Abstract
Objective
Depression and brief periods of (hypo)mania are linked to an increased risk of progression to bipolar I or II disorder (BD) in children of bipolar parents. This randomized trial examined the effects of a 4-month family-focused therapy (FFT) program on the 1-year course of mood symptoms in youth at high familial risk for BD, and explored its comparative benefits among youth in families with high vs. low expressed emotion (EE).
Method
Participants were 40 youth (mean 12.3 ± 2.8 years, range 9–17) with BD not otherwise specified, major depressive disorder, or cyclothymic disorder who had a first-degree relative with BD I or II and active mood symptoms (Young Mania Rating Scale [YMRS] > 11 or Child Depression Rating Scale > 29). Participants were randomly allocated to FFT–High Risk version (FFT-HR; 12 sessions of psychoeducation and training in communication and problem-solving skills) or an education control (EC; 1–2 family sessions).
Results
Youth in FFT-HR had more rapid recovery from their initial mood symptoms (hazard ratio = 2.69, p = .047), more weeks in remission, and a more favorable trajectory of YMRS scores over 1 year than youth in EC. The magnitude of treatment effect was greater among youth in high-EE (vs. low-EE) families.
Conclusions
FFT-HR may hasten and help sustain recovery from mood symptoms among youth at high risk for BD. Longer follow-up will be necessary to determine if early family intervention has downstream effects that contribute to the delay or prevention of full manic episodes in vulnerable youth. Clinical trial registration information—Early Family-Focused Treatment for Youth at Risk for Bipolar Disorder; http://www.clinicaltrials.gov/; NCT00943085.
Keywords: expressed emotion, high risk, early warning signs, psychoeducation, psychosocial intervention
INTRODUCTION
Bipolar I and II disorder (BD) affect approximately 2.5% of US adolescents, with the risk for syndromal mania doubling from the early to the late teen years.1 There is increasing agreement on clinical phenotypes of children and adolescents who are at elevated risk for progression to fully syndromal BD.2–3 Among children of parents with BD, subthreshold “high-risk” forms of the disorder can be detected as many as 10 years prior to onset of BD I or II.3
Significant controversies exist about the diagnostic boundaries of early-onset BD, but agreement is substantial that BD and its subthreshold antecedents have a considerable impact on general functioning and quality of life.4 Youth who meet a stringent operational definition of BD Not Otherwise Specified (BD-NOS)—characterized by brief, recurrent subthreshold (hypo)manic and depressive periods with a clear change in functioning—have cumulative affective morbidity, impairment, suicidal ideation, and comorbidity comparable to or exceeding that of youth with BD I.2,5 When combined with a family history of mania in first- or second-degree relatives, more than 50% of youth with BD-NOS progress to fully syndromal BD I or II over 5 years.6
Children or adolescents with major depressive disorder (MDD) also have elevated risk of conversion to BD I/II (15%– 49%) in the 2–4 years after onset of their first depressive episode.3,7 Conversion to BD I or II is highest among depressed patients with a family history of mania, early onset of symptoms, subthreshold hypomania, psychosis, or episodic mood lability (e.g., 8,9).
At present, little empirical evidence exists to guide the treatment risk individuals at high risk for BD. Pharmacological studies during the phases preceding fully syndromal BD have not produced conclusive results.10,11 Currently, high-risk youth tend to be treated with a wide variety of medications and therapies, or do not receive any treatment.5 Delays to first treatment of BD are associated with greater depressive morbidity and less time euthymic in adulthood.12 Without early intervention, the social, intellectual, and emotional development of youth at high risk for BD may be seriously compromised.
Well-timed psychosocial interventions in young high-risk individuals may allow for a more normative acquisition of life skills, such as achieving personal autonomy, academic success, and strong peer relationships before the more debilitating bipolar syndrome begins. Furthermore, environmental-contextual factors that may increase risk for mood symptoms—such as whether parents exhibit high expressed emotion (EE) attitudes toward the child (i.e., high levels of criticism, hostility, or emotional over-involvement)—may be most amenable to change early in the course of the disorder.7 Few studies, however, have examined the effects of early psychosocial intervention on youth with high-risk phenotypes. One 12-month waitlist trial found that multi-family psychoeducation groups protected against conversion to bipolar spectrum disorders in a sample of school-aged children with depressive spectrum disorders.13
Family-focused treatment (FFT) is a manual-based psychoeducational intervention designed to reduce familial stress, conflict, and affective arousal by enhancing communication and problem-solving among patients and caregivers. In a two-site randomized clinical trial (RCT) of adolescents with BD I and II, 9 months of FFT and best-practice pharmacotherapy were associated with more rapid and complete remission from depressive episodes over 2 years than brief psychoeducation and pharmacotherapy.14 Similar protective effects of FFT on patients’ symptomatic and functional outcomes have been observed in four RCTs of adults with BD.15 Further, in two trials, adolescent or adult patients in high EE families had a greater magnitude of treatment response compared to patients in low EE families.16,17
This randomized trial examined whether a brief (4-month) early family intervention (FFT–High Risk protocol [FFT-HR]) was effective in stabilizing mood symptoms among youth who were at high-risk for BD. In a previous open trial of FFT-HR, offspring of parents with BD I or II who were diagnosed with MDD or BD-NOS showed significant improvements in depression and hypomania scores and psychosocial functioning over 1 year.18 We hypothesized that the 12-session FFT-HR protocol would promote more rapid symptom recovery, more time in remission, and greater improvement in depressive and hypomanic symptoms over 1 year compared to a family education control (EC). A secondary aim was to explore whether high-risk youth from high-EE parental households showed a greater magnitude of response to FFT-HR than those from low-EE households.
METHOD
Participants
Children between ages 9 years, 0 months and 17 years, 11 months who had a first-degree relative with BD I or II were recruited at the University of Colorado or the Stanford University School of Medicine between June 2008 and August 2010. Referrals originated from community practitioners, parent support groups, e-mail advertisements, and inpatient settings. Following a full explanation of the procedures, children and parents read and signed University Institutional Review Board–approved assent and consent forms.
Eligibility criteria included: (1) English speaking; (2) at least one first-degree relative met DSM-IV-TR criteria for BD I or II, based on the Mini-International Neuropsychiatric Interview (MINl);19 (3) the child met DSM-IV-TR criteria for a lifetime diagnosis of BD-NOS, MDD, or cyclothymic disorder, and had significant current affective symptoms (1-week Young Mania Rating Scale [YMRS] score > 11 or 2-week Children’s Depression Rating Scale, Revised [CDRS-R] score > 29).20,21 The diagnostic criteria for BD-NOS were the same as those used in the Course and Outcome in Bipolar Youth (COBY) study and included: a distinct period of abnormally elevated, expansive, or irritable mood plus two (three, if irritable only) DSM-IV-TR symptoms of mania that caused a change in functioning, lasted ≥ 4 hours in a day, and occurred for a total of 4 or more days across the child’s lifetime.2 If the main diagnosis was MDD, the youth must have had a full DSM-IV major depressive episode (MDE) within the previous 2 years. Cyclothymic disorder required that, in the preceding year, the child had multiple brief (i.e., 1–2 day) periods of hypomanic, depressive or subsyndromal mixed symptoms and no more than a 2-month period without mood symptoms, without meeting the DSM-IV criteria for MDD or the COBY criteria for BD-NOS.
Procedures: Diagnostic Evaluation
Trained MA/MD/PhD level diagnosticians with at least 2 years of experience administered the Washington University Schedule for Affective Disorders and Schizophrenia for Children (K-SADS).22 The K-SADS interviews were conducted separately with the youth and at least one parent; when discrepancies between reports occurred, they were interviewed conjointly. Current and lifetime K-SADS ratings were based on the most severe 1–2 weeks in the prior 4 months and the most severe 1–2 weeks lifetime, respectively. Board certified psychiatrists conducted separate evaluations with the youth and at least one parent. Final diagnoses were based on information obtained from all sources.
Interrater reliability between three expert diagnosticians at Stanford was .90 (intraclass r) on K-SADS mood items and .75 (Kappa) on the presence/absence of lifetime comorbid disorders. Interrater reliability (intraclass rs) among 11 Colorado raters for K-SADS depression items was .89 and for mania items was .97; and .70 (Kappa) for lifetime comorbid disorders. A cross-site reliability evaluation (7 raters at Colorado, 6 at Stanford; 54 ratings) yielded the following intraclass rs between raters for K-SADS items: depressed mood, .77; elevated mood, .90; irritability, .85; and grandiosity, .81 (all ps < .0001).
At the initial or a second evaluation visit, we administered the MINI diagnostic interview to all available first-degree relatives. Interrater reliabilities at the two sites for diagnoses of relatives ranged from .76–.87 (Kappas) for presence/absence of mood disorders.
Expressed Emotion
Five-Minute Speech Samples (FMSSs) were obtained from parents (or, in one case, a grandparent) who were primary caregivers (i.e., lived with and had a minimum of 4 hrs/week of contact with the child). Research staff members instructed the parent to “talk for 5 minutes about what kind of person (your child) is and how the two of you get along together.” In cases where two parents were available, FMSSs were obtained individually from each parent. FMSS audiotapes were coded by Ana Magana-Amato, M.A., who developed the FMSS-EE coding system.23 Following conventions, families were classified as high in EE if during the FMSSs one or both parents made at least one critical comment (e.g., “I resent his oppositional attitude”) or showed evidence of hostility or emotional overinvolvement. Families in which neither parent met the high-EE criteria were classified as low-EE. Single-parent families were assigned the EE rating of the individual parent. Reliability between Ms. Magana-Amato and 12 trained raters, each of whom completed 10 independent ratings, averaged .95 for agreement on classifications of parents as high or low EE, and .84 on the EE components (e.g., critical comments).
Psychosocial and Pharmacological Treatments
A data analyst who was not otherwise involved in the study randomly assigned participants to a treatment condition in a 50:50 split (FFT-HR or EC) using a modification of Efron’s biased coin toss.24 The assignments were balanced within sites on age (< 13 years or ≥ 13 years), initial diagnosis (BD-NOS/cyclothymic vs. MDD), and medications (mood stabilizer/atypical antipsychotic or not). Youth were not required to take medications to participate; 40% were medication-free at entry (Table 1).
Table 1.
Demographic and Illness History Variables of 40 Youth at High Risk for Bipolar Disorder (BD)
| Variablea | Family-Focused Treatment (n=21) | Education Control (n = 19) |
|---|---|---|
| Age, years, mean ± (SD) | 12.2 (2.8) | 12.3 (2.9) |
| 9–12 years, n (%) | 11 (52.4) | 10 (52.6) |
| 13–17 years, n (%) | 10 (47.6) | 9 (47.4) |
| Female sex, n (%) | 10 (47.6) | 7 (36.8) |
| Race, non-white, n (%) | 3 (14.3) | 1 (5.3) |
| Hispanic ethnicity, n (%) | 1 (4.8) | 1 (5.3) |
| Socioeconomic status, Hollingshead Class I–V, m ± (SD) | 1.5 (.69) | 1.61 (0.78) |
| Child Depression Rating Scale, Entry, m ± (SD)b | 38.3 (13.6) | 41.2 (11.8) |
| Young Mania Rating Scale, Entry, m ± (SD)b | 18.3 (7.6) | 13.9 (5.4) |
| Child’s Global Assessment Scale, m ± (SD)b | ||
| Most severe past episode | 44.6 (13.0) | 45.7 (13.9) |
| Highest in prior year | 66.6 (12.6) | 65.1 (11.2) |
| Living situation, n (%) | ||
| both biological parents | 14 (66.7) | 7 (36.8) |
| both biological parents, joint custody | 2 (9.5) | 5 (26.3) |
| One biological parent only | 5 (23.8) | 5 (26.3) |
| Other | 0 (0.0) | 2 (10.5) |
| Index Mood Episode, n (%)c | ||
| Depression (syndromal) | 3 (14.3) | 6 (31.6) |
| Depression (subsyndromal) | 6 (28.6) | 7 (36.8) |
| Hypomania (syndromal) | 2 (9.5) | 0 (0.0) |
| Hypomania (subsyndromal) | 1 (4.8) | 0 (0.0) |
| Mixed (subsyndromal) | 4 (19.0) | 3 (21.1) |
| Remitted | 4 (19.0) | 3 (21.1) |
| Missing | 1 (4.8) | 0 (0.0) |
| Primary diagnosis, n (%) | ||
| Major depressive disorder | 2 (9.5) | 6 (31.6) |
| Bipolar disorder NOS | 19 (90.5) | 10 (52.6) |
| Cyclothymic disorder | 0 (0.0) | 3 (15.8) |
| Comorbid disorders, any, n (%) | 13 (65.0) | 13 (72.2) |
| Anxiety | 6 (30.0) | 10 (55.6) |
| ADHD | 11 (55.0) | 13 (72.2) |
| ODD | 8 (40.0) | 8 (44.4) |
| CD | 0 (0.0) | 2 (11.1) |
| Any psychiatric medication, yes, n (%) | 10 (47.6) | 14 (73.7) |
| Mood stabilizers | 4 (19.0) | 5 (26.3) |
| Atypical antipsychotics | 7 (33.3) | 6 (31.6) |
| Antidepressant | 3 (14.3) | 4 (21.1) |
| Antidepressant only | 1 (4.8) | 2 (10.5) |
| Psychostimulants | 4 (19.0) | 8 (42.1) |
| Psychostimulant only | 2 (9.5) | 1 (5.3) |
| Parent or Sibling Diagnosis, n (%)d | ||
| Bipolar I disorder, mother | 7 (33.3) | 7 (36.8) |
| Bipolar II disorder, mother | 4 (19.0) | 5 (26.3) |
| Bipolar I disorder, father | 4 (19.0) | 3 (15.8) |
| Bipolar II disorder, father | 4 (19.0) | 4 (21.1) |
| Bipolar I disorder, sibling | 2 (9.5) | 1 (5.3) |
| Bipolar II disorder, sibling | 1 (4.8) | 0 (0.0) |
Abbreviations: Comorbidity data were missing from 1 Family-Focused Therapy–High Risk version (FFT-HR) participant and 1 enhanced care (EC) participant. ADHD = attention deficit hyperactivity disorder; CD= conduct disorder; NOS = not otherwise specified; ODD = oppositional defiant disorder.
None of these variables significantly distinguished between the psychosocial treatment groups, based on either χ2 tests or univariate analyses of variance.
Refers to scores collected at intake into the study, covering the 2 weeks (for the Child Depression Rating Scale) or the 1 week (for the Young Mania Rating Scale) prior to random assignment.
Based on weekly Adolescent Longitudinal Interval Follow-up Psychiatric Status Ratings (PSRs) for the 4 weeks prior to random assignment. Youth with syndromal mood episodes had at least 2 weeks (for depression) or 1 week (for hypomania) with relevant PSR scores ≥ 5. Youth in remission had 4 continuous weeks with PSR depression and hypomania scores ≤ 2. Youth with subsyndromal status had at least one week prior to randomization with a PSR depression or hypomania score between 3 and 4, or a PSR depression score of 5 for a maximum of 1 week. Baseline PSR scores were missing for one participant.
In one family, both parents were diagnosed with bipolar I disorder; in one other family, one parent had BD I and the other had BD II.
When families and youth opted for medication management through the study, psychiatrists saw them weekly for the first month, and then a minimum of once per month for the study year. Physicians followed a pharmacotherapy algorithm that specified first-, second-, and third-line agents, dosage adjustments, and rescue medications. Treatments for depression included citalopram, sertraline, and buproprion as first-, second-, or third-line treatments or lamotrigine if the child had a history of antidepressant-induced mania. Treatment of mania symptoms included aripiprazole, quetiapine, risperidone or lithium as first-, second-, third-, or fourth-line treatments. Treatment of comorbid attention-deficit/hyperactivity disorder (ADHD) included stimulants as first-line agents and atomoxetine as second-line, whereas treatment of comorbid anxiety disorders included citalopram, sertraline, and clonazepam as first-, second-, and third-line treatments, respectively. Study psychiatrists carefully monitored patients on antidepressants for signs of mood switching or increased cycling, and discontinued antidepressants when warranted.
FFT-HR was administered to the youth, parent(s), and available siblings in 12 hour-long sessions over 4 months (8 weekly, 4 biweekly) by trained therapists who followed a session-by-session manual (available at www.semel.ucla.edu/champ/resources). The goals of FFT-HR are to assist youth and family members to recognize and intervene early with symptoms of mood episodes and enhance intrafamilial communication and problem-solving. In the first 4 sessions (psychoeducation), the child and family develop an individualized mood management plan involving a summary of stress triggers, signs of worsening mood (as revealed through daily monitoring), and regulation strategies (e.g., regulating sleep cycles, removing oneself from volatile situations, or arranging for medication changes). In the second module (sessions 5–8), communication enhancement training, participants learn to down-regulate impulsive expressions of negative emotions through practicing expressing positive feelings, active listening, and diplomatically requesting changes in each other’s behavior. In the problem-solving module (sessions 9–12), participants learn to break down large problems (e.g., “We can’t get along”) into smaller ones (e.g., “We need to use lower tones of voice.”), generate and evaluate the pros and cons of various solutions, and select solutions to implement (e.g., alerting each other to aggressive voice tones).
EC consisted of 1–2 family sessions in which clinicians summarized the results of the diagnostic assessment, introduced daily mood monitoring, and offered recommendations and instructional handouts on managing mood swings. Families in both treatments could request additional crisis sessions from study clinicians during the post-therapy interval, or referrals for additional psychosocial services if needed. Children continued to see their study psychiatrists for the full year.
Following an initial training workshop, two of the authors (D.J.M. and E.L.G.) provided biweekly or monthly supervision for clinicians, and made quality control ratings of audiotapes of FFT-HR and EC sessions using the Therapist Competence and Adherence Scales, Revised (TCAS-R).14,25 The TCAS-R includes 13 clinician adherence/skill items rated 1 (poor) to 7 (excellent). Interrater reliabilities for TCAS-R items ranged from .61–.86 (intraclass rs) among four independent raters. Clinicians had to maintain overall TCAS ratings of ≥ 5.0 (very good) on > 75% of sessions to work in each modality. Colorado and Stanford therapists had nearly identical fidelity ratings across the treatment conditions (Colorado: M= 5.9 ± 1.3; Stanford: M = 5.8 ± 1.4; 49 ratings, p =.65).
Developmental Considerations in FFT-HR
A broad age range (9–17 years) was chosen because (a) the cases referred to our clinics often included younger children and (b) FFT-HR had been adapted to school-aged children during the open trial.18 FFT-HR included simplified handouts that emphasized visual stimuli (e.g., mood charts with facial images portraying emotional states), flipcharts to explain key concepts, and flexible session structures. Younger children responded better to training in communication and problem-solving when the tasks resembled games (e.g., choosing skills to practice out of a hat). Parents played more active roles in directing younger children during in-session skill rehearsals, whereas adolescents were given more autonomy in these tasks.
Outcome Assessments
Independent evaluators (IEs) interviewed the youth and at least one parent at baseline (covering the prior 4 months) and again at 4, 8, and 12 months post-randomization. The IEs remained “blind” to treatment assignments by instructing patients and family members not to disclose their treatment condition during follow-up interviews, communicating clinical concerns to the PI instead of the therapists, and scheduling follow-up interviews at different times than therapy sessions.
IEs administered the YMRS and CDRS-R at each evaluation and rated the prior 1 or 2 weeks based on a consensus of parent and child reports. Next, the IE rated each week of the 4-month interval using the Adolescent Longitudinal Interval Follow-up Evaluation (A-LIFE) Psychiatric Status Ratings (PSRs) which cover the severity of depression, mania, and hypomania symptoms on 1–6 point scales of severity.2,26 PSRs are numeric values linked to DSM-IV criteria: ratings of 1–2 indicate asymptomatic or mildly symptomatic status with no or minimal impairment; 3 or 4, subsyndromal symptoms with mild-to-moderate impairment; and 5–6, fully syndromal states with severe impairment. Weekly PSRs, based on inquiries regarding symptom intensity and change points, were used to clarify the timing of recovery, onsets of new episodes, and weeks in remission (all PSR mood scales ≤ 2). Cross-site reliability for PSR ratings was 0.74 (12 ratings; intraclass r; p < .01).
Remission status at baseline was assigned if the youth’s depression or hypomania PSRs were rated ≤ 2 for each of the 4 weeks prior to randomization. Depressive or hypomanic status at baseline was assigned if the youth had ≥ 2 weeks with PSR depression scores ≥ 5 or ≥ 1-week with hypomania scores ≥ 5. Baseline status was classified as subthreshold if the youth was neither in remission nor in episode in the prior 4 weeks, but had a PSR mood score between 3–4 for ≥ 1-week. For the 1-year prospective period, we identified (a) the first 8-week period with PSR depression, mania, and hypomania scores ≤ 2 (recovery), (b) the first occurrence of a ≥ 2-week depressive or ≥ 1-week (hypo)manic episode (PSRs ≥ 5), or (c) the first occurrence of a subthreshold hypomanic episode of at least moderate intensity (PSR score of 4 lasting ≥ 1 week) following a period of recovery. 2
Data Analysis
All analyses were by intent-to-treat. Time to recovery was calculated as the number of weeks from the date of randomization to the beginning of the first 8-week recovery period. We used Kaplan-Meier models to compare the group survival curves on weeks to full mood recovery (all PSR scales ≤ 2), with separate calculations for time to recovery from baseline depressive and (hypo)manic symptoms. We used Cox Proportional Hazards models to estimate the independent effects of treatment group on time to recovery with each of the following covariates: family EE status (low, high), site, sex, age, baseline clinical status, diagnosis, and baseline medications. Patients who had been remitted for the 4 weeks prior to randomization were not included in the analyses of time to recovery. Those who did not recover or left the study prematurely were “censored: at the point of their final assessment.
The secondary hypothesis was that youth in FFT-HR would spend proportionately more weeks in remission from all mood symptoms than youth in EC during the 1-year study. This hypothesis was tested in a Poisson regression model that adjusted for total number of weeks in the study, site, age, sex, family EE, and the percentage of weeks with subthreshold or threshold symptoms (PSR depression or hypomania scores ≥ 3) in the 4 months prior to randomization. Finally, mixed effects regression (growth curve) models with random subject effects were used to explore the effects of treatments on CDRS-R and YMRS scores from baseline to 4, 8, and 12 month follow-ups.
Secondary analyses explored whether (a) comorbid disorders explained the effects of treatment condition on the primary outcome variables once concurrent mood states were covaried; and (b) the effects of FFT-HR remained robust when covarying medication regimens. With 40 subjects, a one-sided log rank test of survival curves had 78% power (p < .05) to detect a 25% difference in recovery rates over 1 year, assuming 20% attrition. With three follow-up points, mixed effects regression models had 80% power to detect a 0.92 standard deviation (SD) group difference in means at follow-up (adjusted for baseline scores).
RESULTS
Sample Composition
The mean age ± SD of the 40 participants was 12.3 ± 2.8 years (Table 1); 17 (42.5%) were female. Participants were most frequently diagnosed with BD-NOS (n=20, 50%) or MDD (n=17, 42.5%); 3 (7.5%) had cyclothymic disorder. Although there was variability in clinical status in the month prior to randomization, no participant met criteria for a DSM-IV-TR manic or mixed episode. One patient did not have PSR ratings and was dropped from further analyses. Patients assigned to FFT-HR and EC did not differ on any of the demographic, clinical, or illness history variables in Table 1, nor were there any treatment × site interactions on these variables (all ps > .10).
Psychiatric Diagnoses and Expressed Emotion Among First-Degree Relatives
MINI diagnostic interviews were conducted with 82 first-degree relatives. Lifetime diagnoses of BD were observed in 15 fathers (7 with BD I, 8 with BD II), 23 biological mothers (14 with BD I, 9 with BD II) (Table 1), and 4 full siblings of the index child; 40 relatives had no lifetime history of mood disorder. Thirty-five of 40 (87.5%) relatives with BDI or II were living with the high-risk child, as were 34 of 37 (91.9%) relatives with no mood disorder (data on living situation were missing on 5 relatives).
A total of 42 relatives (12 fathers, 29 mothers, 1 grandmother) of 35 youths met the caregiver inclusion criteria for the FMSS-EE assessment. In 5 families, EE ratings were unavailable because of inaudible tapes. Of the 42 caregivers, 17 (40.48%) were rated high in EE (3/12 fathers [25.0%], 13/29 mothers [44.8%], and 1 grandmother) and 25 (59.5%) were rated low in EE. EE classifications did not differ between relatives with and without BD (χ2[2] = 2.16, P = .34). Using the conventional EE classification criteria (above), 17 families (48.6%) were rated high-EE, a rate almost identical to the rate previously observed in families of adolescents with BD I or II (46.2%).16 Family EE status was unrelated to whether the family had two parents (n = 23) or a single parent (n = 17) (χ2[1] =.27, p = .60), or whether there were two biological parents (n = 21) or a single biological parent living in the home (n = 19; χ2[1] =.03, P = .86).
Treatment and Study Completion
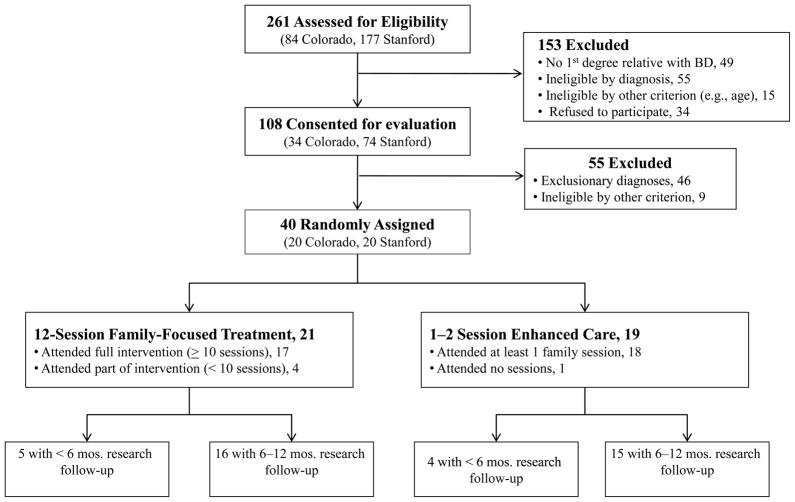
Treatment completion was equivalent in the two conditions (Figure 1): The mean number of FFT-HR sessions was 12.43 ± 5.75 (range 0–18), whereas the mean number of EC sessions was 2.26 ± 1.19 (range 0–5). The number of sessions exceeded protocol specifications when families requested crisis sessions during the year. The groups did not differ on the number of extra sessions (F [1,38] = 0.48, p = .49), or on use of nonprotocol outpatient or school-based services at follow-up (for all, p > .10). Participants in FFT-HR completed an average of 45.0 + 12.44 weeks of research follow-up, whereas participants in EC completed 44.88 ± 14.7 weeks (t[32] = .03, p = .98; Figure 1).
Figure 1.
CONsolidated Standards of Reporting Trials (CONSORT) diagram.
Time to Recovery from Baseline Symptoms
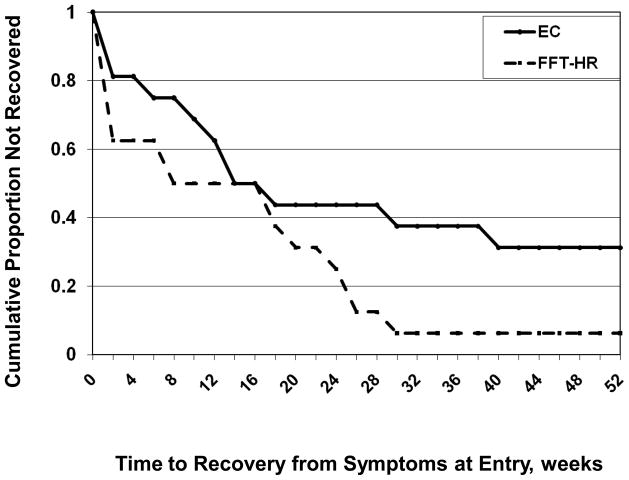
Of the 32 patients who were not remitted at baseline, 26 (81.25%) recovered (all PSR mood scales ≤ 2 for ≥ 8 weeks) during the 1-year study. Of the 6 who did not recover, 5 (83.3%) were in EC and 1 (16.7%) was in FFT-HR. Patients in FFT-HR recovered in an average of 13.0 weeks (SE= 2.93; 25% quartile = 1 week, 95% confidence interval [CI] 0 to 8) and in the EC group, 21.25 weeks (SE = 4.22; 25% quartile = 7.5 weeks, 95% CI 0 to 14), a significant difference (χ2[1] = 5.24, p = .022; Kaplan-Meier model, Figure 2). The effects of FFT-HR on time to recovery were robust (χ2[1] = 3.96, p = .047; HR = 2.69) when baseline hypomania status (χ2[1] = 16.22, p < .0001; HR = 5.88) and baseline depression status (χ2[1] = 7.52, p = .006; HR = 3.0) were included in a Cox Proportional Hazards model. The treatment effect was marginally significant (χ2[1] = 2.71, p = .099; HR = 2.02) when the child’s living status (with both biological parents or not) was covaried, although there was no independent effect of living status on time to recovery (χ2[1] = .31, p = .58; HR = .79).
Figure 2.
1-year survival curves for participants in family-focused treatment, high-risk version (FFT-HR) and enhanced care (EC).
In the subgroup of patients with subthreshold (n = 20) or syndromal (n= 9) depressive symptoms at baseline, FFT-HR was associated with faster recovery from depressed mood (M = 9.2 weeks, SE = 2.98; 25% quartile = 1 week, 95% CI 0 to 8) than EC (M = 21.38 weeks, SE = 4.05; 25% quartile = 11 wks, 95% CI 6 to 18) (χ2(1) = 7.69, p = .006; HR = 2.63). A similar analysis could not be conducted for recovery from hypomania because only 10 participants entered with at least subthreshold hypomania symptoms (PSR scores ≥ 3).
Regardless of treatment assignment, patients in high-EE households took significantly longer to recover from their initial mood symptoms (M = 21.2 weeks, SE = 3.32) than patients in low-EE households (M = 11.38 weeks, SE = 3.29) and were more likely to remain symptomatic for the full follow-up (5/14 [35.7%] vs. 1/16 [6.25%]) (χ2 = 9.62, p = .002). In a Cox Proportional Hazards model, separate main effects were observed for treatment group (χ2 = 4.14, p = .042; HR= 2.47) and EE (χ2 = 7.37, p = .007, HR = .29). Although Cox models were underpowered to detect an interaction between treatment group and EE, it is notable that the hazard ratio for time to recovery (favoring FFT-HR) was numerically larger in the high-EE subgroup (HR = 4.59, p = .014; n = 14) than in the low-EE subgroup (HR = 1.46; p = .11; n = 16).
Weeks in Remission and in Episode at Follow-up
Over the 1-year, participants spent an average of 25.15 ± 14.75 weeks in remission from all mood symptoms (all PSR scores ≤ 2), 17.15 weeks ± 12.75 in subthreshold states (PSR scores of 3 or 4) and 2.65 ± 4.32 weeks in subthreshold states (at least one PSR scale ≥ 5). In a Poisson regression model that included site, sex, age, treatment, weeks followed, family EE, and percentage of weeks with subthreshold or threshold symptoms in the 4 months before randomization, youth in FFT-HR had significantly more weeks in full remission (M = 26.80 weeks, 95% CI 24.03 to 29.89) than youth in EC (M = 19.50 weeks, 95% CI 17.19 to 22.11) (χ2[1] = 15.80, p < .0001). The treatment group x EE interaction on weeks in remission also was significant (χ2[1] = 7.62, p = .006), indicating that participants in high-EE families who received EC had fewer remitted weeks at follow-up (M =11.95 weeks, 95% CI 9.53 to 14.99) than participants in high-EE families who received FFT-HR (M = 26.58 weeks, 95% CI 22.95 to 30.78), or youth in low-EE families who received FFT-HR (M = 27.20 weeks, 95% CI 23.14 to 31.96) or EC (M = 26.18 weeks, 95% CI 22.67 to 30.24).
A separate Poisson model with the same predictors and baseline covariates examined treatment in relation to weeks in subthreshold states. Consistent with the remission findings, there was a main effect of treatment group (χ2[1] = 4.49, p = .034) and a treatment x EE interaction (χ2[1] = 4.98, p = .026), indicating more subthreshold weeks among youth in high-EE families who received EC (M = 26.29 weeks ± 15.82) than among youth in high-EE families who received FFT-HR (M= 14.88 ± 13.33).
Over the 1-year follow-up, new MDEs after a period of mood recovery were observed in 8 of 32 patients (25.0%), of whom 5/8 (62.5%) were in EC and 3/8 (37.5%) were in FFT-HR (χ2 [1] = .64, p =.42). Following mood recovery, 7 participants developed subsyndromal hypomanic episodes of moderate severity (PSR = 4) that lasted at least one week. Of these 7, 3 (42.8%) were in EC and 4 (57.1%) were in FFT-HR (χ2 [1] = .06, p =.80). One FFT-HR participant with BD-NOS entered the study in a MDE and, at 5-weeks post-randomization, developed a 5-week fully syndromal hypomanic episode (PSR = 5). This participant was judged by two independent raters to have “converted” to BD II. The remaining participants retained their initial diagnoses at follow-up.
Longitudinal Trajectory of Mood Symptoms: YMRS and CDRS-R Scores
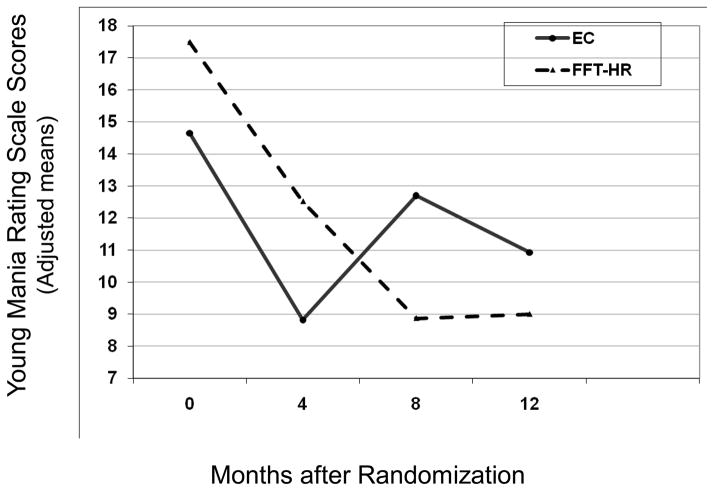
The treatment by time (study visit) interaction on follow-up CDRS-R scores was nonsignificant (F[1, 84.1] = 1.52, p = .22). There was, however, a treatment x time interaction on YMRS scores (Figure 3; F[1, 98] = 4.55, p = .035), indicating a greater improvement slope for YMRS scores from baseline to 12 months among youth in FFT-HR (M = −8.73 ± 10.92) as compared to youth in EC (M = −4.38 ± 6.10) (Cohen’s d = .49). The effects of treatment group on YMRS scores remained significant when baseline YMRS score, pretreatment hypomania status (PSR scale), diagnosis (BD-NOS/cyclothymic or MDD), sex, or age were included in mixed regression models. There was no effect of EE or an interaction between treatment group and EE on CDRS-R or YMRS scores (all ps > .10).
Figure 3.
Young Mania Rating Scale scores over 12 months for participants in family-focused treatment, high-risk version (FFT-HR) and enhanced care (EC).
Effects of Comorbid Disorders on Recovery and Symptom Trajectories
Comorbid disorders were common in this sample (Table 1). Individual comorbid disorders were not more common among youth with BD-NOS/cyclothymic disorder than among youth with MDD (χ2[4] = 1.78, p = .78). To determine the relation of comorbidity to the primary and secondary outcomes, we created an ordinal variable based on number of comorbid disorders: none, (n = 10; 26.3%), one (n = 10, 26.3%), two (n = 6, 15.8%) or three (n = 12, 31.6%) (comorbidity data were missing for 2 cases). In a Cox Proportional Hazards model, comorbid disorders were not related to time to full mood recovery (χ2[1] = 0.14, p = .70; HR = 0.93) when baseline hypomania (χ2[1] = 14.98, p <.0001; HR = 5.88) and depression status (χ2[1] = 5.51, p = .019; HR = 2.76) were covaried. FFT-HR retained its association (HR = 2.85) with time to recovery in this model, although at a lower level of significance (χ2[1] = 3.21, p = .07). In a mixed effects regression model, number of comorbid disorders were not associated with YMRS scores over time (F[1,32] = 2.19, p = .15), but the treatment group by time interaction remained significant (F[1,93.8] = 4.40, p = .04), indicating more improvement in YMRS scores in the FFT-HR group. These results were virtually identical when comorbidity was operationalized in terms of subtype (externalizing [attention deficit disorder (ADD), oppositional defiant disorder (ODD), or conduct disorder, n = 6], internalizing [anxiety only, n = 2], or both internalizing and externalizing [n = 20]).
Effects of Medication Regimens
At study entry, 60% of the participants were taking at least one psychiatric medication (Table 1). Of the 40 participants, 30 (75.0%) received ongoing medication sessions from study psychiatrists (mean 6.27 ± 3.34 visits, range 2–14). Patients in FFT-HR and EC did not differ at randomization on use of mood stabilizers, atypical antipsychotics, psychostimulants, or antidepressants (all ps > .10) and were equally likely to receive follow-up medication management from a study psychiatrist (χ2[1] = 0.03, p = .86).
In most cases study psychiatrists did not significantly alter medication regimens over the study year. Of 10 patients in FFT who began on a psychiatric medication, 8 remained treated at 1 year (80%); of 14 in EC, 10 remained treated (71.4%). Log-linear models indicated that changes in prescriptions for mood stabilizers, atypical antipsychotics, psychostimulants or antidepressants over the year were unrelated to treatment group (all ps > .10). When number of mood stabilizers and/or atypical antipsychotics at baseline were covaried, youth in FFT-HR still spent more weeks in full remission over the year than youth in EC (χ2 [1] = 24.30, p < .0001). An independent effect of number of mood stabilizers/atypicals (χ2 [1] = 26.81, p < .0001) indicated fewer weeks in remission among those youth who entered the study with more complex medication regimens.
DISCUSSION
Although delayed diagnosis and treatment are often cited as reasons for the poor prognosis of BD in adulthood,12 controlled research on early interventions for high-risk youth is scant.11,13 To our knowledge, this is the first blinded, randomized early psychosocial intervention study of symptomatic youth at high risk for BD I or II. Relative to the brief EC treatment, FFT-HR was associated with more rapid recovery from initial mood symptoms, more weeks in remission over 1 year, and a more favorable trajectory of dimensional hypomania (but not depression) scores. These group differences were not explained by differences in initial clinical state, comorbid disorders, or pharmacotherapy regimens. The results add to a body of literature indicating that family psychoeducation is an effective adjunct to pharmacotherapy in children or adolescents with BD spectrum disorders,13,14 psychosis,27 obsessive–compulsive disorder (OCD),28 and depression,29 and chronic medical illnesses including diabetes and asthma. 30–31
Identifying subgroups of patients who are most likely to respond to an early intervention may significantly reduce the costs of treatment delivery in community care settings. In this study, the effects of FFT-HR were more pronounced among youth in high EE families than in low-EE families. The moderating effects of EE on patients’ responses to FFT also have been observed in RCTs of adolescents and adults with BD.16–17 Unfortunately, the costs of administering and scoring standardized EE assessments have prevented the use of EE as a criterion for treatment selection in community practice. Parents’ scores on brief relationship questionnaires—such as the Perceived Criticism Scale or the Conflict Behavior Questionnaire—may serve as useful proxies for EE, although data on their prognostic validity in early-onset mood disorders are limited.32–34
We acknowledge the small sample size and exploratory nature of the current trial, which was underpowered to identify potential moderators of treatment effects. Additionally, this study was conducted with families who sought treatment, which may have oversampled participants who were more responsive to interventions aimed at improving relationships. Early intervention programs for mood disorders may undersample families in which first-degree relatives have had negative experiences with their own treatment, or those in which parents fear the “self-fulfilling prophecy” of treating their offspring for a disorder that has not yet fully developed.
We cannot determine whether the effects of FFT-HR compared to EC were due to its specific content or its greater number of contact hours, leading to more opportunities for clinicians to intervene early with mood exacerbations. Nonetheless, the brevity of EC mirrors the intensity of psychosocial interventions often given to patients with BD in community care.35 Studies of FFT with active comparators matched for treatment intensity, with repeated measurements of potential mediators (e.g., changes in communication effectiveness) and nonspecific factors (e.g., working alliance) will help address these design limitations.
Although randomization to psychosocial treatments was balanced on entry medications, and changes in regimens did not vary with psychosocial treatments, it is possible that individual differences in drug dosages or adherence to medicines contributed to participants’ mood severity scores at follow-up. Furthermore, not requiring that participants take mood stabilizers may have selected for youth who were more open to participating in psychotherapy. Future studies should examine whether early psychosocial interventions reduce the need for complex pharmacotherapy through stabilizing depressive symptoms and increasing drug adherence.
Rates of conversion from MDD or BD-NOS to BD I/II are highest in the first 4 to 5 years after initial diagnosis, with the incidence of new onsets doubling in the late teens.1–3,6,7 The duration of follow-up in this study was too short to determine whether the short-term effects of FFT-HR on mood recovery translated into a lower risk of later conversion to BD I or II. Future studies may establish whether early interventions that hasten and sustain remission from mood episodes among high-risk youth—particularly those in high conflict families—have downstream effects that contribute to the delay or prevention of the full bipolar syndrome.
Clinical Guidance.
Offspring of parents with bipolar disorder often develop significant mood disturbances and functional impairment in late childhood or early adolescence. These youth may require treatment for major depressive episodes or brief and recurrent periods of mania or hypomania.
Youth with early-onset mood disorders may benefit from early psychosocial interventions. In this randomized trial, 40 youth with mood disorders who also had first-degree relatives with bipolar disorder were randomly assigned to family-focused treatment, a 12-session psychoeducational therapy, or an education control (1–2 sessions). Over 1 year, those who received family-focused treatment had more rapid recoveries from mood symptoms and longer periods of remission than youth in the education control.
Future studies of high-risk populations may be able to determine whether early psychosocial interventions can delay the onset of bipolar disorder or minimize its functional consequences.
Acknowledgments
Financial support was provided by National Institute of Mental Health (NIMH) grants MH077856 and MH073871 and a National Association for Research on Schizophrenia and Depression (NARSAD) Distinguished Investigator Award to Dr. Miklowitz; and NIMH grant MH077047 and a NARSAD Independent Investigator Award to Dr. Chang. The authors thank David Axelson, M.D., of the University of Pittsburgh School of Medicine for consultation on study design; Julia Maximon, M.D., Kathleen Kovner-Kline, M.D., Kimberly Kelsay, M.D., and Marianne Wamboldt, M.D., of the University of Colorado Denver Health Sciences Center for serving as study physicians; Aimee Sullivan, M.A., Dana Elkun, M.A., Christopher Hawkey, M.A., Jedediah Bopp, M.A., and Jessica Lunsford, M.A., and Zachary Millman of the University of Colorado, Boulder for serving as study therapists and research assistants; and Tenah Acquaye, Erica Marie Sanders, and Erica Weitz of Stanford University School of Medicine for serving as research assistants. Dr. Dickinson of the University of Colorado Denver Health Sciences Center was the statistical consultant.
Footnotes
Disclosures
Dr. Miklowitz has received research funding from the National Institute of Mental Health (NIMH), the National Association for Research on Schizophrenia and Depression (NARSAD), the Danny Alberts Foundation, the Attias Family Foundation, and the Robert L. Sutherland Foundation; and royalties from Guilford Press and John Wiley and Sons. Dr. Schneck has received funding from the Crowne Family Foundation. Dr. Cosgrove has received funding from the Lucille Packard Foundation for Children’s Health, Spectrum Child Health, and the Klingenstein Third Generation Foundation. Dr. Garber has received funding from NIMH and the William T. Grant Foundation. Dr. Chang has received research funding from NIMH, NARSAD, Merck, and GlaxoSmithKline; and is a non-paid consultant for GlaxoSmithKline, Eli Lilly and Company, Bristol-Myers Squibb, and Merck. Drs. Singh, Taylor, George, Dickinson, and Ms. Howe report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. David J. Miklowitz, University of California–Los Angeles School of Medicine
Dr. Christopher D. Schneck, University of Colorado–Denver Health Sciences Center
Dr. Manpreet K. Singh, Stanford University School of Medicine
Dr. Dawn O. Taylor, University of Colorado–Boulder
Dr. Elizabeth L. George, University of Colorado–Boulder
Dr. Victoria E. Cosgrove, Stanford University School of Medicine
Meghan E. Howe, University of Colorado–Denver Health Sciences Center
Dr. L. Miriam Dickinson, University of Colorado–Denver Health Sciences Center
Dr. Judy Garber, Vanderbilt University
Dr. Kiki D. Chang, Stanford University School of Medicine
References
- 1.Merikangas KR, Cui L, Kattan G, Carlson GA, Youngstrom EA, Angst J. Mania with and without depression in a community sample of US adolescents. Arch Gen Psychiatry. 2012;69(9):943–951. doi: 10.1001/archgenpsychiatry.2012.38. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luby JL, Navsaria N. Pediatric bipolar disorder: evidence for prodromal states and early markers. J Child Psychol Psychiatry. 2010;51(4):459–471. doi: 10.1111/j.1469-7610.2010.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson GA, Findling RL, Post RM, et al. AACAP 2006 Research Forum--Advancing research in early-onset bipolar disorder: barriers and suggestions. J Child Adol Psychopharmacol. 2009;19(1):3–12. doi: 10.1089/cap.2008.100. [DOI] [PubMed] [Google Scholar]
- 5.Axelson DA, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 6.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1001–1016. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miklowitz DJ, Chang KD. Prevention of bipolar disorder in at-risk children: theoretical assumptions and empirical foundations. Dev Psychopathol. 2008;20(3):881–897. doi: 10.1017/S0954579408000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry. 2011;168(1):40–48. doi: 10.1176/appi.ajp.2010.10030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nusslock R, Frank E. Subthreshold bipolarity: diagnostic issues and challenges. Bipolar Disord. 2011;13:587–603. doi: 10.1111/j.1399-5618.2011.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Findling RL, Frazier TW, Youngstrom EA, et al. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry. 2007;68(5):781–788. doi: 10.4088/jcp.v68n0519. [DOI] [PubMed] [Google Scholar]
- 12.Post RM, Leverich GS, Kupka RW, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71(7):864–872. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni RB, Fristad MA. Clinical course of children with a depressive spectrum disorder and transient manic symptoms. Bipolar Disord. 2010;12:494–503. doi: 10.1111/j.1399-5618.2010.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miklowitz DJ, Axelson DA, Birmaher B, et al. Family-focused treatment for adolescents with bipolar disorder: results of a 2-year randomized trial. Arch Gen Psychiatry. 2008;65(9):1053–1061. doi: 10.1001/archpsyc.65.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miklowitz DJ, Scott J. Psychosocial treatments for bipolar disorder: Cost-effectiveness, mediating mechanisms, and future directions. Bipolar Disord. 2009;11:110–122. doi: 10.1111/j.1399-5618.2009.00715.x. [DOI] [PubMed] [Google Scholar]
- 16.Miklowitz DJ, Axelson DA, George EL, et al. Expressed emotion moderates the effects of family-focused treatment for bipolar adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:643–651. doi: 10.1097/CHI.0b013e3181a0ab9d. [DOI] [PubMed] [Google Scholar]
- 17.Kim EY, Miklowitz DJ. Expressed emotion as a predictor of outcome among bipolar patients undergoing family therapy. J Affect Disord. 2004;82:343–352. doi: 10.1016/j.jad.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Miklowitz DJ, Chang KD, Taylor DO, et al. Early psychosocial intervention for youth at risk for bipolar disorder: a 1-year treatment development trial. Bipolar Disord. 2011;13(1):67–75. doi: 10.1111/j.1399-5618.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 20.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 21.Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- 22.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40(4):450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Magana AB, Goldstein MJ, Karno M, Miklowitz DJ, Jenkins J, Falloon IRH. A brief method for assessing expressed emotion in relatives of psychiatric patients. Psychiatr Res. 1986;17:203–212. doi: 10.1016/0165-1781(86)90049-1. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36:81–90. [PubMed] [Google Scholar]
- 25.Weisman AG, Okazaki S, Gregory J, et al. Evaluating therapist competency and adherence to behavioral family management with bipolar patients. Fam Process. 1998;37:107–121. doi: 10.1111/j.1545-5300.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 26.Keller MB, Lavori PW, Friedman B, et al. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 27.Lucksted A, McFarlane W, Downing D, Dixon L. Recent developments in family psychoeducation as an evidence-based practice. J Marital Fam Therapy. 2012;38(1):101–121. doi: 10.1111/j.1752-0606.2011.00256.x. [DOI] [PubMed] [Google Scholar]
- 28.Piacentini J, Bergman RL, Chang S, et al. Controlled comparison of family cognitive behavioral therapy and psychoeducation/relaxation training for child obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1149–1161. doi: 10.1016/j.jaac.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanford M, Boyle M, McCleary L, et al. A pilot study of adjunctive family psychoeducation in adolescent major depression: Feasibility and treatment effect. J Am Acad Child Adolesc Psychiatry. 2006;45(4):386–395. doi: 10.1097/01.chi.0000198595.68820.10. [DOI] [PubMed] [Google Scholar]
- 30.Hampson SE, Skinner TC, Hart J, et al. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review. Health Technol Assess. 2001;5:1–79. doi: 10.3310/hta5100. [DOI] [PubMed] [Google Scholar]
- 31.Brown JV, Bakeman R, Celano MP, Demi AS, Kobrynski L, Wilson SR. Home-based asthma education of young low-income children and their families. J Pediatric Psychol. 2002;27:677–688. doi: 10.1093/jpepsy/27.8.677. [DOI] [PubMed] [Google Scholar]
- 32.Hooley JM, Parker HA. Measuring expressed emotion: an evaluation of the shortcuts. J Family Psychol. 2006;20(3):386–396. doi: 10.1037/0893-3200.20.3.386. [DOI] [PubMed] [Google Scholar]
- 33.Schubiner H, Robin A. Screening adolescents for depression and parent-teenager conflict in an ambulatory medical setting: a preliminary investigation. Pediatrics. 1990;85:813–818. [PubMed] [Google Scholar]
- 34.Sullivan AE, Judd CM, Axelson DA, Miklowitz DJ. Family functioning and the course of adolescent bipolar disorder. Behav Therapy. 2012;43(4):837–847. doi: 10.1016/j.beth.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lembke A, Miklowitz DJ, Otto MW, et al. Psychosocial service utilization by patients with bipolar disorders: data from the first 500 participants in the Systematic Treatment Enhancement Program. J Psychiatr Pract. 2004;10:81–87. doi: 10.1097/00131746-200403000-00002. [DOI] [PubMed] [Google Scholar]