SUMMARY
Hepatitis C virus (HCV) infects more than 3% of the world’s population, leading to an increased risk of cirrhosis and hepatocellular carcinoma. The current standard of care, a combination of pegylated interferon alfa and ribavirin, is poorly tolerated and often ineffective against the most prevalent genotype of the virus, genotype 1. The very recent approval of boceprevir and telaprevir, two HCV protease inhibitors, promises to significantly improve treatment options and outcomes. In addition to the viral protease NS3 and the viral polymerase NS5B, direct-acting antivirals are now in development against NS5A. A multifunctional phosphoprotein, NS5A is essential to HCV genome replication, but has no known enzymatic function. Here we report how the design of small-molecule inhibitors against NS5A has evolved from promising monomers to highly potent dimeric compounds effective against many HCV genotypes. We also highlight recent clinical data and how the inhibitors may bind to NS5A, itself capable of forming dimers.
HEPATITIS C VIRUS (HCV): AN INTERNATIONAL HEALTH CONCERN
Hepatitis C virus (HCV) infects approximately 170 million individuals, with an estimated 2.3-4.7 million new infections each year (1). The primary mode of transmission of HCV is via exposure to infected blood, including transfusions from infected donors, and through injection drug use. It is estimated that 15-30% of all HCV infections will spontaneously clear, but the remaining 70-85% of infections will develop into chronic hepatitis (2, 3). Chronic infections can subsequently lead to steatosis, cirrhosis and hepatocellular carcinoma (4). Among all recognized positive-strand RNA viruses, the ability to establish a chronic infection is exclusive to HCV (5), although how the virus mediates persistence remains unknown.
Current treatment options for HCV are relatively poor. The standard of care is often a grueling 48-week combination of pegylated interferon alfa (IFN-α) and the nucleoside analogue ribavirin. Effective clearance of the virus is achieved in less than 50% of genotype 1 infections, the most prevalent strain of HCV worldwide. Moreover, the regimen often causes significant recurring side effects, including flu-like symptoms and fatigue. Recent studies suggest that both the genotype of the virus (3, 6) and individual host polymorphisms (7) have a significant influence on the success rate of current therapies. Direct-acting antivirals designed to block specific HCV enzymatic functions have been intensely studied over the last decade (8), as have small-molecule inhibitors targeted against host factors utilized by the virus for replication (9). The heterogeneous nature of HCV across the infected population has made the development of effective direct-acting antivirals difficult, and the creation of a universal vaccine impossible thus far.
MOLECULAR VIROLOGY OF HCV
As a member of the Flaviviridae, the general life cycle of HCV is similar to other viruses of this family. The virus attaches to the host hepatocyte through interactions with a number of cellular receptors. Clathrin-mediated endocytosis of the HCV particles permits the release of the viral genome into the cytoplasm of the target cell (10). Mimicking a host mRNA, the positive-strand genome is translated by the hijacked cellular machinery through the use of an internal ribosome entry site (IRES) (11). The polyprotein undergoes subsequent processing by both cellular and viral factors (12, 13). Viral proteins involved in the replication of the HCV genome assemble into replicase complexes, where the viral RNA is transcribed (14). The endoplasmic reticulum (ER) serves as the assembly site for HCV virions (15), which are likely released from the cell via the secretory pathway.
The 9.6-kb, single-strand, positive-strand RNA genome of HCV includes many features similar to other viruses within the Flaviviridae family (Fig. 1). Untranslated RNA elements flank a single open reading frame encoding a polyprotein of approximately 3,000 amino acids. From the amino to the carboxy-terminus of the polyprotein, three proteins (core, E1 and E2) serve as the major structural components of the HCV virion, two proteins (p7 and NS2) are involved in viral morphogenesis and five proteins (NS3, NS4A, NS4B, NS5A and NS5B) are required for HCV RNA replication.
Figure 1.
The HCV genome is typical of other positive-strand RNA viruses. The HCV genome contains structural proteins (C, E1, E2), proteins involved in virion morphogenesis (p7, NS2) and nonstructural proteins responsible for HCV genome replication (NS3, NS4A, NS4B, NS5A and NS5B). NS5A stands out as a unique feature of HCV compared to other positive-strand RNA viruses.
NON-STRUCTURAL PROTEIN 5A (NS5A): SWISS ARMY KNIFE OF HCV
Interacting with a myriad of cellular and viral factors, non-structural protein 5A (NS5A) is a promiscuous phosphoprotein comprised of three domains separated by two linker regions (Fig. 2). While the protein is known to be essential to HCV genome replication, the specific role of NS5A in this process remains undefined.
Figure 2.
NS5A interacts with multiple viral and cellular proteins. HCV proteins (blue) and cellular proteins (black) have been mapped to interact with specific regions of the three domains of NS5A. Other interactions (grey boxes) remain unmapped to specific regions of NS5A. NS5A also binds to RNA (red) and interacts with a number of cellular kinases (green box).
Structure
The first 32 amino acids at the amino-terminus of NS5A comprise the amphipathic α-helix, responsible for anchoring NS5A to the ER and ER-derived membranes, including lipid droplets (16, 17). Disruption of the α-helix inhibits HCV genome replication (18).
The only portion of the viral protein to be successfully crystallized, domain I (residues 33-245), coordinates a single zinc atom via four cysteines (19) and can homodimerize into at least two unique conformations (19, 20). The first dimer reported forms a groove 33 Å long and 16 Å wide between the two monomers, sufficient to accommodate single- and double-strand RNA based on sterics and electrostatic potential (19). This finding validates the NS5A-RNA interaction published prior to the elucidation of the crystal structure (21).
The remainder of NS5A is considered to be intrinsically disordered (22, 23). Intrinsically disordered proteins are proteins or regions of proteins that do not adopt a stable secondary or tertiary structure based on classic biophysical techniques. This flexibility may be conducive to the numerous interactions mapped to this region of NS5A (Fig. 2), as well as accessibility to putative phosphorylation sites.
Interactions
NS5A interacts with many factors, including viral and host proteins and RNA, making it one of the most connected of the HCV proteins (Tables I and II).
Table I. Interactions between NS5A and HCV proteins.
| Protein | NS5A interaction residues | Functional role | Ref. |
|---|---|---|---|
| Core | 456, 458, 461 | Formation of HCV particles | 24 |
| NS2 | Production of infectious virus | 25 | |
| NS3 | Modulation of NS5A phosphorylation | 28 | |
| NS4A | 163-167 | Modulation of NS5A phosphorylation | 29 |
| NS4B | 28 | ||
| NS5A | 36-198 | Promotion of RNA binding | 19, 32 |
| NS5B | 105-162, 277-334 | Modulation of NS5A phosphorylation | 34 |
Table II. Interactions between NS5A and cellular proteins.
| Protein | NS5A interaction residues | Functional role | Ref. |
|---|---|---|---|
| 2-5A synthase | 1-148 | Inhibition of IFN signaling | 87 |
| Actin | Movement of replication complexes | 27 | |
| Akt | 38 | ||
| Amphiphysin II | 350-356 | Modulation of NS5A phosphorylation | 88, 89 |
| Annexin A2 | domain 3 | Assembly of infectious virus | 90 |
| Apo-AI | Promotion of HCV-induced steatosis | 17 | |
| Apo-E | 205-280 | Assembly of infectious virus | 91 |
| BAX | 262-277 | Inhibition of apoptosis | 92 |
| β-Catenin | 1-144 | Inhibition of apoptosis | 93 |
| Myc box-dependent- interacting protein 1 |
348-356 | Inhibition of apoptosis | 94 |
| CDK1 | Modulation of cell cycle | 95 | |
| CKI-α | Phosphorylation of NS5A | 39, 49 | |
| CK II | carboxy-terminus | Phosphorylation of NS5A | 40 |
| Cyclophilin A | 304-320 | 96 | |
| Cyclophilin B | 310-315 | 96 | |
| FKBP38 | 148-236 | Inhibition of apoptosis | 97, 98 |
| FKBP8 | 121 | Stimulation of HCV RNA replication | 99 |
| Fyn | 350-356 | HCV replication and pathogenesis | 100 |
| GRB2 | 350-356 | Disruption of signal transduction pathway | 101 |
| hB-ind1 | Stimulation of HCV RNA replication | 102 | |
| HCK | 350-356 | HCV replication and pathogenesis | 100 |
| HSP 27 | 1-181 | 103 | |
| Heat shock 70 kDa protein 2 |
221-302 | Stimulation of HCV RNA replication | 104 |
| hTAFII32 | 175-179 | 105 | |
| hVAP-33 | Membrane association of replication complexes | 106 | |
| hVAP-A | 213, 215 | Stimulation of HCV RNA replication | 107 |
| hVAP-B | 66-70, 341-344 | Stimulation of HCV RNA replication | 108 |
| ISG15 | 379 | ISGylation of NS5A | 109 |
| Karyopherin β-3 | 1-200 | 110 | |
| La | 1-83 | 111 | |
| Lck | 350-356 | HCV replication and pathogenesis | 100 |
| Lyn | 343-356 | HCV replication and pathogenesis | 100 |
| MEK 1 | 38 | ||
| MEK 6 | 38 | ||
| MyD88 | 240-280 | Modulation of TLR signaling pathway | 112 |
| OSBP-related protein | 1-147 | HCV particle maturation and release | 113 |
| p53 | 1-150 | Modulation of cell cycle | 114 |
| p70 S6K | 38 | ||
| PI3K | 270-300 | Inhibition of apoptosis | 115 |
| PIP4KIII-α | 116 | ||
| PKA | Phosphorylation of NS5A | 41 | |
| PKR | 237-276 | Inhibition of IFN signaling | 117 |
| PLK1 | Phosphorylation of NS5A | 42 | |
| PTX1 | 133-220 | Modulation of IFN activity | 118 |
| Raf-1 | Stimulation of HCV RNA replication | 119 | |
| SRCAP | Modulation of cell cycle | 120 | |
| STAT1 | Inhibition of IFN signaling | 121 | |
| SYK | 1-175, 237-302 | Inhibition of tumor suppression | 122 |
| TBC1 domain family member 20 | 1-100 | Stimulation of HCV RNA replication | 123 |
| TBP | 124 | ||
| TRADD | 125 | ||
| TRAF2 | 148-301 | HCV pathogenesis | 126 |
| Tubulin | Movement of replication complexes | 27 | |
| TβR-I | 148-238 | Modulation of TGF-β signaling | 127 |
Viral proteins
NS5A is involved in the production of virus particles via its interaction with the HCV core nucleocapsid on lipid droplets. Three serine residues within the carboxy-terminus of domain III have been reported to be important for NS5A–core interactions (24).
Functioning as an organizer of HCV virion assembly, NS2 participates in a number of protein–protein interactions, including association with NS5A (25). Co-immunoprecipitation and subcellular colocalization studies have confirmed the NS2–NS5A interaction on lipid droplets (26).
Both NS3 and NS5A colocalize at HCV replication complexes and mediate complex movement via association with microtubules and actin filaments (27). Although NS3 and NS5A have been shown to interact in vitro (28), it remains unclear whether NS5A associates with the protease domain, the helicase domain or both domains of NS3. Domain I of NS5A contains a five-amino-acid stretch (Pro163-Pro167) that associates with NS4A (29). Neutralization of acidic residues in the carboxy-terminus of NS4A allows the non-structural protein to adopt an α-helical conformation, supporting its interaction with the basic stretch on NS5A (30).
The NS4B–NS5A interaction remains poorly understood. Despite colocalizing with NS5A at HCV replication complexes (31), NS4B has yet to be shown to interact with a specific region on NS5A. Domain I of NS5A can dimerize with itself into at least two unique conformations (19, 20). The function of this interaction appears to promote RNA binding, as NS5A dimerization is stimulated in the presence of G/U-rich RNA (32).
Two discontinuous regions of NS5A located in domain I and domain II have been identified as independently essential to binding NS5B (33). NS5A modulates the HCV polymerase in vitro, with NS5B RNA-dependent RNA polymerase (RdRP) activity inhibited by NS5A in a concentration-dependent manner (34).
Cellular proteins
Interactions between NS5A and cellular proteins with a defined function can be grouped into three main categories: those which inhibit the host immune response, those which modulate the host cell cycle and those which stimulate the HCV life cycle. These interactions are listed in Table II.
RNA
NS5A represents a novel structural class of RNA-binding proteins (21, 32), with the optimal RNA-binding region mapping to domain I and the first low-complexity sequence of the viral protein, together referred to as domain I-plus (32). In stable cell lines expressing the HCV replicon, NS5A colocalizes with viral RNA (14). G/U-rich RNA, or G/U-rich elements (GREs), five to six nucleotides in length has high-affinity binding to NS5A and promotes dimerization of domain I-plus. This is consistent with the NS5A dimer reported by the Rice laboratory (19), with the groove of positive electrostatic potential of sufficient size to bind RNA, containing residues capable of hydrogen bonding to the keto and imino moieties of guanine and uracil bases. One putative viral RNA-binding site for NS5A is the poly(rU) tract in the 3′-UTR of the HCV genome. Insertion of cytidylate or adenylate residues every four to six nucleotides in this region impairs replication of HCV RNA, perhaps via disruption of NS5A binding (35). The role of NS5A binding in this region, however, is unclear. NS5A also binds to regions of the HCV IRES. Domains III and IV of the IRES contain GREs and bind tightly to NS5A. In contrast, domain II of the IRES does not contain GREs and binds NS5A less tightly, supporting the GRE-binding preference of NS5A (36). As the HCV IRES regulates interferon-induced, double-stranded RNA-activated protein kinase (PKR) activity, interactions between NS5A and the IRES may modulate the function of the viral RNA.
Phosphorylation
NS5A exists in two forms based on electrophoretic mobility, designated p56 and p58 (37). The p56 form can either be unphosphorylated or basally phosphorylated by a number of kinases in different regions (38-48). The formation of p58 is thought to arise from hyperphosphorylation of a phosphorylated p56 form by casein kinase I isoform alpha (CKI-α) (39, 49), although the modifications that ultimately comprise p58 are unclear. It is only through loss of p58 via adaptive mutations in the replicon that efficient replication of the HCV subgenomic replicon can occur (50, 51), suggesting that only p56 is required for HCV genome replication and that phosphorylation may toggle NS5A between different functions.
SMALL-MOLECULE NS5A INHIBITORS: FROM MONOMERS TO DIMERS
NS5A is an essential protein for HCV replication. Although the exact role(s) it plays during the viral life cycle has yet to be elucidated, the necessity of NS5A, with its multiple interactions, makes it an attractive target for direct-acting antivirals (DAAs).
In 2004, Bristol-Myers Squibb (BMS) disclosed substituted iminothiazolidinone-based HCV inhibitors believed to target NS5A, as mutations within the protein conferred resistance to these compounds. Molecules in this class exhibited significant potency against HCV genotype 1b in replicon cells, but were essentially inactive against genotype 1a. Further optimization of a screening hit, BMS-858, resulted in more potent analogues, such as BMS-824 (Fig. 3) (52, 53).
Figure 3.

Early NS5A inhibitors from BMS.
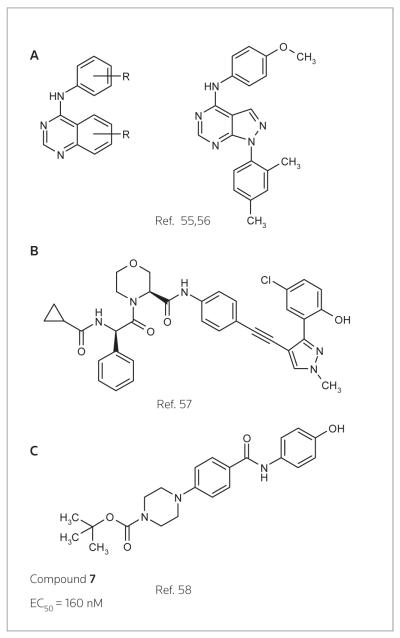
Early NS5A inhibitors have been reviewed (54). Briefly, Arrow Therapeutics disclosed patent applications covering 4-aminoquinazolines and pyrazolopyrimidines (Fig. 4A) (55, 56). Arrow subsequently performed phase I studies with AZD-2836 and AZD-7295. Presidio Pharmaceuticals and XTL Biopharmaceuticals disclosed a series of phenyl-acetylene-pyrazoles (Fig. 4B) (57), the current status of which is unknown. In a recent publication, Merck scientists described piperazine-aryl scaffold-based NS5A inhibitors (Fig. 4C) (58). Mutations that arose to treatment with the most potent inhibitor in the series, compound 7 (EC50 = 160 nM), localized to NS5A, implicating the viral protein as the macromolecular target. Interestingly, these mutations mapped to the homodimer interface in the crystal structure of NS5A, suggesting that the dimerization may have significant biological consequences. Compound 7 was also modeled in domain IA of the NS5A homodimer. Two potential binding modes were possible, but the preferential fit involved 7 binding across the dimer interface.
Figure 4.
NS5A inhibitors from Arrow Therapeutics (A), Presidio Pharmaceuticals and XTL Biopharmaceuticals (B), and compound 7 from Merck (C).
Genelabs and GlaxoSmithKline (GSK) scientists disclosed novel thiazoles in their NS5A program (Fig. 5), which were potent against HCV genotype 1b. One of the key structure-activity relationship (SAR) findings in this series was that replacement of the carboxybenzyl (Cbz) group in GL-100953 by the morpholinophenylglycine moiety in GL-103905 resulted in nearly a 4,000-fold increase in potency against genotype 1b. The GL-100953 SAR was also explored in a different fashion, as exemplified by GL-102709. These and other SAR findings subsequently led to the bis-aryl GL-104646, which was highly potent not only against genotpye 1b, but also genotype 1a (59-65).
Figure 5.
GSK & Genelabs NS5A compounds.
While evaluating the iminothiazolidinone class, BMS scientists noticed their chemical instability and the formation of a potent dimeric byproduct, 48 (EC50 = 2 nM), from BMS-824 (Fig. 6). Removal of the iminothiazolidinone moiety from 48 paved the way to the discovery of a very potent trans-stilbene, 55 (EC50 = 0.1 nM) (53). Subsequent optimization of 55 led to BMS-790052, which entered the clinic in 2008. BMS-790052 exhibited increased potency against genotype 1a of > 200,000 times compared to 55 (53, 66).
Figure 6.
BMS conversion from monomeric to dimeric NS5A inhibitor.
NS5A INHIBITOR CHEMICAL SPACE
The current trend in NS5A inhibitor designs utilizes a dimeric pharmacophore, featuring a linear conjugated bis-biaryl core terminated by peptidic caps. Imidazole–proline coupling is the predominant junction between the core and caps. The mean length of the core, defined by the distance between the imidazole junction with proline, is approximately 16.5 Å, with a standard deviation of 1.5 Å. Since current NS5A inhibitors are dimeric by design, their physical properties often exceed optimal lead-like parameters for small molecules by roughly a factor of two. The median molecular weight, cLogP and cLogD are approximately 787 Da, 4.9 and 4.6, respectively, which can present a challenge for achieving in vivo oral bioavailability. Nonetheless, encouraging clinical results for BMS-790052 have demonstrated the viability of this pharmacophore for treating HCV and highlight the caveats of strictly imposing widely accepted, yet crude, cutoff that can negate potential therapeutics.
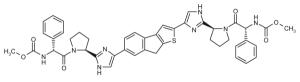
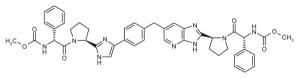
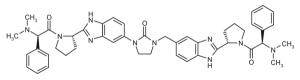
Representative dimeric NS5A inhibitors, selected from 35 HCV patents and 11 companies (2008 to the present), are showcased in Table III and Figure 7. Compared to the relatively conserved peptidic caps, the chemical composition of the core linkers varies widely, although their lengths tend to be within the range of 15-18 Å. There is a preference for conjugated aryl groups, including alkyne isosteres, to bridge the midsection of the cores, although saturated linkers, with and without heteroatoms, have been exemplified (e.g., ABT, BMS, ENANTA). Imidazole is the predominant terminus of the core, while amide coupling to the caps is a distant minority. There are many examples of alkyl-fused tricyclic ring systems in the mid-section of the core that restrict the biaryl rotational degrees of freedom (BMS_32, ENANTA_10, GILEAD_2 & 4, PRESIDIO_1), in addition to a few aryl tricyclic systems (BMS_1).
Table III.
Representative inhibitors from the HCV NS5A patent space. The juxtaposition of these compounds is represented in Figure 7, with corresponding compound labels and company symbols. Chirality is S, except for phenylglycines.
| Symbol | Id Patent | Symbol | Id Patent |
|---|---|---|---|
| ● | ABT_1 WO2010144646 | ● | ABT_2 WO2010120935 |

|

|
||
|
| |||
| ■ | ARROW_1 WO2010094977 | ◆ | BMS_1 WO2010120621 |

|

|
||
|
| |||
| ◆ | BMS_10 WO2008021927 | ◆ | BMS_11 WO2008021927 |
|
|
||
|
| |||
| ◆ | BMS_12 WO2008021927 | ◆ | BMS_14 WO2010117635 |

|
|
||
|
| |||
| ◆ | BMS_15 WO2010117635 | ◆ | BMS_16 WO2010117635 |

|

|
||
|
| |||
| ◆ | BMS_18 WO2009102318 | ◆ | BMS_19 WO2009102318 |

|
|
||
|
| |||
| ◆ | BMS_21 WO2010117704 | ◆ | BMS_23 WO2010017401 |

|

|
||
|
| |||
| ◆ | BMS_24 WO2010017401 | ◆ | BMS_26 WO2010117977 |

|
|
||
|
| |||
| ◆ | BMS_28 WO2009102694 | ◆ | BMS_3 WO2010096302 |
|

|
||
|
| |||
| ◆ | BMS_30 WO2010138488 | ◆ | BMS_32 US20090202483 |

|

|
||
|
| |||
| ◆ | BMS_4 WO2010096302 | ◆ | BMS_6 US20100249190 |

|

|
||
|
| |||
| ◆ | BMS_8 WO2010138368 | ▲ | ENANTA_10 WO2010096462 |
|
|
||
|
| |||
| ▲ | ENANTA_12 WO2010099527 | ▲ | ENANTA_13 WO2010099527 |
|

|
||
|
| |||
| ▲ | ENANTA_15 WO2010091413 | ▲ | ENANTA_16 WO2010091413 |

|
|
||
|
| |||
| ▲ | ENANTA_3 WO2010148006 | ▲ | ENANTA_4 WO2010148006 |

|

|
||
|
| |||
| ▲ | ENANTA_6 WO2011031904 | ▲ | ENANTA_7 WO2011031904 |

|

|
||
|
| |||
| ▲ | ENANTA_9 WO2010096462 | ▼ | GILEAD_1 WO2010132601 |

|

|
||
|
| |||
| ▼ | GILEAD_2 WO2010132601 | ▼ | GILEAD_3 WO2010132601 |

|

|
||
|
| |||
| ▼ | GILEAD_4 WO2010132601 | ◀ | GSK_1 WO2011028596 |

|

|
||
|
| |||
| ▶ | MRK_SGP_1 WO2010138790 | ✦ | PFE_1 WO2011004276 |

|

|
||
|
| |||
| ★ | PRESIDIO_1 WO2010111534 | ★ | PRESIDIO_2 WO2010111534 |

|

|
||
|
| |||
| ★ | PRESIDIO_4 WO2010111673 |
|
TIBOTEC_1 WO2011015657 |

|

|
||
|
| |||
|
|
TIBOTEC_3 WO2010122162 |
|
TIBOTEC_6 WO2011015658 |

|

|
||
|
| |||
|
|
TIBOTEC_8 WO2011026920 | ■ | VTX_1 WO2011009084 |

|

|
||
|
| |||
| ■ | VTX_2 WO2011009084 | ■ | VTX_3 WO2011009084 |

|

|
||
Figure 7.
The patent landscape of HCV NS5A inhibitors. Compound labels and symbols, designating company, correspond to Table III with the chemical structures. Relative compound proximity is determined by chemical similarity; X and Y axes are in Tanimoto distance units. BMS-790052 (BMS_11 in figure) is found near the center of the densest cluster of competitive compounds, also near the center of the overall patent landscape.
The most popular caps feature a Pro-Val-carbamate motif (Fig. 7, blue), followed by a Pro-phenyl-Gly motif (Fig. 7, green). Ether and alcohol derivatives are also exemplified at the Val and terminal positions (PRESIDIO_2, TIBOTEC_6 & 8). Although symmetric presentation of caps on each side of the core is common, asymmetric cap combinations are also popular. Proline is by far the most popular peptide junction to the core, which kinks the caps out of the plane of the core. In some cases, open-ring proline variants are featured (WO 2008021936, WO 2009102318, WO 2010017401), while in more recent patents, substitution of the proline ring, including fused-ring and spiro systems, has evolved (BMS_15 &16, ENANTA_6, GILEAD_3 & 4, GSK_1, VTX_2). Relatively few six-membered proline ring variants have been exemplified (ENANTA_6, ENANTA_7, PRESIDIO_4). Azo coupling has also been used to mimic proline (WO 2010117977, BMS_26), as well as cap termini (ENANTA_3 & 4).
PUTATIVE NS5A INHIBITOR INTERACTIONS
The dimeric symmetry of NS5A inhibitors suggests a corresponding symmetry in NS5A. Published structures of NS5A (19, 20) reveal the formation of a homodimer between the zinc finger (ZnF) domains, although the relative arrangement of the monomer units is quite different, which has been attributed to multiple protein–protein and protein–RNA interaction roles for NS5A (20). Resistant mutants of BMS-790052 arise in the loop region (e.g., HCV genotype 1a Met28Thr, Gln30His, Leu31Val) that flanks the dimer interface and connects the NS5A ZnF domain to the amino-terminal amphiphilic α-helix, including Tyr93Cys at the edge of the ZnF dimer interface (Fig. 8). The O–O distance between Tyr93 residues in the monomer units is about 19 Å, which serves as a rough estimate of the width of the dimer interface. Notably, this distance is also similar to the length spanned by dimeric inhibitor cores. This suggests that dimeric inhibitors may interact with NS5A by straddling the ZnF dimer interface, positioning the caps in proximity to either NS5A loop where mutational resistance is prevalent.
Figure 8.
Putative binding modes of NS5A inhibitors with NS5A gt 1b domain I. Monomer units of the NS5A homodimer are color-coded red and cyan. BMS-790052-resistant mutant sites (gt 1a loop region: Met28, Gln30, Leu31; dimer interface: Tyr93) are color-coded blue. (A) Dimeric interaction mode of BMS-790052 looking down the C2 symmetry axis of the NS5A homodimer. The bis-biaryl core of BMS-790052 spans the NS5A dimer interface, positioning the caps at the zinc finger (ZnF) domain and α-helix “hinge” loop interfaces. (B) Monomeric interaction mode of GL-103905 looking down the C2 symmetry axis of the NS5A homodimer. (C) Same as (A) but rotated 90°, looking down the plane of the dimer interface, and BMS-790052 depicted as CPK spheres. The Pro-Val-carbamate cap docks between the ZnF domain and the helix loop. (D) Same as (B) but rotated 90° with GL-103905 depicted as CPK spheres. GL-103905 occupies the crevice formed between the ZnF domain and loop interface.
Docking studies using a full-length homology model (67) of NS5A domain I constructed from 3FQM (20) and 1R7C (68) suggest a dimeric interaction mode, whereby the core lies in the solventexposed groove of the dimer interface, while the caps make buried contacts in crevices formed between the loops and ZnF elements (Fig. 8). In this scenario, the core functions mainly as a spacer, simultaneously delivering the caps to interact with the loop regions on either side of the dimer interface. As fragments, if it is assumed that independently the caps interact only weakly with the loop region (Kd,mono ~ 10 μM), then by linking the caps to bind cooperatively inhibition grows exponentially (Kd,dimer ~ Kd,mono•Kd,mono ~ 0.1 nM = exp[−ΔGdimer/RT] ~ exp[−2ΔGmono/RT]). This may explain the picomolar potency of dimeric NS5A inhibitors and the abrupt SAR that can be observed as dimeric NS5A inhibitors are truncated into monomeric analogues.
In the HCV genotype 1b homology model, loop residues Leu28, Arg30 and Leu31 are in close proximity to Tyr93 at the dimer interface. Together, the locus of these residues suggests a “hinge” site that regulates the orientation and motion of the α-helix relative to the ZnF domain. The flexibility of the loop can enable a wide range of helix orientations. By occupying the space between the loop and dimer interface, the caps can restrict the movement of the helix relative to the ZnF domain, thereby limiting NS5A function.
Monomeric NS5A inhibitors have been reported to be highly potent against genotype 1b, but not 1a. However, resistant mutations have arisen at similar sites as dimeric inhibitors, including Met28, Gln30, Leu31 and Tyr93, implying similar interaction modes. Docking of monomeric inhibitors like GL-103905 suggests that monomers can occupy the same site as the BMS-790052 caps. The putative interaction mode of GL-103905 with NS5A genotype 1b (Fig. 8) illustrates that the ligand clearly cannot span the dimer interface, but GL-103905 can more fully occupy the loop–hinge crevice. In this manner, GL-103905 could similarly restrict the hinge motion of the loop region, like BMS-790052. Figure 9 illustrates the overlapping pharmacophore elements of BMS-790052 and GL-103905. Although there are residue differences between genotype 1a and 1b at loop residues 28, 30, 34 and 37 that may contribute to the selectivity of genotype 1b versus genotype 1a for GL-103905, modeling is at a loss to explain the 100,000-fold divergence in potency, or the hypersen-sitivity of NS5A dimeric inhibitors to gentoype 1a mutations compared to genotype 1b.
Figure 9.
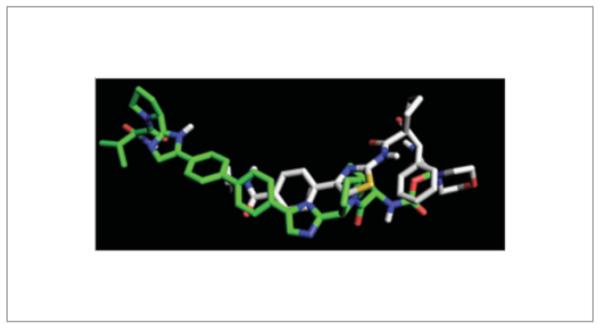
Overlapping pharmacophore elements of BMS-790052 (green carbons) and GL-103905 (white carbons), as docked in the NS5A gt 1b homology model of Figure 8.
To date, no NS5A inhibitor co-crystal structures, nor photoaffinity labeling studies, have been published. Thus, the direct binding modes of NS5A inhibitors, dimeric or monomeric, remain elusive. Conceivably, the absence of the connecting loop between helix and ZnF domains, where mutations arise, could preclude the formation of a co-crystal complex with protein constructs used thus far in structural biology. Given the multifaceted functionality of NS5A reported in the literature, multiple inhibitory mechanisms of action could also be contributing and complicating factors in interpreting NS5A inhibitor interactions. Future experimental structure-based studies, as well as NS5A functional studies, will hopefully shed light on the intriguing SAR and roles of NS5A.
BMS-790052
Through the use of the cell-based HCV replicon system, compounds with a thiazolidinone core were high-throughput screened by BMS to test for inhibition of HCV replication (69). The resulting candidate compound, the above-mentioned BMS-824 (Fig. 6), effectively inhibited HCV genotype 1b replication with an EC50 of 6 nM, but had no inhibitory effect against NS3 protease or NS5B polymerase activity. The nonstructural coding regions from the polyprotein of HCV RNA-replicating cell lines resistant to the compound were sequenced to determine the viral target. An amino acid substitution at Tyr2065 of the polyprotein, corresponding to Tyr93 of NS5A, to either His or Cys was found in all resistant cell lines. A Tyr93His NS5A derivative was engineered into the HCV replicon and verified to be sufficient to confer resistance to the compound. It was also found that BMS-824 inhibited the phosphorylation of NS5A, required for p58 formation, although it had no effect on p58 that had already been formed. The Tyr93His-resistant phenotype displayed no inhibition of p58 formation when treated with the compound. Whether the decrease in NS5A p58 is directly related to the mechanism of action of the compound or is a secondary phenotype has yet to be explored.
Further optimization of BMS-824 led to the discovery of BMS-790052 (Fig. 6), a molecule currently being tested in clinical trials for the treatment of chronic HCV infection. The proof-of-concept study involved administering a single dose of 1-100 mg BMS-790052 to genotype 1 treatment-naive HCV patients. The level of HCV RNA was measured for 7 days post-dosing. Patients receiving doses of 1, 10 and 100 mg of BMS-790052 showed a rapid decline in HCV RNA; 1.8 log10, 3.2 log10 and 3.3 log10 reductions, respectively, in HCV RNA levels were observed for doses of 1, 10 and 100 mg (66). The compound is less potent against genotype 1a than 1b replicons (EC50 = 50 and 9 pM, respectively), and the study results showed that genotype 1b patients had an increased reduction in HCV RNA levels compared to equivalently dosed gentopye 1a patients. The genotype 1b patients also showed a more sustained response of 5 days. The compound has since been shown to be inhibitory against HCV genotypes 1a, 1b, 2a, 3a, 4a and 5a, with EC50 values of 9-146 pM. Although the treatment population is small (N = 16 on active compound), the data suggest that there is a correlation between in vitro potency and in vivo effect for inhibitors targeting NS5A.
HCV resistance is an important consideration for any HCV therapy, as the virus grows to high titers and has a high mutation rate. The previously described preclinical characterization of BMS-790052 demonstrated that resistance to the compound mapped to the first 100 amino acids of NS5A in both genotype 1a and 1b (66). BMS analyzed the HCV sequences from the patients treated with BMS-790052 in this single-dose trial and identified mutations at residues known to be important for drug potency in vitro. Further trials are needed to ascertain if the presence of these mutations confers resistance in vivo.
BMS also performed a multiple-dose proof-of-concept study in HCV genotype 1-infected patients. Patients were dosed for 14 days with 1, 10, 30, 60 or 100 mg BMS-790052 once a day, or 30 mg twice daily. Four patients with a mix of genotype 1a and 1b were included in each active drug cohort, and data from the study are therefore based on a small number of patients. The maximum decline in HCV RNA levels for genotype 1a-infected patients was 3.8 log10 at the dose of 60 mg once daily and the minimum decline was 2.4 log10 with the dose of 1 mg once daily (70). Twice-daily dosing with 30 mg BMS-790052 gave a maximum reduction in HCV RNA of 5.7 log10. The maximum decline for once-daily dosing in genotype 1b patients was 4.3 log10 and the 10-mg dose gave the lowest decline, 3.3 log10. Although a small population was treated in this study, the data show that genotype 1b patients respond better to therapy, as seen by the greater declines in HCV RNA. The antiviral effect was not sustained for the entire dosing period, supporting its potential inclusion as part of a combination therapy, but highlighting its inability to serve as a monotherapy due to the emergence of resistant viruses.
Further clinical studies have evaluated the impact of BMS-790052 on HCV RNA levels in combination with IFN-α and ribavirin. This dose-ranging (3, 10 or 60 mg once daily plus standard of care) phase IIa study in genotype 1-naive patients compared BMS-790052 to standard of care alone for 48 weeks. Analysis of viral load declines at week 12 post-treatment showed that the combination of 10 or 60 mg BMS-790052 with standard of care improved sustained virological response (SVR12) rates when compared to standard of care alone, where SVR12 is defined as undetectable HCV RNA at week 12 post-treatment. SVR12 rates for the 10- and 60-mg combinations were 92% and 83%, respectively, versus 25% for standard of care (71). The 3-mg dose of BMS-790052 in combination with standard of care gave an SVR12 rate of 40%, more than standard of care alone but far less than that achieved with the higher doses of the inhibitor. There were two cases of virological breakthrough for the 3-mg dose group and one case in the 60-mg dose group. Additionally, two patients experienced a post-treatment relapse in the 3-mg dose group and one in the 60-mg dose group. The cohort size for this study was 12 patients per group, and thus the data are based on a small number of patients.
An additional study was performed to determine if two direct-acting antivirals alone could “cure” HCV infection in infected individuals. This was a proof-of-concept study, as there is currently no consensus in the field as to how many direct-acting antivirals would be needed to eliminate HCV infection. Thus, several companies are studying different direct-acting antiviral combinations to determine if any such combinations could be successful. The BMS trial addressing this point involved combining the NS5A inhibitor BMS-790052 and the BMS inhibitor targeting the NS3 protease, BMS-650032 (72). The molecules had non-overlapping resistance patterns and preclinical combination studies suggested that they would not be antagonistic. A phase IIa study enrolling genotype 1 prior non-responders for 24 weeks of treatment was completed, examining the combination of the two direct-acting antivirals alone compared to the two direct-acting antivirals in combination with standard of care. The doses of the two direct-acting antivirals chosen were 60 mg once daily of BMS-790052 and 600 mg twice daily of BMS-650032, and the data reported were SVR24, defined as undetectable RNA at 24 weeks post-treatment. When the two direct-acting antivirals were given to non-responders, only 36% achieved SVR24. This is compared to 90% who achieved SVR24 on quadruple therapy of standard of care with the two direct-acting antivirals. The study was small, but the data suggest that the four-regimen therapy was effective at reducing HCV RNA levels, while the dual therapy only had a similar impact on a smaller percentage of patients (36% SVR24 rate). Although the SVR24 rate was low, this study did demonstrate that SVR24 is achievable in a treatment regimen lacking IFN-α and ribavirin. Follow-up of the patients who had “breakthroughs” in HCV RNA levels showed that mutations were present that would impact the potencies of both direct-acting antivirals (73). Patients will be followed for 48 weeks.
The resistance profile of BMS-790052 was extended in a later publication, but the critical residues originally identified, whether alone or in combination, remained the key sources of resistance (74). Cell culture experiments with biotinylated analogues from this series were used to isolate proteins binding to the compound and showed that NS5A interacted either directly or indirectly with an active compound from the series, while an inactive compound failed to show binding (66). The binding was visualized by Western blotting with an NS5A antibody. Further experimentation is needed to identify the exact mechanism of action for this series, but the resistance pattern and published cell culture experiments suggest that the compound works via an NS5A-mediated mechanism, potentially by binding directly to NS5A.
BMS-790052 has been shown to alter the subcellular localization and biochemical fractionation of NS5A (75, 76). Upon treatment of replicon-containing cells with the inhibitor, NS5A cytoplasmic distribution shifted to centralization at lipid droplets. Co-localization of NS5A and NS5B was also impaired (75).
NS5A INHIBITOR CLINICAL DATA
Gilead Sciences also has an NS5A inhibitor in the clinic. GS-5885 is a potent inhibitor of HCV genotype 1 replicons, with EC50 values of 34 and 4 pM, respectively, for genotypes 1a and 1b (77). Recently, the results of a 3-day dose-ranging study in HCV genotype 1 patients were reported. HCV patients were dosed once daily for 3 days with 1-90 mg of GS-5885 and reductions in viral load were measured for 7 days. Most patients enrolled in this study were genotype 1a and thus discussion will be divided between the responses of the two subtypes. Median change from baseline in genotype 1a patients was 2.3 log10 for the 1-mg dose, and between 3.1 and 3.3 log10 for the 3-, 10-, 30- and 90-mg doses, suggesting that the impact of the compound on viral load had peaked (78). One cohort of 12 genotype 1b patients (10 active, 2 placebo) was dosed once daily for 3 days with 10 mg GS-5885 and the decline in HCV RNA was also tracked for 7 days. Median change from baseline in genotype 1b patients was 3.3 log10. Similar maximum viral load declines were observed for genotype 1a and 1b, but the genotype 1b patients appeared to have a more sustained response, as they showed a delayed return to baseline. This could possibly be due to the differences in genotype 1a and 1b potencies and resistance patterns. It is also noteworthy that one patient in the 90-mg dose group did not respond to therapy. This molecule continues under development.
Presidio Pharmaceuticals has also advanced a compound into phase I studies. PPI-461 is a small-molecule inhibitor with picomolar potency against genotype 1a and 1b replicons. EC50 values were 200 and 10 pM, respectively, against genotype 1a and 1b (79). The molecule is active across multiple genotypes, although potency is substantially reduced in chimeric replicons containing sequences from genotypes 3a and 6a. Preclinical profiling of resistance to PPI-461 demonstrated that it is susceptible to mutations in the first 100 amino acids of the NS5A protein, similar to BMS. Presidio reported single or double mutations that led to a high level of drug resistance, and these mutations are combinations of single mutations identified by BMS in their resistance profiling of BMS-790052, suggesting that both molecules work through a similar mechanism of action. Presidio also did resistance passaging with PPI-461 on chimeric genotype 1b replicons containing NS5A sequences from genotypes 2a-6a. The resistance mutations identified for each genotype demonstrate that resistance is limited to residues in the first 100 amino acids of NS5A and include the same critical positions −28, 30, 31 and 93– that are involved in resistance in genotypes 1a and 1b, as well as identified by BMS as important for BMS-790052 activity. Presidio has reported the results of the phase 1a trial involving healthy volunteers and is currently running the phase 1b proof of concept in HCV patients.
Several other companies have also reported preclinical characterization of small-molecule inhibitors that work via an NS5A-mediated mechanism. GSK has presented preclinical data for GSK2336805 (see GSK_1, Table III). The compound has picomolar activity against genotype 1a, 1b and 2a (JFH-1) replicons, as well as genotype 2a chimeric replicons containing NS5A sequences from genotypes 4a, 5a and 6ab (63, 80). These chimeric replicons, as well as those alluded to below, contain NS5A sequences derived from public databases and represent a consensus of input sequences, or the closest patient to that consensus. GSK2336805 showed reduced activity on chimeric replicons containing NS5A patient or consensus sequences from genotypes 2a, 3a, 6cg and 6hn. On these replicons, potency is shifted into the nanomolar range. Resistance profiling of the compound on genotype 1a and 1b replicons showed that the compound is sensitive to the same residues reported by BMS and Presidio −28, 30, 31 and 93–, suggesting that these inhibitors may share a common mechanism of action. It is noteworthy that this compound has picomolar activity on the JFH-1 genotype 2a replicon, but nanomolar activity on chimeric replicons containing genotype 2a NS5A patient or consensus sequences. Sequence analysis of the replicons showed that the JFH-1 replicon contains a minority variant at position 31 of NS5A. Position 31 in JFH-1 is Leu, while the majority residue is Met, present in both the genotype 2a NS5A consensus and patient sequences in the chimeric replicons. GSK mutated NS5A position 31 in the JFH-1 replicon to Met and showed that this mutation caused a loss in activity for BMS-790052 and analogues in the GSK2336805 series. These data were supported from a study by Scheel et al. (81). This group also demonstrated that BMS-790052 suffers a loss of potency on genotype 2a replicons containing Met at position 31, supporting the GSK data on BMS-790052 replicons and further strengthening the theory that these compounds are acting via a similar mechanism. It also raises the question as to the activity of the other NS5A inhibitors on the replicons containing the majority amino acid at position 31. GSK2336805 is currently in phase IIa clinical trials.
Achillion Pharmaceuticals has reported a potent small-molecule inhibitor targeting NS5A. The EC50 value for ACH-2928 for a genotype 1a replicon was 46 pM and values for two different genotype 1b replicons were 2.1-2.8 pM (82). The compound had EC50 values of < 103 pM against genotype 2-6 replicons and maintained activity on chimeric replicons containing genotype 1a or 1b patient sequences. The genotype 2a replicon sequence was not specified, but the data for BMS-790052 reported in parallel suggest it contains the minority variant at position 31. Resistance profiling of this compound was not reported. This compound has been selected for clinical development.
Indenix Pharmaceuticals has also reported preclinical data on two compounds impacting HCV replication via effects on NS5A. Both IDX-380 and IDX-719 have EC50 values of < 50 pM against genotype 1-5 replicons, with CC50 values of > 100 μM, yielding a good selectivity index (83). Both compounds are sensitive to some of the key residues critical for other compounds targeting NS5A –positions 30, 31 and 93. The compounds were shifted between 310- and 8,601-fold on Gln30Glu, Leu31Val and Tyr93His mutations in genotype 1a replicons. Similar mutations in the genotype 1b replicon, namely Leu31Val and Tyr93His, shifted the potency of both compounds < 100-fold. These genetic data suggest that the molecule may target a similar mechanism as the inhibitors described above. The compounds are being further evaluated to identify the clinical candidate.
InterMune reported a structure-based approach that identified a series of compounds targeting NS5A. Data reported on five compounds show that they are active on genotypes 1a and 1b (6-140 pM), with consistently higher activity on genotype 1b (84). One compound, ITMN-9959, was also tested in a mouse model of HCV replication. Mice with humanized livers chronically infected with HCV were treated for 14 days with 30 mg/kg of the compound, resulting in a 2.2 log10 drop in HCV RNA levels. Resistance profiles were not reported. The molecules are currently being characterized.
Enanta Pharmaceuticals released data on a small-molecule inhibitor of HCV that targets NS5A. EDP-239 showed picomolar activity on genotype 1a and 1b replicons, with EC50 values of 31.2 and 7.3 pM, respectively (85). The compound also showed a small shift (< 4-fold) in the presence of 40% human serum. Resistance profiling was not reported, nor were there reports of pan-genotype activity. In vitro safety and in vivo animal pharmacokinetic studies suggest that the compound may have favorable characteristics for once-daily, low-dose regimens in humans.
In summary, small molecules targeting NS5A have proven to be potent inhibitors of HCV replication in vitro and in vivo. The current clinical compounds share similar profiles of inhibition in terms of resistance, suggesting similar mechanisms, although further work is required to define the exact mode(s) of action. It is also worthwhile to note that, although the current clinical compounds inhibit multiple HCV genotypes, some suffer losses of potency on genotype 2 due to a variant at residue 31. There is a later report from BMS on another series of small-molecule inhibitors targeting NS5A with more potent activity across all genotypes (including genotype 2) and improved resistance profiles, suggesting that future generations of molecules could tackle some of the weaknesses of the current compounds being tested (86).
Footnotes
DISCLOSURES
The authors state no conflicts of interest.
REFERENCES
- 1.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamal SM, Fouly AE, Kamel RR, et al. Peginterferon alfa-2b therapy in acute hepatitis C: Impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130(3):632–8. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J Med Virol. 2004;73(3):387–91. doi: 10.1002/jmv.20103. [DOI] [PubMed] [Google Scholar]
- 4.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–6. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 5.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–89. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asselah T, Bieche I, Narguet S, et al. Liver gene expression signature to predict response to pegylated interferon plus ribavirin combination therapy in patients with chronic hepatitis C. Gut. 2008;57(4):516–24. doi: 10.1136/gut.2007.128611. [DOI] [PubMed] [Google Scholar]
- 7.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 8.Asselah T, Benhamou Y, Marcellin P. Protease and polymerase inhibitors for the treatment of hepatitis C. Liver Int. 2009;29(Suppl. 1):57–67. doi: 10.1111/j.1478-3231.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 9.Poenisch M, Bartenschlager R. New insights into structure and replication of the hepatitis C virus and clinical implications. Semin Liver Dis. 2010;30(4):333–47. doi: 10.1055/s-0030-1267535. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrinmediated endocytosis. J Virol. 2006;80(14):6964–72. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda M, Beard MR, Ping LH, Lemon SM. A phylogenetically conserved stem-loop structure at the 5′ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J Virol. 1999;73(2):1165–74. doi: 10.1128/jvi.73.2.1165-1174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67(3):1385–95. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hijikata M, Mizushima H, Tanji Y, et al. Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci U S A. 1993;90(22):10773–7. doi: 10.1073/pnas.90.22.10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosert R, Egger D, Lohmann V, Bartenschlager R, Blum HE, Bienz K, Moradpour D. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J Virol. 2003;77(9):5487–92. doi: 10.1128/JVI.77.9.5487-5492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartenschlager R, Penin F, Lohmann V, Andre P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19(2):95–103. doi: 10.1016/j.tim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Brass V, Bieck E, Montserret R, et al. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus non-structural protein 5A. J Biol Chem. 2002;277(10):8130–9. doi: 10.1074/jbc.M111289200. [DOI] [PubMed] [Google Scholar]
- 17.Shi ST, Polyak SJ, Tu H, Taylor DR, Gretch DR, Lai MM. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology. 2002;292(2):198–210. doi: 10.1006/viro.2001.1225. [DOI] [PubMed] [Google Scholar]
- 18.Elazar M, Cheong KH, Liu P, Greenberg HB, Rice CM, Glenn JS. Amphipathic helix-dependent localization of NS5A mediates hepatitis C virus RNA replication. J Virol. 2003;77(10):6055–61. doi: 10.1128/JVI.77.10.6055-6061.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tellinghuisen TL, Marcotrigiano J, Rice CM. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature. 2005;435(7040):374–9. doi: 10.1038/nature03580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love RA, Brodsky O, Hickey MJ, Wells PA, Cronin CN. Crystal structure of a novel dimeric form of NS5A domain I protein from hepatitis C virus. J Virol. 2009;83(9):4395–403. doi: 10.1128/JVI.02352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Hwang J, Sharma SD. Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein. J Biol Chem. 2005;280(43):36417–28. doi: 10.1074/jbc.M508175200. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Kang CB, Yoon HS. Molecular and structural characterization of the domain 2 of hepatitis C virus non-structural protein 5A. Mol Cells. 2006;22(1):13–20. [PubMed] [Google Scholar]
- 23.Hanoulle X, Verdegem D, Badillo A, Wieruszeski JM, Penin F, Lippens G. Domain 3 of non-structural protein 5A from hepatitis C virus is natively unfolded. Biochem Biophys Res Commun. 2009;381(4):634–8. doi: 10.1016/j.bbrc.2009.02.108. [DOI] [PubMed] [Google Scholar]
- 24.Masaki T, Suzuki R, Murakami K, et al. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J Virol. 2008;82(16):7964–76. doi: 10.1128/JVI.00826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Anantpadma M, Timpe JM, Shanmugam S, Singh SM, Lemon SM, Yi M. Hepatitis C virus NS2 protein serves as a scaffold for virus assembly by interacting with both structural and nonstructural proteins. J Virol. 2011;85(1):86–97. doi: 10.1128/JVI.01070-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, Penin F, Bartenschlager R. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog. 2010;6:e1001233. doi: 10.1371/journal.ppat.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai CK, Jeng KS, Machida K, Lai MM. Association of hepatitis C virus replication complexes with microtubules and actin filaments is dependent on the interaction of NS3 and NS5A. J Virol. 2008;82(17):8838–48. doi: 10.1128/JVI.00398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitrova M, Imbert I, Kieny MP, Schuster C. Protein-protein interactions between hepatitis C virus nonstructural proteins. J Virol. 2003;77(9):5401–14. doi: 10.1128/JVI.77.9.5401-5414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asabe SI, Tanji Y, Satoh S, Kaneko T, Kimura K, Shimotohno K. The N-terminal region of hepatitis C virus-encoded NS5A is important for NS4A-dependent phosphorylation. J Virol. 1997;71(1):790–6. doi: 10.1128/jvi.71.1.790-796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindenbach BD, Pragai BM, Montserret R, Beran RK, Pyle AM, Penin F, Rice CM. The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication. J Virol. 2007;81(17):8905–18. doi: 10.1128/JVI.00937-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aligo J, Jia S, Manna D, Konan KV. Formation and function of hepatitis C virus replication complexes require residues in the carboxy-terminal domain of NS4B protein. Virology. 2009;393(1):68–83. doi: 10.1016/j.virol.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang J, Huang L, Cordek DG, et al. Hepatitis C virus nonstructural protein 5A: Biochemical characterization of a novel structural class of RNA-binding proteins. J Virol. 2010;84(24):12480–91. doi: 10.1128/JVI.01319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita T, Kaneko S, Shirota Y, Qin W, Nomura T, Kobayashi K, Murakami S. RNA-dependent RNA polymerase activity of the soluble recombinant hepatitis C virus NS5B protein truncated at the C-terminal region. J Biol Chem. 1998;273(25):15479–86. doi: 10.1074/jbc.273.25.15479. [DOI] [PubMed] [Google Scholar]
- 34.Shirota Y, Luo H, Qin W, Kaneko S, Yamashita T, Kobayashi K, Murakami S. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (RdRP) NS5B and modulates RNA-dependent RNA polymerase activity. J Biol Chem. 2002;277(13):11149–55. doi: 10.1074/jbc.M111392200. [DOI] [PubMed] [Google Scholar]
- 35.You S, Rice CM. 3′ RNA elements in hepatitis C virus replication: Kissing partners and long poly(U) J Virol. 2008;82(1):184–95. doi: 10.1128/JVI.01796-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toroney R, Nallagatla SR, Boyer JA, Cameron CE, Bevilacqua PC. Regulation of PKR by HCV IRES RNA: Importance of domain II and NS5A. J Mol Biol. 2010;400(3):393–412. doi: 10.1016/j.jmb.2010.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko T, Tanji Y, Satoh S, Hijikata M, Asabe S, Kimura K, Shimotohno K. Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem Biophys Res Commun. 1994;205(1):320–6. doi: 10.1006/bbrc.1994.2667. [DOI] [PubMed] [Google Scholar]
- 38.Coito C, Diamond DL, Neddermann P, Korth MJ, Katze MG. High-throughput screening of the yeast kinome: Identification of human serine/threonine protein kinases that phosphorylate the hepatitis C virus NS5A protein. J Virol. 2004;78(7):3502–13. doi: 10.1128/JVI.78.7.3502-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintavalle M, Sambucini S, Di Pietro C, De Francesco R, Neddermann P. The alpha isoform of protein kinase CKI is responsible for hepatitis C virus NS5A hyperphosphorylation. J Virol. 2006;80(22):11305–12. doi: 10.1128/JVI.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Lee D, Choe J. Hepatitis C virus NS5A protein is phosphorylated by casein kinase II. Biochem Biophys Res Commun. 1999;257(3):777–81. doi: 10.1006/bbrc.1999.0460. [DOI] [PubMed] [Google Scholar]
- 41.Ide Y, Tanimoto A, Sasaguri Y, Padmanabhan R. Hepatitis C virus NS5A protein is phosphorylated in vitro by a stably bound protein kinase from HeLa cells and by cAMP-dependent protein kinase A-alpha catalytic subunit. Gene. 1997;201(1-2):151–8. doi: 10.1016/s0378-1119(97)00440-x. [DOI] [PubMed] [Google Scholar]
- 42.Chen YC, Su WC, Huang JY, Chao TC, Jeng KS, Machida K, Lai MM. Polo-like kinase 1 is involved in hepatitis C virus replication by hyperphosphorylating NS5A. J Virol. 2010;84(16):7983–93. doi: 10.1128/JVI.00068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed KE, Rice CM. Identification of the major phosphorylation site of the hepatitis C virus H strain NS5A protein as serine 2321. J Biol Chem. 1999;274(39):28011–8. doi: 10.1074/jbc.274.39.28011. [DOI] [PubMed] [Google Scholar]
- 44.Katze MG, Kwieciszewski B, Goodlett DR, Blakely CM, Neddermann P, Tan SL, Aebersold R. Ser(2194) is a highly conserved major phosphorylation site of the hepatitis C virus nonstructural protein NS5A. Virology. 2000;278(2):501–13. doi: 10.1006/viro.2000.0662. [DOI] [PubMed] [Google Scholar]
- 45.Huang L, Sineva EV, Hargittai MR, Sharma SD, Suthar M, Raney KD, Cameron CE. Purification and characterization of hepatitis C virus non-structural protein 5A expressed in Escherichia coli. Protein Expr Purif. 2004;37(1):144–53. doi: 10.1016/j.pep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Appel N, Pietschmann T, Bartenschlager R. Mutational analysis of hepatitis C virus nonstructural protein 5A: Potential role of differential phosphorylation in RNA replication and identification of a genetically flexible domain. J Virol. 2005;79(5):3187–94. doi: 10.1128/JVI.79.5.3187-3194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tellinghuisen TL, Foss KL, Treadaway JC, Rice CM. Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein. J Virol. 2008;82(3):1073–83. doi: 10.1128/JVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordle Gilliver A, Griffin S, Harris M. Identification of a novel phosphorylation site in hepatitis C virus NS5A. J Gen Virol. 2010;91(Pt. 10):2428–32. doi: 10.1099/vir.0.023614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quintavalle M, Sambucini S, Summa V, Orsatti L, Talamo F, De Francesco R, Neddermann P. Hepatitis C virus NS5A is a direct substrate of casein kinase I-alpha, a cellular kinase identified by inhibitor affinity chromatography using specific NS5A hyperphosphorylation inhibitors. J Biol Chem. 2007;282(8):5536–44. doi: 10.1074/jbc.M610486200. [DOI] [PubMed] [Google Scholar]
- 50.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285(5424):110–3. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 51.Lohmann V, Korner F, Dobierzewska A, Bartenschlager R. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J Virol. 2001;75(3):1437–49. doi: 10.1128/JVI.75.3.1437-1449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colonno R, Lemm J, O’Boyle D, et al. Bristol-Myers Squibb Co. Preparation of iminothiazolidinone amino acid derivatives as combination pharmaceutical agents for use as inhibitors of HCV replication. WO 2004014313
- 53.Romine JL, St. Laurent DR, Leet JE, et al. Inhibitors of HCV NS5A: From iminothiazolidinones to symmetrical stilbenes. ACS Med Chem Lett. 2011;2(3):224–9. doi: 10.1021/ml1002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitz U, Tan SL. NS5A—From obscurity to new target for HCV therapy. Recent Pat Antiinfect Drug Discov. 2008;3(2):77–92. doi: 10.2174/157489108784746597. [DOI] [PubMed] [Google Scholar]
- 55.Tiberghien N, Lumley J, Reynolds K, et al. Arrow Therapeutics, Ltd. Pyrazolopyrimidines as anti-hepatitis C agents, their preparation, pharmaceutical compositions and use. WO 2005047288
- 56.Dennison H, Mathews N, Barnes M, Chana S, Arrow Therapeutics, Ltd. Morpholinylanilinoquinazoline derivatives for use as antiviral agents. WO 2005105761
- 57.Li G, Fathi R, Yang Z, et al. XTL Biopharmaceuticals, Inc. Preparation of aryl substituted pyrazoles, especially N-[4-[[1H-pyrazol-4-yl]ethynyl]phenyl]dipeptide derivatives, as HCV antiviral agents and their use in the treatment of hepatitis C infection. WO 2008048589
- 58.Conte I, Giuliano C, Ercolani C, et al. Synthesis and SAR of piperazinyl-N-phenylbenzamides as inhibitors of hepatitis C virus RNA replication in cell culture. Bioorg Med Chem Lett. 2009;19(6):1779–83. doi: 10.1016/j.bmcl.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz FU, Roberts CD, Abadi ADM, Griffith RC, Leivers MR, Slobodov I, Rai R, Genelabs Technologies, Inc. N-Heterocyclylpyrrolidine carboxamide derivatives, processes for preparing them, pharmaceutical compositions containing them, and their use as antiviral agents. WO 2007070600
- 60.Schmitz FU, Rai R, Griffith R, Roberts CD, GlaxoSmithKline, Inc. Preparation of N-heterocyclylpyrrolidinecarboxamides and related compounds, pharmaceutical compositions containing them, and their use as antiviral agents. WO 2010027564
- 61.Leivers MR, Schmitz FU, Griffith RC, et al. Genelabs Technologies, Inc. Preparation of thiazolidine derivatives as antiviral agents. WO 2008064218
- 62.Schmitz FU, Rai R, Roberts CD, Kazmierski W, Grimes R, GlaxoSmithKline, Inc. Preparation of biphenyls and biheteroaryls endcapped with amino acid or peptide derivatives as antivirals for treating Flaviviridae family virus infection. WO 2010062821
- 63.Chen P, Couch R, Duan M, et al. GlaxoSmithKline, Inc. Preparation of biphenyls end capped with amino acids or peptide derivatives for treating HCV infection. WO 2011028596
- 64.Baskaran S, Dickerson SH, Duan M, Kazmierski WM, McFadyen RB, GlaxoSmithKline, Inc. Preparation of naphthalenylene, quinolinylene and isoquinolinylene linked benzimidazole-imidazoles and analogs end capped with peptide derivatives for treating HCV infection. WO 2011091446
- 65.Baskaran S, Botyanszki J, Cooper JP, Duan M, Kazmierski WM, McFadyen RB, Redman A, GlaxoSmithKline, Inc. Preparation of phenylene, pyridinylene, furanylene and thiofuranylene linked benzimidazoles end derivatives end capped with peptide derivatives for treating HCV infection. WO 2011050146
- 66.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465(7294):96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maynard AT, et al. A full-length homology model of HCV NS5A domain 1. To be published. [Google Scholar]
- 68.Penin F, Brass V, Appel N, et al. Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J Biol Chem. 2004;279(39):40835–43. doi: 10.1074/jbc.M404761200. [DOI] [PubMed] [Google Scholar]
- 69.Lemm JA, O’Boyle D, Liu M, et al. Identification of hepatitis C virus NS5A inhibitors. J Virol. 2010;84(1):482–91. doi: 10.1128/JVI.01360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nettles RE, Seveksy H, Chung E, et al. BMS-790052, a first-in-class potent hepatitis C virus NS5A inhibitor, demonstrates multiple-dose proof-of-concept in subjects with chronic gt1 HCV infection; 61st Annu Meet Am Assoc Study Liver Dis (Oct 29-Nov 2, Boston); 2010; Abst 1881. [Google Scholar]
- 71.Pol S, Ghaliv RH, Rustgi VK, et al. First report of SVR12 for a NS5A replication complex inhibitor, BMS-790052 in combination with PED-IFN-2A and RBV: Phase 2a trial in treatment naïve HCV genotype 1 infected subjects; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1373. [Google Scholar]
- 72.Lok A, Gardiner D, Lawitz E, et al. Quadruple therapy with BMS-790052, BMS-650032, and PEG-IFN/RBV for 24 weeks results in 100% SVR12 in HCV genotype 1 null responders; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1356. [Google Scholar]
- 73.McPhee F, Hernandez D, Yu F, et al. Characterization of virologic escape in HCV genotype 1 null responders receiving a combination of the NS3 protease inhibitor BMS-650032 and NS5A inhibitor BMS-790052; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 63. [Google Scholar]
- 74.Fridell RA, Qiu D, Wang C, Valera L, Gao M. Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system. Antimicrob Agents Chemother. 2011;54(9):3641–50. doi: 10.1128/AAC.00556-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Targett-Adams P, Graham EJ, Middleton J, et al. Small molecules targeting hepatitis C virus-encoded NS5A cause subcellular redistribution of their target: Insights into compound mode of action. J Virol. 2011;85(13):6353–68. doi: 10.1128/JVI.00215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee C, Ma H, Hang JQ, et al. The hepatitis C virus NS5A inhibitor (BMS-790052) alters the subcellular localization of the NS5A non-structural viral protein. Virology. 2011;414(1):10–8. doi: 10.1016/j.virol.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Link J, Bannister R, Beilke L, et al. Nonclinical profile and phase I results in healthy volunteers of the novel and potent HCV NS5A inhibitor GS-5885; 61st Annu Meet Am Assoc Study Liver Dis (Oct 29-Nov 2, Boston); 2010; Abst 1883. [Google Scholar]
- 78.Lawitz E, Gruener D, Hill J, et al. Three-day, dose-ranging study of the HCV NS5A inhibitor GS-5885; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1219. [Google Scholar]
- 79.Colonno R, Huang Q, Huang N, et al. Comprehensive analysis shows conserved pathways to resistance across all major HCV genotypes for a panel of NS5A inhibitors; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1199. [Google Scholar]
- 80.Bechtel J, Crosby R, Wang A, et al. In vitro profiling of GSK2336805, a potent and selective inhibitor of HCV NS5A; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 764. [Google Scholar]
- 81.Scheel TKH, Gottwein JM, Mikkelsen LS, Jensen TB, Bukh J. Recombinant HCV variant with NS5A from genotypes 1-7 have different sensitivities to an NS5A inhibitor but not interferon-alpha. Gastroenterology. 2011;140(3):1032–42. doi: 10.1053/j.gastro.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 82.Huang M, Yang G, Patel D, et al. ACH-2928: A novel highly potent HCV NS5A inhibitor with favorable preclinical characteristics; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1212. [Google Scholar]
- 83.Cousson CB, Chapron C, Standring D, et al. Idenix NS5A HCV replication inhibitors with low picomolar, pan-genotypic in vitro antiviral activity; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 815. [Google Scholar]
- 84.Nicholas JB, Buckman BO, Serebryany V, et al. Characterization of novel, highly potent NS5A inhibitors with QD dosing potential and robust activity in an HCV chimeric animal model; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1228. [Google Scholar]
- 85.Jiang LJ, Liu S, Phan T, et al. A highly potent HCV NS5A inhibitor EDP-239 with favorable preclinical pharmacokinetics; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 1213. [Google Scholar]
- 86.Gao M, Fridell R, Wang C, et al. BMS-766, a novel HCV NS5A inhibitor with enhanced resistance coverage; Int Liver Congr (March 30-April 3, Berlin); 2011; Abst 787. [Google Scholar]
- 87.Taguchi T, Nagano-Fujii M, Akutsu M, Kadoya H, Ohgimoto S, Ishido S, Hotta H. Hepatitis C virus NS5A protein interacts with 2′,5′-oligoadenylate synthetase and inhibits antiviral activity of IFN in an IFN sensitivity-determining region-independent manner. J Gen Virol. 2004;85(Pt. 4):959–69. doi: 10.1099/vir.0.19513-0. [DOI] [PubMed] [Google Scholar]
- 88.Zech B, Kurtenbach A, Krieger N, et al. Identification and characterization of amphiphysin II as a novel cellular interaction partner of the hepatitis C virus NS5A protein. J Gen Virol. 2003;84(Pt. 3):555–60. doi: 10.1099/vir.0.18801-0. [DOI] [PubMed] [Google Scholar]
- 89.Masumi A, Aizaki H, Suzuki T, DuHadaway JB, Prendergast GC, Komuro K, Fukazawa H. Reduction of hepatitis C virus NS5A phosphorylation through its interaction with amphiphysin II. Biochem Biophys Res Commun. 2005;336(2):572–8. doi: 10.1016/j.bbrc.2005.08.142. [DOI] [PubMed] [Google Scholar]
- 90.Backes P, Quinkert D, Reiss S, et al. Role of annexin A2 in the production of infectious hepatitis C virus particles. J Virol. 2010;84(11):5775–89. doi: 10.1128/JVI.02343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cun W, Jiang J, Luo G. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol. 2010;84(21):11532–41. doi: 10.1128/JVI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung YL, Sheu ML, Yen SH. Hepatitis C virus NS5A as a potential viral Bcl-2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int J Cancer. 2003;107(1):65–73. doi: 10.1002/ijc.11303. [DOI] [PubMed] [Google Scholar]
- 93.Milward A, Mankouri J, Harris M. Hepatitis C virus NS5A protein interacts with beta-catenin and stimulates its transcriptional activity in a phosphoinositide-3 kinase-dependent fashion. J Gen Virol. 2010;91(Pt. 2):373–81. doi: 10.1099/vir.0.015305-0. [DOI] [PubMed] [Google Scholar]
- 94.Nanda SK, Herion D, Liang TJ. The SH3 binding motif of HCV [corrected] NS5A protein interacts with Bin1 and is important for apoptosis and infectivity. Gastroenterology. 2006;130(3):794–809. doi: 10.1053/j.gastro.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 95.Arima N, Kao CY, Licht T, Padmanabhan R, Sasaguri Y. Modulation of cell growth by the hepatitis C virus nonstructural protein NS5A. J Biol Chem. 2001;276(16):12675–84. doi: 10.1074/jbc.M008329200. [DOI] [PubMed] [Google Scholar]
- 96.Hanoulle X, Badillo A, Wieruszeski JM, et al. Hepatitis C virus NS5A protein is a substrate for the peptidyl-prolyl cis/trans isomerase activity of cyclophilins A and B. J Biol Chem. 2009;284(20):13589–601. doi: 10.1074/jbc.M809244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Tong W, Zhang X, et al. Hepatitis C virus non-structural protein NS5A interacts with FKBP38 and inhibits apoptosis in Huh7 hepatoma cells. FEBS Lett. 2006;580(18):4392–400. doi: 10.1016/j.febslet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Peng L, Liang D, Tong W, Li J, Yuan Z. Hepatitis C virus NS5A activates the mammalian target of rapamycin (mTOR) pathway, contributing to cell survival by disrupting the interaction between FK506-binding protein 38 (FKBP38) and mTOR. J Biol Chem. 2010;285(27):20870–81. doi: 10.1074/jbc.M110.112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okamoto T, Nishimura Y, Ichimura T, et al. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J. 2006;25(20):5015–25. doi: 10.1038/sj.emboj.7601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macdonald A, Crowder K, Street A, McCormick C, Harris M. The hepatitis C virus NS5A protein binds to members of the Src family of tyrosine kinases and regulates kinase activity. J Gen Virol. 2004;85(Pt. 3):721–9. doi: 10.1099/vir.0.19691-0. [DOI] [PubMed] [Google Scholar]
- 101.Tan SL, Nakao H, He Y, et al. NS5A, a nonstructural protein of hepatitis C virus, binds growth factor receptor-bound protein 2 adaptor protein in a Src homology 3 domain/ligand-dependent manner and perturbs mitogenic signaling. Proc Natl Acad Sci U S A. 1999;96(10):5533–8. doi: 10.1073/pnas.96.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taguwa S, Okamoto T, Abe T, Mori Y, Suzuki T, Moriishi K, Matsuura Y. Human butyrate-induced transcript 1 interacts with hepatitis C virus NS5A and regulates viral replication. J Virol. 2008;82(6):2631–41. doi: 10.1128/JVI.02153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi YW, Tan YJ, Lim SG, Hong W, Goh PY. Proteomic approach identifies HSP27 as an interacting partner of the hepatitis C virus NS5A protein. Biochem Biophys Res Commun. 2004;318(2):514–9. doi: 10.1016/j.bbrc.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 104.Chen YJ, Chen YH, Chow LP, et al. Heat shock protein 72 is associated with the hepatitis C virus replicase complex and enhances viral RNA replication. J Biol Chem. 2010;285(36):28183–90. doi: 10.1074/jbc.M110.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lan KH, Sheu ML, Hwang SJ, et al. HCV NS5A interacts with p53 and inhibits p53-mediated apoptosis. Oncogene. 2002;21(31):4801–11. doi: 10.1038/sj.onc.1205589. [DOI] [PubMed] [Google Scholar]
- 106.Tu H, Gao L, Shi ST, et al. Hepatitis C virus RNA polymerase and NS5A complex with a SNARE-like protein. Virology. 1999;263:30–41. doi: 10.1006/viro.1999.9893. [DOI] [PubMed] [Google Scholar]
- 107.Evans MJ, Rice CM, Goff SP. Phosphorylation of hepatitis C virus non-structural protein 5A modulates its protein interactions and viral RNA replication. Proc Natl Acad Sci U S A. 2004;101(35):13038–43. doi: 10.1073/pnas.0405152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamamoto I, Nishimura Y, Okamoto T, et al. Human VAP-B is involved in hepatitis C virus replication through interaction with NS5A and NS5B. J Virol. 2005;79(21):13473–82. doi: 10.1128/JVI.79.21.13473-13482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim MJ, Yoo JY. Inhibition of hepatitis C virus replication by IFN-mediated ISGylation of HCV-NS5A. J Immunol. 2010;185(7):4311–8. doi: 10.4049/jimmunol.1000098. [DOI] [PubMed] [Google Scholar]
- 110.Chung KM, Lee J, Kim JE, et al. Nonstructural protein 5A of hepatitis C virus inhibits the function of karyopherin beta3. J Virol. 2000;74(11):5233–41. doi: 10.1128/jvi.74.11.5233-5241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Houshmand H, Bergqvist A. Interaction of hepatitis C virus NS5A with La protein revealed by T7 phage display. Biochem Biophys Res Commun. 2003;309(3):695–701. doi: 10.1016/j.bbrc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 112.Abe T, Kaname Y, Hamamoto I, et al. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81(17):8953–66. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amako Y, Sarkeshik A, Hotta H, Yates J, Siddiqui A. Role of oxysterol binding protein in hepatitis C virus infection. J Virol. 2009;83(18):9237–46. doi: 10.1128/JVI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Majumder M, Ghosh AK, Steele R, Ray R, Ray RB. Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J Virol. 2001;75(3):1401–7. doi: 10.1128/JVI.75.3.1401-1407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Street A, Macdonald A, Crowder K, Harris M. The hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279(13):12232–41. doi: 10.1074/jbc.M312245200. [DOI] [PubMed] [Google Scholar]
- 116.Reiss S, Rebhan I, Backes P, et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9(1):32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gale MJ, Jr., Korth MJ, Tang NM, et al. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230(2):217–27. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 118.Ghosh AK, Majumder M, Steele R, Ray R, Ray RB. Modulation of interferon expression by hepatitis C virus NS5A protein and human homeodomain protein PTX1. Virology. 2003;306(1):51–9. doi: 10.1016/s0042-6822(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 119.Burckstummer T, Kriegs M, Lupberger J, Pauli EK, Schmittel S, Hildt E. Raf-1 kinase associates with hepatitis C virus NS5A and regulates viral replication. FEBS Lett. 2006;580(2):575–80. doi: 10.1016/j.febslet.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 120.Ghosh AK, Majumder M, Steele R, Yaciuk P, Chrivia J, Ray R, Ray RB. Hepatitis C virus NS5A protein modulates transcription through a novel cellular transcription factor SRCAP. J Biol Chem. 2000;275(10):7184–8. doi: 10.1074/jbc.275.10.7184. [DOI] [PubMed] [Google Scholar]
- 121.Lan KH, Lan KL, Lee WP, et al. HCV NS5A inhibits interferon-alpha signaling through suppression of STAT1 phosphorylation in hepatocytederived cell lines. J Hepatol. 2007;46(5):759–67. doi: 10.1016/j.jhep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 122.Inubushi S, Nagano-Fujii M, Kitayama K, et al. Hepatitis C virus NS5A protein interacts with and negatively regulates the non-receptor protein tyrosine kinase Syk. J Gen Virol. 2008;89(Pt. 5):1231–42. doi: 10.1099/vir.0.83510-0. [DOI] [PubMed] [Google Scholar]
- 123.Sklan EH, Staschke K, Oakes TM, et al. A Rab-GAP TBC domain protein binds hepatitis C virus NS5A and mediates viral replication. J Virol. 2007;81(20):11096–105. doi: 10.1128/JVI.01249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qadri I, Iwahashi M, Simon F. Hepatitis C virus NS5A protein binds TBP and p53, inhibiting their DNA binding and p53 interactions with TBP and ERCC3. Biochim Biophys Acta. 2002;1592(2):193–204. doi: 10.1016/s0167-4889(02)00315-4. [DOI] [PubMed] [Google Scholar]
- 125.Majumder M, Ghosh AK, Steele R, Zhou XY, Phillips NJ, Ray R, Ray RB. Hepatitis C virus NS5A protein impairs TNF-mediated hepatic apoptosis, but not by an anti-FAS antibody, in transgenic mice. Virology. 2002;294(1):94–105. doi: 10.1006/viro.2001.1309. [DOI] [PubMed] [Google Scholar]
- 126.Park KJ, Choi SH, Lee SY, Hwang SB, Lai MM. Nonstructural 5A protein of hepatitis C virus modulates tumor necrosis factor alpha-stimulated nuclear factor kappa B activation. J Biol Chem. 2002;277(15):13122–8. doi: 10.1074/jbc.M111599200. [DOI] [PubMed] [Google Scholar]
- 127.Choi SH, Hwang SB. Modulation of the transforming growth factorbeta signal transduction pathway by hepatitis C virus nonstructural 5A protein. J Biol Chem. 2006;281(11):7468–78. doi: 10.1074/jbc.M512438200. [DOI] [PubMed] [Google Scholar]