a main player in the clearance of Hb from the extracellular environment. Normally, Hp is produced primarily in liver and secreted into blood circulation, though tissue-specific expression of Hp exists in the brain, lung and kidney [30–32]. In brain, Hp was identified in the neural retina [33] and oligodendrocytes [34]. In response to hemolysis, including after ICH, sequestration of Hb by Hp may cause the level of Hp in the blood or affected tissue to decrease, leading to hypohaptoglobinemia (HHp) [34–35]. At this stage, until Hp is re-synthesized the clearance of Hb could be compromised, leading to prolonged and more robust Hb cytotoxicity [36]. In context of ICH, HHp and Hp deficiency could result in more severe brain damage. In our recent study, we demonstrated that after ICH the levels of Hp in blood plasma and in ICH-affected brain were robustly increased in animals that were treated with Nrf2 activator, sulforaphane (SF)[34]. We also identified that the most likely source of Hp in brain are oligodendroglia, cells in which the robust synthesis and secretion of Hp takes place in response to Nrf2 activation with SF or tert-butylhydroquinone (tBHQ).
Hemopexin (Hx)
Similar to free-Hb, extracellular heme is highly cytotoxic. Specifically, heme can intercalate into cell membranes and other lipophilic structures, causing cell damage and pathologic inflammatory conditions through the potent pro-oxidative effects [37–39]. Hx is a 60-kDa protein that binds heme with the highest affinity out of any known protein-protein interactions [12, 40]. The formation of heme-Hx complexes could occlude the strong pro-oxidative features of free heme [41–42]. Heme-Hx dimer may be readily removed from extracellular space via microglia/macrophages expressing CD91 scavenging receptor [10, 14]. Hx, similar to Hp, is primarily produced by liver [40]; however, its production by the CNS, retina, and peripheral nerves has also been suggested [43, 33]. Regard to ICH, we found that Hx is upregulated in the peri-hematoma area, mainly in neurons and phagocytes (microglia/macrophages). We propose that this increase in Hx production forms the auxiliary line of defense against hemolysate-mediated oxidative damage [44]. The complementary role of Hx and Hp in models of acute hemolysis was demonstrated by showing that mice with both Hp and Hx inactivated are much more sensitive to hemolytic stress than mice with single Hp or Hx deficiency [45]. Interestingly, the strong evidence exists for Hx expression increase in response to Nrf2 activation [46–47], including our results (fig 2), suggesting that activation of this transcription factor can assist in elimination not only Hb, but also heme.
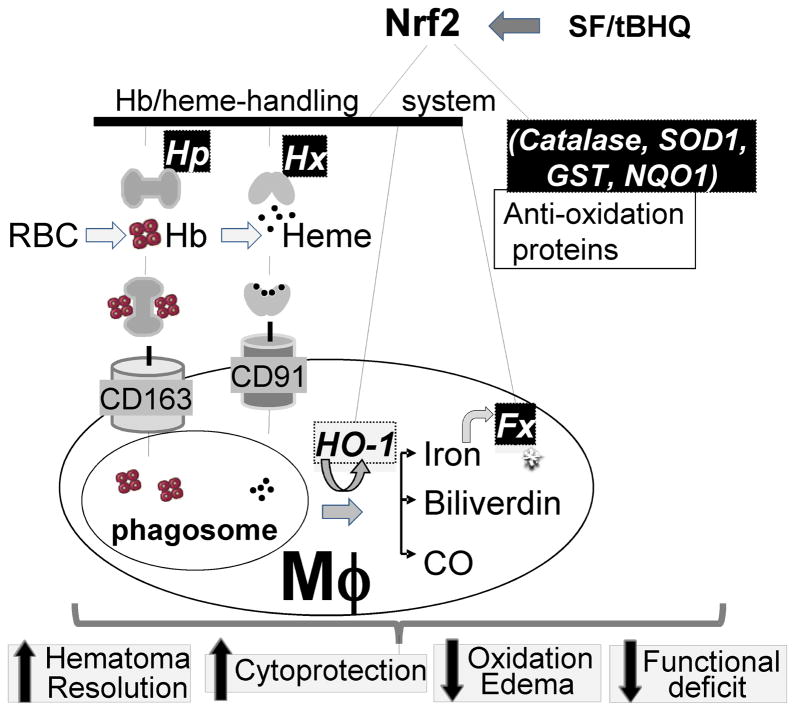
Model 1. The endogenous detoxification system for hemolytic products (Hb, heme and iron).
It is proposed that the cell-free Hb and heme are initially neutralized by binding to Hp and Hx, respectively, forming stable Hb-Hp and heme-Hx complexes. The complexes can be endocytosed by Mφ through the Hb scavenger receptor CD163 [68] and the heme scavenger receptor CD91 [14], respectively. Heme in Mφ is catabolized by HO-1 to biliverdin, carbon monoxide (CO), and free iron [52]. The latter one is sequestrated within Mφ by intracellular iron binding protein, ferritin (Fx). Activation of Nrf2 with SF or tBHQ may upregulate production of: (A) enzymes constituting the antioxidant/cytoprotective defense system (e.g. SOD1, catalase, γGCS, GR, TG, Prxs, GST and NQO1) [69], and (B) detoxification enzymes (e.g. Hp, Hx, HO-1 and Fx) for preventing the toxic effect of hemolytic products. Thus, approaches aiming at stimulating Nrf2 prior to hematoma hemolysis may ameliorate oxidative stress directly by controlling production of anti-oxidant enzymes and indirectly by upregulating components capable of blocking toxic effect of iron-containing hemolytic products.
Heme oxygenase-1 (HO-1)
In contrast to HO-2 that is expressed constitutively, HO-1 gene is inducible and transcriptionally regulated via oxidative stress signals and Nrf2 [48–50]. HO is the rate limiting enzyme participating in catabolism of Hb/heme to biliverdin, free iron, and carbon monoxide [51–52]. Whereas inhibition of HO-1 with SnPP may effectively reduce generation of the pro-oxidative iron in the hematoma, SnPP may have adverse effect on clearance of RBC from the hematoma (our unpublished data). HO-1 is protective to many cell types in various models of injury, including excitotoxicity and cerebral ischemia [53–56]. Interestingly, HO-1 in ICH may have distinct roles, as numerous studies suggest that HO inhibitors (ZnPP or SnPP) could benefit hemorrhagic brain by reducing edema after ICH [57–60].
Ferritin (Fx)
Fx is a ubiquitous and highly conserved iron storage protein, with the function in maintaining iron homeostasis and preventing free iron toxicity [61]. In phagocytes iron is stored by Fx-containing hemosiderin which is particularly abundant in phagocytic cells after hemorrhage. Fx synthesis normally occurs in Mφ depending on the local concentration of iron and the presence of iron regulatory proteins [17]. While Fx expression is primarily regulated at the translational level, the additional transcriptional regulation of Fx expression is also accepted and is an important protective mechanism in some pathological situations such as in ischemia [62]. Nrf2 plays an important role in the transcriptional regulation of Fx expression [63–64] and Fig 2. Overall, Fx represents a key component of the detoxification system for Hb/heme downstream from Hp and Hx. Based on the promising studies with deferoxamine (an iron chelator that is evaluated as treatment for ICH), additional strategies aiming at iron chelating would have great potential in preventing hemolytic products-mediated toxicity after ICH [65–67].
In conclusion, in contrast to ischemic stroke, a considerably longer therapeutic window may exist for preventing the secondary injury caused by the toxic hemolytic products after ICH. As such, we postulate that the pre-conditioning by activating Nrf2 within several hours after ICH (prior to hemolytic events are initiated) could prime the ICH-affected brain to better handle the noxious hemolytic products. This Nrf2-mediated priming may include: (1) upregulation of anti-oxidant enzymes in all the affected brain cells to increase their resistant to oxidative stress and, (2) upregulation of detoxification proteins to neutralize the toxic hemolysis products around hematoma.
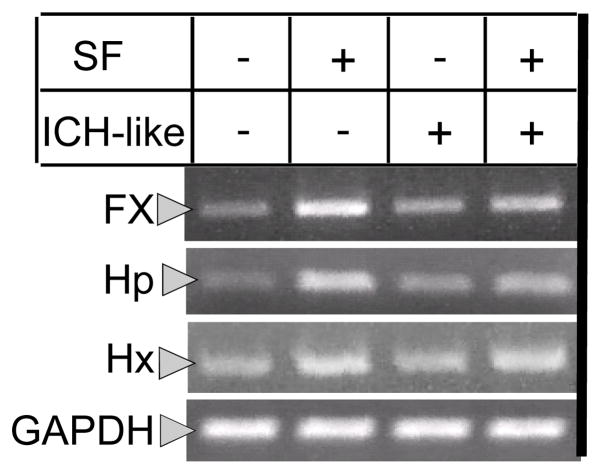
Fig. 1. Photograph of RT-PCR products in rat brain cortical neuron-glial co-cultures.
The expression of Fx, Hp and Hx is up-regulated in the SF-treated cells with or without the presence of lysed RBCs. The GAPDH served as the internal control. SF was pre-incubated for 30min and the gene products are checked at 6h after exposure to lysed RBCs. “ICH” --- “ICH-like” injury (Lysed RBCs).
Acknowledgments
This work was partially supported by National Institutes of Health, NINDS grants NS060768 and NS064109.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest
References
- 1.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–6. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi G, Fewel ME, Hua Y, Thompson BG, Jr, Hoff JT, Keep RF. Intracerebral hemorrhage: pathophysiology and therapy. Neurocrit Care. 2004;1(1):5–18. doi: 10.1385/ncc:1:1:5. [DOI] [PubMed] [Google Scholar]
- 5.Wagner KR, Dwyer BE. Hematoma removal, heme, and heme oxygenase following hemorrhagic stroke. Ann N Y Acad Sci. 2004;1012:237–51. doi: 10.1196/annals.1306.020. [DOI] [PubMed] [Google Scholar]
- 6.Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23(6):629–52. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- 7.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27(3):268–79. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 8.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96(2):287–93. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953(1–2):45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- 10.Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, et al. Hemoglobin and heme scavenging. IUBMB Life. 2005;57(11):749–59. doi: 10.1080/15216540500380871. [DOI] [PubMed] [Google Scholar]
- 11.Schaer DJ, Alayash AI, Buehler PW. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid Redox Signal. 2007;9(7):991–9. doi: 10.1089/ars.2007.1576. [DOI] [PubMed] [Google Scholar]
- 12.Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA and cell biology. 2002;21(4):297–306. doi: 10.1089/104454902753759717. [DOI] [PubMed] [Google Scholar]
- 13.Muller-Eberhard U. Hemopexin. The New England journal of medicine. 1970;283(20):1090–4. doi: 10.1056/NEJM197011122832007. [DOI] [PubMed] [Google Scholar]
- 14.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7):2572–9. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 15.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. American journal of physiology. 2006;290(3):F563–71. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 16.Roskams AJ, Connor JR. Iron, transferrin, and ferritin in the rat brain during development and aging. J Neurochem. 1994;63(2):709–16. doi: 10.1046/j.1471-4159.1994.63020709.x. [DOI] [PubMed] [Google Scholar]
- 17.Rogers J, Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987;84(8):2277–81. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4(3):267–81. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 19.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280(37):32485–92. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38(12):3280–6. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43(3):408–14. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278(39):37948–56. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278(14):12029–38. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114(2):237–46. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wada T, Oara H, Watanabe K, Kinoshita H, Yachi A. Autoradiographic study on the site of uptake of the haptoglobin-hemoglobin complex. J Reticuloendothel Soc. 1970;8(2):185–93. [PubMed] [Google Scholar]
- 27.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89(6):991–6. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke. 2002;33(7):1882–8. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- 29.Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984;259(23):14354–6. [PubMed] [Google Scholar]
- 30.Lee MY, Kim SY, Choi JS, Lee IH, Choi YS, Jin JY, et al. Upregulation of haptoglobin in reactive astrocytes after transient forebrain ischemia in rats. J Cereb Blood Flow Metab. 2002;22(10):1176–80. doi: 10.1097/01.wcb.0000037989.07114.d1. [DOI] [PubMed] [Google Scholar]
- 31.Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest. 1993;91(3):977–85. doi: 10.1172/JCI116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang F, Haile DJ, Berger FG, Herbert DC, Van Beveren E, Ghio AJ. Haptoglobin reduces lung injury associated with exposure to blood. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L402–9. doi: 10.1152/ajplung.00115.2002. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Lu H, Dutt K, Smith A, Hunt DM, Hunt RC. Expression of the protective proteins hemopexin and haptoglobin by cells of the neural retina. Exp Eye Res. 1998;67(1):83–93. doi: 10.1006/exer.1998.0494. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci. 2009;29(50):15819–27. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panter SS, Sadrzadeh SM, Hallaway PE, Haines J, Anderson VE, Eaton JW. Hypohaptoglobinemia: a possible predisposition to epilepsy. Trans Assoc Am Physicians. 1984;97:56–62. [PubMed] [Google Scholar]
- 36.Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36(40):12189–98. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. J Neurosci Res. 2003;73(1):113–21. doi: 10.1002/jnr.10633. [DOI] [PubMed] [Google Scholar]
- 38.Camejo G, Halberg C, Manschik-Lundin A, Hurt-Camejo E, Rosengren B, Olsson H, et al. Hemin binding and oxidation of lipoproteins in serum: mechanisms and effect on the interaction of LDL with human macrophages. J Lipid Res. 1998;39(4):755–66. [PubMed] [Google Scholar]
- 39.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Eaton JW, et al. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res. 2005;49(11):1030–43. doi: 10.1002/mnfr.200500076. [DOI] [PubMed] [Google Scholar]
- 40.Nikkila H, Gitlin JD, Muller-Eberhard U. Rat hemopexin. Molecular cloning, primary structural characterization, and analysis of gene expression. Biochemistry. 1991;30(3):823–9. doi: 10.1021/bi00217a036. [DOI] [PubMed] [Google Scholar]
- 41.Grinberg LN, O’Brien PJ, Hrkal Z. The effects of heme-binding proteins on the peroxidative and catalatic activities of hemin. Free Radic Biol Med. 1999;27(1–2):214–9. doi: 10.1016/s0891-5849(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 42.Hunt RC, Hunt DM, Gaur N, Smith A. Hemopexin in the human retina: protection of the retina against heme-mediated toxicity. J Cell Physiol. 1996;168(1):71–80. doi: 10.1002/(SICI)1097-4652(199607)168:1<71::AID-JCP9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Tolosano E, Cutufia MA, Hirsch E, Silengo L, Altruda F. Specific expression in brain and liver driven by the hemopexin promoter in transgenic mice. Biochem Biophys Res Commun. 1996;218(3):694–703. doi: 10.1006/bbrc.1996.0124. [DOI] [PubMed] [Google Scholar]
- 44.Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin Chim Acta. 2001;312(1–2):13–23. doi: 10.1016/s0009-8981(01)00586-1. [DOI] [PubMed] [Google Scholar]
- 45.Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, et al. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100(12):4201–8. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- 46.Kristiansson MH, Bhat VB, Babu IR, Wishnok JS, Tannenbaum SR. Comparative time-dependent analysis of potential inflammation biomarkers in lymphoma-bearing SJL mice. J Proteome Res. 2007;6(5):1735–44. doi: 10.1021/pr060497x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5(1):39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- 48.Li N, Venkatesan MI, Miguel A, Kaplan R, Gujuluva C, Alam J, et al. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J Immunol. 2000;165(6):3393–401. doi: 10.4049/jimmunol.165.6.3393. [DOI] [PubMed] [Google Scholar]
- 49.Turner CP, Bergeron M, Matz P, Zegna A, Noble LJ, Panter SS, et al. Heme oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. J Cereb Blood Flow Metab. 1998;18(3):257–73. doi: 10.1097/00004647-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Ewing JF, Haber SN, Maines MD. Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32) in rat brain: hyperthermia causes rapid induction of mRNA and protein. J Neurochem. 1992;58(3):1140–9. doi: 10.1111/j.1471-4159.1992.tb09373.x. [DOI] [PubMed] [Google Scholar]
- 51.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 52.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y, Vreman HJ, Wong RJ, Tjoa T, Yamauchi T, Noble-Haeusslein LJ. Heme oxygenase-1 stabilizes the blood-spinal cord barrier and limits oxidative stress and white matter damage in the acutely injured murine spinal cord. J Cereb Blood Flow Metab. 2007;27(5):1010–21. doi: 10.1038/sj.jcbfm.9600412. [DOI] [PubMed] [Google Scholar]
- 54.Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, et al. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol. 2006;290(5):C1399–410. doi: 10.1152/ajpcell.00386.2005. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad AS, Zhuang H, Dore S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141(4):1703–8. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 56.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72(3):1187–203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 57.Wagener FA, van Beurden HE, von den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system: a molecular switch in wound healing. Blood. 2003;102(2):521–8. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 58.Gong Y, Tian H, Xi G, Keep RF, Hoff JT, Hua Y. Systemic zinc protoporphyrin administration reduces intracerebral hemorrhage-induced brain injury. Acta Neurochir Suppl. 2006;96:232–6. doi: 10.1007/3-211-30714-1_50. [DOI] [PubMed] [Google Scholar]
- 59.Wagner KR, Hua Y, de Courten-Myers GM, Broderick JP, Nishimura RN, Lu SY, et al. Tin-mesoporphyrin, a potent heme oxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies. Cell Mol Biol (Noisy-le-grand) 2000;46(3):597–608. [PubMed] [Google Scholar]
- 60.Koeppen AH, Dickson AC, Smith J. Heme oxygenase in experimental intracerebral hemorrhage: the benefit of tin-mesoporphyrin. J Neuropathol Exp Neurol. 2004;63(6):587–97. doi: 10.1093/jnen/63.6.587. [DOI] [PubMed] [Google Scholar]
- 61.Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63(3):793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 62.Chi SI, Wang CK, Chen JJ, Chau LY, Lin TN. Differential regulation of H- and L-ferritin messenger RNA subunits, ferritin protein and iron following focal cerebral ischemia-reperfusion. Neuroscience. 2000;100(3):475–84. doi: 10.1016/s0306-4522(00)00317-1. [DOI] [PubMed] [Google Scholar]
- 63.Yanagawa T, Itoh K, Uwayama J, Shibata Y, Yamaguchi A, Sano T, et al. Nrf2 deficiency causes tooth decolourization due to iron transport disorder in enamel organ. Genes Cells. 2004;9(7):641–51. doi: 10.1111/j.1356-9597.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- 64.Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278(4):2361–9. doi: 10.1074/jbc.M210664200. [DOI] [PubMed] [Google Scholar]
- 65.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104(2):305–12. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100(4):672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 67.Wan S, Hua Y, Keep RF, Hoff JT, Xi G. Deferoxamine reduces CSF free iron levels following intracerebral hemorrhage. Acta Neurochir Suppl. 2006;96:199–202. doi: 10.1007/3-211-30714-1_43. [DOI] [PubMed] [Google Scholar]
- 68.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 69.Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, et al. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310(1):263–71. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]