Abstract
We investigated the relationship between epicuticular and internal hydrocarbons in the adult house fly, Musca domestica and the distribution of hydrocarbons, including the female sex pheromone component, (Z)-9-tricosene, in tissues. Internal hydrocarbons increased dramatically in relation to sexual maturation and were found in the hemolymph, ovaries, digestive tract, and fat body. (Z)-9-Tricosene comprised a relatively large fraction of the hydrocarbons in the female carcass and hemolymph, and less so in other tissues, while other hydrocarbons were represented in greater amounts in the ovaries than in other tissues. It therefore appears that certain hydrocarbons were selectively provisioned to certain tissues such as the ovaries, from which pheromone was relatively excluded. Both KBr gradient ultracentrifugation and specific immunoprecipitation indicated that > 90% of hemolymph hydrocarbons were associated with a high-density lipophorin (density = 1.09 g ml−1), composed of two apoproteins under denaturing conditions, apolipophorin I (∼240 kD) and apolipophorin II (∼85 kD). Our results support a predicted model (Chino, 1985) that lipophorin is involved in the transport of sex pheromone in M. domestica. In addition to delivering hydrocarbons and sex pheromones to the cuticular surface, we suggest that lipophorin may play an important role in an active mechanism that selectively deposits certain subsets of hydrocarbons at specific tissues.
Keywords: Musca domestica, house fly, sex pheromone, hydrocarbon, lipophorin, wax transport
Introduction
Mate recognition in the house fly, Musca domestica, is guided by visual and chemical cues (Adams et al., 1995). Close-range sexual recognition is mediated primarily by sex-specific chemical signals. The female produces (Z)-9-tricosene (muscalure, Z9-C23:1) as the main sex pheromone component (Carlson et al., 1971), whereas the epicuticle of adult males contains large amounts of (Z)-9-heptacosene (Uebel et al., 1976). These compounds are only part of a complex chemical profile that includes many other cuticular lipids (Mpuru et al., 2001). For example, (Z)-14-tricosene-10-one, (Z)-9,10-epoxytricosane, and a series of methyl branched alkanes with chain lengths from 28 to 30 appear to facilitate the sexual responses of males to Z9-C23:1 (Uebel et al., 1976, 1978; Adams and Holt, 1987; Adams et al., 1995).
The epicuticular hydrocarbons of the house fly, including sex pheromone components, are synthesized in the abdominal integument (Dillwith et al., 1981; Dillwith and Blomquist, 1982). Microsomal preparations from the abdominal integument synthesize hydrocarbons whereas preparations from the fat body do not (Blomquist et al., 1993). In his review of lipid transport by lipophorin, Chino (1985) predicted that the house fly pheromone, being a component of the epicuticular hydrocarbons, might be transported by hemolymph lipophorin. Indeed, several studies have reported large amounts of “internal” hydrocarbons in surface-extracted flies (Ahmad et al., 1989; Mpuru et al., 2001). Yet, the tissues with which internal hydrocarbons associate remained unknown.
In cockroaches, locusts, termites, beetles, and fruit flies most hemolymph hydrocarbons are carried by lipophorin (Chino and Kitazawa, 1981; Katase and Chino, 1982, 1984; Katagiri and de Kort, 1991; Gu et al., 1995; Pho et al., 1996; Young et al., 1999; Sevala et al., 1999, 2000). But the transport of hydrocarbon pheromones through the hemolymph is less well documented. In the female German cockroach, Blattella germanica, long-chain methyl ketone derivatives of cuticular hydrocarbons serve as sex pheromone components (Nishida and Fukami, 1983; Schal et al., 1990; Chase et al., 1992). The cuticular hydrocarbons and pheromones are produced by abdominal oenocytes and are shuttled through the hemolymph by high-density lipophorin (Gu et al., 1995; Schal et al., 1998b; Sevala et al., 1999). Likewise, a heptacosadiene female sex pheromone is transported by lipophorin in Drosophila melanogaster (Pho et al., 1996). Short-chain volatile hydrocarbon pheromone components are also transported by high-density lipophorin in the tiger moth, Holomelina aurantiaca (Schal et al., 1998a).
Here we report on the relationship between epicuticular and internal hydrocarbons in the house fly, examine the distribution of hydrocarbons in internal tissues, and validate Chino's (1985) prediction that lipophorin is involved in the transport of sex pheromone in the house fly.
Methods and Materials
Insects
M. domestica pupae of the Fales 1958 T-II strain were shipped overnight from S. C. Johnson Wax (Racine, WI). Pupae and adults were maintained at 27 ± 0.5°C and a 12:12 light:dark photoperiodic regime. Newly eclosed adults were separated daily (within 12 h of eclosion) under cold anesthesia and maintained separately by sex and age in moist cages with access to water, sucrose, and condensed milk.
Chemicals
All chemicals were purchased from Sigma (St. Louis, MO). Precast gels and molecular weight markers were obtained from the Bio-Rad (Richmond, CA). Pansorbin Cells used in immunoprecipitation were obtained from Calbiochem (San Diego, CA).
Collection of hemolymph
Hemolymph was obtained from adult males and females by puncturing the cervical region with a fine needle and collecting the hemolymph in a drop of cold saline containing protease inhibitors (0.05 M sodium phosphate buffer, pH 7.0, containing 0.15 M NaCl, 10 mM ethylenediamide tetra acetic acid, 5 mM glutathione, 2 mM phenylmethyl sulfonyl fluoride, leupeptin [10 µg ml−1], and pepstatin [10 µg ml−1]). Hemolymph was immediately collected into a chilled microcentrifuge tube and centrifuged at 735g for 10 min at 4°C to remove hemocytes; the supernatant was stored at −80°C.
Fractionation of hemolymph proteins
Male and female plasma proteins were fractionated by the method of Shapiro et al. (1984) with minor modifications (Sevala et al., 1997). Briefly, plasma in saline was mixed with 2.57 g of KBr and adjusted to a final volume of 5.8 ml, transferred to a Beckman Quickseal tube (Fullerton, CA) and overlaid with 0.9% NaCl. Tubes were sealed and centrifuged with slow acceleration and deceleration at 285,000g in a Beckman L8-70M ultracentrifuge with a 70.1 Ti fixed angle rotor for 22 h at 4°C. Fractions (400 µl) were collected from the top of the tube and the absorbance was measured at 280 and 455 nm in a Beckman DV-64 spectrophotometer. The density of each fraction was determined gravimetrically. The purity of each fraction was checked by 4 – 20% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (1970). Gels were stained with Coomassie brilliant blue G-250 to detect proteins.
Lipophorin immunoprecipitation
Polyclonal antibodies against larval M. domestica lipophorin, raised in rabbits by Bianchi et al. (1987), were used in the present study. Hemolymph was collected from 6-day-old female flies, the hemocytes were removed (735g for 10 min), and lipophorin was immunoprecipitated by addition of excess anti-lipophorin rabbit serum followed by Pansorbin cells. Lipophorin was pelleted (10,000g for 10 min) and lipids were extracted and hydrocarbons purified from the supernatant using the modified Bligh and Dyer (1959) procedure, as detailed below.
Quantification of hydrocarbons by gas chromatography (GC)
Two procedures were used, one for qualitative GC-MS analysis of hydrocarbons of 0 – 6-day-old females and another for quantitative GC analyses. The GC-MS procedures were as described in Mpuru et al. (2001). External (epicuticular) lipids were extracted from groups of 5 females with three washes of 1 ml hexane. The resulting extracts were pooled, and concentrated to 1 ml under a gentle stream of N2, and 400 ng of n-nonadecane (n-C19) was added as internal standard.
For extraction of internal lipids, females that had already been washed with hexane to remove external hydrocarbons, were immersed in 3 ml of chloroform/methanol (1:2 v/v) for 1 h in an ultrasonic bath with ice, before extracting the internal lipids by the procedure of Bligh and Dyer (1959). The chloroform layer was evaporated under a gentle stream of N2, the lipids taken up in 1 ml hexane, and 400 ng of n-C19 was added as an internal standard. Prior to GC-MS analyses, the 5 replicates for each age were pooled.
Analyses were conducted by GC-MS on a HP5890 GC Series II (Hewlett-Packard, Palo Alto, CA) equipped with a CP-Sil-5CB WCOT apolar capillary column (Chrompack, 25 m, 0.25 mm ID) using a splitless injector at 280°C. Oven temperature was programmed from 70 to 150°C at 30°C/min and from 150 to 320°C at 5°C/min, and held at 320°C for 5 min. The carrier gas was helium. The interface to the mass spectrometer, a HP 5989A MS Engine, and a HPUX Chemsystem control unit, was maintained at 300°C. The MS was operated in the electron impact mode at 70 eV and scanned from m/z 35 to 600 with about one scan per sec, with source temperature held at 240°C and quadrupole at 100°C.
For quantitative analyses, the epicuticular hydrocarbons of individual 0 – 6-day-old male and female flies were extracted for 5 min in 1 ml hexane containing 1 µg 1-docosene as an internal standard, followed by a 5 min rinse in clean hexane and a third hexane dip. After removal of surface hydrocarbons, whole flies were macerated with scissors and homogenized by ultrasonication for 30 sec (Kontes micro ultrasonic cell disrupter, Vineland, NJ) and internal hydrocarbons extracted by a modification of the Bligh and Dyer (1959) procedure, replacing chloroform with hexane. Hemolymph and KBr fractions were similarly extracted. The hexane fraction, containing hydrocarbons, was loaded onto silicic acid mini-columns made in Pasteur pipets (particle size 100–200; Selecto Scientific, Norcross, GA). Hydrocarbons were eluted with 7 ml hexane and the solvent evaporated in conical vials with a slow stream of high purity N2. Samples (1–2 µl) were injected splitless into a 25 m × 0.32 mm × 1 µm HP-1 capillary column in a HP5890II GC equipped with a flame-ionization detector and interfaced with a HP3365II ChemStation. The column was operated at 150°C for 2 min, the purge valve opened, and oven temperature increased at 10°C min−1 to 300°C and held for 10 min. Helium was used as the carrier at a flow rate of 30 cm sec−1 and the injector and detector were held at 300°C and 320°C, respectively.
Tissue distribution of hydrocarbons
A fresh group of 6-day-old female flies was extracted with hexane to remove epicuticular lipids. Individual flies were processed as follows in 1 ml PBS: The ovaries were excised, and adhering fat body and tracheae carefully removed. The fat body and digestive tract were similarly removed and each tissue, as well as the remaining carcass, was separately washed 3X in 0.5 ml PBS and then homogenized (Polytron, Brinkmann, Westbury, NY), extracted, and the purified hydrocarbon fractions were quantified by GC. The hemolymph constituted the saline in which dissections were conducted plus the washes of all internal tissues. In males, we were concerned with the distribution of hydrocarbons in the hemolymph. Thus, the fat body, digestive tract, and reproductive system were washed and discarded, and the hemolymph and the rest of the body (carcass) were homogenized and extracted, as above.
Statistics
Comparisons of the relative amounts of various compounds in different tissues were conducted with analysis of variance on arcsine square-root transformed percentages at α = 0.05. Multiple comparisons of means were conducted with Fisher's least significant difference test (StatView 5.0, SAS Institute, Cary, NC). All means are presented with standard error of the mean (SEM).
Results
Cuticular hydrocarbons and their relative amounts
The cuticular lipids of M. domestica are characterized by a marked sexual dimorphism (Nelson et al., 1981; Mpuru et al., 2001). Newly eclosed females emerged with only 2.3 ± 0.2 ng (n = 5) Z9-C23:1 on their cuticular surface. The amount of Z9-C23:1 on the female's epicuticle increased significantly from 0.05% of the total hydrocarbons soon after the imaginal molt to 16.9 ± 2.0% on day 6 (Figures 1, 3A; Table 1), as previously shown (Ahmad et al., 1989; Mpuru et al., 2001). Most other hydrocarbon constituents, as well as the total hydrocarbons on the cuticle increased significantly as the female matured sexually (Table 1). Z9-C27:1, on the other hand, constituted 23.2 ± 2.0% of all epicuticular hydrocarbons on day 2, but declined dramatically to only 5.4 ± 2.5% of the external hydrocarbons by day 6.
Figure 1.
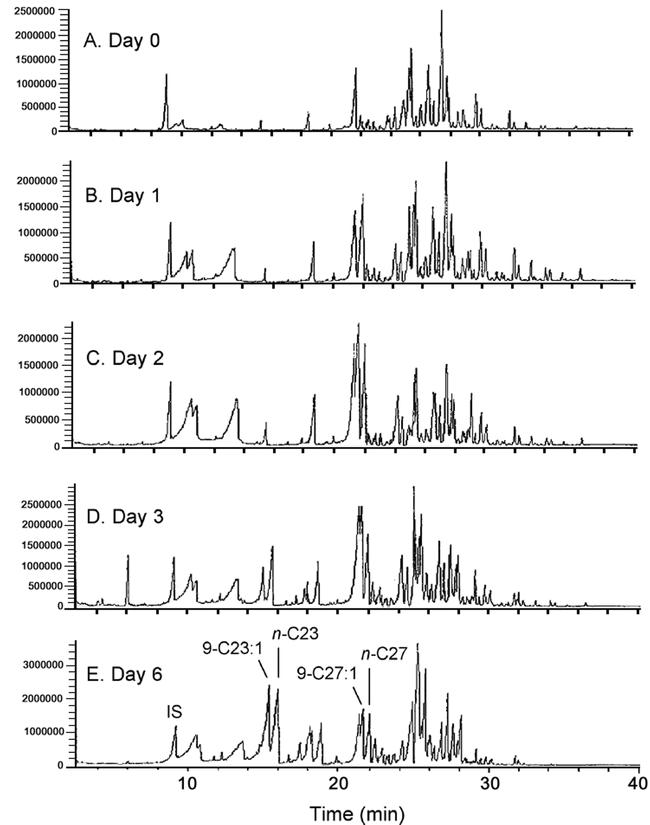
Total ion gas chromatograms of hydrocarbons extracted from the epicuticle of Musca domestica females of various ages. The external (epicuticular) lipids were extracted from 5 groups of 5 females each and 400 ng of n-nonadecane (n-C19) was added as internal standard to each. Prior to GC-MS analysis the 5 groups were pooled.
Figure 3.
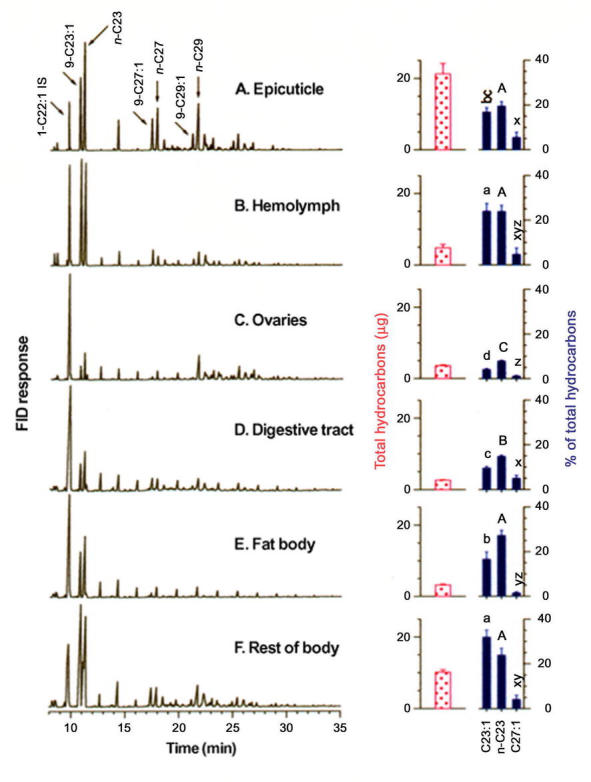
Gas chromatograms (FID) of hydrocarbons extracted from the epicuticle and various tissues of 6-day-old Musca domestica females. In each chromatogram 1 µg 1-C22:1 represents an internal standard and each chromatogram is scaled to its tallest peak. Above the bars on the right, different letters represent statistically significant differences (P < 0.05) in an analysis of variance followed by LSD of arcsine square-root transformed percentages. Lower case letters a–d represent an ANOVA of Z9-C23:1 in different tissues, capital letters A–C an ANOVA of n-C23 in different tissues, and x–z an ANOVA of Z9-C27:1 in various tissues. n = 5 males or females per mean. Bars represent mean ± SEM.
Table 1.
Amount of hydrocarbons recovered from various tissues of 6-day-old Musca domestica females and males
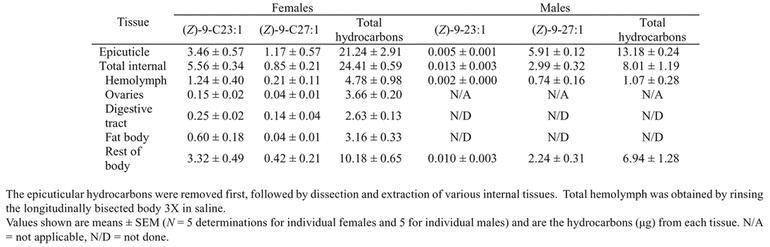
The converse was the case in adult males: While Z9-C23:1 was barely detectable on the epicuticle of 6-day-old males (< 5 ng, 0.03% of the total hydrocarbons), the C27 homolog increased to 5.9 ± 0.1 µg, representing 44.8 ± 0.3% of the total cuticular hydrocarbons.
Internal hydrocarbons and their tissue distribution
As flies matured, the amounts of internal hydrocarbons in both females and males increased dramatically. On day 6, flies contained as much internal as external hydrocarbons, and in females the amount of Z9-C23:1 was approximately similar internally and externally. Other hydrocarbons, however, were significantly under-represented in internal extractions (Figures 2, 3).
Figure 2.
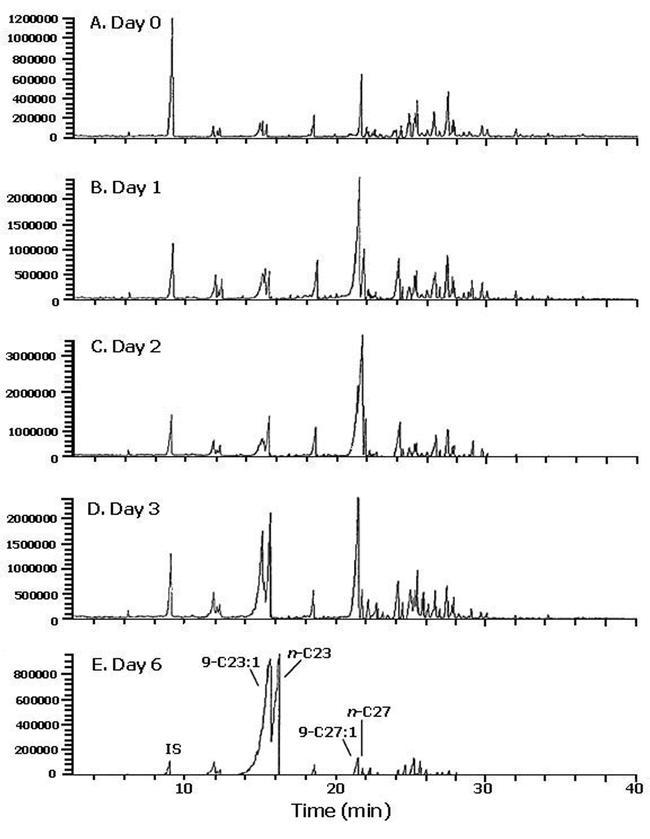
Total ion gas chromatograms of the internal hydrocarbons extracted from Musca domestica females of various ages. For extraction of internal lipids, insects that had already been washed with hexane to remove external hydrocarbons, were immersed in 3 ml of chloroform/methanol (1:2 v/v) for 1 hour in an ultrasonic bath with ice, before extracting the internal lipids by the procedure of Bligh and Dyer (1959). The chloroform layer was evaporated under a gentle stream of nitrogen and the lipids taken up in 1 ml hexane to which 400 ng of n-C19 was added as an internal standard. Prior to GC-MS analysis the 5 samples from each age were pooled.
We aimed to determine which internal tissues contained hydrocarbons. As a preliminary investigation, after extracting the epicuticle with hexane, the head/thorax and abdomen of females were extracted separately. Surprisingly, the head and thorax contained 40.8 ± 1.8% (n = 5) total internal hydrocarbons, and 38.6 ± 3.4% of the internal Z9-C23:1, suggesting an association of hydrocarbons with either fat body or hemolymph, or both. In another set of 6-day-old females, the ventral sclerites of the abdomen were longitudinally sectioned and the internal tissues washed extensively with phosphate buffered saline. The resulting pooled saline was centrifuged and the supernatant contained most of the hemolymph. The ovaries were then removed, as were the fat body and digestive tract, and hydrocarbons were then extracted from these tissues and from the rest of the body (carcass). The hemolymph contained 1.24 µg of Z9-C23:1, representing 24.0% of the 4.78 µg total hydrocarbons in the hemolymph (Figure 3B, Table 1). Hydrocarbons were also recovered from the ovaries, but interestingly, Z9-C23:1 represented only 4.1 ± 0.4% of ovarian hydrocarbons compared with 9.6, 16.7, 16.9, 24.0, and 32.0% in the digestive tract, fat body, epicuticle, hemolymph, and the rest of the carcass, respectively (Figure 3, Table 1). Other hydrocarbons were therefore represented in greater proportion in the ovaries than in other tissues. Z9-C23:1 comprised a relatively large fraction of the hydrocarbons in the female carcass and hemolymph, and less so in other tissues, including the epicuticle (Figures 1, 3). It therefore appears that hydrocarbons were selectively provisioned to certain tissues such as the ovaries, from which pheromone was relatively excluded.
In adult males, Z9-27:1 represented a major hydrocarbon (44.8, 74.3, and 35.1% in the epicuticle, hemolymph, and carcass, respectively) and only negligible amounts of Z9-23:1 were found in both external and internal tissues. As in females, the hemolymph contained 13.3% of the total internal hydrocarbons, while hemolymph Z9-27:1 comprised 24.8% of the total internal Z9-27:1 (Table 1). We obtained similar results when males were lightly homogenized in 3 ml saline with only 3 strokes of a pestle in a glass homogenizer, and centrifuged. The supernatant, which included the hemolymph, contained 11.6 ± 1.0% (n = 5 determinations of 5 males each) of the internal hydrocarbons while the pellet contained the remaining 88.4 ± 1.0%.
Association of hemolymph hydrocarbons with lipoproteins
High density lipophorin has been implicated in shuttling hydrocarbons through insect hemolymph (Chino, 1985; Schal et al., 1998a, b). Therefore, we took two approaches in our efforts to localize hemolymph hydrocarbons to specific carrier proteins: Immunoprecipitation with lipophorin antibodies, and fractionation of hemolymph proteins by KBr gradient ultracentrifugation. The immunoprecipitate of male plasma contained 98.9% of hemolymph Z9-27:1. In female plasma, 93.6% of hemolymph Z9-23:1 was immunoprecipitated and therefore, lipophorin-bound. The precipitated hydrocarbons were qualitatively identical to the hemolymph hydrocarbons (Figure 4).
Figure 4.
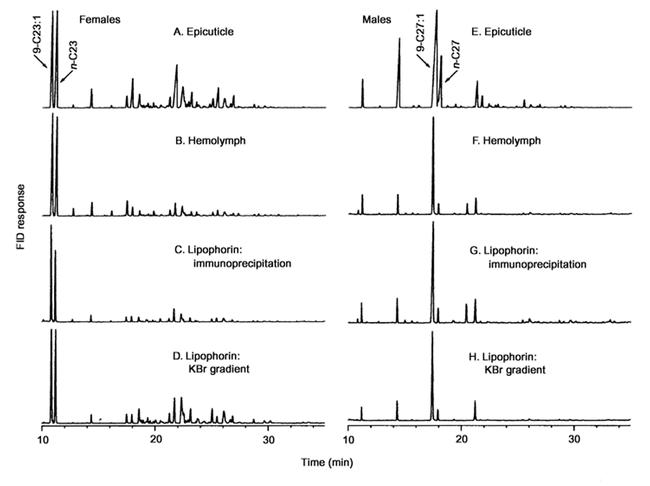
Epicuticular, hemolymph, and lipophorin hydrocarbons of Musca domestica females and males, as determined by gas chromatography (FID). Lipophorin was purified by immunoprecipitation and by KBr gradient ultracentrifugation.
To identify hydrocarbon carrier protein(s) we fractionated plasma proteins from 6-day-old females by KBr density-gradient ultracentrifugation. Most of the Z9-23:1 (94.8%) associated with two high-density lipoproteins, and only trace amounts were found in adjacent fractions and in a fat layer on top of the gradient (Figure 5). The less dense lipoprotein (density = 1.097 g ml−1) contained 62.6% of the hemolymph Z9-23:1, whereas the denser lipoprotein (density = 1.137 g ml−1) contained 32.2% of the hemolymph Z9-23:1. Under denaturing conditions, both lipoproteins from female hemolymph appeared identical and were composed of two apoproteins (not shown). The same two apoproteins, apolipophorin I (∼240 kD) and apolipophorin II (∼85 kD), were isolated from purified lipophorin of d = 1.097 from both males and females (Figure 6). We found no evidence of a smaller apolipophorin III, which is typically found in adult lepidopterans and orthopterans (Kanost et al., 1990; Van der Horst et al., 1993).
Figure 5.
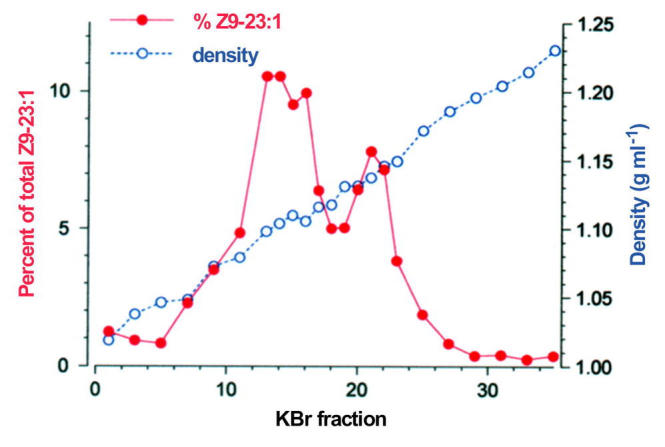
Fractionation of Musca domestica female hemolymph proteins by KBr gradient ultracentrifugation. Hemolymph was collected from 6-day-old adult females and the plasma subjected to fractionation. Fractions (400 µl) were collected from the top to the bottom of the gradient. The density of each fraction was determined gravimetrically. Z9-C23:1 content of each hemolymph KBr fraction was determined by GC and is represented as a percentage of the total Z9-C23:1 in all fractions.
Figure 6.
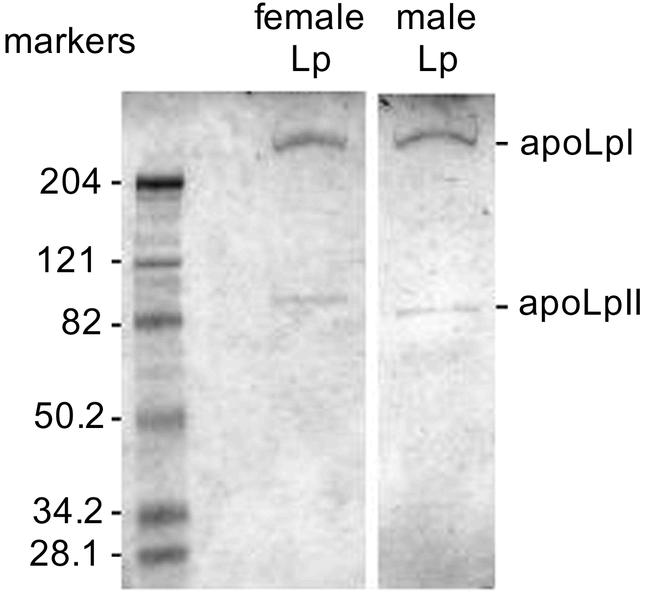
SDS-PAGE of the combined lipophorin fractions 12–17 of KBr gradients of hemolymph proteins from Musca domestica females and males. The positions of the molecular weight markers (kDa) are indicated on the left.
Discussion
In the last two decades tremendous progress has been realized in the chemical characterization of insect hydrocarbons, their biogenesis, information content as semiochemicals, and utility in chemotaxonomy (Blomquist and Dillwith, 1985; Blomquist et al., 1987, 1993; Lockey, 1988, 1991; Bagnères et al., 1990, 1996; Howard, 1993; Nelson, 1993; Nelson and Blomquist, 1995). Notwithstanding, early seminal investigations concerning the sites of hydrocarbon biosynthesis (see Romer, 1991) and hydrocarbon transport (see Chino, 1985) have not been extensively followed up. The pathway(s) that hydrocarbons traverse to storage depots, and from there to deposition sites have received relatively little attention. There is an increasing body of evidence that hydrocarbons, as well as relatively apolar pheromones derived from hydrocarbons, are shuttled by high density lipoproteins from biosynthetic to storage and deposition sites.
Our results with M. domestica support this model and indicate that in addition to covering the cuticular surface, hydrocarbons are found in various internal tissues as well. Moreover, because different proportions of the same hydrocarbons occur at different tissues, these results also suggest an active mechanism that selectively delivers certain subsets of hydrocarbons to specific tissues.
Hydrocarbons of the house fly and their tissue localization
Nelson et al. (1981) identified the cuticular hydrocarbons of 4-day-old female and male flies, raised at 25°C, and concluded that both sexes had essentially the same hydrocarbon components. Each female had 27.8 µg cuticular hydrocarbons but, in contrast to males, females had 14% alkenes and 37% methylalkanes; Z9-C23:1 represented 4.3% of the total hydrocarbons in the female. The presence of Z9-C23:1 and methyl-branched alkanes in females is consistent with their role as components of the sex pheromone (Uebel et al., 1976, 1978; Adams and Holt, 1987).
It appears that the amount of Z9-C23:1 on the cuticular surface of female flies varies considerably, probably because of differences in the rate of oogenesis as well as strain differences. In the house fly, copulation and the onset of pheromone production, as well as vitellogenesis occur around day 2 (Dillwith et al., 1983), resulting in dynamic changes in the pattern of hydrocarbon accumulation as the fly matures (Mpuru et al., 2001). Thus, while we recovered 3.46 µg of Z9-C23:1 (21.2 µg total hydrocarbons) from the cuticle of 6-day-old female flies, Dillwith and Blomquist (1982) found 4 µg on the cuticle of 5-day-old females, whereas Nelson et al. (1981) extracted 1.2 µg from the cuticle of 4-day-old females and Dillwith et al. (1983) found up to 1.5 µg per female. With a different strain, Ahmad et al. (1989) found > 1 µg Z9-C23:1 on the epicuticle of 6-day-old females.
Flies with stage-2 and stage-3 ovaries (< 36 h old) contained no detectable quantities of Z9-C23:1, C23 epoxide, or C23 ketone; however, the cuticular surface carried 4.6 µg of methyl-branched alkanes (Dillwith et al., 1983). A linear increase in Z9-C23:1 after stage 4 coincided with the production of ovarian ecdysteroids and with the progression of vitellogenesis (Dillwith et al., 1983). Dillwith et al. (1983) also showed an ontogenetic increase in the incorporation of injected sodium [1-14C]propionate into methyl branched hydrocarbons in adult female flies; synthesis of hydrocarbons increased more than 10-fold between days 0 and 5. Although the deposition of newly synthesized hydrocarbons on the cuticle (measured by incorporation of sodium [1-14C]acetate) was relatively constant in reproductive females between ovarian stages 2 and 10, there were dramatic changes in the types of hydrocarbons these females synthesized (Dillwith et al., 1983). Previtellogenic females made predominantly C27:1 and C29:1, whereas vitellogenic females made more Z9-C23:1. Ovariectomy eliminated this shift, and 20-hydroxyecdysone injections promoted it (Adams et al., 1984). It appears that ovarian ecdysteroids repress the synthesis of specific elongases, resulting in the accumulation of 24:1-CoAs, which are in turn converted to Z9-C23:1 (Tillman-Wall et al., 1992) by reduction to the aldehyde and a cytochrome P450-catalyzed decarboxylation (Reed et al., 1994, 1995; Blomquist et al., 1995).
Male hydrocarbons contain 59% unsaturated components, with large amounts of Z9-C27:1, no detectable Z9-23:1, and only 4% branched alkanes (Nelson et al., 1981). However, implantation of ovaries or 20-hydroxyecdysone injections into males induce the production of Z9-C23:1 and its metabolites, the epoxide and ketone (Blomquist et al., 1984), which is consistent with the idea that ecdysteroids modify the production of specific elongases rather than effect the chain length specificity of the enzymes that convert acyl-CoAs to aldehydes and then to hydrocarbon (Reed et al., 1994, 1995, 1996).
Our results are consistent with these patterns, showing an elevation in female epicuticular Z9-C27:1 until day 2, followed by a sharp decline in Z9-C27:1 and a concomitant increase in Z9-C23:1; by day 6 Z9-C23:1 accounts for 16.9% of the hydrocarbons and this value increases further as the female ages (data not shown). Although we found minor amounts of Z9-23:1 in 6-day-old males and only 13.2 µg of cuticular hydrocarbons (5.9 µg Z9-C27:1 compared with 20.3 µg in Nelson et al. [1981]), in general the results are consistent.
Lipophorin shuttles hydrocarbons and sex pheromones through the hemolymph
Ahmad et al. (1989) showed that the amount of Z9-23:1 found internally always exceeded that found on the epicuticle; in day 6 females 10 µg was found internally and only 1–2 µg externally. We found more similar amounts of Z9-23:1 internally and externally, 5.6 and 3.5 µg respectively, using the same strain of house fly (Figure 3, Table 1). Slight variations in diets and temperature may account for these differences. We also replaced the chloroform with hexane in the Bligh and Dyer (1959) extraction, because both solvents were equally efficient at extracting hydrocarbons (personal observation).
There is a tacit assumption that internal hydrocarbons largely represent hemolymph hydrocarbons. Yet, our results show that only 19.6% of internal hydrocarbons are in the hemolymph, and thus suggest that hydrocarbons may play important physiological roles in various internal tissues, in addition to their functions in water balance and as semiochemicals on the cuticular surface.
Notably, the ovaries contained 3.7 µg hydrocarbons, suggesting that female flies may provision their eggs with hydrocarbons that are later used by either the embryo or neonates, or both. A similar allocation of hydrocarbons to embryos has been demonstrated in the cockroach B. germanica (Schal et al., 1994; Young et al., 1999). The ovarian hydrocarbons in the house fly are significantly deficient in alkenes compared with other tissues. The relative abundance of ovarian Z9-23:1 was low (4.1% of total ovarian hydrocarbons) compared with the epicuticle (16.9%) and hemolymph (24.0%). Likewise, Z9-27:1 was 5-fold more prevalent on the cuticle than in the ovaries. The bulk of the ovarian hydrocarbons are therefore n-alkanes and methyl branched alkanes, which would confer on the embryos greater protection against desiccation (Gibbs et al., 1995; Gibbs, 1998). This is further compelling evidence that different hydrocarbons are selectively shuttled to different tissues. In support of this conclusion, the carcass contained the highest proportion of Z9-23:1 of any tissue (32%). Although the carcass may contain some adhering fat body, it presumably represents the oenocytes that synthesize hydrocarbons and the epidermis which hydrocarbons traverse as they are transported to the exterior.
Interestingly, de Bianchi et al. (1987) did not detect any lipophorin in either ovarian or egg soluble proteins by immunodiffusion assays. Yet, the ovaries contain hydrocarbons (Table 1). It is therefore likely that in M. domestica, lipophorin serves as a reusable shuttle, unloading its lipid cargo without entering the oocyte, as it does at muscle and epidermal cells (Chino, 1985).
In both females and males, hydrocarbons were found in the hemolymph and in both, the sex-specific pheromone was over-represented in the hemolymph compared to its proportion on the cuticle. In females, 24% of plasma hydrocarbons were Z9-C23:1 compared with 16.9% on the cuticle (P < 0.05). Likewise, in males, Z9-C27:1 constituted 74.3% of plasma hydrocarbons, but only 44.8% of cuticular hydrocarbons (P < 0.05). In females, the synthesis of Z9-C23:1 rapidly increases 2 days after adult emergence, and higher or lower amounts of this component in certain pools may also represent the rate of transport of newly synthesized hydrocarbon to a given location. Thus, if hydrocarbons are synthesized in the epidermal tissue and are first secreted into the hemolymph, by day 6, Z9-C23:1 should be a major component in the hemolymph, as was observed, as it is the major component being synthesized by day-6 females.
Both immunoprecipitation with larval lipophorin-specific antibodies and fractionation of hemolymph proteins by KBr gradient ultracentrifugation demonstrated that only high density lipophorins carried hydrocarbons. These results confirm Chino's (1985) hypothesis that some internal Z9-C23:1 may be associated with hemolymph lipophorin or with the abdominal integument that synthesized it. Oddly, however, in the adult fly, hydrocarbons appear to associate with two lipophorins of different densities (Figure 5). The lower density particle, which contains most of the Z9-C23:1 in hemolymph, corresponds to adult lipophorin, previously purified as a d = 1.106 g/ml lipoprotein (Bianchi et al., 1987). The identity of the more dense (d=1.137 g/ml) lipophorin is unknown. It may be a remnant of pupal lipophorin, reported by Bianchi et al. (1987) as having a density of 1.142 g/ml. Fly pupae contain about 3-fold more lipophorin than the adults (Bianchi et al., 1987), and it is possible that some pupal lipophorin is present in the adult hemolymph. Alternatively, newly synthesized M. domestica lipophorin has a higher density (1.131 g/ml), at least in vitro (Capurro and Bianchi, 1990), suggesting that the higher density lipophorin may be either newly synthesized or more lipid depleted. However, these particles would be expected to rapidly equilibrate in vivo to a single predominant density. Importantly, both lipophorins are immunologically reactive with the antibodies that we used and both have identical apoprotein composition. Therefore, they are unrelated to an unusual hydrocarbon-carrying larval-specific lipoprotein of the house fly, which is formed by four apoproteins (20 – 26 kDa), has no immunological similarity to larval or adult lipophorin, and disappears within 2 days after the pupal molt (Bianch and Capurro, 1991).
Early studies on endocrine regulation of pheromone production relied heavily on behavioral assays (Barth and Lester, 1973, Schal and Smith, 1990). Unfortunately, extractions of whole insects for behavioral assays fail to distinguish pheromone synthesis from release. More recent studies have employed isotope-tracer techniques that more closely measure pheromone synthesis (Prestwich and Blomquist, 1987). Yet, in light of the emerging model that some pheromones, especially hydrocarbons, are shuttled through the hemolymph, it appears that both approaches study the overall process of synthesis and release. The regulation of pheromone transport through the hemolymph and cuticle should receive more attention in future studies because it appears that these processes impart some specificity to the epicuticular semiochemical profile.
Acknowledgments
We thank Y. Fan, C. Gemeno, M. Trabalon, and L. Zurek for their critical comments on the manuscript, S. C. Johnson Wax for supplying the flies, and Bridget Deasy for technical assistance. This work was supported in part by the Blanton J. Whitmire Endowment, the W. M. Keck Center for Behavioral Biology at North Carolina State University, and by grants from the NSF (IBN-9817075 [CS] and IBN-9630916 [GJB]) and the Fondation Simone et Cino Del Duca (AGB).
Glossary
| Abbreviation: | |
|---|---|
| GC-MS | gas chromatography-mass spectroscopy |
| IS | internal standard |
| Z9-C23:1 | (Z)-9-tricosene, muscalure, female sex pheromone |
| Z9-C27:1 | (Z)-9-heptacosene, male-specific cuticular hydrocarbon |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
References
- Adams TS, Holt GG. Effect of pheromone components when applied to different models on male sexual behavior in the housefly Musca domestica. Journal of Insect Physiology. 1987;33:9–18. [Google Scholar]
- Adams TS, Dillwith JW, Blomquist GJ. The role of 20-hydroxyecdysone in housefly sex pheromone biosynthesis. Journal of Insect Physiology. 1984;30:287–294. [Google Scholar]
- Adams TS, Nelson DR, Blomquist GJ. Effect of endocrine organs and hormones on (Z)9-tricosene levels in the internal and external lipids of female houseflies, Musca domestica. Journal of Insect Physiology. 1995;41:609–615. [Google Scholar]
- Ahmad S, Mackay ME, Blomquist GJ. Accumulation of the female sex pheromone and its transfer and metabolism in the male housefly, Musca domestica L., during courtship and mating. Journal of Insect Physiology. 1989;35:775–780. [Google Scholar]
- Bagnères AG, Clément JL, Blum MS, Severson RF, Joulie C, Lange C. Cuticular hydrocarbons and defensive compounds of Reticulitermes flavipes (Kollar) and R. santonensis (Feytaud): Polymorphism and chemotaxonomy. Journal of Chemical Ecology. 1990;16:3213–3244. doi: 10.1007/BF00982094. [DOI] [PubMed] [Google Scholar]
- Bagnères AG, Lorenzi MC, Dusticier G, Turillazzi S, Clement JL. Chemical usurpation of a nest by paper wasp parasites. Science. 1996;272:889–892. doi: 10.1126/science.272.5263.889. [DOI] [PubMed] [Google Scholar]
- Barth RH, Lester LJ. Neuro-hormonal control of sexual behavior in insects. Annual Review of Entomology. 1973;18:445–472. doi: 10.1146/annurev.en.18.010173.002305. [DOI] [PubMed] [Google Scholar]
- Bianchi AG de, Capurro ML de. Musca domestica larval lipoprotein. Archives of Insect Biochemistry and Physiology. 1991;17:15–27. doi: 10.1002/arch.940170104. [DOI] [PubMed] [Google Scholar]
- Bianchi AG de, Capurro ML de, Marinotti O. Lipophorin in the larval and adult stages of Musca domestica. Archives of Insect Biochemistry and Physiology. 1987;6:39–48. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;39:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blomquist GJ, Adams TS, Dillwith JW. Induction of female sex pheromone production in male houseflies by ovary implants or 20-hydroxyecdysone. Journal of Insect Physiology. 1984;30:295–302. [Google Scholar]
- Blomquist GJ, Dillwith JW. 1985 Cuticular Lipids. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 3. 117–154.Oxford: Pergamon Press. [Google Scholar]
- Blomquist GJ, Dillwith JW, and Adams TS. 1987 Biosynthesis and endocrine regulation of sex pheromone production in Diptera. In: Prestwich GD, Blomquist GJ, editors. Pheromone Biochemistry. 217–250.New York: Academic Press. [Google Scholar]
- Blomquist GJ, Tillman-Wall JA, Guo L, Quilici D, Gu P, and Schal C. 1993 Hydrocarbon and hydrocarbon derived sex pheromones in insects: biochemistry and endocrine regulation. In: Stanley-Samuelson DW, Nelson DR, editors. Insect Lipids: Chemistry and Biology. 317–351.Lincoln: University of Nebraska Press. [Google Scholar]
- Blomquist GJ, Tillman JA, Reed JR, Gu P, Vanderwel D, Choi S, Reitz RC. Regulation of enzymatic activity involved in sex pheromone production in the housefly, Musca domestica. Insect Biochemistry and Molecular Biology. 1995;25:751–757. doi: 10.1016/0965-1748(95)00015-n. [DOI] [PubMed] [Google Scholar]
- Capurro ML de, Bianchi AG de. Larval Musca domestica lipophorin biosynthesis. Comparative Biochemistry and Physiology. 1990;97B:655–659. [Google Scholar]
- Carlson DA, Mayer MS, Silhacek DL, James JD, Beroza M, Bierl BA. Sex attractant pheromone of the housefly: Isolation, identification and synthesis. Science. 1971;174:76–78. doi: 10.1126/science.174.4004.76. [DOI] [PubMed] [Google Scholar]
- Chase J, Touhara K, Prestwich GD, Schal C, Blomquist GJ. Biosynthesis and endocrine control of the production of the German cockroach sex pheromone 3,11-dimethylnonacosan-2-one. Proceedings of the National Academy of Science USA. 1992;89:6050–6054. doi: 10.1073/pnas.89.13.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino H. 1985 Lipid transport: biochemistry of hemolymph lipophorin. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 10. 115–135.Oxford: Pergamon Press. [Google Scholar]
- Chino H, Kitazawa K. Diacylglycerol-carrying lipoprotein of hemolymph of the locust and some insects. Journal of Lipid Research. 1981;22:1042–1052. [PubMed] [Google Scholar]
- Dillwith JW, Blomquist GJ. Site of sex pheromone biosynthesis in the female housefly, Musca domestica. Experientia. 1982;38:471–473. [Google Scholar]
- Dillwith JW, Blomquist GJ, Nelson DR. Biosynthesis of the hydrocarbon components of the sex pheromone of the housefly, Musca domestica L. Insect Biochemistry. 1981;11:247–253. [Google Scholar]
- Dillwith JW, Adams TS, Blomquist GJ. Correlation of housefly sex pheromone production with ovarian development. Journal of Insect Physiology. 1983;29:377–386. [Google Scholar]
- Gibbs A. Water-proofing properties of cuticular lipids. Amer Zool. 1998;38:471–482. [Google Scholar]
- Gibbs A, Kuenzli M, Blomquist GJ. Sex- and age-related changes in the biophysical properties of cuticular lipids in the housefly, Musca domestica. Archives of Insect Biochemistry and Physiology. 1995;29:87–97. doi: 10.1002/arch.940290108. [DOI] [PubMed] [Google Scholar]
- Gu X, Quilici D, Juarez P, Blomquist GJ, Schal C. Biosynthesis of hydrocarbons and contact sex pheromone and their transport by lipophorin in females of the German cockroach Blattella germanica. Journal of Insect Physiology. 1995;41:257–267. [Google Scholar]
- Howard RW. 1993 Cuticular hydrocarbons and chemical communication. In: Stanley-Samuelson DW, Nelson DR, editors. Insect Lipids: Chemistry and Biology. 176–226.Lincoln: University of Nebraska Press. [Google Scholar]
- Katagiri C, de Kort S. Characterization of Colorado potato beetle lipophorin: a hydrocarbon-rich diacylglycerol-poor lipophorin. Comparative Biochemistry and Physiology. 1991;100B:149–152. [Google Scholar]
- Katase H, Chino H. Transport of hydrocarbons by the lipophorin of insect hemolymph. Biochimistry Biophysics Acta. 1982;710:341–348. [Google Scholar]
- Katase H, Chino H. Transport of hydrocarbons by haemolymph lipophorin in Locusta migratoria. Insect Biochemistry. 1984;14:1–6. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lockey KH. Lipids of the insect cuticle: origin, composition and function. Comparative Biochemistry and Physiology. 1988;89B:595–645. [Google Scholar]
- Lockey KH. Insect hydrocarbon classes: implications for chemotaxonomy. Insect Biochemistry. 1991;21:91–97. [Google Scholar]
- Mpuru S, Blomquist GJ, Schal C, Kuenzli M, Dusticier G, Roux M, Bagnères AG. Effect of age and sex on the production of internal and external hydrocarbons and pheromones in the housefly, Musca domestica. Insect Biochemistry and Molecular Biology. 2001;31:139–155. doi: 10.1016/s0965-1748(00)00098-9. [DOI] [PubMed] [Google Scholar]
- Nelson DR. 1993 Methyl-branched lipids in insects. In: Stanley-Samuelson DW, Nelson DR, editors. Insect Lipids: Chemistry and Biology. 271–315.Lincoln: University of Nebraska Press. [Google Scholar]
- Nelson DR, Blomquist GJ. 1995 Insect Waxes. In: Hamilton RJ, editor. Waxes: Chemistry, Molecular Biology and Functions. 1–90.England: WW Christie, The Oily Press. [Google Scholar]
- Nelson DR, Dillwith JW, Blomquist GJ. Cuticular hydrocarbons of the house fly, Musca domestica. Insect Biochemistry. 1981;11:187–197. [Google Scholar]
- Nishida R, Fukami H. Female sex pheromone of the German cockroach, Blattella germanica. Memoirs of the College of Agriculture, Kyoto University. 1983;122:1–24. [Google Scholar]
- Pho DB, Pennanec'h M, Jallon JM. Purification of adult Drosophila melanogaster lipophorin and its role in hydrocarbon transport. Archives of Insect Biochemistry and Physiology. 1996;31:289–303. doi: 10.1002/(SICI)1520-6327(1996)31:3<289::AID-ARCH4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Prestwich GD, Blomquist GJ. 1987 Pheromone Biochemistry. 584. pp. New York: Academic Press. [Google Scholar]
- Reed JR, Vanderwel D, Choi S, Pomonis JG, Reitz RC, Blomquist GJ. Unusual mechanism of hydrocarbon formation in the housefly: cytochrome P450 converts aldehyde to the sex pheromone component (Z)-9 tricosene and CO2. Proceedings of the National Academy of Science USA. 1994;91:10000–10004. doi: 10.1073/pnas.91.21.10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JR, Quilici DR, Blomquist GJ, Reitz RC. Proposed mechanism for the cytochrome(c)P450 catalyzed conversion of aldehydes to hydrocarbons in the housefly, Musca domestica. Biochemistry. 1995;34:26221–26227. doi: 10.1021/bi00049a038. [DOI] [PubMed] [Google Scholar]
- Reed JR, Hernandez P, Blomquist GJ, Feyereisen R, Reitz RC. Hydrocarbon biosynthesis in the housefly, Musca domestica: substrate specificity and cofactor requirement of P450hyd. Insect Biochemistry and Molecular Biology. 1996;26:267–276. [Google Scholar]
- Romer F. 1991 The oenocytes of insects: differentiation, changes during molting, and their possible involvement in the secretion of molting hormone. In: Gupta AP (editor). Morphogenetic Hormones of Arthopods: Roles in Histogenesis, Organogenesis, and Morphogenesis. 542–567.New Brunswick: Rutgers University Press. [Google Scholar]
- Schal C, Smith A. 1990 Neuroendocrine regulation of pheromone synthesis and release in cockroaches. In: Huber I, Rao BR, Masler EP, editors. Cockroaches As Models for Neurobiology: Applications in Biomedical Research. Vol. 2. 179–200.Boca Raton: CRC Press. [Google Scholar]
- Schal C, Burns EL, Jurenka RA, Blomquist GJ. A new component of the sex pheromone of Blattella germanica (Dictyoptera: Blattellidae), and interaction with other pheromone components. Journal of Chemical Ecology. 1990;16:1997–2008. doi: 10.1007/BF01020511. [DOI] [PubMed] [Google Scholar]
- Schal C, Gu X, Burns EL, Blomquist GJ. Patterns of biosynthesis and accumulation of hydrocarbons and contact sex pheromone in the female German cockroach, Blattella germanica. Archives of Insect Biochemistry and Physiology. 1994;25:375–391. doi: 10.1002/arch.940250411. [DOI] [PubMed] [Google Scholar]
- Schal C, Sevala VL, Cardé RT. Novel and highly specific transport of a volatile sex pheromone by hemolymph lipophorin in moths. Naturwissenchaften. 1998a;85:339–342. [Google Scholar]
- Schal C, Sevala VL, Young H, Bachmann JAS. Synthesis and transport of hydrocarbons: Cuticle and ovary as target tissues. American Zoologist. 1998b;38:382–393. [Google Scholar]
- Sevala VL, Bachmann JAS, Schal C. Lipophorin: a hemolymph juvenile hormone binding protein in the German cockroach, Blattella germanica. Insect Biochemistry and Molecular Biology. 1997;27:663–670. [Google Scholar]
- Sevala V, Shu S, Ramaswamy SB, Schal C. Lipophorin of female Blattella germanica: Characterization and relation to hemolymph titers of juvenile hormone and hydrocarbons. Journal of Insect Physiology. 1999;45:431–441. doi: 10.1016/s0022-1910(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Sevala V, Bagnères A-G, Kuenzli M, Blomquist GJ, Schal C. Cuticular hydrocarbons of the termite Zootermopsis Nevadensis (Hagen): Caste differences and role of lipophorin in transport of hydrocarbons and hydrocarbon metabolites. J Chem Ecol. 2000;26:765–789. [Google Scholar]
- Shapiro JP, Keim PS, Law JH. Structural studies on lipophorin, an insect lipoprotein. Journal of Biological Chemistry. 1984;259:3680–3685. [PubMed] [Google Scholar]
- Tillman-Wall JA, Vanderwel D, Kuenzli ME, Reitz RC, Blomquist GJ. Regulation of sex pheromone biosynthesis in the housefly, Musca domestica: Relative contribution of the elongation and reductive step. Archives of Insect Biochemistry and Physiology. 1992;299:92–99. doi: 10.1016/0003-9861(92)90248-u. [DOI] [PubMed] [Google Scholar]
- Uebel EC, Sonnet PE, Miller RW. House fly sex pheromone: Enhancement of mating strike activity by combination of (Z)-9-tricosene with branched saturated hydrocarbons. Environmental Entomology. 1976;5:905–908. [Google Scholar]
- Uebel EC, Schwarz M, Lusby WR, Miller RW, Sonnet PE. Cuticular non hydrocarbons of the female housefly and their evaluation as mating stimulants. Lloydia-The Journal of Natural Products. 1978;41:63–67. [Google Scholar]
- Young HP, Bachmann JAS, Sevala V, Schal C. Site of synthesis, tissue distribution, and lipophorin transport of hydrocarbons in Blattella germanica (L) nymphs. Journal of Insect Physiology. 1999;45:305–31. doi: 10.1016/s0022-1910(98)00128-0. [DOI] [PubMed] [Google Scholar]


